Abstract
The polymersome, a fully synthetic cell mimetic, is a tunable platform for drug delivery vehicles to detect and treat disease (theranostics). Here, we design a leuko-polymersome, a polymersome with the adhesive properties of leukocytes, which can effectively bind to inflammatory sites under flow. We hypothesize that optimal leukocyte adhesion can be recreated with ligands that mimic receptors of the two major leukocyte molecular adhesion pathways, the selectins and the integrins. Polymersomes functionalized with sialyl Lewis X and an antibody against ICAM-1 adhere avidly and selectively to surfaces coated with inflammatory adhesion molecules P-selectin and ICAM-1 under flow. We find that maximal adhesion occurs at intermediate densities of both sialyl Lewis X and anti-ICAM-1, owing to synergistic binding effects between the two ligands. Leuko-polymersomes bearing these two receptor mimetics adhere under physiological shear rates to inflamed endothelium in an in vitro flow chamber at rate 7.5 times higher than to uninflamed endothelium. This work clearly demonstrates that polymersomes bearing only a single ligand bind less avidly and with lower selectivity, thus suggesting proper mimicry of leukocyte adhesion requires contributions from both pathways. This work establishes a basis for the design of polymersomes for targeted drug delivery in inflammation.
Keywords: ICAM-1, P-selectin, sialyl Lewis X, drug delivery, theranostics
Introduction
Inflammation is the process by which the body recruits and activates leukocytes at sites of infection, but an overzealous inflammatory response can create deleterious physiological effects. Therefore significant effort has been made toward developing targeted therapies to treat inflammation 1, 2. The two major classes of adhesion molecules upregulated during inflammation, adhesion molecules and selectins, are natural targets for diagnostic and therapeutic particles 3–7, but particles must be designed to bind sites of inflammation selectively 8. Intercellular adhesion molecule-1 (ICAM-1), which is upregulated during inflammation, is expressed at low levels throughout uninflamed endothelium 9, so targeting this molecule alone with a high affinity probe would result in binding to healthy endothelium. P-selectin-mediated adhesion plays a major role in leukocyte recruitment 10, and unlike ICAM-1, is only present in inflamed tissues. Selectin mediated bonds, however, are fast, weak catch-slip interactions that do not typically mediate firm adhesion by themselves 11–13. In this paper, we explore the design of a colloidal mimetic of leukocytes that combines two molecules and thus is designed to preferentially bind to inflamed tissues that express P-selectin and upregulate ICAM-1 with specificity and yield.
Because blood cells, such as neutrophils, lymphocytes, and platelets, have evolved to use two adhesion molecules simultaneously, one can question if there is an inherent advantage for using two adhesion molecules rather than one. Our laboratory previously showed that the simultaneous targeting of both selectins and ICAM-1 results in super adhesion of porous polymeric particles compared with particles targeting one molecule alone 14. For example, it was shown that firm adhesion to surfaces coated with P-selectin and ICAM-1 could be greatly enhanced with particles that bore the same concentration of anti-ICAM-1 antibody if sialyl Lewis X (sLex), a carbohydrate that mediates rolling adhesion, was added to the particles. The concept that rolling can mechanistically facilitate firm adhesion has also been predicted by computer simulations of adhesion in our laboratory 15.
Here, we describe the preparation and performance of leuko-polymersomes, in which two adhesion molecules are attached to a polymersome. Polymersomes, fully synthetic and biocompatible analogs of liposomes assembled from block co-polymers, are an ideal choice as the underlying colloid for a leukocyte mimetic. Polymersomes have been used as an in vivo imaging agent and drug carrier 16–20. Polymersomes are significantly stronger and have much thicker membranes than liposomes 21, allowing them to carry large amounts of hydrophobic cargo 22, 23 within the membrane core, as well as aqueously soluble agents within the vesicle lumen. Ligands, such as antibodies 24 and peptides 25, can be attached to the exterior of these vesicles without destruction of the vesicular structure. Storage of large proteins and activated release of contents 26–28 have also been demonstrated in polymersome systems.
In this work, we show that the ratio of rolling and firm adhesion ligands on the polymersome surface can be tuned and that we can adjust the adhesivity of a leuko-polymersome to a specific substrate by adjusting this ratio of ligands on the vesicle surface. We demonstrate how our tunable design allows us to increase the adhesivity of a vesicle to endothelium bearing inflammatory molecules while simultaneously decreasing the adhesivity of these particles for uninflamed endothelium. Finally, we show that one of our optimal leuko-polymersome constructs binds selectively to inflamed HUVECs compared to uninflamed cells in vitro under hydrodynamic flow.
Materials and Methods
Polymersome Assembly
The polymersomes were prepared as described previously 29. Briefly, the biocytin terminated copolymer (PEO(1300)-b-PBD(2500), Polymer Source, Inc., Montreal Quebec) and the fluorophores (PZn2) 30 were dissolved in methylene chloride at a 7.5:1 molar ratio of polymer to fluorophore. The solution was then deposited onto a roughened Teflon square and dried overnight under vacuum. 2 mL of 290 mOsm sucrose was then added to a glass vial containing the film; the vial was then sealed and allowed to hydrate at 65°C for 24 hours. Solutions were vortexed after heating yielding a solution of vesicles ranging in size from approximately 800 nm to 30 μm diameter. Vesicles used for HUVEC experiments were hydrated with 2 mL of 290 mOsm sucrose + 20 μL 10 mg/ml Alexa Fluor-488-labeled 3000 Da dextran (Invitrogen, Carlsbad, CA).
Vesicles used as size standards for flow cytometry were further prepared with serial extrusion using a Liposofast Basic hand-held extruder equipped with 5 μm, 1 μm, and 400 nm polycarbonate membranes (Avestin Inc., Ottawa, Ontario). Relative concentrations of polymersomes were determined based on the Beer’s Law and established extinction coefficient of PZn2 at 485 and 708 nm (εPZn2(485) = 294 875 M−1 cm−1, εPZn2(708) = 59 000 M−1 cm−1) 22.
Synthesis of Biocytin Modified Polymer
The terminal end of the PEO block was modified to display biocytin through a two step synthesis previously described 29. Briefly, 4-fluoro-3-nitrobenzoic acid was attached to the hydroxyl polymer terminus through an esterification in methylene chloride. After HPLC purification, biocytin was attached to the modified polymer through a nucleophilic aromatic substitution in 50% THF/50% DI water. This polymer was purified after the reaction using HPLC separation. Characterization of the final polymer using NMR showed that 88% of the polymer was modified with biocytin.
Association of Ligands and Separation of Free Ligand
1 mL volumes of polymersomes post assembly (approx. 0.1 mM polymer) were diluted in 9 mL PBS + 1% BSA. 50 μL NeutrAvidin at 10 mg/ml in DI water were added to solution, and vesicles were allowed to bind 1 hour in tube rotator at RT. Vesicles were then separated twice. First by adding a cushion (20% Optiprep density gradient medium, Sigma-Aldrich, 80% 270 mOsm sucrose) to the bottom of a centrifuge tube containing diluted polymersomes. Tubes spun 30 min at 7500 rpm and 1–2 conc. vesicles removed. 9 mL density gradients ranging from 100% Optiprep/sucrose to 100% PBS were made. Conc. vesicles were separated again at 7500 rpm for 30 min. Vesicles diluted to 10 mL in PBS + 1% BSA, and α-ICAM-1 and/or sLex was added to solution. Binding and separation steps were identical to the steps described for association/separation of NeutrAvidin. Vesicles were dialyzed in 2L DPBS + Mg2+ + Ca2+ with 2 changes of buffer over a period of 36 hours before adhesion experiments. Concentrations were verified using absorbance of PZn2 in membrane bilayer prior to experiments. Vesicles used for HUVEC experiments were diluted to approx. 4.8 × 106 particles/mL for all experiments; vesicles were diluted at a ratio of 2:1 media to dialyzed vesicles using L-15 media (Lonza, Walkersville, MD).
Flow Cytometry
Ligand coated vesicles were assembled according to method described in this work. 0.5 mL vesicles were diluted to 5 mL in PBS + 1% BSA and either 20 μL of 0.5 mg/ml mouse anti-human CD15s (BD Pharmingen) or 20 μL of 0.5 mg/ml FITC-rat anti-mouse Ig, κ light chain monoclonal antibody (BD Pharmingen) added to the solution and allowed to bind 1 hr in tube rotator. For sLex vesicles labeled with anti-human CD15s, vesicles were separated once in centrifuge using Optiprep/sucrose cushion. Vesicles were immediately diluted to 5 mL in ice cold PBS + 1% BSA, and 20 μL of 0.5 mg/ml FITC-rat anti-mouse Ig, κ light chain monoclonal antibody (BD Pharmingen) added to the solution, and allowed to bind 1 hr in tube rotator at 4°C. Vesicles were separated again using Optiprep/sucrose cushion.
Flow cytometry was performed on a Guava EasyCyte flow cytometer (Guava Technologies, Hayward CA., CyteSoft version 3.6 software) immediately after separation. Samples diluted to approx. 500 vesicles/μL in PBS, and 20,000 points were collected for each sample. Extruded samples of known size distributions analyzed to calibrate forward scatter with approx. vesicle sizes. Quantum FITC MESF High Level beads (Bang’s Laboratories, Inc., Fishers, IN) used to calibrate fluorescent signal. Analysis performed using FlowJo software.
Size distributions of extruded (NeutrAvidin coated) vesicle samples were determined using dynamic light scattering (DLS). Particles were mixed well in low-volume disposable cuvettes using a pipette. Three runs of 13–15 measurements on a Zetasizer Nano-S Instrument (Malvern Instruments, Southborough, MA). These runs were averaged using accompanying DTS software (Malvern Instruments, Southborough, MA), and an intensity transformation was used to determine particle size distribution.
Confocal Light Scanning Microscopy (CLSM)
Experiments carried out on an Olympus Fluoview FV1000 confocal microscope (Center Valley, PA), equipped with a UPLFLN 40x oil objective lens. 488 nm laser was used to image FITC and Alexa-488 labeled proteins, and 633 nm laser was used to image PZn2-loaded polymersome membrane. Typical scan speeds were between 10 μs/pixel and 20 μs/pixel, and 3–4 scans using a Kahlman filter were used to acquire final images.
Adhesive Substrate Preparation
Preparation of receptor coated substrates described previously 29. Briefly, double well, rectangular, flexiperm gaskets (Sigma-Aldrich, Vivascience) placed on untreated polystyrene slides. Surfaces then washed with 0.1 M NaHCO3 buffer adjusted to pH 9.2 (binding buffer). 375 μL of ICAM-1/Fc at either 0, 5, or 10 μg/mL in binding buffer added to lower well, and 375 μL binding buffer added to upper well. Receptor allowed to bind on laboratory rocker for 2 hr at RT. Slides placed in refrigerator (4°C) overnight. Substrates allowed to warm on rocker at RT 1 hr. Buffer then aspirated, and 1 mL binding buffer used to wash each well (except 100% ICAM-1 surface). 375 μL P-selectin/Fc at 10 μg/mL in binding buffer added to bottom wells, and 375 μL binding buffer added to top wells (except 100% ICAM-1 surface). Receptor was allowed to bind 2 hr on laboratory rocker at RT.
Substrate Site Density Determination
Surface ELISA method used to characterize site densities of P-selectin/Fc and ICAM-1/Fc. Flexiperm gaskets placed in wells of 12-well untreated polystyrene plates. Substrates prepared as previously described using 165 μL receptor solution rather than 375 μL. After substrate prep, each well then washed 2X with 1 mL ice-cold PBS, and gaskets were removed. Each well then washed 1X with 2 mL ice-cold PBS. 2 mL ice-cold StartingBlock protein blocking buffer (Pierce Biotechnology) added to each well and aspirated. Wells washed again 2X with 2mL ice-cold PBS. 0.5 mL of 5 μg/mL antibody in PBS (mouse anti-human P-selectin monoclonal ab or mouse anti-human ICAM-1 monoclonal ab, R&D Systems) added to each well. Antibodies allowed to bind 1 hr on laboratory rocker at 4°C. Antibody solution aspirated, and each well washed with ice-cold PBS 3X (1 mL 1X, 2 mL 2X). 0.5 mL of HRP rat-anti-mouse IgG monoclonal antibody (50:1 dilution in PBS, BD Pharmingen) added to each well. Antibody allowed to bind 1 hr on laboratory rocker at 4°C. Buffer aspirated, and surfaces washed 3X (1 mL PBS 1X, 2 mL PBS 2X). Added 100 μL PBS + 300 μL TMB substrate (TMB Substrate Kit, Pierce Biotechnology) to each well. Reactions allowed to proceed 10 min 20 sec on laboratory rocker at RT. Quenched reaction with 1 mL 1N H2SO4. Read absorbance on plate reader (Tecan Infinite M200 Männedorf, Switzerland) at 450 nm. 12-well tissue culture treated polystyrene plates were used to create calibration curve. Wells blocked with 2 mL StartingBlock then washed with 2 mL PBS per well 4X. 100 μL HRP biotin (Pierce Biotechnology) added to wells at concentrations between 0 and 0.1 μg/mL. 300 μL TMB substrate was added to each well. Reactions allowed to proceed 10 min 20 sec on a laboratory rocker at RT. Reactions then quenched with 1 mL 1N H2SO4. Read absorbance on plate reader at 450 nm.
Cell Culture and Activation
Human umbilical vein endothelial cells (HUVECs) were cultured in EGM Endothelial Growth Media (Lonza, Walkersville, MD) supplemented with 0.4% bovine brain extract (BBE) with heparin, 0.1% h-EGF, 0.1% hydrocortisone, 0.1% gentamicin sulfate (GA-1000), and 2% fetal bovine serum (FBS). Cells were maintained in plastic culture flasks at 37°C in a humidified atmosphere containing 5% CO2 in air and subcultured when the flasks were 70% to 90% confluent. HUVECs were used between passages 5–7. Cells were activated by incubating with recombinant TNF-α (R&D Systems) at 10 ng/ml for 6 hours. This incubation causes HUVEC cells to upregulate expression of inflammatory markers 31.
ICAM Immunostaining and Upregulation Quantification
HUVECs were seeded into 10 wells of a black 96 well plate (PerkinElmer, Bridgeville, PA) at a density of 10,000 cells per well and allowed to grow to a confluent monolayer for two days. Cells in 5 wells were then treated as described with recombinant TNF-α (R&D Systems) at 10 ng/ml for 6 hours, and the media was changed on the 5 control wells. The wells were then washed with PBS and fixed with 4% PFA for 15 minutes. The wells were blocked with 2% BSA for 30 minutes at 37°C and then incubated with 10 μg/ml mouse α-ICAM for 15 minutes at 37°C. The cells were washed and incubated with goat anti-mouse IgG PE-Cy7 for 15 minutes at 37°C (Santa Cruz Biotechnology, Santa Cruz, CA). The fluorescence intensity was then read on a LICOR Odyssey and analyzed with the LICOR Odyssey analysis software version 2.1; background fluorescence was subtracted from all signals. HUVECs treated with recombinant TNF-α had an adjusted fluorescence intensity of 0.946, and the untreated HUVECs had an adjusted fluorescence intensity of 0.748. A Student t-test with a p-value = 0.022 shows that incubation with TNF-α statistically increases the level of ICAM expressed by HUVECs above the basal level.
Monolayer Assembly
A culture surface was created by attaching a 30×5 mm flexiPERM slide to a clean 75×25 mm microscope slide. The chamber was then filled with 0.5 ml of 0.1 mg/ml fibronectin (Sigma-Aldrich, St. Louis, MO) in PBS and incubated at room temperature for 1.5 hours. The fibronectin solution was then aspirated, and 90,000 cells/chamber were plated and incubated for 2 days to create a confluent monolayer.
Parallel Plate Flow Assay
Substrates blocked with SuperBlock protein blocking buffer (Pierce Biotechnology), 1mL per well 3X. A parallel-plate flow chamber similar to those described previously 14, mounted on the stage of a Nikon Diaphot inverted microscope (Nikon, Tokyo, Japan) used for laminar flow assays. Flow initiated using a syringe pump (Harvard Apparatus, South Natick, MA), and positions in the flow chamber monitored using Nikon stage calipers (Nikon Inc., Melville, NY). Experiments recorded using a Cool-Snap HQ cooled CCD camera and Sony SVO-9500MD S-VHS recorder (Sony Medical Systems, Montvale, NJ). For each experiment, chamber height (gap width) and flow rate were measured to calculate the wall shear stress obtained. Shear rate of 130 s−1 was used for all experiments because this is representative of flow within postcapillary venules 32. Vesicle interactions with the surface observed along the center axis of the flow chamber and recorded for later analysis. Experiments with HUVEC monolayers performed using the same flow chambers and microscope (cells maintained at 37°C in L-15 media buffered for use in non-CO2 equilibrated environments). 4 or 5 fields of view chosen for each experiment, and confluent regions of cells (at similar locations within the chamber for each experiment) were chosen. Fluorescent imaging was used so that vesicles could be easily identified. Images collected every 6 seconds.
Image Analysis and Particle Tracking
For HUVEC experiments, firmly adhered vesicles were counted. The particle tracking algorithm (used for all adhesion experiments except those performed on cell monolayers) is based in the MATLAB software suite, using the image processing toolbox. Two minute avi files were created for each location in the flow chamber observed during an adhesion experiment: three locations on the functionalized surface, two locations on the control surface. Particles were identified in each frame by first thresholding to create a binary image and then using intrinsic MATLAB functions to count and determine properties for each particle (i.e. diameter, eccentricity, solidity). Each particle in frame n was then compared to each particle in frame n+1 to construct trajectories and classify the type of movement (firm adhesion, rolling, transient adhesion) based on the particle size and free stream velocity at the vesicle centroid. After particle tracking was complete, broken trajectories were reconstructed and noise was filtered by eliminating any particle that interacted for less than 30 frames (1 second) or did not roll or firmly adhere during the trajectory. Firm binding is classified as the centroid of a particle moving less than 1.5 pixel between frames for 150 consecutive frames or more (5 seconds). Stable rolling is classified as a particle centroid moving more than 1.5 pixel but less than 45% the free stream velocity at the particle centroid (calculated based on Poiseuille flow) for greater then 10% of the entire trajectory of the particle. Transient rolling is classified as a particle that interacts for at least 30 frames but roll for less then 10% of the trajectory of the particle. Rolling + binding vesicles are classified as particle that meets the criteria for firm binding and makes rolling movements during the trajectory.
Results and Discussion
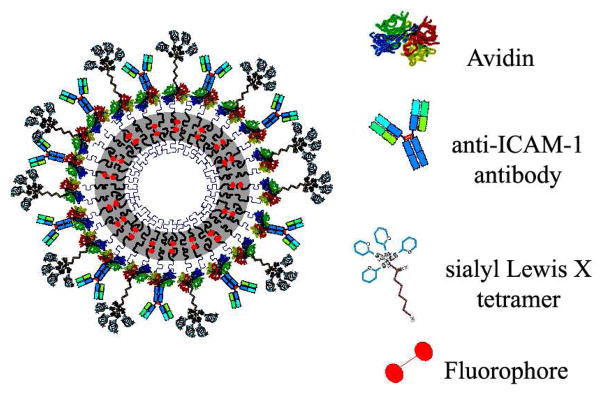
Ligand coated emissive polymersomes22 were built by first assembling vesicles from biotin-terminated block copolymer and PZn2 fluorophore 30, then saturating the surfaces with NeutrAvidin (referred to as ‘avidin’) and biotinylated ligands in subsequent steps, as illustrated in Figure 1. A previously published reaction – an esterification followed by an aromatic substitution followed (supplemental data) – is used to attach biotin to the hydrophilic (polyethylene-oxide) end of the copolymer 24, 29. The final reaction efficiency was determined to be 88% by NMR. Aliquots of this product (biotin-polyethyleneoxide-b-polybutadiene) was used, without further modification or blending, for all experiments in order to ensure consistency between samples, and synthesis of a biotin-terminated copolymer allows for the assembly of an effectively fully biotinylated polymersome surface. Confocal light scanning microscopy was used to confirm the presence of both avidin and targeting ligand on vesicle surfaces, and there was no evidence of ligand clustering when both ligands were attached to the vesicle surface (supplemental data).
Fig. 1.
Schematic illustrating the avidin-coated polymersome used for all experiments. Anti-ICAM-1 ab and sLex polymer were titrated onto the surface of this vesicle at varied ratios. Use of avidin-coated vesicles ensures a similar particle size distribution of vesicles for all experiments, and super-saturating conditions during association of ligands ensures similar surface site densities for all experiments.
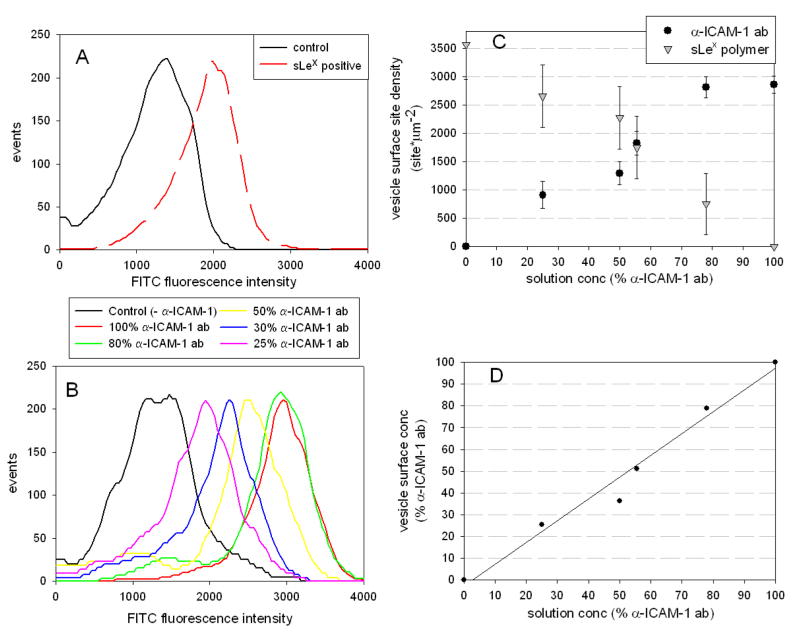
Quantitative surface site-density measurement of the targeting ligands sialyl Lewis X (PSGL-1 analog) or anti-ICAM-1 antibody (LFA-1 analog) on avidin-coated vesicles was determined using flow cytometry. First, the total number of accessible biotin-binding pockets on avidin-coated vesicles was determined by binding FITC-tagged 3000 Da biotinylated dextran to a population of vesicles and comparing to calibrated fluorescent standards 33. Second, a FITC-labeled κ-light chain specific monoclonal antibody was bound to 100% α-ICAM-1 coated vesicles to determine the site density of α-ICAM-1 antibody on the vesicle surfaces. These measurements yield site densities of 3560 ± 760 sites/μm2 and 2890 ± 150 sites/μm2 for the 100% dextran and 100% α-ICAM-1 antibody-coated polymersomes, respectively. This density is approximately 50% the maximum possible biotin-binding sites, based on closest packing of avidin molecules on a flat surface 34. The maximum sLex site density is approximated to be 3560 ± 760 sites/μm2, the measured maximum site density of dextran. Sialyl Lewis X site densities for α-ICAM-1/sLex vesicles were calculated using site density balances (Figure 2c) – while we saw positive anti-sLex antibody signals (Figure 2a), we were unable to achieve strong enough binding of antibodies to sLex to specifically quantify the number of sLex sugars on the vesicle surface. These maximal achievable site densities are far greater than those measured for activated leukocytes, which have site densities of approximately 50 – 350 sites/μm2 LFA-1 and 60 – 200 sites/μm2 PSGL-1 35–37. Having an excess in ligand density on our leuko-polymersome is warranted since leukocytes can cluster adhesion ligands which leads to more effective adhesion than uniformly-coated cells would achieve 38. Histograms of FITC signals from populations of α-ICAM-1/sLex vesicles in which the α-ICAM-1 antibodies were labeled are shown in Figure 2b; this figure clearly shows an increased signal from the secondary antibody against α-ICAM-1 as the amount of α-ICAM-1 on the vesicle is increased. The average total site density of each population is reported as the site density for each mixed ligand vesicle evaluated, shown in Figure 2c. These results confirm that multiple ligands can be attached to the surface of these vesicles and that the ratio of α-ICAM-1 and sLex on the vesicle surface can be set using the concentration of each biotinylated ligand in solution (Figure 2d). A histogram showing the size distribution of vesicles that adhered in these experiments is shown in the supplemental data (Figure S2).
Fig. 2.
FITC intensity histogram for a fully sLex coated polymersome labeled with a primary monoclonal mouse antibody against sLex and a secondary FITC-labeled κ-light chain specific antibody confirms sLex is present on vesicle surfaces (A). FITC intensity histogram for various α-ICAM-1 + sLex coated vesicles tagged with a FITC-labeled κ-light chain specific antibody. Results show an increased fluorescent signal corresponding to increased concentrations of α-ICAM-1 on polymersome surfaces (B). Site densities for these different populations were determined based on the mean fluorescence intensities of the various samples and a calibration curve based on FITC-coated beads (C), and these site densities show that the ratio of ligands on the polymersome surfaces closely correlates to the ratio of ligand in solution during association of biotinylated ligand with avidin-coated polymersomes (D). Data represent the mean ± SEM for 8 experiments.
Using a parallel plate flow chamber (supplemental data), the adhesiveness of leuko-polymersomes was measured at a physiological shear rate of 130 s−1 32 with 24 combinations of vesicle/substrate functionalities. Substrate site densities were determined using surface ELISA. The specific site densities for the four substrates used in this study are indicated in Table 1.
These four surfaces provide a range of inflammatory marker concentrations similar to those measured for endothelial cells during cytokine induced inflammation 9, 39, 40. In addition to quantifying the total amount of adhesion, we quantified the amounts of rolling, firm adhesion, and transient adhesion (or saltation 41) to gain insight into the mechanisms of adhesion. The use of these substrates facilitated repeatable experiments with uniform ligand site density and uniform shear force, and the simplicity and cleanliness of the substrates allowed for the collection of accurate rates of transient and rolling adhesion. The total rates of binding for all vesicle/substrates combinations tested are shown in Figure 3 (note: ordinate scales are different for each panel so that details are not obscured). Supplemental Figures S5 and S6 show aggregates of the data in Figure 3 and representative trajectories, respectively.
Fig. 3.
Binding flux specified by adhesive event for different vesicles (represented by each bar) on different substrates (indicated by title of each plot) at γ = 130 s−1 (note: ordinate scales are different for each panel so that details are not obscured).
The inclusion of sLex on vesicles is required for significant adhesion on any substrate that bears P-selectin. It is known that under physiological conditions, sLex-P-selectin interactions mediate tethering and rolling owing to the fast on-rate for sLex with P-selectin which facilitates these interactions 11, 13. Therefore it is not surprising that the 100% sLex vesicles bind at a higher rate on P-selectin than any other vesicle and that sLex is required for significant vesicle accumulation. Indeed, the absence of sLex (on 100% α-ICAM-1 vesicles) shows that the characteristic interactions between antibody and ICAM-1 are a significant impediment to binding under flow 14. 100% α-ICAM-1 vesicles only bound in significant numbers on high ICAM-1 density substrates, but even small decreases in the site density of α-ICAM-1 significantly impair the ability of vesicles to bind to this substrate, as demonstrated by the 20% and 35% sLex vesicles (80% and 65% α-ICAM-1). In fact, these two vesicles interacted sparsely on most substrates because neither the site densities of sLex were high enough to promote rolling on P-selectin substrates nor were the α-ICAM-1 site densities high enough to promote firm adhesion on high density ICAM-1 substrates.
As expected, interactions between α-ICAM-1 antibody and ICAM-1 promote predominantly firm adhesion, and sLex-P-selectin interactions result in primarily rolling interactions. Perhaps owing to the extremely high density of sLex achievable on leuko-polymersomes, 100% sLex vesicles firmly bind to 100% P-selectin and 50% P-selectin substrates. Firm adhesion is not often observed in selectin-mediated adhesion. Although the sLex polymer used is a tetramer, it is unlikely that more than one sugar on each polymer could bind a P-selectin molecule simultaneously because of steric hindrances. The close proximity of multiple sugars to any sLex-P-selectin bond, however, increases the association rate of a new sLex-P-selectin bond as mature bonds break, thus facilitating the observed rates of firm adhesion.
The goal of tuning the adhesion of a population of vesicles for a target substrate using a specific combination of two ligands is demonstrated in the adhesion of mixed-ligand vesicles. Vesicles made with either 50% sLex or 65% sLex exhibit the highest rates of binding for substrates that imitate inflamed tissue. The 50% sLex vesicle showed a high rate of firm adhesion to the 30% P-selectin substrate, presumably because of a high number of α-ICAM-ICAM-1 interactions, while the 65% sLex vesicles bound most frequently to the 50% P-selectin substrate, presumably because the increased sLex could more effectively engage P-selectin. The main difference between these two vesicles is the increased rate of rolling adhesion observed between the 65% sLex vesicles and high P-selectin density substrates. It is clear from these experiments that there is a threshold number of α-ICAM-ICAM-1 bonds required to bring a slow rolling vesicle to firm arrest, but once enough α-ICAM-1 is present on this vesicle, sLex-P-selectin bonds are more important in increasing the total binding flux. On the 30% P-selectin substrate, in which the 50% sLex vesicles bind most frequently, a threshold number of sLex-P-selectin bonds are required to slow the vesicle, but the concentration of P-selectin on the substrate is not sufficiently high to stop vesicles at a high rate. In this case, α-ICAM-1-ICAM-1 interactions are needed to promote significant firm adhesion. These results indicate that the composition of the biometitic surface has to be carefully tuned to maximize adhesion to a target substrate.
In order to evaluate our hypothesis that two-ligand vesicles adhere at a higher rate and with more selectively than vesicles bearing single ligands, we tested the rate of binding for several populations of vesicles on monolayers of HUVEC cells. Two populations of cells were used, a control population of uninflamed cells, and an inflamed population of cells that had been stimulated with TNF-α to activate the inflammatory pathway according to established methods31. Three vesicular populations, 50% sLex/50% α-ICAM-1, 100% α-ICAM-1, and 100% sLex, were evaluated for total binding events at γ = 130 s−1 in a parallel plate flow chamber. These results are shown in Figure 4. An additional fluorophores was encapsulated in the aqueous luman of these vesicles to allow accurate identification of vesicles on the cell substrates. Fluorescent image capture, however, was too slow to capture fast events such as transient and rolling adhesion, so firm adhesion was used as a measure of binding flux. The best binder was the 50% sLex vesicle with a 7.5-fold increase in binding rate to inflamed versus uninflamed cells. Vesicles coated with 100% α-ICAM-1 bound at a high rate to the inflamed cells (approximately 66% the rate of the 50% sLex vesicle), but as expected, binding was not selective because these high density α-ICAM-1 vesicles bound to uninflamed and inflamed cells at equal rates. Additionally, there was a wide distribution in the rate of polymersome binding in different locations on the monolayer, suggesting that local shear rate (dependent on cell confluence) plays a significant role in the binding of these vesicles. It is hypothesized that selectivity could be achieved with α-ICAM-1 vesicles by decreasing the site density of antibody on the vesicle surface, but this treatment would also cause a decrease in the rate of adhesion to inflamed vesicles. The 100% sLex vesicles bound selectively, with a 5.6-fold selectivity in binding between uniflamed and inflamed cells, but these vesicles bound at the lowest rate. Additionally, the shear rate used for these experiments is at the low end of venous shear rates, so we expect that binding rates for 100% sLex vesicles will decrease with increased shear rate, in the mid range of venous shear rates seen in circulation, as sLex-P-selectin bonds are weak and tend not to promote firm binding.
Fig. 4.
50% sLex/50% α-ICAM-1, 100% α-ICAM-1, and 100% sLex vesicles binding flux on uninflamed and inflamed HUVEC cell monolayers at γ = 130 s−1. Data represents mean ± SEM for 4 or 8 experiments. A two-sided t-test was performed on each pair; * indicates p = 0.00001, ** indicates p = 0.002
Conclusions
We have described the construction and characterization of a leuko-polymersome, a polymersome with the adhesive properties of leukocytes. Through the biotin-avidin chemistry, multiple ligands can be titrated onto the surface of these vesicles to create a multi-functional leukocyte mimetic.
Additionally, this work demonstrates the simultaneous use of two adhesive ligands, which mimic the adhesive ligands on activated leukocytes, as a strategy to selectively mediate binding of vesicles to inflammatory substrates. The synergy, employed in nature, of fast, weak selectin-mediated bonds in combination with strong adhesion molecule-mediated bonds, allows firm binding to occur on relevant substrates at physiological shear rates. By tuning the ratio of ligands, the rate and type of adhesive interaction can be tuned to the adhesive characteristics of a specific substrate. Binding can be enhanced on certain substrates and simultaneously diminished on other substrates by tuning the ligand ratio on the leuko-polymersome. Finally, we show, using HUVECs, that leuko-polymersomes characterized on synthetic surfaces display selective binding to inflamed endothelium under flow.
The adhesiveness of this polymersome can be combined with other unique features of polymersome technology, such as their ability to encapsulate drugs and image contrast agents to create a theranostic particle for inflammation that can image and deliver drugs to inflammatory sites.
Supplementary Material
Tab. 1.
Substrate site densities determined by surface ELISA.
| Substrate Name | sites/μm2 | |
|---|---|---|
| 100% ICAM-1 | ICAM-1/Fc | 861 ± 21# |
| P-sel/Fc | 0 | |
| 50% P-selectin | ICAM-1/Fc | 547 ± 188* |
| Psel/Fc | 542 ± 135* | |
| 30% P-selectin | ICAM-1/Fc | 970 ± 163* |
| P-sel/Fc | 424 ± 30* | |
| 100% P-selectin | ICAM-1/Fc | 0 |
| P-sel/Fc | 476 ± 37# |
Data points represent the mean ± SEM for 4 experiments (#) or 2 experiments (*).
Acknowledgments
We thank Prof. John C. Crocker for many helpful discussions concerning particle tracking and physics. We thank Eric Johnston for valuable technical support, and we thank Joshua Katz, Dr. Michael Beste, and Dr. Andrew Trister for helpful discussions about chemistry and leukocyte biology. We also acknowledge our funding sources: NIH EB003457, NIH CA115229, and the MRSEC Program of the NSF DMR-0520020.
References
- 1.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24(12):640– 647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. P Natl Acad Sci USA. 2003;100(26):15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration: The Multistep Paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909– 1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 6.Mrowietz U, Boehncke W-H. Leukocyte Adhesion: A Suitable Target for Anti-Inflammatory Drugs. Curr Pharm Design. 2006;12:2825– 2831. doi: 10.2174/138161206777947768. [DOI] [PubMed] [Google Scholar]
- 7.Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26:661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Haun JB, Hammer DA. Quantifying Nanoparticle Adhesion Mediated by Specific Molecular Interactions. Langmuir. 2008;24(16):8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 9.Dustin ML, Springer TA. Lymphocyte Function-associated Antigen-1 (LFA-1) Interaction with Intercellular Adhesion Molecule-1 (ICAM-1) is One of At Least Three Mechanisms for Lymphocyte Adhesion to Cultured Endothelial Cells. J Cell Biol. 1988;107:321– 331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Cullere M, Wagner DD, Hynes RO. Multiple, targeted deficiencies in selectins reveals a prodominant role for P-selectin in leukocyte recruitment. P Natl Acad Sci USA. 1999;96:11452– 11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to force in hydrodynamic flow. Nature. 1995;374:539– 542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia SK, Swers JS, Camphausen RT, Wittrup KD, Hammer DA. Rolling Adhesion Kinematics of Yeast Engineered To Express Selectins. Biotechnol Prog. 2003;19:1033– 1037. doi: 10.1021/bp025756b. [DOI] [PubMed] [Google Scholar]
- 13.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190– 193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 14.Eniola AO, Wilcox PJ, Hammer DA. Interplay between Rolling and Firm Adhesion Elucidated with a Cell-Free System Engineered with Two Distinct Receptor-Ligand Pairs. Biophys J. 2003;85:2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia SK, King MR, Hammer DA. The state diagram for cell adhesion mediated by two receptors. Biophys J. 2003;84(4):2671– 2690. doi: 10.1016/S0006-3495(03)75073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Tewari M, Pajerowski JD, Cai S, Sen S, Williams J, Sirsi S, Lutz G, Discher DE. Polymersome delivery of siRNA and antisense oligonucleotides. J Control Release. 2009;134(2):132– 140. doi: 10.1016/j.jconrel.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang Z, Lu W, Gao H, Hu K, Chen J, Zhang C, Gao X, Jiang X, Zhu C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release. 2008;128(2):120– 127. doi: 10.1016/j.jconrel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Levine DH, Ghoroghchian PP, Freudenberg J, Zhang G, Therien MJ, Greene MI, Hammer DA, Murali R. Polymersomes: A new multi-functional tool for cancer diagnosis and therapy. Methods. 2008;46:25– 32. doi: 10.1016/j.ymeth.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Z, Tsourkas A. Paramagnetic Porous Polymersomes. Langmuir. 2008;24(15):8169– 8173. doi: 10.1021/la801027q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian NA, Benencia F, Milone MC, Li G, Frail PR, Therien MJ, Coukos G, Hammer DA. In vivo dendritic cell tracking using fluorescence lifetime imaging and near-infrared-emissive polymersomes. Mol Imaging Biol. 2009;11(3):167–177. doi: 10.1007/s11307-008-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Discher BM, Won Y-Y, Ege DS, Lee JC-M, Bates FS, Discher DE, Hammer DA. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 22.Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS, Chance B, Hammer DA, Therien MJ. Near-infrared-emissive polymersomes: Self-assembled soft matter for in vivo optical imaging. P Natl Acad Sci USA. 2005;102(8):2922–2927. doi: 10.1073/pnas.0409394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular Weight Dependence of Polymersome Membrane Structure, Elasticity, and Stability. Macromolecules. 2002;35:8203–8208. [Google Scholar]
- 24.Lin JJ, Ghoroghchian PP, Zhang Y, Hammer DA. Adhesion of Antibody-Functionalized Polymersomes. Langmuir. 2006;22(9):3975– 3979. doi: 10.1021/la052445c. [DOI] [PubMed] [Google Scholar]
- 25.Demirgoz D, Pangburn TO, Davis KP, Lee S, Bates FS, Kokkoli E. PR_b-targeted delivery of tumor necrosis factor-a by polymersomes for the treatment of prostate cancer. Soft Matter. 2009;5:2011– 2019. [Google Scholar]
- 26.Robbins GP, Jimbo M, Swift J, Therien MJ, Hammer DA, Dmochowski IJ. Photoinitiated Destruction of Composite Porphyrin-Protein Polymersomes. J Am Chem Soc. 2009;131(11):3872–3874. doi: 10.1021/ja808586q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabrouk E, Cuvelier D, Brochard-Wyart F, Nassay P, Li M-H. Bursting of sensitive polymersomes induced by curling. P Natl Acad Sci USA. 2009;106(18):7294– 7298. doi: 10.1073/pnas.0813157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghoroghchian PP, Li G, Levine DH, Davis KP, Bates FS, Hammer DA, Therien MJ. Bioresorbable Vesicles Formed through Spontaneous Self-Assembly of Amphiphilic Poly(ethylene oxide)-block-polycaprolactone. Macromolecules. 2006;39:1673– 1675. doi: 10.1021/ma0519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammer DA, Robbins GP, Haun JB, Lin JJ, Qi W, Smith LA, Ghoroghchian PP, Therien MJ, Bates FS. Leuko-polymersomes. Faraday Discuss. 2008;139:129–141. doi: 10.1039/b717821b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan T, Susumu K, Sinks L, Therien M. Exceptional Near Infrared Fluorescence Quantum Yields and Excited-State Absorptivity of Conjugated Porphyrin Arrays. J Am Chem Soc. 2006;128:9000–9001. doi: 10.1021/ja061897o. [DOI] [PubMed] [Google Scholar]
- 31.Tavintharan S, Lim SC, Sum CF. Effects of Niacin on Cell Adhesion and Early Atherogenesis: Biochemical and Functional Findings in Endothelial Cells. Basic Clin Pharmacol. 2009;104:206– 210. doi: 10.1111/j.1742-7843.2008.00364.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemostasis. 1986;55(3):415– 435. [PubMed] [Google Scholar]
- 33.Vogt RF, Cross GD, Henderson DL, Phillips DL. Model system evaluating fluorescein-labeled microbeads as internal standards to calibrate fluorescence intensity of flow cytometers. Cytometry. 1989;10:294–302. doi: 10.1002/cyto.990100308. [DOI] [PubMed] [Google Scholar]
- 34.Gu Z, Patterson G, Cao R, Armitage B. Self-assembled supramolecular microgels: Fractal structure and aggregation mechanism. J Polym Sci pol Phys. 2003;41(23):3037– 3046. [Google Scholar]
- 35.Norman KE, Katopodis AG, Thoma G, Kolbinger F, Hicks AE, Cotter MJ, Pockley AG, Hellewell PG. P-selectin glycoprotein ligand-1 supports rolling on E- and P-selectin in vivo. Blood. 2000;96(10):3585–3591. [PubMed] [Google Scholar]
- 36.Goebel MU, Mills PJ. Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and density. Psychosom Med. 2000;62:664–670. doi: 10.1097/00006842-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Springer TA, Dustin ML, Kishimoto TK, Marlin SD. The Lymphocyte Function-Associated LFA-1, CD2, and LFA-3 Molecules - Cell-Adhesion Receptors of the Immune-System. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 38.Korn C, Schwartz US. Efficiency of Initiating Cell Adhesion in Hydrodynamic Flow. Phys Rev Lett. 2006;97(13):138103(1)– 138103(4). doi: 10.1103/PhysRevLett.97.138103. [DOI] [PubMed] [Google Scholar]
- 39.Lomakina EB, Waugh RE. Micromechanical Tests of Adhesion Dynamics between Neutrophils and Immobilized ICAM-1. Biophys J. 2004;86:1223– 1233. doi: 10.1016/S0006-3495(04)74196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 41.Schade AJ, Arya M, Gao S, Diz-Küçükkaya R, Anvari B, McIntire LV, López JA, Dong J-f. Cytoplasmic Truncation of Glycoprotein Ibα Weakens Its Interaction with von Willebrand Factor and Impairs Cell Adhesion. Biochemistry. 2003;42(7):2245–2251. doi: 10.1021/bi026549n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.