Abstract
Purpose of review
This paper reviews current work investigating the neural bases of autism spectrum disorder (ASD) within the discipline of electrophysiological brain research. The manuscript focuses primarily on advances in understanding related to social information processing and interconnectivity among brain systems in ASD.
Recent findings
Recent research indicates anomalous function of social brain regions in ASD and highlights the specificity of processing problems to these systems. Atypical activity in this circuitry may reflect genetic susceptibility for ASD, with increased activity in compensatory areas marking the distinction between developing or not developing the disorder. Advances in understanding connectivity in ASD are highlighted by novel work providing initial evidence of atypical interconnectivity in infancy.
Summary
Emerging understanding of neural dysfunction in ASD indicates consistent but heterogeneous dysfunction across brain systems in ASD. Key objectives for the immediate future include: the use of multimethod approaches that encompass temporal and spatial imaging; behavioral phenotyping carried out in developmental context to reveal subgroups defined uniquely by trajectories; and individual-specific profiles of behavioral performance and brain function.
Keywords: Autism Spectrum Disorder, social neuroscience, electrophysiology, EEG, ERP
Introduction
Autism spectrum disorder (ASD) is an early-onset neurodevelopmental disorder characterized by difficulties in social interaction, communication, and repetitive or restricted interests and behaviors [1]. Despite great phenotypic heterogeneity and presumed etiologic diversity in ASD, social dysfunction has been the hallmark and unifying feature of ASD since its original description [2], affecting both simple (e.g., shared gaze) and complex social behaviors (e.g., triadic attention sharing). Anomalies of social perception, unlike communication problems or repetitive behaviors which are present in numerous disorders (e.g., anxiety, expressive language impairment), are unique to ASD and are documented across sensory modalities [3,4]. Social deficits in ASD tend to precede impairment in other domains [5], emerging within the first year of life [6]. The current review focuses on understanding underlying neural systems subserving social perception and information processing in ASD. We review conceptual background and highlight recent advances. We also address recent work investigating an alternative interpretation of autistic dysfunction, problems with interconnectivity and consequent difficulties with sophisticated information processing.
Social perception and information processing
Given the predominance and universality of social dysfunction in the autistic phenotype, neuropathology in brain systems subserving social information processing has become a key focus in autism research. This theoretical framework for understanding development in ASD posits that (a) specific brain systems evolved to process information pertaining to other humans [7] and (b) autistic dysfunction originates in these brain systems, exerting secondary, peripheral impacts through developmental effects. For example, the social motivation hypothesis posits that that reduced social drive leads to inattention to people and consequent failure of developmental specialization in experience-driven brain systems, such as the face perception system [8].
Several specific systems and corresponding anatomical regions have been posited to comprise the neural underpinnings for social behavior [9]. These systems are displayed in Figure 1 and include: biological motion perception, linked to the superior temporal sulcus (STS) [3]; face perception, linked to the fusiform gyrus, or fusiform face area (FFA) [11]; the action-perception system, linked to the inferior frontal gyrus and inferior parietal lobe (IPL) [11]; perception of emotional states and emotional experience, linked to the amygdala (AMY) and limbic system [12]; visual perception of the human body, linked to the extrastriate body area in lateral occipitotemporal cortex (EBA) [13]; social reward and reinforcement, linked to the orbitofrontal cortex (OFC) and ventrolateral prefrontal cortex (VLPFC) [14]; and theory of mind, linked to prefrontal cortex (MPFC) [15]. Rather than a collection of modules, this system reflects a true network with some degree of specialization at individual nodes and emergent functionality via integrated processing across nodes.
Figure 1.
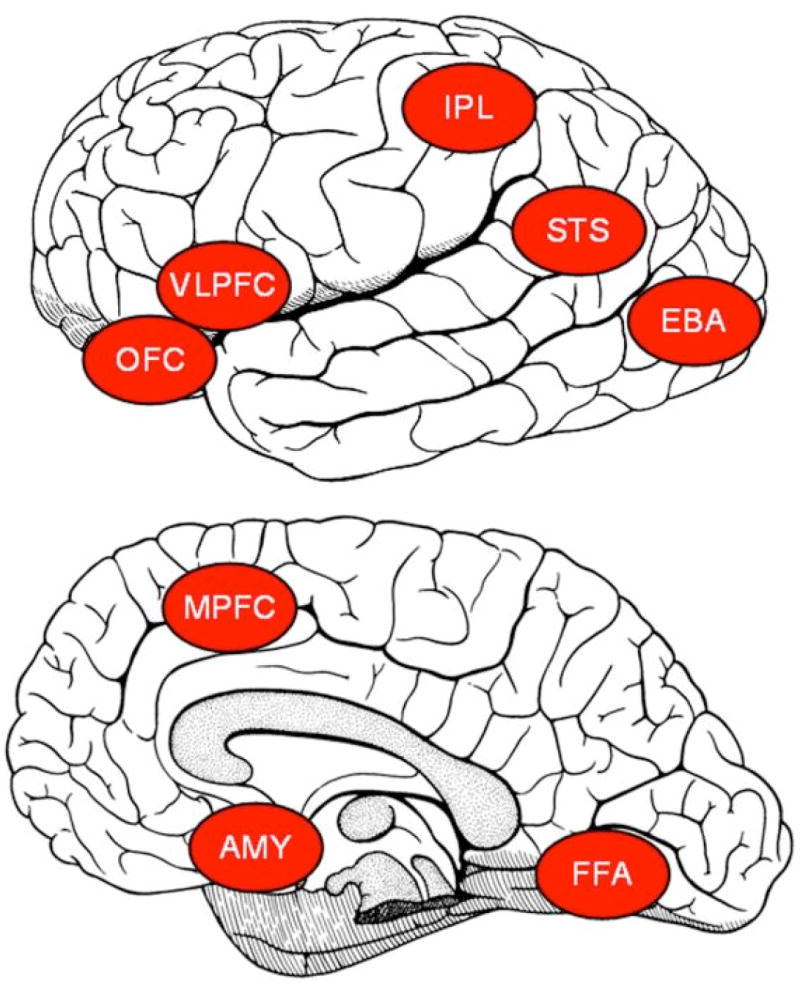
Brain regions subserving social perception and information processing in the human brain: biological motion perception, linked to the superior temporal sulcus (STS); face perception, linked to fusiform face area (FFA); the action-perception system, linked to the inferior frontal gyrus and inferior parietal lobe (IPL); perception of emotional states and emotional experience, linked to the amygdala (AMY) and limbic system; visual perception of the human body, linked to the extrastriate body area in lateral occipitotemporal cortex (EBA); social reward and reinforcement, linked to the orbitofrontal cortex (OFC) and ventrolateral prefrontal cortex (VLPFC); and theory of mind, linked to prefrontal cortex (MPFC). Reprinted with permission from Pelphrey, K. A., & Carter, E. J. (2008). Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci, 1145, 283-299.
A recent area of advanced understanding in ASD is perception of biological motion. Human infants can detect biological motion in the first days of life independent of visual experience, as evidenced by differentiation and preferential attention [16]. From these sparse stimuli, complex attributes about identity, activity, and emotional state can be inferred [17]. Distinction of biological motion occurs rapidly; by 200 milliseconds, the brain distinguishes biological motion from other forms of movement [18,19]. Behavioral studies have shown that from very early in life, children with ASD display reduced sensitivity to biological motion [20]. Recent neuroimaging research demonstrates reduced activity in portions of the STS during biological motion perception in individuals with ASD. This same work investigated unaffected siblings of autistic children, screened to ensure no subthreshold autistic symptomatology was present, and revealed increased compensatory activity in complementary brain regions [21*]. These results suggest that some differences in social brain systems may be reflective of both the state and trait of having ASD, but compensatory activation in other brain systems may prevent development of the disorder itself.
Face perception is also an intensely studied social function in ASD [22]. Humans preferentially attend to faces and recognize individual faces in early infancy [23]. Neural specialization for face perception is evident by three months of age [24] and throughout the lifespan [10, 26]. Children with ASD display reduced attention to faces in the first year of life [6] with persisting differences in attention to and processing of faces [22, 26, 27]. Children with autism show reduced activity in the FFA during free viewing of faces [22, 28] that may reflect underlying differences in visual attention, i.e. that people with autism tend to fixate on different parts of the faces, such as the mouth rather than the eyes [29]. People with ASD also show slowed processing of faces [30], a finding that has also been observed in parents of children with ASD [10] and infants at-risk for ASD [31]. Social attentional factors likely contribute to these differences, as individuals with ASD show reduced attentional modulation of face perception [32*].
A matter of debate regarding face perceptual difficulties in ASD is whether they reflect a specific problem with social perception or a gross perceptual problem [33]. For example, slowed processing of faces could be interpreted as a reflection of atypical social perception or a broader problem with connectivity among visual areas. Evidence in support of the former account has been provided by recent work contrasting analogous, experience-driven brain networks involved in perception of social and non-social information. Like faces, letters from an alphabet in which one is literate elicit a rapid ERP component (N170) over occipitotemporal scalp [34]. McPartland and colleagues [35*] presented high-functioning individuals with ASD and typical controls with letters of the Roman alphabet and a confabulated alphabet of pseudoletters. Although individuals with ASD displayed a characteristic delay in neural response to faces, they did not demonstrate delays in the analogous response to letters, drawing upon a comparably complex neural network. These findings provide strong evidence that the nature of social information, per se, is relevant in understanding the brain bases of ASD.
Connectivity in neural systems
An alternative account for the core difficulties observed in ASD suggests altered connectivity among distributed brain regions [36]. Interconnectivity theories of ASD, in contrast to social information processing theories, generally cite non-specific brain processes in which the nature of the information processed is relevant only insofar as it requires distributed brain function [37, 38]. For example, it has been posited that, due to poor long range connectivity, simple, local processing is intact, while complex and distributed information processing is impaired in ASD [36]. Because social interaction tends to be complex, these theories suggest that these interactions are particularly vulnerable to disruption due to underconnectivity. Because ASD is associated with a wide range of cognitive, social, and perceptual difficulties, disruption in neural connectivity has been suggested as a parsimonious account for these varied findings [39, 40]. Several studies have directly measured connectivity through imaging of white matter tracts connecting different brain regions, demonstrating atypical patterns of connective tissue in ASD [41-43]; however, most evidence for atypical interconnectivity in ASD has relied on fMRI to examine covariation in activity in distal brain regions. This approach has demonstrated atypical connectivity at rest [44,45] and during a wide range of tasks, including face perception [46], attribution of mental states during viewing of animations [47], language processing [44], executive function [48], visual-motor action [49], and response inhibition [50]. This body of research reveals inconsistent patterns of results across studies, including underconnectivity, overconnectivity, and typical patterns of connectivity, suggesting that connectivity problems may not be a universal feature of ASD [47].
Recent work has focused upon EEG complexity as a marker of connectivity in ASD. Catarino et al. [51**] employed a metric of complexity in biological systems, multiscale entropy, to demonstrate reduced complexity in adults with ASD during a visual recognition task. The developmental nature of connectivity problems in ASD has remained largely unexplored, raising the possibility that, given the import of network feedback in developing long range brain connections, localized problems in early development could manifest as connectivity issues in studies of children and adults. Bosl and colleagues [52**] recently provided initial evidence for a role of connectivity in early development. The authors contrasted EEG complexity in infants at risk for ASD and normal risk controls, revealing reduced complexity in infants at risk. Though this study suggests the role of connectivity in early development in ASD risk, diagnostic outcome was not available for infants in this study, so it is unclear whether connectivity reflects an early marker of disease or autism-risk.
An area of growth for connectivity research in ASD will be increased application of imaging measures with high temporal resolution to enable the study of effective connectivity, in contrast to functional connectivity. Functional connectivity studies test the null hypothesis that activity in two regions shares no mutual information; they are thus model free, largely data driven, and without power to specify directionality of influence [53]. In contrast, effective connectivity examines the direct influence of one neural system on another by testing a causal model, with theoretically constrained connections (in terms of neuroanatomical, neurofunctional, and neuropsychological considerations) specified in advance [54, 55]. Because current models of effective connectivity feature rapid and transient integration of information at both the local and distal level, fMRI studies lack requisite temporal resolution to test effective connectivity [56]. Several recent studies have applied the technique to electrophysiological brain data, which yields the significant benefit of approximating the time scale of actual brain processes [57, 58]. MEG studies have employed effective connectivity to specify a neural path during typical face perception that flows from the occipital lobe to the superior temporal sulcus, inferior parietal lobe, inferior frontal gyrus, and then motor cortex [59, 60], highlighting the role of brain regions involved in face processing (fusiform gyrus [61]) and action-perception (inferior parietal lobe, inferior frontal gyrus [62]). Wicker and colleagues [63] examined effective connectivity in ASD compared to typical controls, revealing patterns of atypical connectivity among social brain regions during emotional face perception. Groups were not matched on cognitive ability, and thus it cannot be determined to what degree functional differences reflected ASD, per se, versus general cognitive impairment or nonspecific developmental disturbance. This nascent area of research suggests that future work investigate effective connectivity using imaging methods, such as EEG and MEG, with acute temporal resolution.
Future directions
Some of the research discussed above is yet to be replicated, and some has failed to replicate universally across samples. Heterogeneity in brain function and the behavioral phenotype in ASD is the rule rather than the exception [63], and parsing this heterogeneity has been a research priority. The research presented here suggests that employment of multimethod studies (i.e., including behavioral measures and spatial and temporal imaging) in the context of longitudinal studies of development will be a critical strategy needed to reveal meaningful differences that may be evident only in trajectory. This integrated research approach will exploit the strengths of each investigative method to enable profiling of function across levels and at individual stages of processing to inform development of specific treatment modalities. Individuals exhibiting anomalous function at processing associated with low-level visual perception would naturally require distinct intervention approaches from those showing problems with subsequent, higher-order processes. We envision a strategy of deep behavior and brain phenoptyping to offer a detailed profile of brain-behavior performance for a given individual for the purpose of subcategorization (e.g. for genetic analysis), treatment selection, and prediction of treatment response. Preliminary work suggests the viability of this strategy in that individuals presenting with complementary behavioral and brain markers of difficulties with emotion regulation show improvements in both measures after cognitive-behavioral therapy targeted to their specific domain of impairment. [64].
Conclusion
Electrophysiological brain measures have provided key insights into the neural bases of ASD. Recent research suggests specificity of dysfunction to brain systems subserving social perception and information processing with preserved function in parallel systems processing nonsocial information. The temporal acuity of electrophysiological recordings reveals decreased processing efficiency in ASD. It remains unclear whether connectivity problems reflect developmental effects of anomalies in social brain systems or core brain dysfunction. The cost effectiveness and broad applicability of EEG measures offers great promise for understanding early development in ASD and creating novel methods for detecting atypical brain function early in life.
Key points.
Specific brain regions involved in social information processing are compromised in ASD.
There is also evidence for altered patterns of brain connectivity accounting for ASD symptomatology.
It remains unclear to what degree observed differences in brain function reflect underlying core brain dysfunctions or developmental effects of having ASD.
Imaging measures with high temporal acuity are necessary to understand patterns of connectivity in ASD and to specify the processing stage at which dysfunction occurs.
Deciphering the heterogeneity in both brain functioning and behavioral characteristics in ASD will require an integrated research approach, combining behavioral measures with spatial and temporal in the context of through longitudinal studies.
Acknowledgments
This work was supported by NIMH K23MH086785 (JM), NIMH R21MH091309 (JM), NARSAD Atherton Young Investigator Award (JM), and NIMH K01MH071284 (KP).
Footnotes
The authors report no conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4. xxxvii. Washington, DC: American Psychiatric Association; 2000. p. 943. [Google Scholar]
- 2.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 3.Pelphrey KA, Mitchell TV, McKeown MJ, et al. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J Neurosci. 2003;23(17):6819–25. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism & Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 5.Zwaigenbaum L, Bryson S, Rogers T, et al. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2-3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Maestro S, Muratori F, Cavallaro MC, et al. Attentional skills during the first 6 months of age in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Brothers L. The social brain: A project for integrating primate behavior and neurophsyiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- 8.Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 9.Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–99. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacoboni M, Woods RP, Brass M, et al. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 12.LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50–7. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- 13.Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293(5539):2470–3. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- 14.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–7. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittrich WH, Troscianko T, Lea SE, Morgan D. Perception of emotion from dynamic point-light displays represented in dance. Perception. 1996;25(6):727–738. doi: 10.1068/p250727. [DOI] [PubMed] [Google Scholar]
- 18.Hirai M, Fukushima H, Hiraki K. An event-related potentials study of biological motion perception in humans. Neuroscience Letters. 2003;344(1):41–44. doi: 10.1016/s0304-3940(03)00413-0. [DOI] [PubMed] [Google Scholar]
- 19.Jokisch D, Daum I, Suchan B, Troje NF. Structural encoding and recognition of biological motion: evidence from event-related potentials and source analysis. Behav Brain Res. 2005;157(2):195–204. doi: 10.1016/j.bbr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. Indicates both neural signatures for the underlying trait of developing ASD, and regions of compensatory activity in unaffected siblings with autism. Holds implications for early identification of risk, diagnosis, and intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2-3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1-2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 24.de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. Int J Psychophysiol. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 25.Bentin S, Allison T, Puce A, et al. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobson R. The autistic child’s appraisal of expressions of emotion. Journal of Child Psychology and Psychiatry. 1986;27(3):321–342. doi: 10.1111/j.1469-7610.1986.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 27.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 28.Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 29.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 31.McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biol Psychiatry. 2009;66(10):950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Churches O, Wheelwright S, Baron-Cohen S, Ring H. The N170 is not modulated by attention in autism spectrum conditions. Neuroreport. 2010;21(6):399–403. doi: 10.1097/wnr.0b013e328334311b. This is the first known ERP study that attempts to experimentally modulate attention. Results demonstrating a modulated N170 in TDs implicate an ability to intervene early with ASD to effect underlying brain mechanisms of face processing. [DOI] [PubMed] [Google Scholar]
- 33.Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends Cogn Sci. 2006;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Wong T, Fung PC, McAlonan GM, Chua SE. Spatiotemporal dipole source localization of face processing ERPs in adolescents: a preliminary study. Behavioral and Brain Functions. 2009;5(1):16. doi: 10.1186/1744-9081-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.McPartland JC, Wu J, Bailey CA, et al. Atypical neural specialization for social percepts in autism spectrum disorder. Soc Neurosci. 2011 doi: 10.1080/17470919.2011.586880. (in press). This ERP study contrasted processing for complex visual stimuli in social and non-social domains. Results showed preserved specialization for non-social information (letters) and atypical specialization for social information (faces) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–50. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45(7):749–55. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- 38.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 39.Belmonte MK, Allen G, Beckel-Mitchener A, et al. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belmonte MK, Cook EH, Anderson GM, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9(7):646–63. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 41.Barnea-Goraly N, Kwon H, Menon V, et al. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55(3):323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 43.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18(1):23–7. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 44.Di Martino A, Shehzad Z, Kelly C, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. American Journal of Psychiatry. 2009;166(8):891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 46.Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 47.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 48.Just MA, Cherkassy VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **51.Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H. Atypical EEG complexity in autism spectrum conditions: A multiscale entropy analysis. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.05.004. (in press). This is the first study to look at EEG complexity in ASD during a task, rather than during resting state EEG and demonstrates higher complexity. This study highlights the importance of looking at connectivity during a face recognition task using tools with high temporal resolution. [DOI] [PubMed] [Google Scholar]
- **52.Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9(11) doi: 10.1186/1741-7015-9-18. This is the first study to look into connectivity changes across time. Shows differences in resting brain state entropy in high risk autism group, possibly indicating a biomarker for risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee L, Harrison LM, Mechelli A. A report of the functional connectivity workshop. Neuroimage. 2003;19(2 Pt 1):457–65. doi: 10.1016/s1053-8119(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 54.Buchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Netw. 2000;13(8-9):871–82. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 55.Friston KJ, Frith CD, Liddle PF, Frackwiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 56.Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol. 2007;63(2):164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Astolfi L, Cincotti F, Mattia D, et al. Estimation of the effective and functional human cortical connectivity with structural equation modeling and directed transfer function applied to high-resolution EEG. Magn Reson Imaging. 2004;22(10):1457–70. doi: 10.1016/j.mri.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Astolfi L, Cincotti F, Babiloni C, et al. Estimation of the cortical connectivity by high-resolution EEG and structural equation modeling: simulations and application to finger tapping data. IEEE Trans Biomed Eng. 2005;52(5):757–68. doi: 10.1109/TBME.2005.845371. [DOI] [PubMed] [Google Scholar]
- 59.Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc Natl Acad Sci U S A. 2000;97(2):913–8. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishitani N, Hari R. Viewing lip forms: cortical dynamics. Neuron. 2002;36(6):1211–20. doi: 10.1016/s0896-6273(02)01089-9. [DOI] [PubMed] [Google Scholar]
- 61.Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage. 2002;15(2):353–72. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- 62.Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15(6):632–7. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Wicker B, et al. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3(2):135–43. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukhodolsky DG, Bolling DZ, Wu J, et al. Cognitive behavior therapy for irritability in high-functioning ASD: Pilot study of neurobiological mechanisms. Paper presented at the International Meeting for Autism Research. 2011 [Google Scholar]


