Abstract
HIV-1 integrase (IN) is indispensable for HIV-1 replication and has become a validated target for developing anti-AIDS agents. In two decades of development of IN inhibition-based anti-HIV therapeutics, a significant number of compounds were identified as IN inhibitors, but only some of them showed antiviral activity. This article reviews a number of patented HIV-1 IN inhibitors, especially those that possess high selectivity for the strand transfer reaction. These compounds generally have a polar coplanar moiety, which is assumed to chelate two magnesium ions in the binding site. Resistance to those compounds, when given to patients, can develop as a result of IN mutations. We refer to those compounds as authentic IN inhibitors. Continued drug development has so far delivered one authentic IN inhibitor to the market (raltegravir in 2007). Current and future attention will be focused on the development of novel authentic IN inhibitors with the goal of overcoming viral resistance.
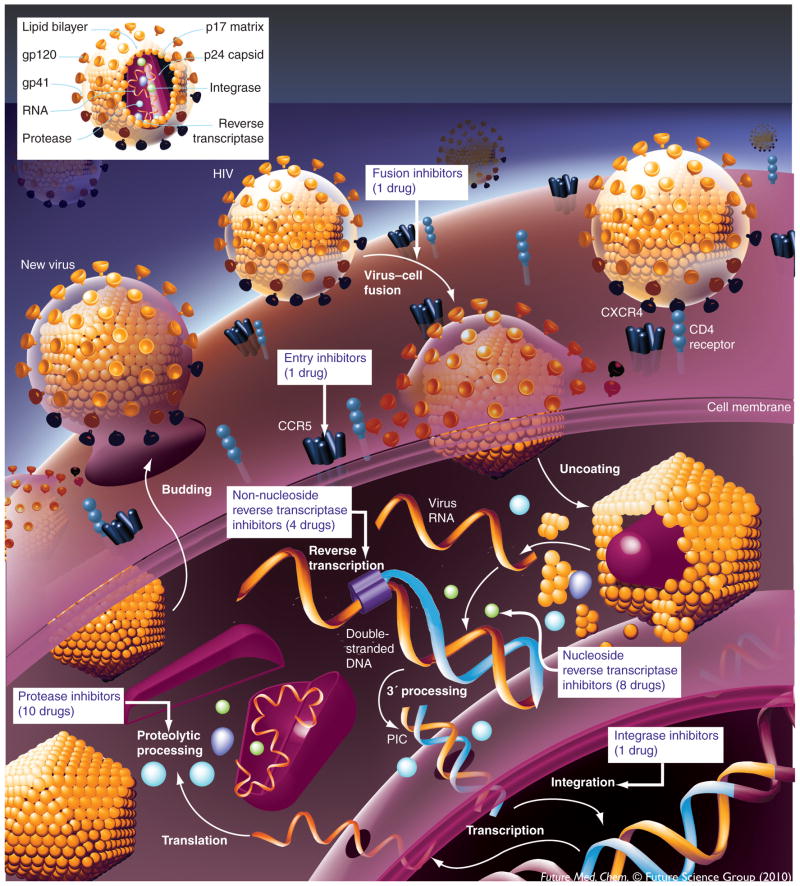
HIV-1: life cycle & anti-HIV drug development
AIDS, a progressive, degenerative disease of the human immune system, which has proven to be one of the world’s most serious health problems since 1981, is generally accepted to be caused by HIV type 1 (HIV-1) [1–3]. AIDS progressively reduces the effectiveness of the immune system and leaves individuals susceptible to opportunistic infections and tumors. The replicative cycle of HIV-1 can be divided into two steps: entry and post entry [4,5], as shown in Figure 1. Entry of HIV-1 into a host cell takes place in three critical steps:
Figure 1.
HIV-1 life cycle and anti-HIV drug development.
The trimeric HIV-1 envelope glycoprotein complex mediates viral entry into susceptible target cells. The virus surface subunit (gp120) attaches to the CD4 receptor of the host cell;
gp120–coreceptor (CXCR4 or CCR5 of the host cell) interaction, which results in the exposure of a coreceptor-binding domain in gp120 on the cell surface;
Subsequent conformational changes within the Env complex, which lead to membrane fusion mediated by the transmembrane subunit (gp41 of the virus).
Post-entry steps involve the viral reverse transcriptase (RT), integrase (IN) and protease (PR) enzymes to complete the viral replication cycle. RT is responsible for the conversion of the single-stranded viral RNA into the double-stranded proviral DNA [6]; IN is required for the integration of proviral DNA into the host genome before replication [7]; and PR cleaves newly synthesized polyproteins at the appropriate places to create the mature protein components of infectious HIV virions [8]. Each of the stages in both the entry and postentry steps can serve as a target for anti-AIDS drug development [9].
The inhibition of enzyme-mediated processes associated with the life cycle of the human HIV-1 has led to great advancements in the treatment of patients suffering from AIDS. Difficulties still persist regarding the best way to manage this disease [10]. To date, there are 25 approved antiretroviral drugs available, which attack four targets: viral entry, RT, PR and IN. There is continued interest in developing new agents in three main areas:
Effective vaccines or comparable preventative strategies;
Better tolerated, more convenient and less expensive treatments;
New agents that do not share cross-resistance and would, thus, not be limited by existing resistance [9].
Currently, the recommended starting regimens for HIV-infected patients generally consist of a non-nucleoside RT inhibitor (NNRTI) or a PR inhibitor (PI) combined with two nucleoside or nucleotide RT inhibitors (NRTIs). Such regimens are commonly referred to as highly active antiretroviral therapy (HAART). For treatment-experienced patients, regimens are more complex and include consideration of all potential agents to which the patient’s virus is sensitive [9]. Novel mechanisms, for example, inhibiting maturation of HIV-1, can be exploited for further anti-AIDS drug development [11].
HARRT has been highly beneficial to many HIV-infected individuals since its introduction in 1996 when the PI-based HAART initially became available [12]. Nevertheless, for many patients, HAART achieves results that are far less than optimal, due to nonadherence to therapy and development of resistance [13]. Targeting IN has become an additional highly promising therapeutic approach since the approval in 2007 of the IN strand-transfer (ST) inhibitor, Raltegravir (Isentress®, MK-0518, RAL) from Merck & Co [14,15]. RAL seems to belong to the class of drugs that act as an interfacial inhibitor by trapping a conformational intermediate of an enzyme [16,17].
Catalytic activities of IN
HIV-1 IN is a 32-kDa protein comprising three structural domains: the N-terminal domain (residues 1–50), the catalytic core domain (residues 51–212), which is highly conserved among retroviruses and the C-terminal domain (residues 213–288) [18]. The atomic structure of each domain separately (or two-domain combinations) has been determined by x-ray crystallography and solution NMR. However, no full-length x-ray or NMR structure of HIV-1 IN has been published to date.
The integration of viral DNA into the host DNA, the step catalyzed by IN, is required for viral replication and chronic infection. Additionally, the stable incorporation of the HIV-1 genome allows the infection to persist asymptomatically within latent viral reservoirs [19]. IN catalyzes two distinct reactions involving phosphate ester modifications [7,16]: 3′-end processing (3′-P) and ST. Following reverse transcription of the HIV-1 genome in the cytoplasm, IN first assembles on the newly synthesized viral DNA and removes two bases, GT, from both 3′-ends of the double-stranded viral DNA (the 3′-P reaction). Subsequently, after transport of the viral DNA into the nucleus within the pre-integration complex (PIC), IN catalyzes the covalent joining of these preprocessed 3′-ends to opposite strands of the host DNA, offset by five base pairs (the ST reaction). The integration is then completed by gap repair and additional steps effected by cellular enzymes. Both the assembly of IN with its DNA substrate and the two catalytic functions of the enzyme require the presence of divalent metals, such as Mn2+ or Mg2+, the latter being assumed to be the physiologically relevant species [20,21]. IN can also catalyze the reverse reaction, disintegration. However, this has only been observed in vitro and its physiological significance is unclear.
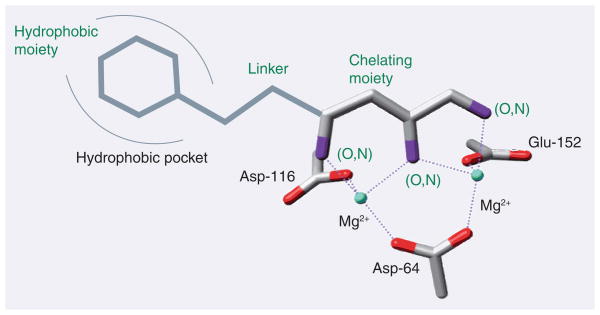
The catalytic core domain of IN contains a canonical three-amino acid DDE motif formed by the catalytic triad Asp-64, Asp-116 and Glu-152, which is highly conserved in all INs and retrotransposases and is supposed to form a coordination complex with two Mg2+ ions and the viral DNA [18,22]. Mutation of any of these three acidic residues abolishes enzymatic activities of IN and viral replication.
Assay methods for integrase inhibitors
Recombinant IN can be used in biochemical assays for the screening of inhibitors [21,23]. The in vitro integration reaction requires recombinant IN, divalent metal (Mg2+ or Mn2+) and short LTR-derived DNA oligonucleotides (U5 or U3). Most of the inhibitors reported to date have been discovered using either gel-or plate-based biochemical assays [16]. Most of these biochemical assays are based on the property of IN to auto-integrate DNA. Assays to measure full-site (concerted) integration, which mimics in vivo integration more closely than auto-integration, have also been developed, using oligonucleotide in addition to longer plasmidic DNA fragments [24,25].
Following the report of the first IN inhibitors in 1993 [26], many potential IN inhibitors have been discovered and reported. Unfortunately, the vast majority of them were neither confirmed as antiviral nor as specific inhibitors of IN. The reasons for this drawback reside in technological challenges imposed by IN. First of all, IN can use either Mn2+ or Mg2+ as a metal cofactor to catalyze integration. It has always been more challenging to obtain good activity in the presence of Mg2+ and, therefore, most of the early inhibitors were reported using Mn2+ as cofactor. It is now commonly admitted that Mg2+ is probably the biological cofactor of HIV-1 IN [16,27]. Mg2+ is a more stringent cofactor compared with Mn2+ and its coordination sphere is more rigid than that of Mn2+ [28]. This impacts directly on the conformation of the IN catalytic site and on the function of its flexible loop (residues 140–149) [28]. Therefore, the screening for IN inhibitors in Mn2+-based assays may have been responsible for a large number of false positives. Second, only a few antiviral assays allow the unambiguous determination of IN as a target of a drug. These assays based on the evaluation of 2-LTR circle formation and the measurement of integrated LTR products via Alu-PCR are technically challenging, allowing only very low-throughput in restricted retrovirology laboratories. [29] Finally, the absence of any reliable information on the 3D structure of the full length IN with its DNA substrates has been an important obstacle to the rational design of specific IN inhibitor.
Overview of the development of integrase inhibitors
The successful development of IN inhibitors as anti-HIV therapeutics has taken more than 20 years [30]. Savarino summarized this ‘saga’ in 2006 in a historical sketch of the discovery and development of IN inhibitors [17]. Briefly, before 1992, inhibition of HIV-1 IN had been considered as a treatment approach, but no specific IN inhibitor had yet been identified. During the period of 1992–1996, researchers laid the basis for modern IN inhibitor discovery with the development of screening assays and description of the first specific inhibitors active in vitro. Nucleic acid-based approaches, including gene therapy, formed a field of intense research and an aptamer, a G-rich nucleotidic sequence that binds specifically to IN, became the first IN inhibitor to be tested in human clinical trials [31]. Several other classes of compounds were identified as IN inhibitors, among which polyphenols served as leads for some investigational drugs studied in subsequent years. Some compounds from natural products, for example fungi, were also identified as IN inhibitors. During the period of 1996–1999, IN inhibitor discovery led to some frustration among researchers since it had become apparent that the identification of a clinical candidate was noticeably more difficult than for other antiretroviral drug classes. During the period of 1999–2002, Merck and Shionogi independently discovered and patented keto-enols acid-type compounds from screening (often referred to as diketo acids), as IN inhibitors. This was a fundamental, innovative step in the history of IN inhibitor discovery. Some compounds conceptually based on these inhibitors, for example with carboxylate groups replaced with isosteres such as a tetrazole group, were soon identified as IN inhibitors [32,33]. A compound from Shionogi/GlaxoSmithKline (GSK), S-1360, was the first IN inhibitor acting specifically by ST inhibition to enter clinical trials [34]. After 2002, IN inhibitors began to be regarded as a valid new class of drugs and a therapeutic strategy worthy of being pursued. The importance of the keto-enol group of ST inhibitors was also in part clarified [35]. A large number of new molecules, in which the carboxylate was mimicked by a suitable heterocycle bearing a lone pair donor atom, were developed as IN inhibitors. In 2007, RAL finally became the first IN inhibitor approved by the US FDA [14,36]. Currently, several other compounds, including Elvitegravir (GS-9137, EVG), a quinolone carboxylic acid that does not possess a keto-enol moiety, are in clinical trial studies [30,37,38].
In the first 10 years of the discovery of IN inhibitors, many compounds belonging to different classes, such as catechols, aurintricarboxylic acids, flavones, flavonoids, curcumins, tyrphostins, lignanolides, cosalanes, triazine derivatives, depsides, depsinoids, styrylquinoline derivatives, thiazolothiazepines, arylamides, salicylhydrazides, integrinic acid derivatives, tetracyclines, diarylsulfones, cobalamin derivatives, nucleotides and analogs, were reported as IN inhibitors [39–43]. However, none of them went on to be developed into an effective anti-HIV agents. Among the many reasons for failures are the facts that some compounds (e.g., catechol-containing compounds) have high toxicities and that some compounds (e.g., sulfonamides) did not exhibit antiviral activity.
Over the past decade, diketo acids and their isosteres, which are assumed to chelate two Mg2+ ions simultaneously (Figure 2) [22,44–47], have remained the prototypical IN inihibitor class. These inhibitors are characterized by great selectivity for the ST reaction. They were almost exclusively developed by pharmaceutical companies and government agencies, notably Merck, Shionogi/GSK, Bristol-Myers Squibb (BMS), Gilead, Japan Tobacco, Pfizer and the NIH. These compounds show not only in vitro activities, but also potent antiviral activities, as a result of the inhibition of viral DNA integration. In this review, we call them authentic IN inhibitors, or IN ST inhibitors (INSTI).
Figure 2.
Putative chelating mode of authentic integrase inhibitors comprising a planar two-metal chelating region, held in place by the catalytic triad Asp-64, Asp-116 and Glu-152.
Structures of some authentic IN inhibitors
All authentic IN inhibitors possess at least two distinct regions: an aromatic hydrophobic region and a chelating region. Except for GS-9137, the chelating region of all these compounds is represented by a diketo acid motif or a bioisostere of diketo acid. In structural terms, this means they have three functional groups in a coplanar conformation, which are assumed to chelate two magnesium ions in the so-called two-metal ion mechanism [22,35,44]. Some compounds, such as L-870,810 and MK-0518, contain a third moiety, which is thought to enhance activity in cell culture by improving cell permeability and reducing binding to cell medium plasma proteins [48].
Diketo acids & their analogues
For the design and optimization of inhibitors against enzymes reliant on a two-metal mechanism of action for endonucleolytic phosphodiester hydrolysis, such as HIV-1 IN, HIV reverse transcriptase RNase H, hepatitis C virus (HCV) polymerase, Tn5 transposase and influenza endo-nuclease and α,γ-diketo acids have often served as starting points [49]. In the presence of Mg2+, the diketo acids are easily deprotonated to yield a dianion, which permits the straightforward chelation of the two Mg2+.
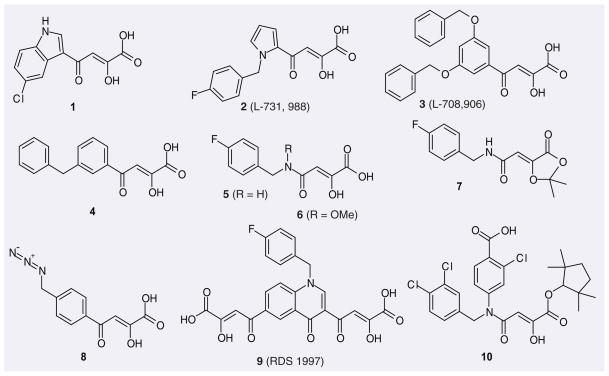
In 1999, Shionogi and Merck almost simultaneously patented α,γ-diketo acids as IN inhibitors. The typical compounds are 1 (from Shionogi) and 2–4 (from Merck) with IC50 values against ST of approximately 100 nM and EC50 values in the micromolar range (Figure 3 & Table 1) [50], which subsequently became the most studied class of IN inhibitors. Compound 5 was developed from 1 [51]; however, its in vitro activity turned out not to be better. Based on the assumption that the hydrogen-bond donating groups may be adversely affecting the transport of the compound into the cell, the corresponding dioxolane prodrug derivative 7 was synthesized. This showed a slight improvement in in vivo activity, probably due to premature hydrolysis of the acetonide ester prior to entering the cell [51]. Methylation of the amide of 5 yielded a tenfold increase in cell culture activity while having only an insignificant effect on in vitro activity. Compound 6, an analog of 5 having a methoxy group on the amide N, also showed good enzyme and cell culture activity. In 2003, the NCI/NIH patented several azido-containing aryl β-diketo acids as IN inhibitors with low cytotoxicity and antiviral activity, of which 8 is a representative structure. In 2005, the NCI/NIH patented a series of bifunctional quinolonyl diketo acids, which contain two diketo acid groups (e.g., 9), as IN inhibitors possessing antiviral activity [52].
Figure 3.
Representative diketo acids and diketo acid analogues patented as HIV-1 integrase inhibitors.
Table 1.
Some patented authentic HIV-1 integrase inhibitors and their biological activities.†
| Compound | Assignee | Patent number | IC50 (ST, μM) | EC50 (μM) | EC95 (μM) | CC50 (μM) |
|---|---|---|---|---|---|---|
| 1 | Shionogi & Co., Ltd | WO 9950245 A1 | 0.07 | |||
| 2 | Merck & Co., Inc. | WO 9962513 A1 | 0.133 | 1.5 | 9.6 | 520 |
| 3 | Merck & Co., Inc. | WO 9962520 A1 | 0.30 | 1–2 | 17.7 | 88.3 |
| 4 | Merck & Co., Inc. | WO 9962520 A1 | <0.10 | 1.053 | ||
| 5 | Bristol-Myers Squibb | WO 200196283 A2 | 0.20 | 5.9 | ||
| 6 | Bristol-Myers Squibb | WO 2003049690 A2 | 99.9% inhibition at 50 μM | 96% inhibition at 1.6 μM | ||
| 7 | Bristol-Myers Squibb | WO 200196283 A2 | 1.2 | |||
| 8 | NIH | WO 2003049695 A2 | 1.53 | 0.5 | ||
| 9 | NIH | WO 2005087759 A1 | 0.017 | 4.29 | >200 | |
| 10 | Japan Tobacco Inc. | WO 2003016266 A1 | 0.0041 | |||
| 11 | Shionogi & Co., Ltd | WO 9950245 A1 | 0.65 | >5 | ||
| 12 | Shionogi & Co., Ltd | WO 200039086 A1 WO 200196329 A1 |
0.02 | 0.2 | 12 | |
| 13 | Merck & Co., Inc. | WO 200100578 A1 | 0.01–5 | 0.01–50 | ||
| 14 | Japan Tobacco Inc. | WO 2003016266 A1 | 0.0043 | |||
| 15 | Shionogi & Co., Ltd | WO 200195905 A1 | 0.247 | |||
| 16 | Shionogi & Co., Ltd | WO 200195905 A1 | 0.059 | 4.8 | ||
| 17 | Shionogi & Co., Ltd | WO 200195905 A1 | 0.55 | >68 | ||
| 18 | Shionogi & Co., Ltd | WO 2003047564 A1 | 2.43 | |||
| 19 | Shionogi & Co., Ltd | WO 2003047564 A1 | 1.57 | |||
| 20 | Pfizer Inc. | WO 2005103003 A2 | 0.084 | 0.00795 | ||
| 21 | Pfizer Inc. | WO 2004067531 A1 | 0.234 | |||
| 22 | Pfizer Inc. | WO 2005103051 A1 | 0.007 | 0.00032 | ||
| 23 | Merck & Co., Inc. | WO 200236734 A2 | 0.01 | 0.39 | >12.50 | |
| 24 | Merck & Co., Inc. | WO 200230931 A2 | 0.040 | 0.002 | 0.250 | >25 |
| 25 | Merck & Co., Inc. | WO 200230931 A2 | 0.010 | 0.003 | 0.102 | 2.1 |
| 26 | BioAlliance Pharma | WO 2003031413 A1 | 1.7 | 1–4 | ||
| 27 | Merck & Co., Inc. | WO 2003062204 A1 | 0.010 | 0.937 | ||
| 28 | GlaxoSmithKline/Shionogi & Co., Ltd | WO 2005077050 A2 | 0.008 | 0.0012 | >2.5 | |
| 29 | Shionogi & Co., Ltd | WO 2004024693 A1 | 0.208 | |||
| 30 | Tibotec Pharmaceuticals Ltd | WO 2004096807 A2 | 0.42 | 0.27 | 2.63 | |
| 31 | GlaxoSmithKline | WO 2004101512 A2 | 0.006 | 0.010 | 12.2 | |
| 32 | Gilead Sciences, Inc. | WO 2006125048 A2 | 0.028 | 0.002 | 4 | |
| 33 | Gilead Sciences, Inc. | WO 2009067541 A2 | ||||
| 34 | IRBM-MRL Rome‡ | WO 2003035076 A1 | 0.01 | |||
| 35 | IRBM-MRL Rome | WO 2003035077 A1 | 0.010 | 0.125 | ||
| 36 | Merck & Co., Inc. | WO 2006060711 A2 | 0.015 | 0.019 | ||
| 37 | Bristol-Myers Squibb | WO 2004096128 A2 | 0.002–2 | |||
| 38 | IRBM-MRL Rome | WO 2006103399 A2 | ||||
| 39 | Bristol-Myers Squibb | WO 2007061714 A1 | 0.001–0.010 | |||
| 40 | IRBM-MRL Rome | WO 2007039218 A1 | 0.019 | 0.062 | ||
| 41 | Shionogi & Co., Ltd | WO 2005061490 A1 | 0.0096 | 0.0096 | ||
| 42 | Merck & Co., Inc. | WO 2005086700 A1 | <1 | <10 | ||
| 43 | Merck & Co., Inc. | WO 2005087768 A1 | <10 | |||
| 44 | Merck & Co., Inc | WO 2005092099 A1 | <10 | |||
| 45 | Merck & Co., Inc. | WO 2008048538 A1 | 0.014 | 0.015 | ||
| 46 | Shionogi & Co., Ltd | WO 2006088173 A1 | 0.0079 | |||
| 47 | Shionogi & Co., Ltd | WO 2006088173 A1 | 0.0022 | |||
| 48 | GlaxoSmithKline/Shionogi & Co., Ltd | WO 2010011812 A1 | 0.0027 | 0.0052 | ||
| 49 | Bristol-Myers Squibb | WO 2004004657 A2 | 99.9% inhibition at 20 μM | |||
| 50 | Shionogi & Co., Ltd | WO 2003016275 A1 | 0.030 | 0.18 | ||
| 51 | Shionogi & Co., Ltd | WO 2003016275 A1 | 0.040 | 0.24 | ||
| 52 | Shionogi & Co., Ltd | WO 2003016275 A1 | 0.079 | 0.55 | ||
| 53 | Merck & Co., Inc. | WO 2004047725 A2 | 0.01 | 0.31 | ||
| 54 | Merck & Co., Inc. | WO 2005120516 A2 | 0.05 | 0.310 | ||
| 55 | Merck & Co., Inc. | WO 2005041664 A1 | <0.01 | 0.16 | ||
| 56 | Merck & Co., Inc. | WO 2005110414 A2 | 0.04 | |||
| 57 | Merck & Co., Inc. | WO 2007050510 A2 | ||||
| 58 | Japan Tobacco Inc. | WO 2004024078 A2 | 0.0072 | 0.0009 | 4.0 | |
| 59 | Japan Tobacco Inc. | WO 2006033422 A1 | <0.01 | |||
| 60 | Shionogi & Co., Ltd | WO 2002070491 A1 | 0.78 | |||
| 61 | Shionogi & Co., Ltd | WO 2002070491 A1 | 2.15 | |||
| 62 | Virochem Pharma, Inc. | WO 2005042524 A1 | <10 | |||
| 63 | Merck & Co., Inc | WO 2004024078 A2 | 0.04 | 0.25 | ||
| 64 | IRM LLC | WO 2008073138 A2 | <0.05 | <0.05 | >0.5, <2 | |
| 65 | IRM LLC | WO 2008073138 A2 | >0.05, <0.5 | >0.05, <0.5 | >2 |
Diketo acid analogues, including esters and amides, have also been patented as IN inhibitors. Generally, the esterification of diketo acids decreases their inhibitory activities against the ST reaction. For example, the ST inhibitory IC50 value of the corresponding ethyl ester of 1 drops 13-fold compared with the former [53]. Nevertheless, some diketo acid esters patented by Japan Tobacco showed very good ST inhibitory activities. The best one is 10 with a remarkable IC50 value of 4.1 nM.
Compounds derived directly from diketo acids
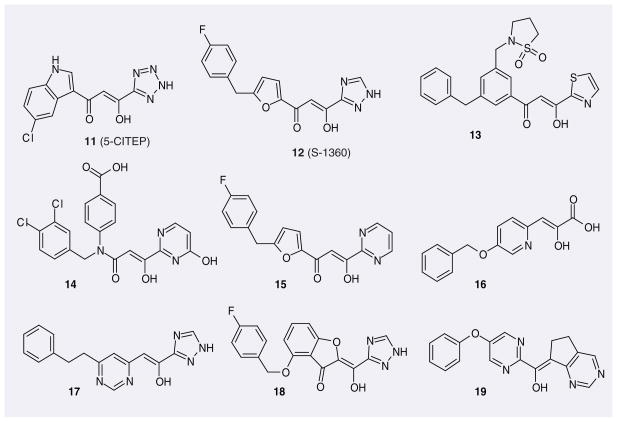
The poor drug-like properties of diketo acids resulted in modest antiviral activity and unfavorable pharmacokinetic properties [54]. This prompted drug developers to replace the acid moiety and/or the carbonyl with an azaheteroaromatic ring, which can provide a lone pair of electrons for the chelation of a metal ion. The replacement of a carboxylic acid by an azaheteroaromatic ring enhances antiviral activity, whereas the replacement of the carbonyl by an azaheteroaromatic ring does not [46,55]. Figure 4 shows some examples of such inhibitors. Among them is 5-CITEP (11) from Shionogi, which was the first and, to this day, remains the only, IN inhibitor co-crystallized in the catalytic site of HIV-1 IN [32,35]. S-1360 (12), also patented by Shionogi but co-developed with GSK, was the first IN ST inhibitor to enter clinical trial. It reached Phase II, however its development was halted in 2003 [34].
Figure 4.
Representative patented integrase inhibitors derived directly from diketo acids.
Pyrrolopyridine hydroxamic acids
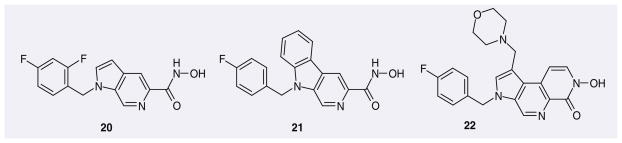
A series of pyrrolopyridine hydroxamic acids (e.g., 20–22), was patented by Pfizer as IN inhibitors (Figure 5). According to the patents, these compounds show excellent inhibition of ST and HIV-1 replication. Nevertheless, for undisclosed reasons, compounds from this structural class do not appear to have been pursued further as HIV-1 IN inhibitors.
Figure 5.
Representative pyrrolopyridine hydroxamic acids patented as HIV-1 integrase inhibitors.
Aza-naphthalenyl carboxamides & related compounds
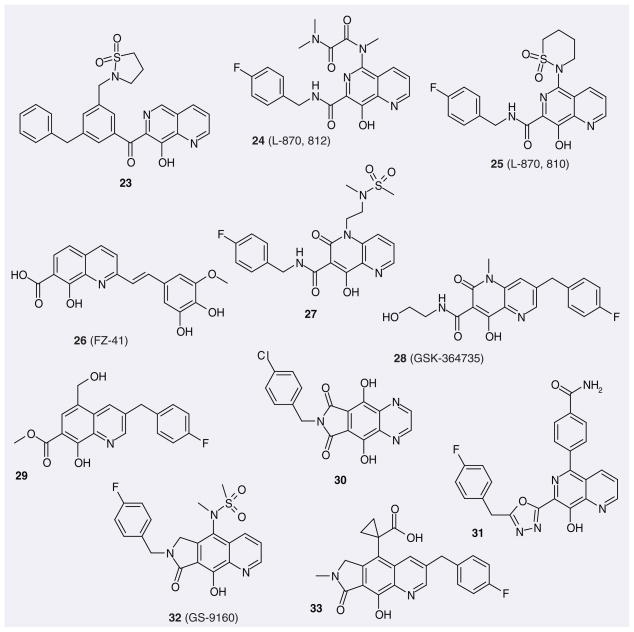
8-hydroxyquinoline and 8-hydroxy-1,6-naph-thyridine are recognized to bind divalent cations. Their carboxamides and related compounds were soon identified and patented as HIV-1 IN inhibitors by Merck, Shionogi, GSK, Gilead, and so forth. The 8-hydroxy-1,6-naph-thyridine 23 (Figure 6) showed excellent potency toward ST and HIV replication [56]. L-870,812 (24) from Merck showed excellent inhibition of ST and HIV replication and only moderate affinity for serum protein [57]. This compound also showed efficacy against Simian immunodeficiency virus, with an IC95 of 350 nM [58]. L-870,810 (25) exhibited improved enzyme inhibitory activity over L-870,812, showed very good pharmacokinetic properties and reached Phase II clinical trials [59]. The 8-hydroxy-quinoline-7-carboxylic acid 26 (FZ-41) is not a selective ST inhibitor (IC50 for 3′-P and ST are 0.7 and 1.7 μM, respectively) [60,61]. Whereas this compound was identified as an in vitro IN inhibitor, the exact in vivo target is still unclear. An alternative template to 1,6-naph-thyridine is 4-hydroxy-2-oxo-1,2-dihydro-1,5-naphthyridine. The typical compound of this group is 27, which was also patented by Merck. GSK-364735 (28), co-patented by GSK and Shionogi, also contains this moiety [62]. It displayed potent antiretroviral activity at nanomolar concentrations and reached Phase II clinical trials. Interestingly, the binding mode of this compound seems to be reversed, in the sense that, for these compounds, the benzyl group is at the C3-position of the quinoline or naphthyridine ring system instead of being connected to the carboxamide group. The orientation of the fluorobenzene of 29 is similar. Tibotec patented a tricycle-based scaffold, containing a 5,8-dihydroxyl-1,4-naphthyridine moiety, as IN inhibitors. A typical compound is 30. GSK used a heterocyclic azole isostere to replace the carboxamide group present in L-870,810 and related analogs, and patented oxadiazole and triazole-substituted naphthyridines as IN inhibitors (e.g., 31), which had impressive biological and toxicological activities [63,64]. Gilead also reported a tricycle-based scaffold containing the 8-hydroxyquinoline moiety as IN inhibitors [65]. Among those, GS-9160 [66] (32) entered Phase I clinical trials but was not pursued further due to unfavorable bio-availability. Compound 33, also patented by Gilead, contains the same tricyclic scaffold but presents reversed benzene ring orientation, as explained above.
Figure 6.
Representative aza-naphthalenyl carboxamides and related compounds patented as HIV-1 integrase inhibitors.
Hydroxypyrimidinone carboxamides & related compounds
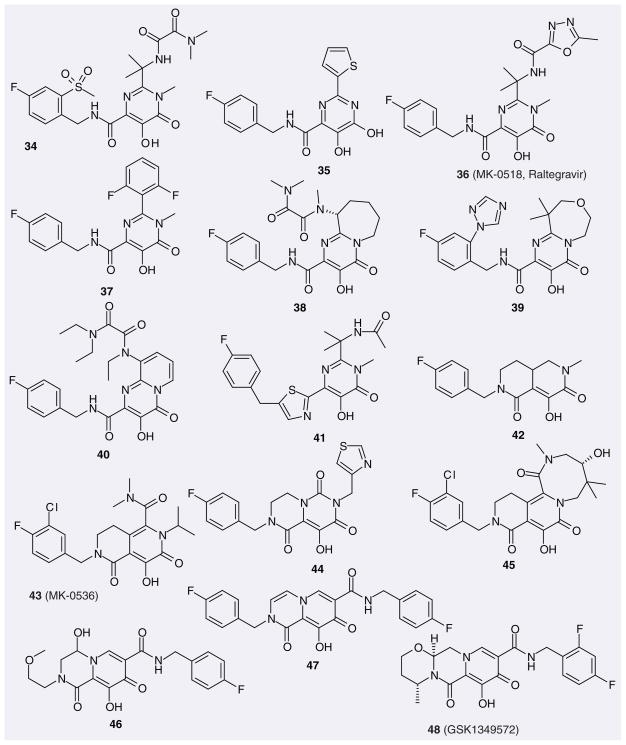
The Istituto Di Ricerche Di Biologia Molecolare (IRBM-MRL Rome) designed N-alkyl-5-hydroxypyrimidinone carboxamides (34; Figure 7; typical compound) and 4,5-dihydroxypyrimidine carboxamides (35; typical compound) as HIV-1 IN inhibitors based on their reported HCV polymerase inhibitors, dihydroxypyrimidine carboxylic acids [67,68]. These are two potent and selective classes of ST inhibitors. Their further evolution included optimization of potency, physicochemical properties and pharmacokinetic profiles led to the discovery and marketing of RAL (MK-0518, 36) [14,36]. BMS also registered a series of patents for inhibitors based on the N-alkyl-5-hydroxypyrimidinone carboxamide scaffold (37 is a representative structure). IRBM-MRL Rome and BMS further modified this scaffold by fusing the alkyl group into a pyrimidinone to form an additional ring (38–40). Shionogi used different azoles to replace the carboxamide group. The resulting compounds retained good inhibition towards ST and viral replication, with IC50 and EC50 values in the nanomolar range (e.g., 41). Merck further incorporated a hydroxypyrimidinone carboxamide moiety into different bicyclic and tricyclic scaffolds (e.g., 42–45), among which 43 (MK-0536) was chosen by Merck as a promising second-generation IN inhibitor owing to its excellent pharmacokinetic profile and improved cross resistance [201]. Shionogi patented a series of bicyclic carbamoylpyridone derivatives as IN inhibitors, in which the hydrophobic fluorobenzene rings of some (e.g., 46) have different orientations, while others (e.g., 47) have two fluorobenzene rings. Interestingly, the latter compounds show better inhibition for ST. In a recently published patent, GSK has disclosed the structure of GSK1349572 (48), which has entered Phase IIB trials. As of the time of writing, this compound is the only once-daily, unboosted IN inhibitor in clinical development [69].
Figure 7.
Representative hydroxypyrimidinone carboxamides and related compounds patented as HIV-1 integrase inhibitors.
Pyrrolecarboxamide & related compounds
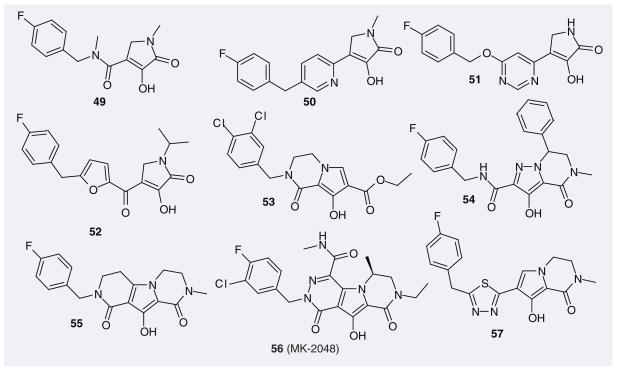
Further scaffolds based on the diketo acid pharmacophore have been designed, leading, for example, to 4-hydroxy-5-pyrrolinone IN inhibitors such as compounds 49–52 (Figure 8) [70,71] IC50 values in the low nanomolar range were found for some 4-hydroxy-5-pyrrolinone-3-carboxamide compounds, some of which, however, lacked cellular activity, possibly due to suboptimal physicochemical properties that could affect cell permeability and/or binding to intracellular proteins and also plasma proteins present in the cell medium [70]. However, when the carboxamide moiety was replaced by an azaheteroaromatic ring, the cellular activities improved dramatically, although the IC50 values dropped. For example, the EC50 values of compounds 50 and 51 from Shionogi are less than 0.25 μM (their IC50 values are 30–40 nM). Shionogi further modified such compounds using a moiety from their inhibitor S-1360 (12), which yielded compounds such as 52. However, their cellular activities were not markedly improved.
Figure 8.
Representative pyrrolecarboxamide and related compounds patented as HIV-1 integrase inhibitors.
Merck incorporated the pyrrolecarboxamide moiety into different bicyclic or tricyclic systems, which yielded clear improvement in antiretroviral activities (53 [72], 54 [73], 55 [72], 56 [74] and 57). Among those, MK-2048 (56) displayed potent antiretroviral activity with an EC95 value of 40 nM in cell culture and a favorable pharmaco-kinetic profile in dog and rat. Additionally, this compound exhibited effectiveness against first-generation IN drug-resistant viral strains and accordingly was chosen by Merck as a valuable second-generation IN inhibitor. Currently, this compound is still in preclinical study.
Quinolone carboxylic acids
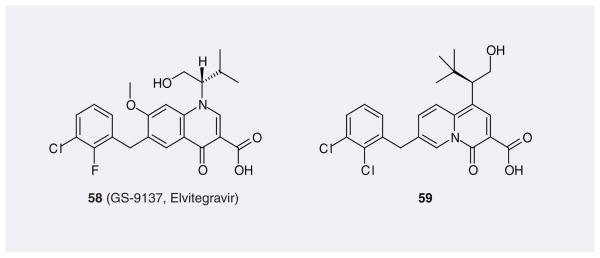
The 4-quinolone-3-glyoxlic acid scaffold was designed by Japan Tobacco, based on the idea that IN inhibitors with this scaffold may maintain the co-planarity of diketo acid functional groups. This scaffold did not show activity; interestingly, however, its precursor 4-quino-lone-3-carboxylic acid had shown IN inhibitory activity [75]. This finally led to the discovery of a very potent IN inhibitor, GS-9137, or EVG (58; Figure 9), which now is in Phase III clinical studies and is co-developed and commercialized by Gilead and Japan Tobacco. Experimental findings and advanced quantum-chemical calculations showed that 4-quinolone-3-carboxylic acid can form three chelating bond by using the carbonyl group and one oxygen atom in the acid group, which is different from the putative chelating mode of diketo acid and its bioisosteres [22]. Japan Tobacco further modified the scaffold structure from 4-quinolone-3-carboxylic acid to 4-oxo-4H-quinolizine-3-carboxylic acid, which also yielded good inhibition towards ST. The representative compound here is 59.
Figure 9.
Representative quinolone carboxylic acids patented as HIV-1 integrase inhibitors.
Others
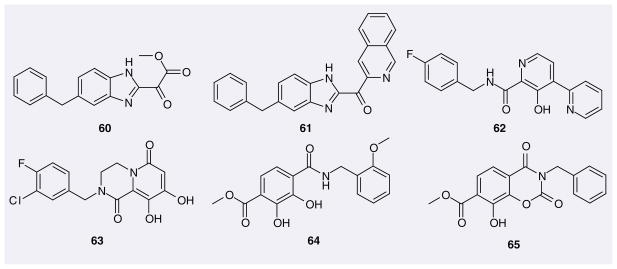
Shionogi has patented (1H-benzimidazol-2-yl)-oxo-acetic acid ester (60; Figure 10) and (1H-benzoimidazol-2-yl)-pyridin-2-yl-methanone (61) as IN inhibitors. Neither of these possess the acidic hydroxyl group. Their reported IC50 values are in the micromolar range.
Figure 10.
Miscellaneous structures patented as HIV-1 integrase inhibitors.
Virochem Pharma patented compounds based on a pyridine carboxamide scaffold as IN inhibitors. A typical compound in this series is 62.
Merck incorporated the dihydroxycarbonyl pharmacophore into a pyridinone scaffold, which led to the dihydroxypyridopyrazine-1,6-diones as novel IN inhibitors [76]. A representative from this series, compound 63, has an IC50 value of 0.04 μM for ST and an EC95 value of 0.25 μM.
IRM LLC patented the scaffold 4-(benzyl-carbamoyl)-2,3-dihydroxy-benzoate for IN inhibitors, whose IC50 and EC50 values are generally nanomolar (e.g., 64 & 65).
Success stories of authentic HIV-1 integrase inhibitors
After more than 25 years of AIDS research, there are currently approximately 25 drugs on the market that are approved for the treatment of HIV infection. In 2007, RAL (36) became the latest anti-HIV drug to be approved by the FDA for the treatment of HIV/AIDS in treatment-experienced patients. With the approval of RAL, the antiretroviral drug arsenal now contains weapons that target all three viral enzymes: RT, PR and IN.
As of early 2010, RAL is the only IN inhibitor approved for the treatment of patients suffering from HIV/AIDS. RAL is the successful result of a long-term research effort by Merck and Co. in the development of IN inhibitors [77]. The approval of RAL represents a major breakthrough in the treatment of HIV/AIDS. This orally administered drug (400 mg twice daily) is highly potent, well tolerated and exhibits excellent pharmacokinetics [37,77–79]. Recently, RAL has been co-administered with NNRTIs (etravirine) and PIs (darunavir or ritonavir) as a salvage therapy for heavily pretreated patients in virological failure with extensive multidrug resistances. In this context, RAL has been shown to achieve virological suppression similar to that observed in treatment-naive patients [80–83]. The robust clinical efficacy and tolerability of RAL instills tremendous hope for many patients who, until recently, were left with almost no treatment alternative. RAL has also been recently reported to be an alternative potential treatment for enfuvirtide-treated patients with stable suppressed viral load [84–87]. Enfuvirtide is an effective antiviral fusion inhibitor administered daily subcutaneously, which may be associated with injection-related side effects.
In July 2009, the FDA approval of RAL was broadened to the treatment of HIV/AIDS in treatment-naive patients. The replacement of the NNRTI efavirenz by RAL has been shown to lead to higher efficiency in the optimized background regimen composed of the NRTIs emtricitabine and tenofovir disoproxil fumarate (Truvada®) [87]. It is very likely that RAL, which has only been studied as a once-daily therapy for treatment-naive patients, will become a keystone of future multidrug cocktails to achieve an oral once-daily highly active antiretroviral therapy [88].
Elvitegravir (58) is, to our knowledge, the compound that is currently the next most advanced in the clinical development pipeline. It has not yet been approved [89]. This quinolone derivative, originally developed by Japan Tobacco Inc. [75], was subsequently licensed to Gilead Sciences under the name GS-9137 for further development. EVG, like RAL, is a potent antiviral agent [75,90–92] but exhibits a potentially higher cytotoxicity in noninfected cells [75]. EVG is also metabolized leading to partial inactivation [77,93], which could be overcome by a co-administration with ritonavir [93]. EVG is also being studied in combination with tenofovir, emtricitabine and cobicistat as a one-pill, once-a-day combination pill for the treatment of treatment-naive patients [202].
Unfortunately, the emergence of resistance leading to treatment failure has already been reported for RAL [77,89–92,94,95]. Three main resistance pathways involving the primary mutations Q148R/H/K, N155H and Y143R/C [90–92], are responsible for virological failure [96–100]. These pathways seem associated with secondary mutations that appear to rescue the viral fitness of those primary mutants: for instance G140S is observed together with Q148H, or G140A with Q148R [96,100]. Recently, EVG’s in vitro resistance profile was found to be similar to that of RAL, suggesting that EVG is unlikely to overcome resistance that has developed to RAL [89,92]. Therefore, continued development work towards novel IN inhibitors capable of overcoming RAL resistance is still very much warranted.
Future perspective
Integrase, which has no counterpart in humans, is now a validated target for the development of anti-HIV agents. However, our knowledge about its structure and function is still incomplete. After diketo acids were identified as ST-specific IN inhibitors and assay methods had matured, more and more compounds have been patented as IN inhibitors by different companies and agencies: to date, more than two hundred patents of, or related to, IN inhibitors have been registered. This effort has yielded one marketed IN inhibitor and several under clinical trial studies, which validates IN as an effective target for the treatment of HIV/AIDS. Both the success and the limitations of RAL (and HAART) clearly indicate the necessity of further development of IN inhibitors. Based on all the known authentic IN inhibitors, some of which have been presented in this review, an analog-based IN inhibitor design would seem to be an effective strategy. The hope and anticipation is that such efforts will lead to additional authentic IN inhibitors being patented in the near future and ultimately made available to patients. Ideally, these new IN inhibitors should successfully address the issues of dosing regimens, and more importantly, viral resistance, which will continue to arise as IN inhibition-based drugs are used.
Most of the ‘authentic’ IN inhibitors presented in this review can be thought of as structural variations on the original ‘diketo acid’ motif, whose mechanisms of action are presumed to involve chelation of catalytic divalent metal ions. These include RAL and other IN inhibitors in clinical trials. The major challenge facing further development of IN inhibitors lies in overcoming resistance to current clinical agents. Accordingly, evaluation of analogues against panels of IN constructs that cover the major patterns of clinical resistance should be an integral component of ongoing medicinal chemistry in this area.
Supplementary Material
Key Terms
- HAART
Therapies based on a combination of antiretroviral drugs – typically three or four – to create multiple obstacles to HIV replication and to increase the number of mutations required for drug resistance
- PIC
Large structure including the HIV viral genome associated with viral and host proteins. The PIC enters the host cell’s nucleus, which is the prerequisite for integration of the viral DNA into the host DNA. It includes the newly synthesized DNA, integrase, nucleocapsid protein, structural protein p6, the accessory protein Vpr, and several copies of matrix protein
- INSTI
Class of HIV-1 IN inhibitors that show higher potency in enzymatic assays against the strand-transfer step of the viral genome integration catalyzed by IN than against the 3′-processing step. Authentic IN inhibitors have exclusively been found among INSTIs so far
- Chelation
Formation or presence of two or more separate bonding interactions between a multiply bonded ligand and a single central metal atom. In the medicinal chemistry context, such ligands (‘chelators’) are organic compounds that inactivate an ion necessary for the normal functioning of an enzyme, for example the Mg2+ ions required for the catalytic activity of IN. Chelation is the mechanism of action of some approved drugs, for example the antibiotic drugs of the tetracycline family are chelators of Ca2+ and Mg2+ ions
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The authors have no potential conflicts with the subject matter or materials discussed in this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Several different identifiers, including standard InChIKey, FICuS [101], SMILES and standard InChI for the compounds presented in this review have been calculated. This material is available free of charge at www.future-science.com.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ of considerable interest
- 1.Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 2.Masur H, Michelis MA, Greene JB, et al. An outbreak of community–acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981;305(24):1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 3.Siegal FP, Lopez C, Hammer GS, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 4.Sanders RW, Dankers MM, Busser E, et al. Evolution of the HIV-1 envelope glycoproteins with a disulfide bond between gp120 and gp41. Retrovirology. 2004;1:3–13. doi: 10.1186/1742-4690-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richman DD. HIV chemotherapy. Nature. 2001;410(6831):995–1001. doi: 10.1038/35073673. [DOI] [PubMed] [Google Scholar]
- 6.Sarafianos SG, Marchand B, Das K, et al. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385(3):693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony NJ. HIV-1 integrase: a target for new AIDS chemotherapeutics. Curr Top Med Chem. 2004;4(9):979–990. doi: 10.2174/1568026043388448. [DOI] [PubMed] [Google Scholar]
- 8.Louis JM, Ishima R, Torchia DA, Weber IT. HIV-1 protease: structure, dynamics, and inhibition. Adv Pharmacol. 2007;55:261–298. doi: 10.1016/S1054-3589(07)55008-8. [DOI] [PubMed] [Google Scholar]
- 9▪▪.Flexner C. HIV drug development: the next 25 years. Nat Rev Drug Discov. 2007;6(12):959–966. doi: 10.1038/nrd2336. Excellent summary of HIV-1 drug development. New opportunities for anti-HIV drug discovery are also discussed. [DOI] [PubMed] [Google Scholar]
- 10▪▪.Nikolopoulos G, Bonovas S, Tsantes A, Sitaras NM. HIV/AIDS: recent advances in antiretroviral agents. Mini Rev Med Chem. 2009;9(8):900–910. doi: 10.2174/138955709788681609. Review summarizing the conventional compounds, as well as describing recently approved agents and taking a comprehensive look at the clinically relevant findings of current research on HIV/AIDS. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Goila-Gaur R, Salzwedel K, et al. PA–457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci. 2003;100(23):13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 13.Becker SL, Dezii CM, Burtcel B, et al. Young HIV-infected adults are at greater risk for medication nonadherence. MedGenMed. 2002;4(3):21. [PubMed] [Google Scholar]
- 14.Anker M, Corales RB. Raltegravir (MK-0518): a novel integrase inhibitor for the treatment of HIV infection. Expert Opin Investig Drugs. 2008;17(1):97–103. doi: 10.1517/13543784.17.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Croxtall JD, Keam SJ. Raltegravir: a review of its use in the management of HIV infection in treatment-experienced patients. Drugs. 2009;69(8):1059–1075. doi: 10.2165/00003495-200969080-00007. [DOI] [PubMed] [Google Scholar]
- 16▪.Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4(3):236–248. doi: 10.1038/nrd1660. Focuses on the molecular basis and rationale of developing HIV-1 integrase (IN) inhibitors. The main classes of lead compounds are also described, as well as the concept of interfacial inhibitors of protein–nucleic acid interactions that might apply to the clinically used strand-transfer inhibitors. [DOI] [PubMed] [Google Scholar]
- 17▪.Savarino A. A historical sketch of the discovery and development of HIV–1 integrase inhibitors. Expert Opin Investig Drugs. 2006;15(12):1507–1522. doi: 10.1517/13543784.15.12.1507. Review of the history of the discovery and development of HIV-1 IN inhibitors. [DOI] [PubMed] [Google Scholar]
- 18.Rice PA, Baker TA. Comparative architecture of transposase and integrase complexes. Nat Struct Biol. 2001;8(5):302–307. doi: 10.1038/86166. [DOI] [PubMed] [Google Scholar]
- 19.Ramratnam B, Mittler JE, Zhang L, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 20.Marchand C, Johnson AA, Karki RG, et al. Metal-dependent inhibition of HIV-1 integrase by β-diketo acids and resistance of the soluble double-mutant (F185K/C280S) Mol Pharmacol. 2003;64(3):600–609. doi: 10.1124/mol.64.3.600. [DOI] [PubMed] [Google Scholar]
- 21.Marchand C, Neamati N, Pommier Y. In vitro human immunodeficiency virus type 1 integrase assays. Methods Enzymol. 2001;340:624–633. doi: 10.1016/s0076-6879(01)40446-0. [DOI] [PubMed] [Google Scholar]
- 22.Liao C, Nicklaus MC. Tautomerism and magnesium chelation of HIV-1 integrase inhibitors: a theoretical study. ChemMedChem. 2010;5:1053–1066. doi: 10.1002/cmdc.201000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debyser Z, Cherepanov P, Pluymers W, De Clercq E. Assays for the evaluation of HIV-1 integrase inhibitors. Methods Mol Biol. 2001;160:139–155. doi: 10.1385/1-59259-233-3:139. [DOI] [PubMed] [Google Scholar]
- 24.Sinha S, Pursley MH, Grandgenett DP. Efficient concerted integration by recombinant human immunodeficiency virus type 1 integrase without cellular or viral cofactors. J Virol. 2002;76(7):3105–3113. doi: 10.1128/JVI.76.7.3105-3113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Mizuuchi M, Burke TR, Jr, Craigie R. Retroviral DNA integration: reaction pathway and critical intermediates. EMBO J. 2006;25(6):1295–1304. doi: 10.1038/sj.emboj.7601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fesen MR, Kohn KW, Leteurtre F, Pommier Y. Inhibitors of human immunodeficiency virus integrase. Proc Natl Acad Sci. 1993;90(6):2399–2403. doi: 10.1073/pnas.90.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu TK, Davies DR. Structure and function of HIV-1 integrase. Curr Top Med Chem. 2004;4(9):965–977. doi: 10.2174/1568026043388547. [DOI] [PubMed] [Google Scholar]
- 28.Semenova EA, Johnson AA, Marchand C, et al. Preferential inhibition of the magnesium-dependent strand transfer reaction of HIV-1 integrase by α-hydroxytropolones. Mol Pharmacol. 2006;69(4):1454–1460. doi: 10.1124/mol.105.020321. [DOI] [PubMed] [Google Scholar]
- 29.Svarovskaia ES, Barr R, Zhang X, Pais GC, et al. Azido-containing diketo acid derivatives inhibit human immunodeficiency virus type 1 integrase in vivo and influence the frequency of deletions at two-long-terminal-repeat-circle junctions. J Virol. 2004;78(7):3210–3222. doi: 10.1128/JVI.78.7.3210-3222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchand C, Maddali K, Metifiot M, Pommier Y. HIV-1 IN inhibitors: 2010 update and perspectives. Curr Top Med Chem. 2009;9(11):1016–1037. doi: 10.2174/156802609789630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Field AK. Oligonucleotides as inhibitors of human immunodeficiency virus. Curr Opin Mol Ther. 1999;1(3):323–331. [PubMed] [Google Scholar]
- 32.Goldgur Y, Craigie R, Cohen GH, et al. Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: a platform for antiviral drug design. Proc Natl Acad Sci. 1999;96(23):13040–13043. doi: 10.1073/pnas.96.23.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neamati N. Patented small molecule inhibitors of HIV-1 integrase: a 10-year saga. Expert Opin Therap Patents. 2002;12(5):709–724. [Google Scholar]
- 34.Billich A. S-1360 Shionogi-GlaxoSmithKline. Curr Opin Investig Drugs. 2003;4:206–209. [PubMed] [Google Scholar]
- 35.Grobler JA, Stillmock K, Hu B, et al. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc Natl Acad Sci. 2002;99(10):6661–6666. doi: 10.1073/pnas.092056199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Summa V, Petrocchi A, Bonelli F, et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem. 2008;51(18):5843–5855. doi: 10.1021/jm800245z. Description from the point of view of medicinal chemistry of how raltegravir was developed. [DOI] [PubMed] [Google Scholar]
- 37▪.Al-Mawsawi LQ, Al-Safi RI, Neamati N. Anti-infectives: clinical progress of HIV-1 integrase inhibitors. Expert Opin Emerg Drugs. 2008;13(2):213–225. doi: 10.1517/14728214.13.2.213. Discusses some HIV-1 IN inhibitors that were/are in clinical studies. [DOI] [PubMed] [Google Scholar]
- 38.Palmisano L. Role of integrase inhibitors in the treatment of HIV disease. Expert Rev Anti Infect Ther. 2007;5(1):67–75. doi: 10.1586/14787210.5.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Pommier Y, Neamati N. Inhibitors of human immunodeficiency virus integrase. Adv Virus Res. 1999;52:427–458. doi: 10.1016/s0065-3527(08)60310-3. [DOI] [PubMed] [Google Scholar]
- 40.Young SD. Inhibition of HIV-1 integrase by small molecules: the potential for a new class of AIDS chemotherapeutics. Curr Opin Drug Discov Devel. 2001;4(4):402–410. [PubMed] [Google Scholar]
- 41.Neamati N, Marchand C, Pommier Y. HIV-1 integrase inhibitors: past, present, and future. Adv Pharmacol. 2000;49:147–165. doi: 10.1016/s1054-3589(00)49026-5. [DOI] [PubMed] [Google Scholar]
- 42.Gupta SP, Nagappa AN. Design and development of integrase inhibitors as anti-HIV agents. Curr Med Chem. 2003;10(18):1779–1794. doi: 10.2174/0929867033456972. [DOI] [PubMed] [Google Scholar]
- 43.Witvrouw M, Van Maele B, Vercammen J, et al. Novel inhibitors of HIV-1 integration. Curr Drug Metab. 2004;5(4):291–304. doi: 10.2174/1389200043335487. [DOI] [PubMed] [Google Scholar]
- 44.Johns BA, Svolto AC. Advances in two-metal chelation inhibitors of HIV integrase. Expert Opin Therap Patents. 2008;18(11):1225–1237. [Google Scholar]
- 45.Barreca ML, Ferro S, Rao A, et al. Pharmacophore-based design of HIV-1 integrase strand-transfer inhibitors. J Med Chem. 2005;48(22):7084–7088. doi: 10.1021/jm050549e. [DOI] [PubMed] [Google Scholar]
- 46.Kawasuji T, Yoshinaga T, Sato A, et al. A platform for designing HIV integrase inhibitors. Part 1: 2-hydroxy-3-heteroaryl acrylic acid derivatives as novel HIV integrase inhibitor and modeling of hydrophilic and hydrophobic pharmacophores. Bioorg Med Chem. 2006;14(24):8430–8445. doi: 10.1016/j.bmc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 47.Deng J, Dayam R, Al-Mawsawi LQ, Neamati N. Design of second generation HIV-1 integrase inhibitors. Curr Pharm Des. 2007;13(2):129–141. doi: 10.2174/138161207779313687. [DOI] [PubMed] [Google Scholar]
- 48.Pace P, Di Francesco ME, Gardelli C, et al. Dihydroxypyrimidine-4-carboxamides as novel potent and selective HIV integrase inhibitors. J Med Chem. 2007;50(9):2225–2239. doi: 10.1021/jm070027u. [DOI] [PubMed] [Google Scholar]
- 49.Kirschberg T, Parrish J. Metal chelators as antiviral agents. Curr Opin Drug Discov Devel. 2007;10(4):460–472. [PubMed] [Google Scholar]
- 50.Wai JS, Egbertson MS, Payne LS, et al. 4-aryl-2,4-dioxobutanoic acid inhibitors of HIV-1 integrase and viral replication in cells. J Med Chem. 2000;43(26):4923–4926. doi: 10.1021/jm000176b. [DOI] [PubMed] [Google Scholar]
- 51.Walker MA, Johnson T, Naidu BN, et al. Benzyl amide-ketoacid inhibitors of HIV-integrase. Bioorg Med Chem Lett. 2007;17(17):4886–4890. doi: 10.1016/j.bmcl.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 52.Di Santo R, Costi R, Roux A, et al. Novel bifunctional quinolonyl diketo acid derivatives as HIV-1 integrase inhibitors: design, synthesis, biological activities, and mechanism of action. J Med Chem. 2006;49(6):1939–1945. doi: 10.1021/jm0511583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pais GC, Zhang X, Marchand C, et al. Structure activity of 3-aryl-1,3-diketo-containing compounds as HIV-1 integrase inhibitors. J Med Chem. 2002;45(15):3184–3194. doi: 10.1021/jm020037p. [DOI] [PubMed] [Google Scholar]
- 54.Pais GCG, Burke TR., Jr Novel aryl diketo-containing inhibitors of HIV–1 integrase. Drugs Future. 2002;27(11):1101–1111. [Google Scholar]
- 55.Kawasuji T, Fuji M, Yoshinaga T, Sato A, et al. A platform for designing HIV integrase inhibitors. Part 2: a two-metal binding model as a potential mechanism of HIV integrase inhibitors. Bioorg Med Chem. 2006;14(24):8420–8429. doi: 10.1016/j.bmc.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang L, Wai JS, Embrey MW, et al. Design and synthesis of 8-hydroxy-[1,6] naphthyridines as novel inhibitors of HIV-1 integrase in vitro and in infected cells. J Med Chem. 2003;46(4):453–456. doi: 10.1021/jm025553u. [DOI] [PubMed] [Google Scholar]
- 57.Guare JP, Wai JS, Gomez RP, et al. A series of 5-aminosubstituted 4-fluorobenzyl-8-hydroxy-[1,6]naphthyridine-7-carboxamide HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2006;16(11):2900–2904. doi: 10.1016/j.bmcl.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Hazuda DJ, Young SD, Guare JP, et al. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305(5683):528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- 59.Hazuda DJ, Anthony NJ, Gomez RP, et al. A naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integrase. Proc Natl Acad Sci. 2004;101(31):11233–11238. doi: 10.1073/pnas.0402357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mousnier A, Leh H, Mouscadet JF, Dargemont C. Nuclear import of HIV-1 integrase is inhibited in vitro by styrylquinoline derivatives. Mol Pharmacol. 2004;66(4):783–788. doi: 10.1124/mol.104.001735. [DOI] [PubMed] [Google Scholar]
- 61.Zouhiri F, Mouscadet JF, Mekouar K, et al. Structure-activity relationships and binding mode of styrylquinolines as potent inhibitors of HIV-1 integrase and replication of HIV-1 in cell culture. J Med Chem. 2000;43(8):1533–1540. doi: 10.1021/jm990467o. [DOI] [PubMed] [Google Scholar]
- 62.Garvey EP, Johns BA, Gartland MJ, et al. The naphthyridinone GSK364735 is a novel, potent human immunodeficiency virus type 1 integrase inhibitor and antiretroviral. Antimicrob Agents Chemother. 2008;52(3):901–908. doi: 10.1128/AAC.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johns BA, Weatherhead JG, Allen SH, et al. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 1: establishing the pharmacophore. Bioorg Med Chem Lett. 2009;19(6):1802–1806. doi: 10.1016/j.bmcl.2009.01.090. [DOI] [PubMed] [Google Scholar]
- 64.Johns BA, Weatherhead JG, Allen SH, et al. 1,3,4-oxadiazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 2: SAR of the C5 position. Bioorg Med Chem Lett. 2009;19(6):1807–1810. doi: 10.1016/j.bmcl.2009.01.089. [DOI] [PubMed] [Google Scholar]
- 65.Metobo SE, Jin H, Tsiang M, Kim CU. Design, synthesis, and biological evaluation of novel tricyclic HIV-1 integrase inhibitors by modification of its pyridine ring. Bioorg Med Chem Lett. 2006;16(15):3985–3988. doi: 10.1016/j.bmcl.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Jones GS, Yu F, Zeynalzadegan A, et al. Preclinical evaluation of GS-9160, a novel inhibitor of human immunodeficiency virus type 1 integrase. Antimicrob Agents Chemother. 2009;53(3):1194–1203. doi: 10.1128/AAC.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summa V, Petrocchi A, Matassa VG, et al. 4,5-dihydroxypyrimidine carboxamides and N-alkyl-5-hydroxypyrimidinone carboxamides are potent, selective HIV integrase inhibitors with good pharmacokinetic profiles in preclinical species. J Med Chem. 2006;49(23):6646–6649. doi: 10.1021/jm060854f. [DOI] [PubMed] [Google Scholar]
- 68.Petrocchi A, Koch U, Matassa VG, et al. From dihydroxypyrimidine carboxylic acids to carboxamide HIV-1 integrase inhibitors: SAR around the amide moiety. Bioorg Med Chem Lett. 2007;17(2):350–353. doi: 10.1016/j.bmcl.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 69.Vandeckerckhove L. GSK-1349572, a novel integrase inhibitor for the treatment of HIV infection. Curr Opin Investig Drugs. 2010;11(2):203–212. [PubMed] [Google Scholar]
- 70.Pace P, Spieser SA, Summa V. 4-hydroxy-5-pyrrolinone-3-carboxamide HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2008;18(14):3865–3869. doi: 10.1016/j.bmcl.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 71.Kawasuji T, Fuji M, Yoshinaga T, et al. 3-hydroxy-1,5-dihydro-pyrrol-2-one derivatives as advanced inhibitors of HIV integrase. Bioorg Med Chem. 2007;15(16):5487–5492. doi: 10.1016/j.bmc.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 72.Fisher TE, Kim B, Staas DD, et al. 8-hydroxy-3,4-dihydropyrrolo[1,2-a]pyrazine-1(2H)-one HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2007;17(23):6511–6515. doi: 10.1016/j.bmcl.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 73.Langford HM, Williams PD, Homnick CF, et al. Design and synthesis of substituted 4-oxo-4,5,6,7-tetrahydropyrazolo[1,5-a] pyrazine-2-carboxamides, novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2008;18(2):721–725. doi: 10.1016/j.bmcl.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 74.Wiscount CM, Williams PD, Tran LO, et al. 10-hydroxy-7,8-dihydropyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazine-1,9(2H,6 H)-diones: potent, orally bioavailable HIV-1 integrase strand-transfer inhibitors with activity against integrase mutants. Bioorg Med Chem Lett. 2008;18(16):4581–4583. doi: 10.1016/j.bmcl.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 75.Sato M, Motomura T, Aramaki H, et al. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J Med Chem. 2006;49(5):1506–1508. doi: 10.1021/jm0600139. [DOI] [PubMed] [Google Scholar]
- 76.Wai JS, Kim B, Fisher TE, et al. Dihydroxypyridopyrazine-1,6-dione HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2007;17(20):5595–5599. doi: 10.1016/j.bmcl.2007.07.092. [DOI] [PubMed] [Google Scholar]
- 77.Pace P, Rowley M. Integrase inhibitors for the treatment of HIV infection. Curr Opin Drug Discov Devel. 2008;11(4):471–479. [PubMed] [Google Scholar]
- 78.Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83(2):293–299. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 79.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46(2):125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 80.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49(9):1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 81.Thuret I, Chaix ML, Tamalet C, Aumaitre H, Blanche S, et al. Raltegravir, etravirine and r-darunavir combination in adolescents with multidrug-resistant virus. AIDS. 2009;23(17):2364–2366. doi: 10.1097/QAD.0b013e328331a456. [DOI] [PubMed] [Google Scholar]
- 82.McKinnell JA, Lin HY, Nevin CN, et al. Early virologic suppression with three-class experienced patients: 24-week effectiveness in the darunavir outcomes study. AIDS. 2009;23(12):1539–1546. doi: 10.1097/QAD.0b013e32832c7b5c. [DOI] [PubMed] [Google Scholar]
- 83.Imaz A, del Saz SV, Ribas MA, et al. Raltegravir, etravirine, and ritonavir-boosted darunavir: a safe and successful rescue regimen for multidrug-resistant HIV-1 infection. J Acquir Immune Defic Syndr. 2009;52(3):382–386. doi: 10.1097/QAI.0b013e3181b17f53. [DOI] [PubMed] [Google Scholar]
- 84.Towner W, Klein D, Kerrigan HL, et al. Virologic outcomes of changing enfuvirtide to raltegravir in HIV-1 patients well controlled on an enfuvirtide based regimen: 24-week results of the CHEER study. J Acquir Immune Defic Syndr. 2009;51(4):367–373. doi: 10.1097/QAI.0b013e3181ae35de. [DOI] [PubMed] [Google Scholar]
- 85.De Castro N, Braun J, Charreau I, Pialoux G, et al. Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trial. Clin Infect Dis. 2009;49(8):1259–1267. doi: 10.1086/605674. [DOI] [PubMed] [Google Scholar]
- 86.Grant PM, Palmer S, Bendavid E, et al. Switch from enfuvirtide to raltegravir in virologically suppressed HIV-1 infected patients: effects on level of residual viremia and quality of life. J Clin Virol. 2009;46(4):305–308. doi: 10.1016/j.jcv.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 88.De Clercq E. A new drug combination therapy for treatment-naive patients with HIV-1 infection, consisting of raltegravir, emtricitabine and tenofovir disoproxil fumarate. Expert Opin Pharmacother. 2009;10(17):2935–2937. doi: 10.1517/14656560903418467. [DOI] [PubMed] [Google Scholar]
- 89.McColl DJ, Chen X. Strand transfer inhibitors of HIV-1 integrase: bringing IN a new era of antiretroviral therapy. Antiviral Res. 2009;85(1):101–118. doi: 10.1016/j.antiviral.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi M, Nakahara K, Seki T, et al. Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res. 2008;80(2):213–222. doi: 10.1016/j.antiviral.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Shimura K, Kodama E, Sakagami Y, et al. Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137) J Virol. 2008;82(2):764–774. doi: 10.1128/JVI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marinello J, Marchand C, Mott BT, et al. Comparison of raltegravir and elvitegravir on HIV-1 integrase catalytic reactions and on a series of drug-resistant integrase mutants. Biochemistry. 2008;47(36):9345–9354. doi: 10.1021/bi800791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramanathan S, Shen G, Hinkle J, et al. Pharmacokinetics of coadmistered ritonavir-boosted elvitegravir and zidovirine, didanosine, stavudine or abacavir. J Acquir Immune Defic Syndr. 2007;46(2):160–166. doi: 10.1097/QAI.0b013e318151fd9a. [DOI] [PubMed] [Google Scholar]
- 94.Buzon MJ, Dalmau J, Puertas MC, et al. The HIV-1 integrase genotype strongly predicts raltegravir susceptibility but not viral fitness of primary virus isolates. AIDS. 2009;24(1):17–25. doi: 10.1097/QAD.0b013e328331c81e. [DOI] [PubMed] [Google Scholar]
- 95.Fransen S, Karmochkine M, Huang W, et al. Longitudinal analysis of raltegravir susceptibility and integrase replication capacity of human immunodeficiency virus type 1 during virologic failure. Antimicrob Agents Chemother. 2009;53(10):4522–4524. doi: 10.1128/AAC.00651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delelis O, Malet I, Na L, et al. The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation. Nucleic Acids Res. 2009;37(4):1193–1201. doi: 10.1093/nar/gkn1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother. 2008;52(4):1351–1358. doi: 10.1128/AAC.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delelis O, Thierry S, Subra F, et al. Impact of Y143 HIV-1 integrase mutations on resistance to raltegravir in vitro and in vivo. Antimicrob Agents Chemother. 2009;54(1):491–501. doi: 10.1128/AAC.01075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sichtig N, Sierra S, Kaiser R, et al. Evolution of raltegravir resistance during therapy. J Antimicrob Chemother. 2009;64(1):25–32. doi: 10.1093/jac/dkp153. [DOI] [PubMed] [Google Scholar]
- 100.Malet I, Delelis O, Soulie C, et al. Quasispecies variant dynamics during emergence of resistance to raltegravir in HIV-1-infected patients. J Antimicrob Chemother. 2009;63(4):795–804. doi: 10.1093/jac/dkp014. [DOI] [PubMed] [Google Scholar]
- 101.Sitzmann M, Filippov IV, Nicklaus MC. Internet resources integrating many small-molecule databases. SAR QSAR Environ Res. 2008;19(1–2):1–9. doi: 10.1080/10629360701843540. [DOI] [PubMed] [Google Scholar]
Websites
- 201.National AIDS treatment advocacy project. www.natap.org/2008/ICAAC/ICAAC_68.htm.
- 202.Clinicaltrials.gov. http://clinicaltrials.gov/ct2/show/NCT00869557.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.