Abstract
Objective
Maternal circulating visfatin concentrations are higher in patients with a small-for-gestational-age (SGA) neonate than in those who delivered an appropriate-for-gestational age AGA neonate or in those with preeclampsia. It has been proposed that enhanced transfer of visfatin from the fetal to maternal circulation may account for the high concentrations of maternal visfatin observed in patients with an SGA neonate. The aims of this study were: 1) to determine whether cord blood visfatin concentrations differ between normal neonates, SGA neonates and newborns of preeclamptic mothers; and 2) to assess the relationship between maternal and fetal circulating visfatin concentrations in patients with an SGA neonate and those with preeclampsia.
Study design
This cross-sectional study included 88 pregnant women and their neonates, as well as 22 preterm neonates in the following groups: 1) 44 normal pregnant women at term and their AGA neonates; 2) 22 normotensive pregnant women and their SGA neonates; 3) 22 women with preeclampsia and their neonates; and 4) 22 preterm neonates delivered following spontaneous preterm labor without funisitis or histologic chorioamnionitis, matched for gestational age with infants of preeclamptic mothers. Maternal plasma and cord blood visfatin concentrations were determined by ELISA. Non-parametric statistics were used for analyses.
Results
1) The median visfatin concentration was lower in umbilical cord blood than in maternal circulation, in normal pregnancy, SGA and preeclampsia groups (p<0.001 for all comparisons); 2) the median cord blood visfatin concentrations did not differ significantly between term AGA or SGA neonates, infants of mothers with preeclampsia and their gestational-age-matched preterm AGA neonates; 3) maternal and cord blood visfatin concentrations correlated only in the normal term group (r= 0.48, p=0.04).
Conclusion
Circulating visfatin concentrations are lower in the fetal than in the maternal circulation and did not significantly differ between the study groups. Thus, it is unlikely that the fetal circulation is the source of the high maternal visfatin concentrations reported in patients with an SGA neonate.
Keywords: visfatin, adipokines, cytokine, pregnancy, fetal growth restriction, AGA, umbilical cord blood
Introduction
Preeclampsia and delivery of a small-for-gestational age (SGA) neonates, two of the “great obstetrical syndromes”[1], share several mechanisms of disease including failure of physiologic transformation of the spiral arteries [2,3], an anti-angiogenic state [4–18], endothelial cell dysfunction [19–21], and an increased maternal intravascular inflammatory response [15,22–32]. Despite these similarities, preeclampsia and pregnancies complicated by an SGA neonate have different clinical manifestations. While preeclampsia is characterized by hypertension, proteinuria and organ damage [33,34], SGA is usually defined as a birthweight below the 10th percentile for gestational age at birth according to the birth weight distribution of a particular population [35]. Hypertension, proteinuria and organ damage are not clinical features of pregnancies with isolated SGA.
Several explanations have been proposed to reconcile this apparent disparity including exposure to infection during pregnancy [36,37], differences in the profile of angiogenic and anti-angiogenic response to intrauterine insults [13,17], altered activity of the coagulation system [38], and changes in the concentrations of placental growth hormone [39] and pro-inflammatory chemokines (CXCL10/IP-10) [15]. Ness and Sibai [19] have proposed that the presence of altered metabolic states (e.g. obesity, insulin resistance, dyslipidemia) predisposes pregnant women to develop preeclampsia, while the absence of these metabolic derangements will result in an SGA neonate.
Visfatin, a newly discovered 52 kDa adipokine, has been implicated in the regulation of glucose homeostasis [40], Type-2 diabetes mellitus (Type-2 DM) [41], gestational diabetes mellitus (GDM) [42–46], as well as in fetal growth [47]. Recently, we have reported that patients with an SGA neinate, but not those with preeclampsia, had a higher maternal plasma visfatin concentration than those with a normal pregnancy [48] suggesting that perturbation of visfatin homeostasis may be implicated in the phenotypic distinction between preeclampsia and SGA. Nevertheless, the source of the higher maternal circulating visfatin concentrations in patients with an SGA neonate has not yet been determined. It has been hypothesized that enhanced transfer of visfatin from the fetal to the maternal circulation can account for the high concentrations of maternal visfatin reported in patients with an SGA neonate. Thus, the aims of this study were: 1) to determine whether cord blood visfatin concentration differ between normal neonates, SGA neonates and newborns of preeclamptic mothers; and 2) to assess the relationship between maternal and fetal circulating visfatin concentrations in patients with an SGA neonate and those with preeclampsia.
Materials and Methods
Study Population
A case-control study was conducted by searching our clinical database and bank of biological samples, and included 88 pregnant women and their neonates, as well as 22 preterm neonates, in the following groups: 1) 44 normal pregnant women at term and their appropriate-for-gestational age (AGA) neonates; 2) 22 normotensive pregnant women and their SGA neonates; 3) 22 women with preeclampsia and their neonates; and 4) 22 preterm AGA neonates delivered following spontaneous preterm labor without funisitis or histologic chorioamnionitis, matched for gestational age with neonates of the preeclampsia group.
Maternal plasma, umbilical cord blood and clinical and demographic data were retrieved from our bank of biological samples and clinical database. Many of these samples have previously been employed to study the biology of inflammation, homeostasis, angiogenesis regulation and adipokines concentrations in normal pregnant women and those with pregnancy complications. Maternal visfatin concentrations for the patients included in the present study have been previously reported [48] and were included in this study in order to provide a complete picture to the reader.
All participating women provided written informed consent prior to enrolment and the collection of blood samples. The collection and use of blood for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital (Santiago, Chile) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS, Bethesda, Maryland, USA).
Definitions
The inclusion criteria for normal pregnancy were: 1) no medical, obstetrical or surgical complications; 2) intact membranes; 3) delivery of a term neonate (>37 weeks) with a birth weight above the 10th percentile;[49] and 4) a normal oral 75-g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation based on the World Health Organization (WHO) criteria [50].
Preeclampsia was defined as the presence of hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) first occurring after 20 weeks of gestation in a woman with previously normal blood pressure, and proteinuria (≥300 mg in a 24-hours urine collection or at least one dipstick measurement ≥1+) [51]. The diagnosis of SGA was based on ultrasonographic estimated fetal weight and confirmed by a birth weight below the 10th percentile for gestational age [49]. The body mass index (BMI) was calculated according to the following formula: weight (kg)/height (m2). Normal weight women were defined as those with a BMI of 18.5–24.9 kg/m2 according to the definition of the WHO [52]. Ponderal Index was calculated according to the following formula: weight (kg)/height (m3).
Sample collection and Human Visfatin C-terminal immunoassay
Maternal blood samples were collected during clinical visits and umbilical cord blood was obtained from the umbilical vein at the time of delivery. Blood was centrifuged at 1300 × g for 10 minutes at 4°C. The plasma obtained was stored at −80°C until analysis.
Comparison between maternal and fetal circulating visfatin concentrations, as well as correlation analysis was conducted only in cases in which the time interval between maternal blood sampling and delivery was less than 48 hours. The 48 hours interval was chosen to maintain a meaningful temporal relationship of visfatin concentrations between umbilical cord blood and maternal blood.
Concentrations of visfatin in maternal and fetal plasma were determined using specific and sensitive enzyme immunoassays (Phoenix Pharmaceuticals, Inc. Belmont, CA, USA). An initial assay validation was performed in our laboratory prior to the conduction of this study. A detailed description of the assay has been previously published [46,53–55]. The calculated inter- and intra-assay coefficients of variation for visfatin C-terminal immunoassays in our laboratory were 5.3% and 2.4%, respectively. The sensitivity was calculated to be 0.04 ng/ml.
Statistical analysis
Normality of the data was tested using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Since maternal plasma and umbilical cord blood visfatin concentrations were not normally distributed, Kruskal–Wallis tests with post-hoc analysis by Mann-Whitney U tests were used for comparisons of continuous variables between the different groups. Comparison of proportions was performed by Fisher's test. Wilcoxon Signed ranks test exact test was used to compare visfatin concentrations between mather-neonate pairs. Spearman rank correlation was utilized to assess correlations between umbilical cord blood visfatin concentrations and birthweight, Ponderal Index, gestational age at delivery, maternal plasma visfatin concentration and maternal BMI. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics of the study groups are presented in Table I. The median gestational age at delivery (i.e. gestational age at cord blood sampling) was significantly lower in neonates of mothers with preeclampsia than in normal term AGA and SGA newborns. There was no significant difference in the median gestational age at delivery between neonate of mothers with preeclampsia and those with preterm labor (Table I).
Table I.
Clinical and demographic characteristics of the study population.
| Normal Pregnancy (n=44) | p | SGA (n=22) | pa | Preeclampsia (n=22) | Pb | Preterm Labor (n=22) | pc | |
|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | 25 (21 – 33) | 0.8 | 28 (21 – 33) | 0.2 | 23 (20 – 29) | 0.3 | 23 (19 – 27) | 0.5 |
| Parity | 1 (0 – 2) | 0.7 | 1 (0 – 1) | 0.7 | 1 (0 – 1) | 0.8 | 1 (0 – 1) | 0.5 |
| Pre-gestational BMI (kg/m2) | 23.4 (21.6 – 26.1) | 0.6 | 23.8 (22.6 – 27) | 0.7 | 23.4 (19.4 – 29.5) | 0.6 | 22.8 (21.1 – 24.1) | 0.6 |
| GA at maternal blood sampling (weeks) | 33.6 (27.9 – 38.5) | 0.007 | 38.1 (37.2 – 38.9) | 0.9 | 33.6 (32.4 – 37.7) | <0.001 | NA | NA |
| GA at delivery (weeks) | 38.6 (38.1 – 38.9) | 0.1 | 38.3 (38.0 – 38.7) | <0.001 | 34.0 (33.0 – 37.7) | 0.003 | 34.0 (33.0 – 34.7) | 0.1 |
| Male Gender | 25 (57%) | 0.4 | 10 (45%) | 0.6 | 11 (50%) | 0.9 | 10 (45%) | 0.9 |
| Birth weight (grams) | 3245 (3102–3500) | <0.001 | 2430 (1962–2595) | <0.001 | 2135 (1632–2575) | 0.2 | 2345 (2017–2547) | 0.3 |
Values expressed as median (interquartile range) or n (%)
SGA- Small-for-gestational-age; GA – Gestational Age; BMI – Body Mass Index; NA – Not available
p: comparison between normal pregnancy and the SGA group
pa: comparison between normal pregnancy and the preeclampsia group
pb: comparison between preeclampsia and the SGA group
pc: comparison between preeclampsia and the preterm labor group
Umbilical cord blood visfatin concentrations in preeclampsia, SGA and preterm labor
The median umbilical cord plasma concentrations did not differ significantly between AGA (7.2 ng/ml, interquartile range [IQR]: 5.8–8.0), SGA newborns (7.1 ng/ml, IQR: 5.7–9.5) and infants of patients with preeclampsia (7.4 ng/ml, IQR: 5.6–8.2) (p=0.8, Kruskal-Wallis; Figure 1).
Figure 1. Comparison between umbilical cord plasma visfatin plasma concentration in normal neonates, SGA newborns, infants of patients with preeclampsia and preterm neonate.
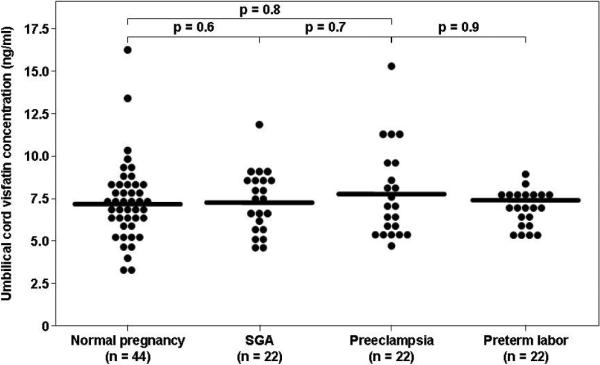
The median umbilical cord plasma concentrations of normal neonates (7.2 ng/ml, interquartile range [IQR] 5.8–8.0), SGA newborns (7.1 ng/ml, IQR: 5.7–9.5) and infants of patients with preeclampsia (7.4 ng/ml, IQR: 5.6–8.2) did not differ significantly (p=0.8, Kruskal-Wallis). Similarly, the median umbilical cord plasma visfatin concentrations did not differ significantly between neonates of patients with preeclampsia and those delivered preterm without preeclampsia matched for gestational age (7.4 ng/ml, IQR: 5.6–8.2 vs. 7.1 ng/ml, IQR: 6.4–7.7).
Since gestational age at umbilical cord sampling was significantly lower in neonate of mothers with preeclampsia, circulating visfatin concentrations were determined in 22 preterm AGA neonates delivered following spontaneous preterm labor without funisitis or histologic chorioamnionitis, matched for gestational age with the infants of patients with preeclampsia. The median umbilical cord visfatin plasma concentrations did not differ significantly between neonates of patients with preeclampsia and those born preterm without preeclampsia (7.4 ng/ml, IQR: 5.6–8.2 vs. 7.1 ng/ml, IQR: 6.4–7.7, p=0.9, Figure 1).
In contrast, the median maternal plasma visfatin concentrations differed significantly among groups (p=0.04, Kruskal-Wallis). The median maternal plasma concentration of visfatin was significantly higher in patients with an SGA neonate than in those with either normal pregnancy (18 ng/ml, IQR: 16.2–23.3 vs. 16.1 ng/ml, IQR: 11.2–20.3; p=0.02) or those with preeclampsia (15.9 ng/ml, IQR: 13–22; p=0.04). The median maternal plasma visfatin concentration did not differ significantly between patients with preeclampsia and those with a normal pregnancy (p=0.7).
Comparison between maternal plasma and umbilical cord blood visfatin concentrations
Comparison between maternal and fetal circulating visfatin concentrations was conducted only in cases in which the time interval between maternal and umbilical cord blood sampling was less than 48 hours. Using this cut-off, maternal-neonatal paired samples were available for 18 patients in the normal pregnancy group, 20 in the SGA group and 19 in the preeclampsia group. The median plasma visfatin concentration was significantly higher in the maternal than in the umbilical blood in the normal pregnancy group (18.7 ng/ml, IQR: 12.7–21.3 vs. 7.3 ng/ml, IQR: 6.1–7.9, p<0.001; Figure 2A), SGA group (18 ng/ml, IQR: 16.4–23 vs. 6.7 ng/ml, IQR: 5.7–8.5, p<0.001; Figure 2B),and in the preeclampsia group (16.5 ng/ml, IQR: 13.1–22.6 vs. 7.6 ng/ml, IQR: 6.4–8.2, p<0.001; Figure 2C).
Figure 2. Comparison between umbilical cord blood and maternal plasma visfatin concentrations in normal gestations (A), pregnancies complicated by an SGA neonate (B) or preeclampsia (C).
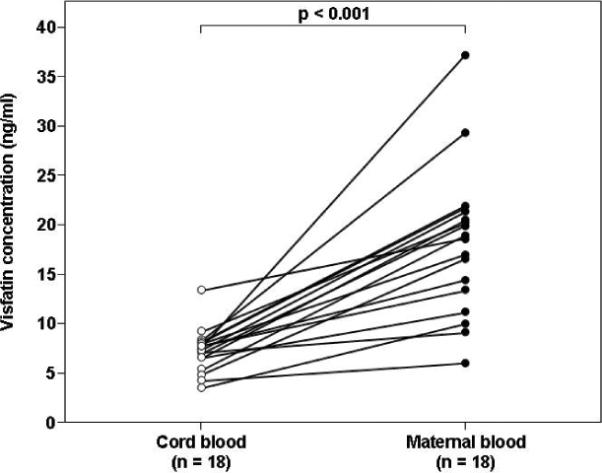
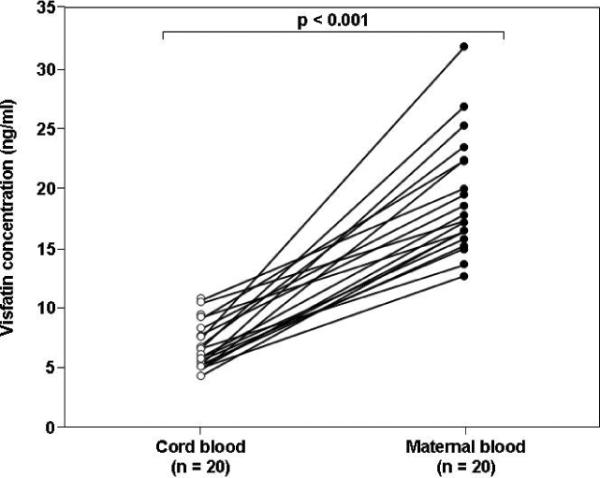
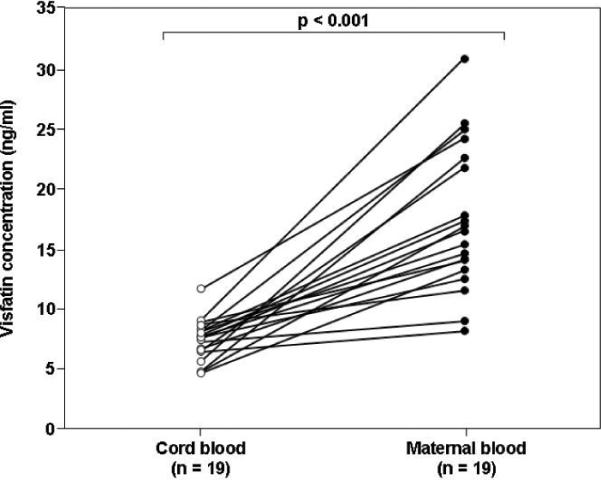
The median maternal plasma visfatin concentration was higher than in the umbilical blood in the normal pregnancy group (18.7 ng/ml, IQR: 12.7–21.3 vs. 7.3 ng/ml, IQR: 6.1–7.9, respectively, p<0.001), SGA group (18.0 ng/ml, IQR: 16.4–23.0 vs. 6.7 ng/ml, IQR: 5.7–8.5, p<0.001),and in the preeclampsia group (16.5 ng/ml, IQR: 13.1–22.6 vs. 7.6 ng/ml, IQR: 6.4–8.2, p<0.001).
The median umbilical cord plasma visfatin concentration did not differ significantly between male and female neonates in a pooled analysis (p=0.1) or within normal neonates (p=0.5), SGA newborns (p=0.8), neonates of mothers with preeclampsia (p=0.1) or preterm neonates (p=0.9, data not shown).
Maternal and cord blood plasma visfatin concentrations correlated only in the normal term group (r= 0.48, p=0.04; Figure 3), but not in the SGA (r= 0.2, p=0.3) or preeclampsia group(r= 0.5, p=0.1). No significant correlation was found between cord blood visfatin concentrations and birthweight, Ponderal Index or gestational age at delivery.
Figure 3. Correlation between umbilical cord blood and maternal plasma visfatin concentrations in the normal gestation.
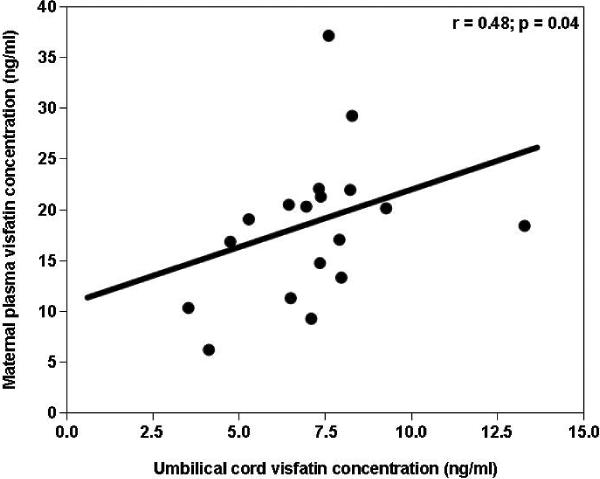
Maternal and cord blood plasma visfatin concentrations correlated only in the normal term group (r= 0.48, p=0.04)
Discussion
Principal findings of the study
1) The median plasma visfatin concentration was significantly lower in cord blood than in the maternal circulation in normal pregnancy, SGA and preeclampsia; 2) the median cord blood visfatin concentrations did not differ significantly between term AGA, SGA and neonates of patients with preeclampsia; 3) maternal and cord blood visfatin concentrations correlated only in the normal term group.
The physiological role of visfatin
Visfatin, also known as Pre-B cell colony-enhancing factor (PBEF), was originally identified as a growth factor for early B cell [56]. Subsequently, it was recognized as a novel adipokine which is preferentially produced by the visceral fat depot.[40] Visfatin/PBEF has been implicated in the regulation of glucose homeostasis. Indeed, in vitro, adipocytes secrete visfatin in response to treatment with glucose[57] and this protein can exert insulin-mimicking effects [58]. In vivo, visfatin-deficient mice have impaired glucose tolerance[59] and a polymorphism in the human visfatin gene promoter is associated with a susceptibility to type-2 DM [60]. Moreover, high circulating concentrations of this adipokine characterize patients with insulin resistance [44–46,61,62].
In addition to its metabolic effects, visfatin has pro-inflammatory properties. In vitro, visfatin synergizes with interleukin (IL)-7 and stem cell factors to promote the growth of B-cell precursors and treatment of human monocytes with visfatin results in an increased secretion of IL-6, tumor necrosis factor-α and IL-1β in a dose-dependent manner.[63] In addition, patients with chronic inflammatory disorders such as inflammatory bowel disease [63] and rheumatoid arthritis [64] have a higher circulating visfatin concentration than normal subjects.
Visfatin in normal gestation and in complications of pregnancy
The rationale to study visfatin concentrations in human pregnancy rests on the association between alterations in adipokines concentrations and adaptations to gestation [55,65–73], as well as with complications of pregnancy such as preeclampsia [48,74–77], preterm labor [53,78], intra-amniotic infection/inflammation [54,79–81], delivery of an SGA neonate [82,83], macrosomia [84,85], GDM [84] and pyelonephritis [86]. Moreover, visfatin is expressed in the placenta, fetal membranes [87–94] and the myometrium [95]. Normal pregnancy is associated with high maternal circulating visfatin concentrations [55,96–100]. In addition, GDM is characterized by alterations in maternal concentrations of this adipokine [42,44–46,101]. Recently, we have reported that intra-amniotic infection/inflammation is associated with higher amniotic fluid concentrations of visfatin than in the absence of infection [54], and that preterm labor is characterized by high maternal circulating concentrations of this adipokine [53].
Visfatin concentrations are lower in the fetal than in the maternal circulation
Only several studies have addressed umbilical cord blood visfatin concentrations [102–106], and a comparison between maternal and neonatal concentrations of this adipokine was included in only two reports [104,105]. The results of the present study indicate that circulating visfatin concentrations are lower in the fetal than in the maternal circulation. This novel finding was demonstrated in all study groups, including normal neonates, SGA newborns and neonates of mothers with preeclampsia. The results reported herein are in contrast to the report by Malamitsi-Puchner et al.[105] in which there was no significant differences between maternal and neonatal circulating visfatin concentrations. Ethnic origin, clinical definitions, and gestational age at enrollment varied between the studies and may account for this discrepancy.
It is not clear why visfatin concentrations are lower in the fetal than in the maternal circulation. Increased production by the larger maternal fat depot compared to the newborn is a plausible explanation. In addition, visfatin, which is expressed in a considerable amount in the term human placenta [97], may be preferentially released into the maternal systemic circulation. Disparity in placental secretion of adipokines into the maternal and fetal circulation has been demonstrated for other adipokines such as leptin [107].
Our findings indicate that maternal and cord blood plasma visfatin concentrations were correlated only in the normal term group. This finding is in agreement with the reports by Ibánez et al.[102] and Malamitsi-Puchner et al.[104,105] we found no gender disparity in umbilical cord visfatin concentrations. Our findings are also in agreement with López-Bermejo et al.[103] who found no association between umbilical cord blood visfatin concentrations and anthropometric indices of the newborn. The present study extends the aforementioned reports by demonstrating these finding not only to term or AGA neonates but also to those of mother with preeclampsia and premature neonates.
The fetal circulation is not the source for the elevated visfatin concentrations of mother with a small-for-gestational-age neonate
Two studies, conducted by Fasshauer et al.[108] and Malamitsi-Puchner et al.[104] have reported higher maternal circulating visfatin concentrations in patients with an SGA neonate than in those who delivered an AGA neonate. In accordance with these findings, we have recently reported that patients with an SGA neonate, but not those with preeclampsia, had a higher maternal plasma visfatin concentration than those with a normal pregnancy suggesting differential involvement of visfatin in SGA and preeclampsia [48]. However, the mechanism by which an SGA neonate affects maternal visfatin circulation is not clear. It has been proposed that enhanced transfer of visfatin from the fetal to the maternal circulation can account for the high concentrations of maternal visfatin reported in patients with an SGA neonate [48]. The results of the present study suggest that the fetal circulation is not the source for the increased maternal visfatin concentrations in patients with an SGA neonate as no significant differences were documented between the cord blood concentrations of the different study groups.
The finding reported herein concerning the lack of difference in umbilical cord blood visfatin concentrations between SGA neonates and those with a normal pregnancy is in contrast to the report by Malamitsi-Puchner et al.[104] in which umbilical cord blood visfatin concentrations were higher in SGA than in AGA neonates matched for gestational age. Differences in the definition of an SGA neonate and ethnic origin may account for this discrepancy. Specifically, in the study by Malamitsi-Puchner et al.[104] the SGA group included patients with preeclampsia, gestational hypertension, iron-deficiency anemia, gestational diabetes, hypothyroidism, as well as cardiac arrhythmias, whereas in the present study mothers with these conditions were excluded from the SGA group.
In conclusion, circulating fetal visfatin concentrations are lower than maternal plasma concentrations. Despite the association between the presence of an SGA neonate and high maternal circulating visfatin concentrations, comparable visfatin concentrations were detected in the umbilical cord blood of normal neonates, SGA newborns, infants of patients with preeclampsia and preterm neonates. Collectively, these observations suggest that fetal-maternal transport of visfatin is not likely to account for the high maternal visfatin concentrations in patients with an SGA neonate.
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 2.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br.J.Obstet.Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 3.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br.J.Obstet.Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 4.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am.J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern.Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 6.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al. A role of the anti-angiogenic factor sVEGFR-1 in the 'mirror syndrome' (Ballantyne's syndrome) J.Matern.Fetal Neonatal Med. 2006;19:607–613. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N.Engl.J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N.Engl.J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J.Clin.Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Goncalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J.Matern.Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 12.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J.Matern.Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J.Matern.Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 15.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, Mittal P, Edwin S, Yoon BH, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J.Matern.Fetal Neonatal Med. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat.Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 17.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J.Matern.Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon CG, Seely EW. Preeclampsia -- searching for the cause. N.Engl.J Med. 2004;350:641–642. doi: 10.1056/NEJMp038241. [DOI] [PubMed] [Google Scholar]
- 19.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Schiff E, Ben-Baruch G, Peleg E, Rosenthal T, Alcalay M, Devir M, Mashiach S. Immunoreactive circulating endothelin-1 in normal and hypertensive pregnancies. Am.J.Obstet.Gynecol. 1992;166:624–628. doi: 10.1016/0002-9378(92)91688-7. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JR, Papayianni A, Brady HR, Darling MR, Walshe JJ. Circulating vascular cell adhesion molecule-1 in pre-eclampsia, gestational hypertension, and normal pregnancy: evidence of selective dysregulation of vascular cell adhesion molecule-1 homeostasis in pre-eclampsia. Am.J.Obstet.Gynecol. 1998;179:464–469. doi: 10.1016/s0002-9378(98)70380-1. [DOI] [PubMed] [Google Scholar]
- 22.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J.Exp.Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiff E, Friedman SA, Baumann P, Sibai BM, Romero R. Tumor necrosis factor-alpha in pregnancies associated with preeclampsia or small-for-gestational-age newborns. Am.J.Obstet.Gynecol. 1994;170:1224–1229. doi: 10.1016/s0002-9378(94)70130-x. [DOI] [PubMed] [Google Scholar]
- 24.Kusanovic JP, Romero R, Hassan SS, Gotsch F, Edwin S, Chaiworapongsa T, Erez O, Mittal P, Mazaki-Tovi S, Soto E, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J.Matern.Fetal Neonatal Med. 2007;20:867–878. doi: 10.1080/14767050701482993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J.Matern.Fetal Neonatal Med. 2008;21:429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am.J.Obstet.Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 27.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am.J.Obstet.Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 28.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am.J.Obstet.Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 29.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am.J.Obstet.Gynecol. 2002;187:889–893. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 30.Than NG, Romero R, Erez O, Kusanovi JP, Tarca AL, Edwin SS, Kim JS, Hassan SS, Espinoza J, Mittal P, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am.J Reprod.Immunol. 2008;60:333–345. doi: 10.1111/j.1600-0897.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmayer N, Sohlberg E, Sundstrom Y, Hamad RR, Berg L, Bremme K, Sverremark-Ekstrom E. Women with pre-eclampsia have an altered NKG2A and NKG2C receptor expression on peripheral blood natural killer cells. Am.J Reprod.Immunol. 2009;62:147–157. doi: 10.1111/j.1600-0897.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Lok CA, Jebbink J, Nieuwland R, Faas MM, Boer K, Sturk A, Van Der Post JA. Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am.J Reprod.Immunol. 2009;61:346–359. doi: 10.1111/j.1600-0897.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 34.Solomon CG, Seely EW. Hypertension in pregnancy. Endocrinol.Metab Clin.North Am. 2006;35:157–71. vii. doi: 10.1016/j.ecl.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Seeds JW. Impaired fetal growth: definition and clinical diagnosis. Obstet.Gynecol. 1984;64:303–310. [PubMed] [Google Scholar]
- 36.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am.J Obstet.Gynecol. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 37.von DP, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet.Gynecol.Scand. 2002;81:642–648. [PubMed] [Google Scholar]
- 38.Erez O, Romero R, Hoppensteadt D, Than NG, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Kim SS, Yoon BH, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J.Matern.Fetal Neonatal Med. 2008;21:855–869. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal P, Espinoza J, Hassan S, Kusanovic JP, Edwin SS, Nien JK, Gotsch F, Than NG, Erez O, Mazaki-Tovi S, et al. Placental growth hormone is increased in the maternal and fetal serum of patients with preeclampsia. J.Matern.Fetal Neonatal Med. 2007;20:651–659. doi: 10.1080/14767050701463571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol.Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J.Clin.Endocrinol.Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 42.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, Tsai EM. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J.Soc.Gynecol.Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Haider DG, Handisurya A, Storka A, Vojtassakova E, Luger A, Pacini G, Tura A, Wolzt M, Kautzky-Willer A. Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes Care. 2007;30:1889–1891. doi: 10.2337/dc07-0013. [DOI] [PubMed] [Google Scholar]
- 44.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, Schernthaner G. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin.Sci.(Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 45.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M, Vatish M, Lewinski A, Prelevic GM, Randeva HS. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 46.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, Chaiworapongsa T, Nhan-Chang CL, Pacora P, Gotsch F, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J Perinat.Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur.J Endocrinol. 2009;160:337–347. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 48.Mazaki-Tovi S, Romero R, Kim SK, Vaisbuch E, Kusanovi JP, Erez O, Chaiwaropongsa T, Gotsch F, Mittal P, Nhan-Chang C, et al. Could alterations in maternal plasma visfatin concentration participate in the phenotype definition of preeclampsia and SGA? The Journal of Maternal-Fetal and Neonatal Medicine. 2009 doi: 10.3109/14767050903301017. PMID: 19900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 50.Prevention of diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech.Rep.Ser. 1994;844:1–100. [PubMed] [Google Scholar]
- 51.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet.Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 52.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech.Rep.Ser. 2003;916:i–149. backcover. [PubMed] [Google Scholar]
- 53.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Chaiwaropongsa T, Mittal P, Kim SK, Pacora P, Gotsch F, Dong Z, et al. Maternal Plasma Visfatin in Preterm Labor. J Matern.Fetal Neonatal Med. 2009;22:693–704. doi: 10.1080/14767050902994788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat.Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, Chaiworapongsa T, Nhan-Chang CL, Pacora P, Gotsch F, et al. Maternal visfatin concentration in normal pregnancy. J Perinat.Med. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol.Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 58.Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY. Insulin-like effects of visfatin on human osteoblasts. Calcif.Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 59.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang YY, Gottardo L, Thompson R, Powers C, Nolan D, Duffy J, Marescotti MC, Avogaro A, Doria A. A visfatin promoter polymorphism is associated with low-grade inflammation and type 2 diabetes. Obesity.(Silver.Spring) 2006;14:2119–2126. doi: 10.1038/oby.2006.247. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R, Ricart W, Fernandez-Real JM. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55:2871–2875. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 62.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–570. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J.Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 64.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann.Rheum.Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr.Diab.Rep. 2005;5:278–281. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 66.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, Sivan E. Maternal serum adiponectin levels during human pregnancy. J.Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 67.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H. Adiponectin in human cord blood: relation to fetal birth weight and gender. J.Clin.Endocrinol.Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 68.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, Shoham A, Sivan E. Determining the source of fetal adiponectin. J.Reprod.Med. 2007;52:774–778. [PubMed] [Google Scholar]
- 69.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, Mittal P, Than GN, Nhan-Chang C, Chaiworapongsa T, et al. Adiponectin multimers in maternal plasma. J.Matern.Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J.Perinat.Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J.Perinat.Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Briana DD, Malamitsi-Puchner A. Adipocytokines in Normal and Complicated Pregnancies. Reprod.Sci. 2009;16:921–937. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 73.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De MS. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 74.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, Nhan-Chang CL, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat.Med. 2009;37:349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Adiponectin in severe preeclampsia. J.Perinat.Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis DF, Canzoneri BJ, Wang Y. Maternal Circulating TNF-alpha Levels are Highly Correlated with IL-10 Levels, but not IL-6 and IL-8 Levels, in Women with Pre-Eclampsia. Am.J Reprod.Immunol. 2009;65:269–274. doi: 10.1111/j.1600-0897.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiworapongsa T, Gotsch F, Than NG, Dong Z, Pacora P, et al. Retinol binding protein 4 - a novel association with early-onset preeclampsia. J Perinat.Med. 2009 doi: 10.1515/JPM.2009.140. DOI: 10.1515/JPM.2009.140: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Dysregulation of maternal serum adiponectin in preterm labor. J.Matern.Fetal Neonatal Med. 2009;22:887–904. doi: 10.1080/14767050902994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than GN, Kim SK, Dong M, Gotsch F, Mittal P, Chaiworapongsa T, et al. Retinol Binding Protein 4: An Adipokine Associated with Intra-amniotic Infection / Inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994739. PMID: 19900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: A novel association with subclinical intrauterine infection/inflammation. J.Matern.Fetal Neonatal Med. 2009 doi: 10.3109/14767050903026481. DOI: 10.1080/14767050903026481: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Maternal Serum Adiponectin Multimers in Patients with a Small-For-Gestational-Age Newborn. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.128. DOI: 10.1515/JPM.2009.128: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, Schiff E, Sivan E. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin.Endocrinol.Metab. 2009;94:892–898. doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 84.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Maternal Serum Adiponectin Multimers in Gestational Diabetes. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.101. DOI: 10.1515/JPM.2009.101: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am.J.Obstet.Gynecol. 2005;193:1238–1242. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 86.Mazaki-Tovi S, Romero R, Vaisbuch E, Chaiworapongsa T, Erez O, Mittal P, Kim SK, Gotsch F, Lamont RF, ogge G, et al. Low circulating maternal adiponectin in patients with pyelonephritis: adiponectin at the crossroads of pregnancy and infection. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.134. DOI:10.1515/JPM.2009.134: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol.Hum.Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 88.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am.J.Obstet.Gynecol. 2000;182:50–59. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 89.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J.Mol.Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 90.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am.J.Obstet.Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 91.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am.J.Obstet.Gynecol. 2003;189:1187–1195. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 92.Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am.J.Obstet.Gynecol. 2005;193:273–282. doi: 10.1016/j.ajog.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am.J.Obstet.Gynecol. 2000;182:60–67. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 94.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta. 2008;29:255–265. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, Adashi EY, Whiting D. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am.J.Obstet.Gynecol. 2005;193:404–413. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 96.Mastorakos G, Valsamakis G, Papatheodorou DC, Barlas I, Margeli A, Boutsiadis A, Kouskouni E, Vitoratos N, Papadimitriou A, Papassotiriou I, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin.Chem. 2007;53:1477–1483. doi: 10.1373/clinchem.2006.084731. [DOI] [PubMed] [Google Scholar]
- 97.Morgan SA, Bringolf JB, Seidel ER. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008;29:1382–1389. doi: 10.1016/j.peptides.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 98.Katwa LC, Seidel ER. Visfatin in pregnancy: proposed mechanism of peptide delivery. Amino.Acids. 2008;37:555–558. doi: 10.1007/s00726-008-0194-7. [DOI] [PubMed] [Google Scholar]
- 99.Szamatowicz J, Kuzmicki M, Telejko B, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M. Serum visfatin concentration is elevated in pregnant women irrespectively of the presence of gestational diabetes. Ginekol.Pol. 2009;80:14–18. [PubMed] [Google Scholar]
- 100.Hu W, Wang Z, Wang H, Huang H, Dong M. Serum visfatin levels in late pregnancy and pre-eclampsia. Acta Obstet.Gynecol.Scand. 2008;87:413–418. doi: 10.1080/00016340801976012. [DOI] [PubMed] [Google Scholar]
- 101.Akturk M, Altinova AE, Mert I, Buyukkagnici U, Sargin A, Arslan M, Danisman N. Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. J.Endocrinol.Invest. 2008;31:610–613. doi: 10.1007/BF03345611. [DOI] [PubMed] [Google Scholar]
- 102.Ibanez L, Sebastiani G, Lopez-Bermejo A, Diaz M, Gomez-Roig MD, de ZF. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J.Clin.Endocrinol.Metab. 2008;93:2774–2778. doi: 10.1210/jc.2008-0526. [DOI] [PubMed] [Google Scholar]
- 103.Lopez-Bermejo A, de ZF, az-Silva M, Vicente MP, Valls C, Ibanez L. Cord serum visfatin at term birth: maternal smoking unmasks the relation to foetal growth. Clin.Endocrinol.(Oxf) 2008;68:77–81. doi: 10.1111/j.1365-2265.2007.03002.x. [DOI] [PubMed] [Google Scholar]
- 104.Malamitsi-Puchner A, Briana DD, Boutsikou M, Kouskouni E, Hassiakos D, Gourgiotis D. Perinatal circulating visfatin levels in intrauterine growth restriction. Pediatrics. 2007;119:e1314–e1318. doi: 10.1542/peds.2006-2589. [DOI] [PubMed] [Google Scholar]
- 105.Malamitsi-Puchner A, Briana DD, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D. Blood visfatin concentrations in normal full-term pregnancies. Acta Paediatr. 2007;96:526–529. doi: 10.1111/j.1651-2227.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 106.Briana DD, Boutsikou M, Gourgiotis D, Kontara L, Baka S, Iacovidou N, Hassiakos D, Malamitsi-Puchner A. Role of visfatin, insulin-like growth factor-I and insulin in fetal growth. J.Perinat.Med. 2007;35:326–329. doi: 10.1515/JPM.2007.071. [DOI] [PubMed] [Google Scholar]
- 107.Hauguel-De MS, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am.J Obstet.Gynecol. 2006;194:1537–1545. doi: 10.1016/j.ajog.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 108.Fasshauer M, Bluher M, Stumvoll M, Tonessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin.Endocrinol.(Oxf) 2007;66:434–439. doi: 10.1111/j.1365-2265.2007.02751.x. [DOI] [PubMed] [Google Scholar]


