Abstract
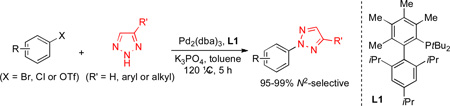
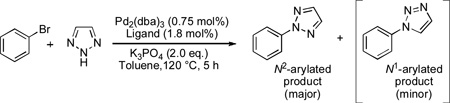
Highly N2-selective arylation of 4,5-unsubstituted and 4-substituted 1,2,3-triazoles was achieved for the first time by Pd/L1 catalyst system. A wide range of N2-aryl-1,2,3-triazoles were prepared from aryl bromides, chlorides and triflates with excellent (95–99%) N2-selectivity. DFT calculations suggest that formation of N2-arylated 1,2,3-triazoles is favored kinetically.
Keywords: palladium; homogeneous catalysis; C-N coupling; 1,2,3-triazole; N-arylation
N-Substituted-1,2,3-triazoles have found widespread applications in material science and medicinal chemistry.[1–2] Due to the importance of this structural motif, many practical synthetic methods have been developed. Among them, the Huisgen azide-alkyne dipolar cycloaddition (AAC) is perhaps the most commonly utilized method for the synthesis of N1-substituted 1,2,3-triazoles.[3] In particular, recent developments in Cu-[4] and Ru-catalyzed[5] AAC reactions have provided a general and regioselective access to 1,4- and 1,5-substituted 1,2,3-triazoles, respectively. On the other hand, regioselective synthesis of N2-substituted 1,2,3-triazoles remains a challenging issue. A particulary interesting subset of these compounds are N2-aryl-1,2,3-triazoles, which are found in biologically active compounds including an orexin receptor antagonist (MK4305), [2a–b] JAK kinase inhibitors[2c] and 2,3-oxidosqualene cyclase inhibitors.[2d] Ideally, the most direct route to N2-aryl-1,2,3-triazoles involves N-arylation of 1,2,3-triazoles.[2a–c, 6–7] However, SNAr and Cu-catalyzed arylation reactions of simple 1,2,3-triazoles generally give mixtures of regioisomers with poor to moderate N2-selectivity.[8] Recently, Shi[9] and Wang[10] reported the highly N2-selective SNAr and Cu-catalyzed arylation reactions using 4,5-disubstituted 1,2,3-triazoles, where C4- and C5-substituents prevent substitution on the N1- and N3-position by steric hindrance.[11] Despite these advances, a highly (>90%) N2-selective arylation method of 4-substituted and 4,5-unsubstituted 1,2,3-triazoles is still lacking. Herein, we report that exceptional levels of N2-selectivity can be obtained in the Pd-catalyzed N-arylation of simple 1,2,3-triazoles by the use of a very bulky biaryl phosphine ligand L1. This method enabled the first highly N2-selective arylation of 4-substituted and 4,5-unsubstituted 1,2,3-triazoles with aryl bromides, chlorides and triflates.
We initiated our study by examining the N-arylation of 1,2,3-triazole with bromobenzene in the presence of Pd2(dba)3 (0.75 mol%) with series of biaryl phosphine ligand L1–L4 (1.8 mol%), (Table 1). Gratifyingly, the Pd-catalyzed reaction of 1,2,3-triazole using L1 furnished N2-arylated product in 90% yield with excellent N2-selectivity (N2:N1 = 97:3) (entry 1).[12] To the best of our knowledge, this is the first Pd-catalyzed and highly N2-selective arylation of 4,5-unsubstituted 1,2,3-triazole. It was important to pre-heat a solution of Pd2(dba)3 and L1 before they were exposed to 1,2,3-triazole, bromobenzene and K3PO4. The reaction was significantly less efficient without catalyst pre-heating (entry 2), presumably due to inhibitory effect of 1,2,3-triazole on the in situ formation of catalytically active Pd(0)-ligand complex. The use of less sterically hindered biaryl phosphines L2–L4 provided, at best, 16% yield of N-arylated product (entries 3–5). This suggests that the nature of the both upper-ring substituents and lower-ring isopropyl groups of L1 are crucial to the present catalyst system.
Table 1.
Ligand effects on the Pd-catalyzed N-arylation of 1,2,3-triazole [a]
 | ||||
|---|---|---|---|---|
| entry | ligand | GC conv. (%) |
GC yield of N2- arylated product (%) |
N2:N1 [b] |
| 1 | L1 | 100 | 93 (90)c | 97:3 |
| 2[d] | L1 | 9 | 7 | N.D. [e] |
| 3 | L2 | <5 | <5 | N.D. |
| 4 | L3 | 20 | 16 | 96:4 |
| 5 | L4 | <5 | <5 | N.D. |
Conditions: bromobenzene (1 mmol), 1,2,3-triazole (1.2 mmol), K3PO4 (2 mmol), Pd2(dba)3 (0.75 mol%), ligand (1.8 mol%), toluene (1 mL), 120 °C, 5 h. Pd2(dba)3 and ligand were premixed in toluene (0.5 mL) at 120 °C for 3 min.
N2-N1 ratio was determined by GC.
Isolated yield.
Reaction was performed without premixing Pd2(dba)3 and L1.
Not determined.
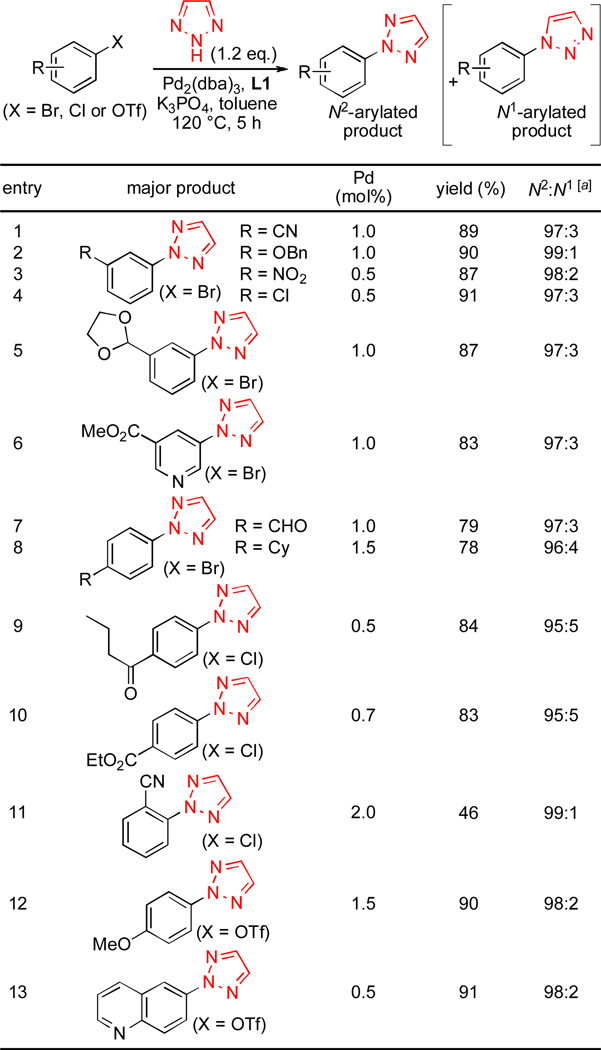
The substrate scope of the N-arylation of 1,2,3-triazole is shown in Scheme 1. A variety of aryl bromides, chlorides and triflates with ester, ketone, aldehyde, acetal, nitro and cyano groups could be employed in the N-arylation reactions. While slightly decreased N2-selectivity was observed for the reactions of aryl chlorides with para-electron withdrawing groups (entries 9 and 10), excellent N2-selectivity (>95% N2-selective) was observed in all other substrates examined. The yield was diminished when the aryl halide bearing an ortho-substituent was employed (46% yield, entry 11) probably due to unfavorable steric interaction between the bulky ligand and the ortho-substituent (entry 11). Lower (0.3–0.7 mol%) Pd loadings could be employed for the electron deficient aryl halides and triflate (entries 3–4, 9–10 and 13).
Scheme 1.
Substrate scope of N2-selective arylation of 4,5-unsubstituted 1,2,3-triazole; Ar-X (1 mmol), 1,2,3-triazole (1.2 mmol), K3PO4 (2 mmol), Pd2(dba)3 (0.25–0.75 mol%), L1 (0.5–1.8 mol%), toluene (1 mL), 120 °C, 5 h. Yields are isolated yield of N2-arylated product (average of two runs). [a] Determined by GC analysis.
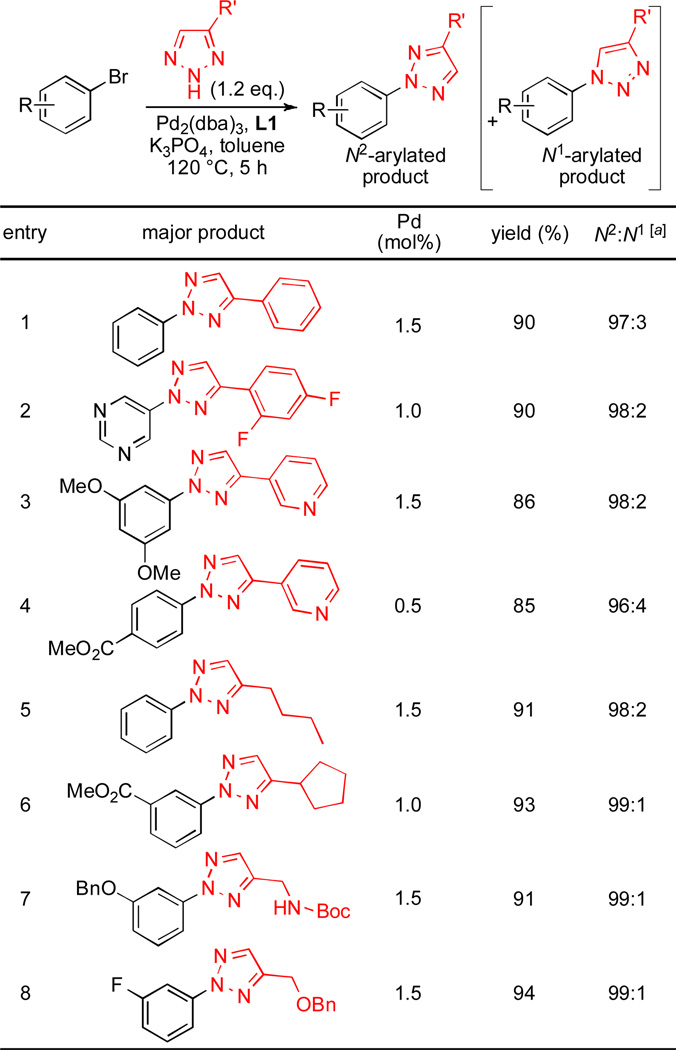
To expand the generality of this process, we examined the N-arylation of 4-substituted 1,2,3-triazoles (Scheme 2). The N-arylation of 4-phenyl-1,2,3-triazole with bromobenzene gave excellent N2/N1 selectivity; the N3-arylated product was not detected by GC-MS or 1H NMR analysis of the crude reaction mixture (entry 1). Similarly, N-arylation of other 4-aryl-substituted 1,2,3-triazoles gave products with 98% N2-selectivity (entries 2–3). These N2-selectivity are higher than selectivity reported for Cu-catalyzed N-arylation (N2:N1=4:1) and SNAr reaction (N2:N1=1.6:1) of 4-aryl-1,2,3-triazoles.7a Reactions of primary alkyl, functionalized primary alkyl and secondary alkyl substituted 1,2,3-triazole also showed excellent N2-selectivities (entries 4–7, 98–99% N2-selective).
Scheme 2.
Substrate scope of N2-selective arylation of 4-substituted 1,2,3-triazoles; Ar-X (1 mmol), 4-substituted 1,2,3-triazole (1.2 mmol), K3PO4 (2 mmol), Pd2(dba)3 (0.5–0.75 mol%), L1 (1.0–1.8 mol%), toluene (1 mL), 120 °C, 5 h. Yields are isolated yield of N2-arylated product (average of two runs). [a] Determined by GC analysis.
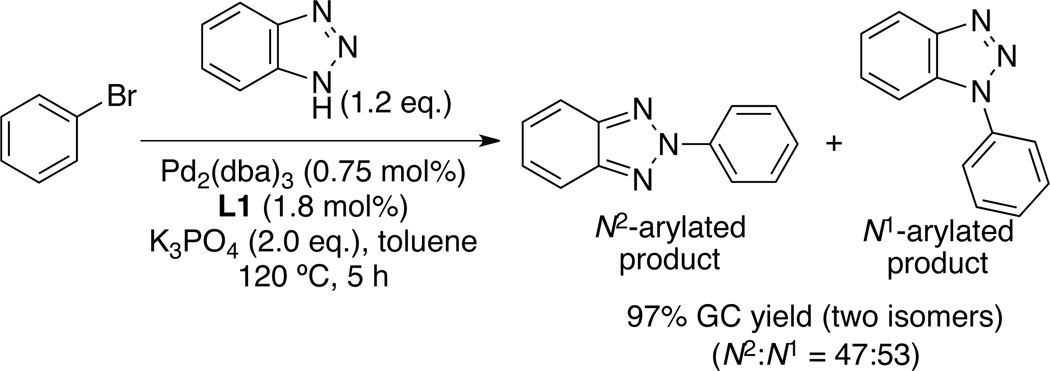
While excellent N2-selectivity was observed for the reactions of 4,5-unsbstituted and 4-substituted 1,2,3-triazoles, we obtained near 1:1 mixture of N1- and N2-aryl isomers for the reaction of benzotriazole with bromobenzene (Scheme 3).
Scheme 3.
Pd-catalyzed N-arylation of benzotriazole
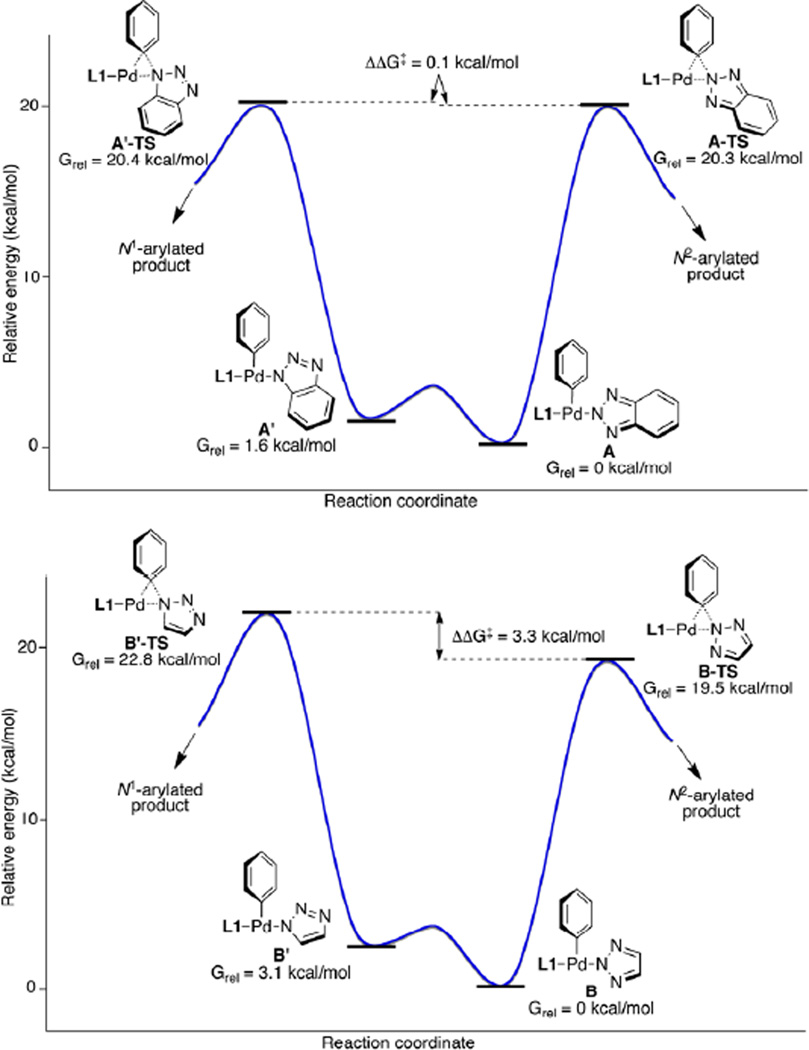
In order to gain insight into the origin of regioselectivity, we performed DFT calculation of the presumed intermediates. For the N-arylations of benzotriazole and 1,2,3-triazole with bromobenzene, transmetallation of the triazolate to the L1Pd(Ph)(Br) complex could provide tautomeric species A/A' and B/B' respectively.[13–14] The relative energies of the key intermediates and the transition states (TSs) are shown in Figure 1. In the benzotriazole case, a small energetic preference (ΔG = 1.6 kcal/mol) for the N2-benzotriazolate complex A over the N1-benzotriazolate A' was observed. Comparison of the two isomeric transition states for the reductive elimination from the benzotriazolate complexes A and A' showed only an insignificant energetic preference exist between the A-TS and A'-TS (ΔΔG‡ = 0.1 kcal/mol). The poor regioselectivity (N2:N1=47:53) observed for the benzotriazole system can be explained by the close relative energies of the A-TS and A'-TS. In the 1,2,3-triazole system, the transition states for the reductive elimination (B-TS and B'-TS) are significantly different (ΔΔG‡ = 3.3 kcal/mol) in favor of the transition state leading to the N2-arylated product, which is in agreement with the observed regioselectivity.
Figure 1.
Energy diagrams for the reductive elimination of benzotriazolate-Pd and 1,2,3-triazolate-Pd complexes
In summary, we have established a highly N2-selective Pd-catalyzed arylation of 4,5-unsubstituted and 4-substituted 1,2,3-triazoles with aryl bromides, chlorides and triflates. Theoretical calculations suggested that highly N2-selective arylation of 1,2,3-triazoles is due to rapid reductive elimination from N2-1,2,3-triazolate-Pd complex B. Together with the well established Cu- and Ru-catalyzed AAC, present Pd-catalyzed system allows straightforward and regioselective preparation of N-aryl-1,2,3-triazoles.
Experimental Section
General procedure: An oven-dried vial was equipped with a magnetic stir bar and charged with Pd2(dba)3 and L1. The vial was sealed with a screw-cap septum, and then evacuated and backfilled with argon (this process was repeated a total of 3 times). Toluene (0.5 mL) was added to the vial via syringe. The resulting dark purple mixture was stirred at 120 °C for 3 min, at this point the color of the mixture turned to dark brown. A second oven-dried vial, which was equipped with a stir bar, was charged with K3PO4 (424 mg, 2.0 mmol) (aryl halides and 1,2,3-triazoles that were solid at room temperature were added at this point). The vial was sealed with a screw-cap septum, and then evacuated and backfilled with argon (this process was repeated a total of 3 times) and then 1,2,3-triazole (1.2 mmol) and aryl halide (1.0 mmol) were added via syringe and the premixed catalyst solution and toluene (0.5 mL) was added by syringe to the second vial (total 1.0 mL toluene). The reaction mixture was heated at 120 °C for 5 h. The reaction was cooled to room temperature, diluted with EtOAc, washed with brine, dried over MgSO4, concentrated in vacuo and purified via flash chromatography to give pure products.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health (GM58160). S.U. thanks the Japan Society for the Promotion of Sciences (JSPS) for a Postdoctral Fellowship for Research Abroad. We thank Dr. Thomas J. Maimone for help with preparation of this manuscript.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Satoshi Ueda, Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge MA 02139 (USA).
Mingjuan Su, Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge MA 02139 (USA).
Stephen L. Buchwald, Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge MA 02139 (USA).
References
- 1.Reviews for N1-substituted-1,2,3-triazoles, see; Kolb HC, Sharpless KB. Drug Discovery Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n..
- 2.Recent applications of N2-aryl-1,2,3-triazoles, see; Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, Roecker AJ, Mercer SP, Bednar RA, Lemaire W, Bruno JG, Reiss DR, Meacham Harrell C, Murphy KL, Garson SL, Doran SM, Prueksaritanont T, Anderson WB, Tang C, Roller S, Cabalu TD, Cui D, Hartman GD, Young SD, Koblan KS, Winrow CJ, Renger JJ, Coleman PJ. J. Med. Chem. 2010;53:5320–5332. doi: 10.1021/jm100541c. Baxter CA, Cleator E, Brands KMJ, Edwards JS, Reamer RA, Sheen FJ, Stewart GW, Strotman NA, Wallace DJ. Org. Process Res. Dev. 2011;15:367–375. Jia ZJ, Venkataramani C, Huang W, Mehrotra M, Song Y, Xu Q, Bauer SM, Pandey A. WO2009136995. 2009 Watanabe T, Umezawa Y, Takahashi Y, Akamatsu Y. Bioorg. Med. Chem. Lett. 2010;20:5807–5810. doi: 10.1016/j.bmcl.2010.07.131..
- 3.Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. New York: Wiley; 1984. pp. 1–176. [Google Scholar]
- 4.a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; b) Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 5.a) Zhang L, Chen X, Xue P, Sun HHY, Williams ID, Sharpless KB, Fokin VV, Jia G. J. Am. Chem. Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]; b) Majireck MM, Weinreb SM. J. Org. Chem. 2006;71:8680–8683. doi: 10.1021/jo061688m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taillefer M, Xia N, Ouali A. Angew. Chem, Int. Ed. 2007;46:934–936. doi: 10.1002/anie.200603173. [DOI] [PubMed] [Google Scholar]
- 7.For synthesis of 2-aryl-1,2,3-triazoles by oxidative cyclization of bisphenylhydrazone; Balachandran KS, Hiryakkanavar I, George MV. Tetrahedron. 1975;31:1171–1177..
- 8.N2:N1=1-4.3:1 was reported for the SNAr and copper-catalyzed N-arylation of 4-substituted and 4,5,-unsubstituted 1,2,3-triazole, see; ref. 2b, 2c, 6 and 7a.
- 9.Liu Y, Yan W, Chen Y, Petersen JL, Shi X. Org. Lett. 2008;10:5389–5392. doi: 10.1021/ol802246q. [DOI] [PubMed] [Google Scholar]
- 10.Wang X-J, Zhang L, Lee H, Haddad N, Krishnamurthy D, Senanayake CH. Org. Lett. 2009;11:5026–5028. doi: 10.1021/ol9019875. [DOI] [PubMed] [Google Scholar]
- 11.For N2-selective alkylation of 4,5-disubstituted 1,2,3-triazoles; Chen Y, Liu Y, Petersen JL, Shi X. Chem. Commun. 2008:3254–3256. doi: 10.1039/b805328f. Wang X-J, Sidhu K, Zhang L, Campbell S, Haddad N, Reeves DC, Krishnamurthy D, Senanayake CH. Org. Lett. 2009;11:5490–5493. doi: 10.1021/ol902334x. Wang X-J, Zhang L, Krishnamurthy D, Senanayake CH, Wipf P. Org. Lett. 2009;11:4632–4635. doi: 10.1021/ol101965a.. For N2-selective allylation of 4 -substituted 1,2,3-triazoles; Kamijo S, Jin T, Huo Z, Yamamoto Y. J. Am. Chem. Soc. 2003;125:7786–7787. doi: 10.1021/ja034191s. Kamijo S, Jin T, Huo Z, Yamamoto. Y. J. Org. Chem. 2004;69:2386–2393. doi: 10.1021/jo035292b.. For N2-selective hydroxymethylation of 4 -substituted 1,2,3-triazoles; Kalisiak J, Sharpless KB, Fokin VV. Org. Lett. 2008;10:3171–3174. doi: 10.1021/ol8006748..
- 12.C-Arylated products were not observed under these conditions. For C-arylation of N1-substituted 1,2,3-triazoles, see; Chuprakov S, Chernyak N, Dudnik AS, Gevorgyan V. Org. Lett. 2007;9:2333–2336. doi: 10.1021/ol070697u..
- 13.Since 1,2,3-triazole has a pKa of 9.26, it could be deprotonated in the presence K3PO4. Hansen LD, West BD, Baca EJ, Blank CL. J. Am. Chem. Soc. 1968;90:6588–6592..
- 14.The possibility that the triazolate first binds to the Pd(0)-L1 complex followed by oxidative addition of PhBr to produce A/A' and B/B' cannot be ruled out.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.