Abstract
The intricate molecular mechanisms that regulate embryonic stem (ES) cell pluripotency are incompletely understood. Prior research indicated that activation of JAK-STAT3 pathway or inhibition of ERK/GSK3 signaling maintains mouse ES cell (mESC) pluripotency. Here we demonstrate that inhibition of protein kinase C (PKC) isoforms maintains mESC pluripotency without the activation of STAT3 or inhibition of ERK/GSK3 signaling pathways. Our analyses revealed that the atypical PKC isoform, PKCζ plays an important role in inducing lineage commitment in mESCs through a PKCζ–NF-κB signaling axis and inhibition of PKC isoforms maintains ES cell-specific epigenetic modifications. Furthermore, inhibition of PKC isoforms permits derivation of germline-competent ES cells from mouse blastocysts and also facilitates reprogramming of mouse embryonic fibroblasts (MEFs) towards induced pluripotent stem cells (iPSCs). Our results indicate that PKC signaling is critical to balancing ES cell self-renewal and lineage commitment.
Keywords: Protein Kinase C, Embryonic Stem cells, Pluripotency, Induced pluripotency
Introduction
Pluripotent stem cells deal with two critical, yet opposing forces, to self-renew and to respond to differentiation signals. Over the years, several culture conditions have been utilized to maintain ES cell pluripotency and to derive new ES and iPS cells 1–6. Interestingly, all of these strategies lead to the establishment of a core transcription factor network that maintains the ES-cell specific chromatin structure and gene expression pattern 7. Induction of ES cell differentiation is associated with the alteration of epigenetic mechanisms leading to expression of developmental regulators, which in turn dictate lineage commitment. Although multiple signaling pathways have been implicated in maintaining ES cell self-renewal vs. differentiation, our understanding regarding the interrelationship among different signaling pathways or mechanisms downstream to a distinct signaling pathway is incomplete.
The PKC family is involved in multiple signaling pathways and consists of several serine/threonine protein kinases 8 that are divided into three subfamilies; (i) classical PKCs (isoforms α, β1, β2, γ, Calcium and phospholipid-dependent), (ii) novel PKCs (isoforms δ, ε, η, θ, Calcium independent and phospholipid-dependent), and (ii) atypical PKCs (isoforms ζ, ι/λ~. Earlier, we found that pharmacological inhibition of PKC signaling by 3-[1-[3-(Dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (Gö6983, henceforth mentioned as PKCi), a highly selective inhibitor of PKC isoforms α, β, γ, δ, ζ, and μ 9, 10, inhibits angiogenic signal-mediated gene expression in endothelial cells 11. Based on this observation, we hypothesized that PKC signaling might be involved in regulating gene expression during formation of the endothelial cell lineage. In investigating the role of PKC during endothelial differentiation, however, we serendipitously found that inhibition of PKC signaling is sufficient to maintain, derive, and propagate pluripotent ES cells and also facilitates reprogramming of differentiated cells to induce pluripotency. Prior to this study, PKC isoforms have been studied during ES cell differentiation in different perspectives 12–16. However, the involvement of the PKC signaling pathway in ES cell pluripotency is largely unknown. Therefore, our study uncovered a yet unknown function of PKC signaling pathway, in which PKC isoforms, specifically PKCζ, induces lineage commitment in ES cells.
Materials and Methods
Inhibitors
PKCi was purchased from three different companies (Sigma, St. Louis, MO # G1918 EMD Chemicals Inc. # 365251, and Tocris Biosciences # 2285). In all experiments except the concentration profile, PKCi was used at 5 µM final concentration. Gö6976 (0.1–2µM, # G1171) was purchased from Sigma. Rottlerin (0.1–5 µM, # 557370), and RO-318425 (0.1–5 µM # 557514) were purchased from EMD Chemicals. PD0325901 (1µM, # 444966) and LY294002 (10µM, # L9908) were from Calbiochem and Sigma, respectively. CHIR99021 (3µM, # 04-0004) was purchased from Stemgent.
ES Cell Cultures
E14Tg2a (E14), R1, and Stat3−/− ES cells were used in this study. Cells maintained for 3–5 days under different culture conditions were used for immunofluorescence study while for other purposes, they were analyzed under steady state condition, i.e., within 8–12h of LIF and other inhibitor treatment. Detailed experimental protocol for ES cell culture under different experimental conditions is described below.
E14 ES Cells
Cells were grown in ES-IMDM media (Lonza, Walkersville, MD) in a feeder-free condition. ES-IMDM was supplemented with 15% serum, 105U/100ml of LIF (ESGRO, Millipore, CA) and 0.0124% monothioglycerol (MTG; Sigma Aldrich). For inducing differentiation in monolayer culture and to determine the effect of PKCi in preventing differentiation, E14 cells were cultured on gelatin-coated plastics for 8 days without LIF. The details of experiments that are done at clonal-density are described below. To maintain E14 cells with PKCi, serum supplemented ES-IMDM containing PKCi was used. For all assays involving elucidation of different signaling mechanism responsible for maintenance of pluripotency, cells from a ~70% confluent ES culture plate were washed two times with 1XPBS, trypsinized and plated on a 6-well tissue culture plate and treated with or without LIF or PKCi or different inhibitors for ~10h followed by preparation of protein lysates and RNA. The PKCζ knocked-down E14 cells that were generated in this study were maintained in serum supplemented ES-IMDM on gelatin-coated plates in the absence of PKCi or LIF or any other inhibitor. We continuously cultured E14 cells for 18 consecutive passages (>58 days) on gelatin-coated plates without significant differentiation.
R1 ES cells
R1 Cells were maintained on MEF feeder in ES-IMDM media supplemented with 15% ES-cell quality serum, 105U/100ml of LIF. Cells were grown for 3–5 days with change of medium every day. For inducing differentiation in monolayer culture and to determine the effect of PKCi, cells were cultured at feeder-free condition and without LIF for 7 days.
Stat3−/− ES cells
Passage 12 Stat3−/− ES cells (Obtained from Dr. Austin Smith, Wellcome Trust Center for Stem Cell Research, Cambridge, UK) were maintained on N2B27 medium with 1µM PD0325901 (MEK inhibitor) and 3µM CHIR99021 (GSK-3 inhibitor) and passaged every 3–4 days. For studies involving PKCi, around 1–2 ×104 cells were plated on 6-well plates having N2B27 medium with or without 5µM PKCi and analyzed for colony morphology and the expression of pluripotency markers.
Quantitative clonal assay
ES and iPS cells were dissociated into single cells using 0.05% trypsin/EDTA and 2–20 cells were plated on each well of a 96-well culture plate. The cells were cultured for 6–7 days, colonies were stained for Nanog, and the number of Nanog positive colonies was counted. For determining maintenance of self-renewal for multiple passages at clonal density with PKCi, E14 cells were cultured at clonal density in 96 well plates with PKCi; cells from undifferentiated colonies were trypsizined after day 6, and again plated at clonal density with PKCi. This procedure was repeated for five consecutive passages (>30 days).
ES cell differentiation on collagen-IV and with retinoic acid
To differentiate ES cells in monolayer culture on collagen IV, ~3×104 cells per well were transferred to collagen IV-coated 6-well plates (354428, BD Biosciences, Franklin Lakes, NJ) cultured for 5 days in ES differentiation medium containing DMEM (Invitrogen), 15% FBS (selected for endothelial differentiation, Stem Cell Technologies, Vancouver, BC), sodium pyruvate and L-glutamine with or without LIF and PKCi, and were recovered by cell dissociation buffer (BD Biosciences, Franklin Lakes, NJ). For culturing multiple passages with PKCi on collagen-IV, the recovered cells were again plated at a density of ~3×104 cells per well and cultured again for 5 days. For our experiments, we continuously cultured E14 cells with PKCi (without LIF) up to 8 consecutive passages on collagen IV plates without any noticeable differentiation. Besides, E14 cells maintained on collagen IV with PKCi for 7 consecutive passages efficiently generated chimeras (showed in Figure 2C). For RA-induced differentiation on monolayer culture, ES cells were treated with all-trans-retinoic acid (R2625-100MG, Sigma, St. Louis, MO) in ethanol at a concentration of 1µM with or without LIF and PKCi for 6 days followed by study of expression of pluripotency markers (by immunofluorescence or western blot or RT-PCR analysis).
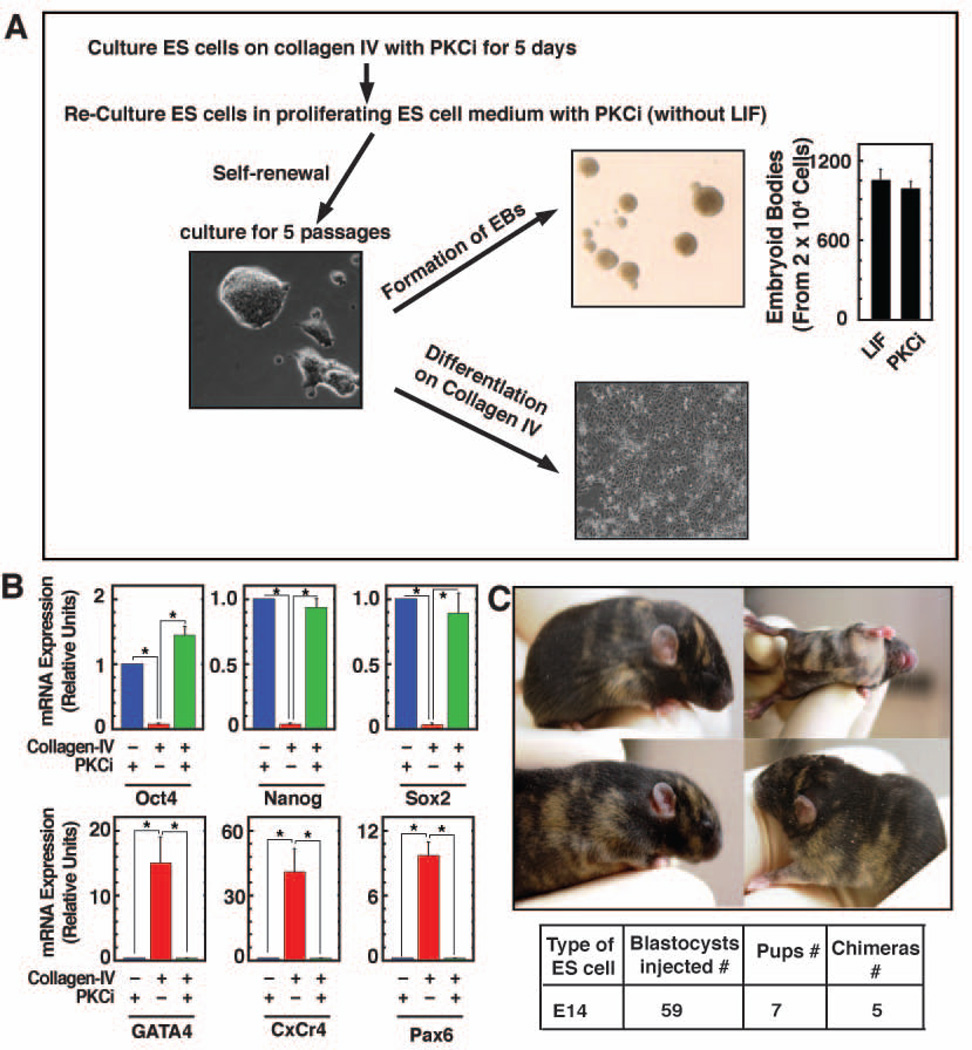
Figure 2. Multi-differentiation and developmental potency of ES cells that are maintained at undifferentiated state by inhibiting PKC isoforms.
A, Experimental strategy to determine the differentiation potency of ES cells that were cultured in the presence of PKCi for multiple passages. The diagram shows that E14 cells, maintained for 5 passages in PKCi, was differentiated on collagen IV (for 5 days) and generated EBs in the absence of PKCi. The bar graph shows the relative efficiency (mean ± standard error; three independent experiments) of EB formation of PKCi-maintained E14 cells in comparison to cells that were maintained with LIF on plastic. B, Multi-differentiation potency on collagen-IV was determined by measuring mRNA expression (mean ± standard error; three independent experiments) of pluripotency and lineage markers. mRNA levels were measured after culturing on collagen IV for 5 days in the absence and or presence of PKCi. The plot shows significant (p≤0.01) loss in pluripotency gene expression and increase in lineage-specific markers in the absence of PKCi. C, Chimeric mice, generated with E14 cells that were cultured for seven passages (35 days) with PKCi on collagen IV. The table below shows number of chimeras obtained.
ES cell culture under serum free condition
E14 cells were maintained in serum free N2B27 medium containing DMEM/F12 (10565, Invitrogen), Neurobasal media (21103, Invitrogen), B27 supplement (17504-044, Invitrogen), N2 supplement (17502-048, Invitrogen), BSA fraction V (15260, Invitrogen), 2-mercaptoethanol (M7522-100ML, Sigma) and LIF/BMP4 (R and D Systems, Minneapolis, MN) with change of medium on alternate days and passed in every 2–4 days. For experiments with PKCi, ~5 ×104 E14 cells were plated on each well of a gelatin-coated 6-well plate having N2B27 medium without LIF/BMP4 and with or without 5µM PKCi and cultured for 3–4 days before passing. Cells were analyzed for expression of different markers (by RT-PCR or immunofluorescence study). For experiments at clonal density, initially, E14 cells were cultured at high density in N2B27 medium with PKCi alone for 4 passages, then ~200 cells were plated in each wells of a 96 well plate with PKCi in N2B27 and cultured for 7 more days. After 7 days, colonies were analyzed for Oct4 staining.
Chimera generation and germ-line transmission
Prepubertal (3 weeks of age) donor C57BL/6 female mice (Jackson Laboratory, Bar Harbor, ME) were superovulated, mated overnight to intact C57BL/6 stud males and euthanized by cervical dislocation on d3.5. Uteri were collected after euthanasia and flushed with M2 medium (Millipore, Bilerica, MA) for the collection of blastocysts. 8–10 ES or iPS cells were injected into the blastocoel of each blastocyst. After injection, the blastocysts were surgically transferred into receipient females that were pseudopregnant by mating with vasectomized males. Recipient females carried the pups to term and nursed until weaning at three weeks. High-level chimeras, generated from newly derived ES cells, were mated with C57BL/6 adult mice to test for germline transmission.
De novo derivation of new ES cells with PKCi
Blastocysts from 6-week-old female 129S2/SvPasCrl (129/Sv) strain (code 476, Charles River Laboratory, Wilmington, MA) mice were isolated at 3.5 day post coitum. Isolated blasotocysts were plated on gelatin coated plastic in medium containing PKCi and FBS and without LIF. To obtain ES cell colonies, blastocyst outgrowths were disaggregated with trypsin and replated on gelatin-coated wells with PKCi and FBS. ES cell colonies were expanded by replating with PKCi at clonal or higher density. For in vitro analysis, cells were cultured following the same protocol mentioned earlier. After six passages with PKCi-condition, chromosome numbers were checked by karyotyping, and injected in C57BL/6 blastocysts for chimera generation.
Generation of iPS cells
iPSCs were derived using lentiviral vectors (Stemgent™ # 00-0004), expressing Oct4, Sox2, Klf4 or c-Myc under the control of the doxycycline (Dox)-inducible tetO operator. 129/Sv MEFs were infected with viral mix following the manufacturer’s protocol. 20h post-transduction, cells were reseeded in ES cell growth medium with either LIF or PKCi at a density of 5×104 cells per well of a 6-well plate and treated with Dox (2µg/ml) for 48 h and then replaced with medium without Dox. For further analysis, iPSC colonies were manually picked at different days starting at day 10. Cells were trypsinized, re-plated at clonal density in medium with PKCi (for PKCi-derived colonies) or LIF (for LIF-derived colonies) and the expression of pluripotent markers Nanog and Rex1 were tested by RT-PCR and immunofluorescence to determine true pluripotent iPSCs. For quantitation, iPSC colonies, which we were able to expand at clonal density and expressed both Nanog and Rex1 were considered. Total number of such colonies obtained with PKCi at day 20 was considered as 100%. RNAs were also analyzed by RT-PCR for the expression of E-Cadherin, Snail, Slug and Twist to determine Epithelial to mesenchymal or Mesenchymal to epithelial (MET) transition during reprogramming of MEFs to iPSCs in the presence of LIF or PKCi. For chimera generation, cells from two different PKCi-derived iPSC colonies were propagated at clonal density with PKCi, expanded for four passages and were injected into blastocysts.
RNA interference and rescue of PKCζ expression in PKCζkd cells
Short hairpin RNAs (shRNAs) targeting mouse PKCζ mRNA were cloned in pLKO1 (Open Biosystems, Huntsville, AL). Lentiviral supernatants were produced in HEK-293T cells as mentioned earlier11, 17. E14 cells were transduced with lentiviral supernatants and were selected by the addition of 1 µg/ml of puromycin (Sigma). Construct with target sequence ATCCCGGTAAGTTCTGTTG, corresponding to the 3’UTR region of PKCζ mRNA, specifically knocked-down PKCζ expression. For rescue of PKCζ expression, PKCζkd cells were re-infected with lentiviral particles having PKCζ cDNA (subcloned from plasmid pMTH PKCζ, Addgene) into the pLKO.3G vector (Addgene) under a hU6 promoter and selected by the expression of EGFP, which is expressed from the same vector under the control of an hPGK promoter (Figure S6). Another construct with target sequence GGACCTCTGTGAGGAAGTG (shRNA2, Figure S5), corresponding to the amino acid coding region of PKCζ mRNA, also specifically knocked-down PKCζ. Construct with target sequence GTTGAGGACGAAGCAAGCC specifically knocked down PKCζ expression.
Additional experimental procedures are mentioned in the “supplemental information”.
Results
Inhibition of PKC isoform signaling is sufficient for maintenance and de novo derivation of ES cells
To understand the function of PKC signaling during ES cell differentiation, we cultured E14 mESCs with PKCi in the absence of LIF. We found that, E14 cells efficiently maintains undifferentiated colony morphology (Figure 1A) when they are propagated at clonal density for five consecutive passages (Figure 1B) with PKCi in the absence of LIF and protein analyses showed that PKCi-treatment maintains expression of pluripotency marker Oct4 (Figure 1C) without induction of differentiation markers (Supplemental Figure 1A (Figure S1A). To further test whether pluripotency is maintained in mESCs for higher passages in PKCi culture condition, we cultured E14 cells for 18 consecutive passages with PKCi in the absence of LIF and tested for colony morphology and expression of pluripotency markers. We found that, through out the culture period, the undifferentiated colony morphology as well expression of pluipotency markers Oct4, Nanog and Sox2 are maintained similar to E14 cells that are cultured with LIF (Figure 1D, E). We found a ~ 30% loss in the Rex1 expression in PKCi cultured cells compared to the LIF-cultured cells. However, in the PKCi condition, the expression of Rex1 was maintained at a significantly higher level compared to cells that were cultured in the absence of both LIF and PKCi (Figure 1E). A concentration profile indicated that PKCi prevents ES cell differentiation in a concentration dependent manner (Figure S1B).
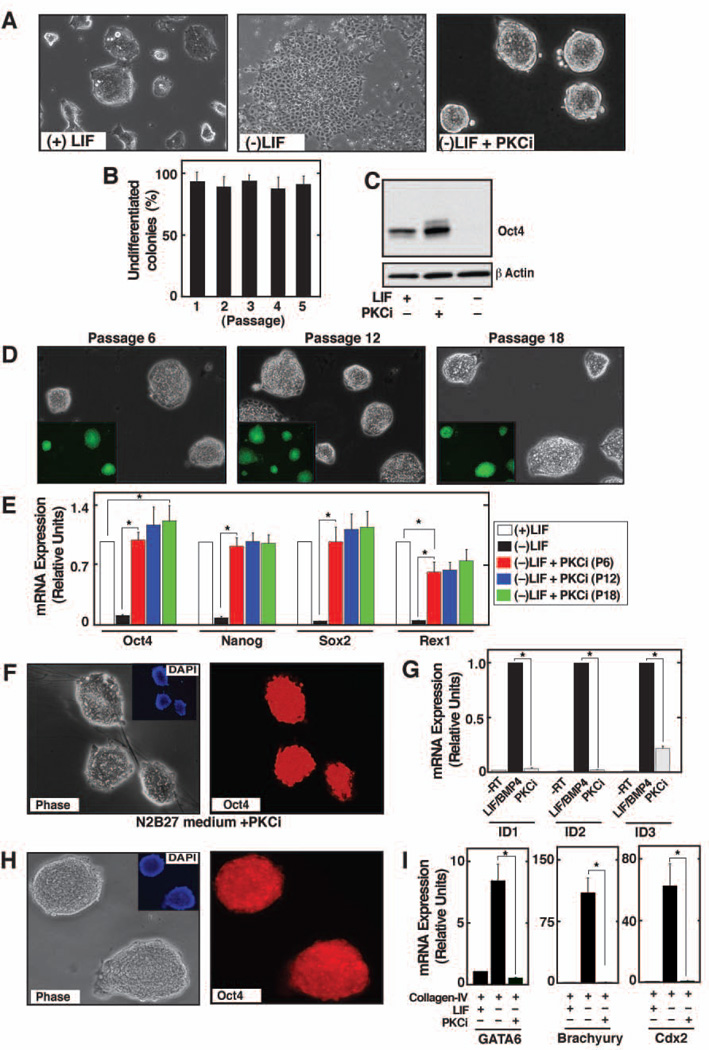
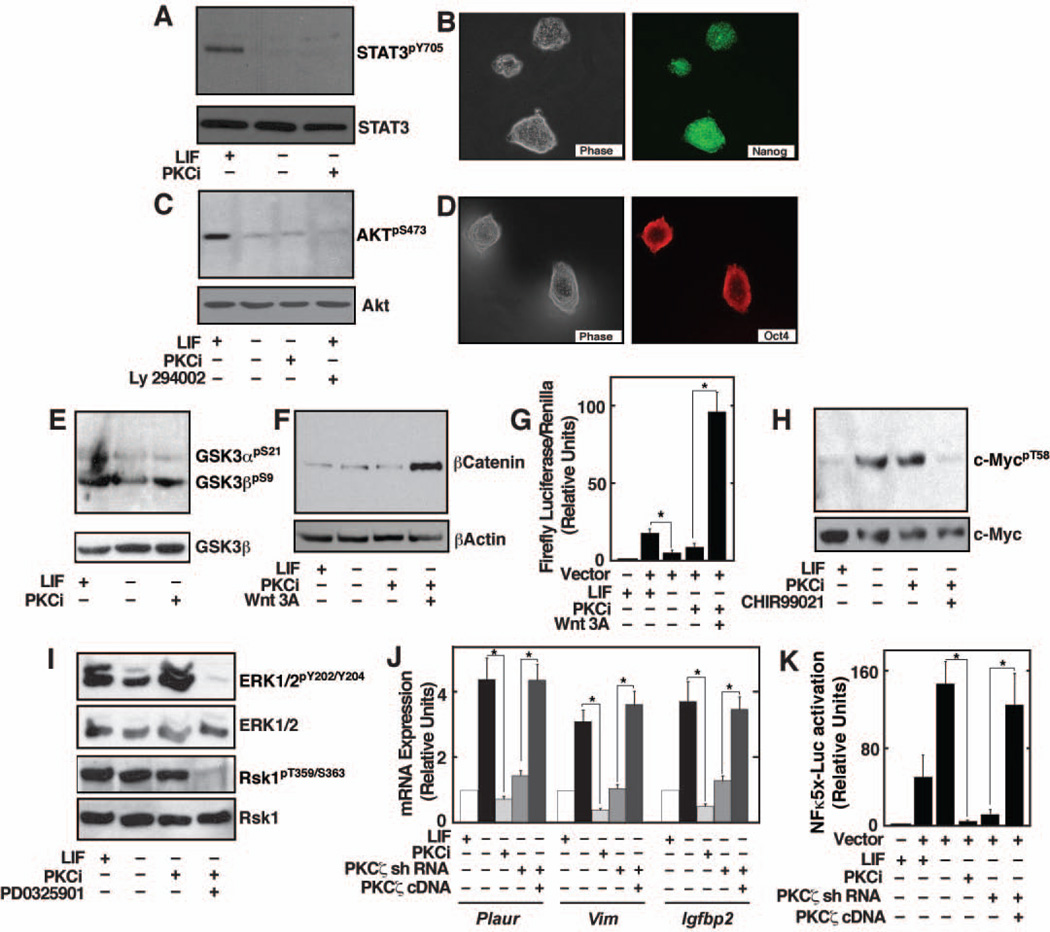
Figure 1. Inhibition of PKC isoform by PKCi supports self-renewal of ES cells.
A, Images of E14 ES cells showing undifferentiated colony morphology with PKCi. B, The plot shows percentage of undifferentiated colonies when E14 cells were cultured at clonal density for 5 consecutive passages with PKCi. C, Western blots showing sustained Oct4 expression in PKCi-treated E14 cells. D, E14 cells were maintained with PKCi for higher passages and the images show the undifferentiated colony morphology and nanog expression (inset) in cells of different passages. E, qRT-PCR analysis (mean ± standard error; three independent experiments) of pluripotency gene expression in E14 cells, maintained for different passages with PKCi. Statistically significant (p≤0.05) changes were indicated in the plot. F, E14 cells were cultured at clonal density in N2B27 with PKCi alone. Images show undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) G, qRT–PCR analysis (mean ± standard error; three independent experiments) of Id gene expression in E14 cells, cultured in N2B27 medium with PKCi alone or LIF/BMP4. H, Oct4 staining in E14 cells after five passages (25 days) on collagen IV with PKCi. I, qRT–PCR analysis (mean ± standard error; three independent experiments) of lineage-specific marker expression in E14 cells that were cultured on collagen IV for 5 days with or without PKCi and LIF. Expression of lineage-specific markers was significantly (p≤0.01) inhibited in PKCi-cultred cells compared to cells that were cultured on collagen IV without LIF and PKCi.
As PKC signaling regulates cell proliferation and survival in multiple contexts 18, we tested the effect of PKC inhibition on mESC proliferation, cell-doubling time, cell cycle distribution pattern, and cell death. We found that at 2.5 – 5 µM concentration of PKCi, which efficiently inhibits ES cell differentiation (Figure S1B), cell proliferation was inhibited by ~30% −40% (Figure S2A). Similarly, we found an increase in cell doubling time in PKCi-cultured cells compared to cells, cultured with LIF (Figure S2B). However, no increase in cell death was observed at 5 µM concentration of PKCi (Figure S2C) and the cell cycle distribution pattern were also very similar between PKCi-cultured and LIF-cultured mESCs (Figure S2D).
We also tested whether PKCi prevents mESC differentiation in the absence of serum. We found that, mESCs can be maintained in an undifferentiated state in serum-free N2B27 medium 4 with PKCi alone (Figure 1F) and this maintenance is not associated with the induction of inhibitor-of-differentiation (Id) genes (Figure 1G).
To test whether PKC inhibition maintains pluripotency independent of mESC types, we used R1 ES cells that are normally maintained at undifferentiated state with LIF on MEF feeder. We found that PKCi also inhibits differentiation of R1 ES cells (Figure S3A). Next, we induced mESC differentiation in the presence of additional differentiation cues by culturing them on collagen IV or treating with retinoic acid (RA)19, 20 and tested whether PKCi prevents mESC differentiation despite the presence of additional differentiation cues. We found that PKCi inhibits mESC differentiation on collagen IV (Figure 1H). Analysis of mRNA expression showed that, similar to culturing with LIF, PKCi treatment completely inhibited induction of lineage-specific gene expression on collagen IV (Figure 1I) and maintained expression of pluripotency markers (Figure S3 B, C). PKCi also efficiently inhibited RA-induced differentiation of mESCs (Figure S3 D, E).
We tested whether mESCs, cultured with PKCi for multiple passages, maintain multi-lineage differentiation potential in vitro and developmental potential in vivo. Intriguingly, we found that upon withdrawal of PKCi, mESCs, maintained for 5 passages with PKCi, readily form embryoid bodies (EBs) and differentiate on collagen IV (Figure 2A) with induction of lineage-specific genes (Figure 2B). Furthermore, when injected into the blastocysts, PKCi-maintained ES cells readily yielded chimeric mice (Figure 2C). These results confirmed that culture in PKCi does not compromise multi-lineage differentiation and developmental potency.
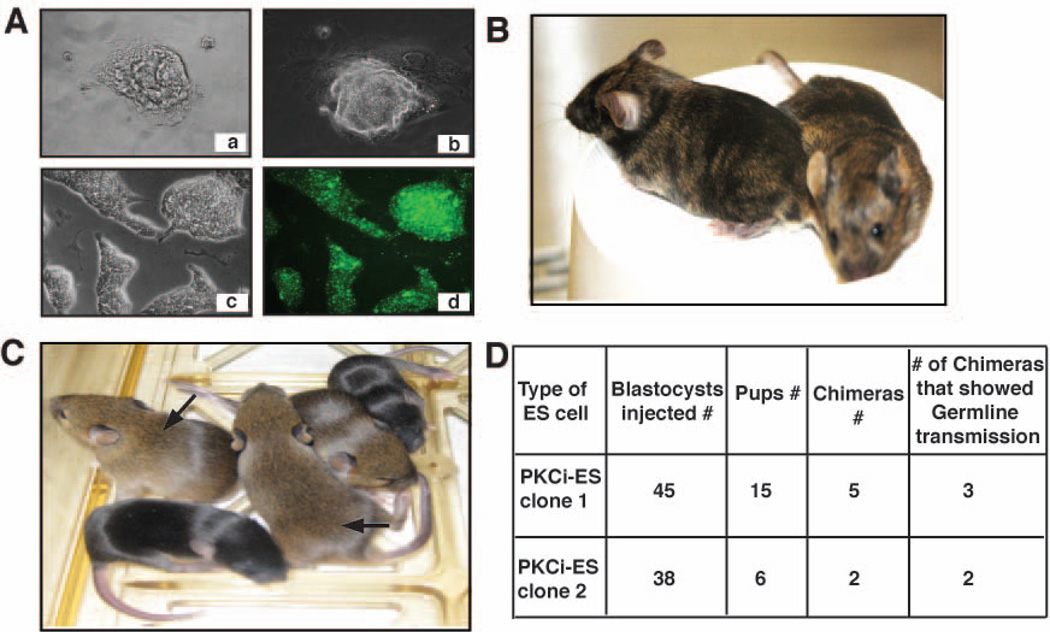
Next, we investigated whether blockade of PKC signaling is sufficient for de-novo derivation of ES cells. We plated embryonic day 3.5 blastocysts from 129/Sv mice on gelatin-coated plates with PKCi and serum and without LIF and feeder cells. We readily derived several ES cell colonies from blastocyst outgrowths (Figure 3A, panel a,b). Although we obtained multiple ES cell colonies from 10 blastocysts in presence of serum and PKCi, we selected 10 ES cell colonies for subsequent analyses. Interestingly, without PKCi, and with serum alone, we were unable to obtain any undifferentiated ES cell colony from >15 blastocysts. The newly PKCi-derived ES cell lines were successfully propagated in an undifferentiated state when cultured at clonal density with PKCi (Figure 3A, panel c,d). After six passages, two different PKCi-derived ES cell lines were injected into the blastocysts to generate chimera (Figure 3B). The PKCi-derived ES cells successfully generated adult chimera, which produced germline offsprings (Figure 3C,D) when crossed with C57BL/6 adults. These results indicate that inhibition of PKC signaling is sufficient to derive germline-competent pluripotent ES cells from mouse blastocyst.
Figure 3. De novo derivation of pluripotent ES cells by inhibiting PKC isoforms.
A, Upper panels: Blastocyst outgrowth (a) and establishment of ES colony (b) from 129/Sv mice with PKCi. Lower panels: phase (c) and fluorescence (d) images showing Nanog expression in 129/Sv ES cells after four passages with PKCi. B, Chimeric mice produced from PKCi-derived 129/Sv ES cells cultured for 6 passages with PKCi. C, Germline offsprings produced from chimeras that were generated from PKCi-derived 129/Sv ES cells. D, The table shows number of chimeras that produced germline offsprings.
Atypical PKC isoform PKCζ promotes mESC differentiation
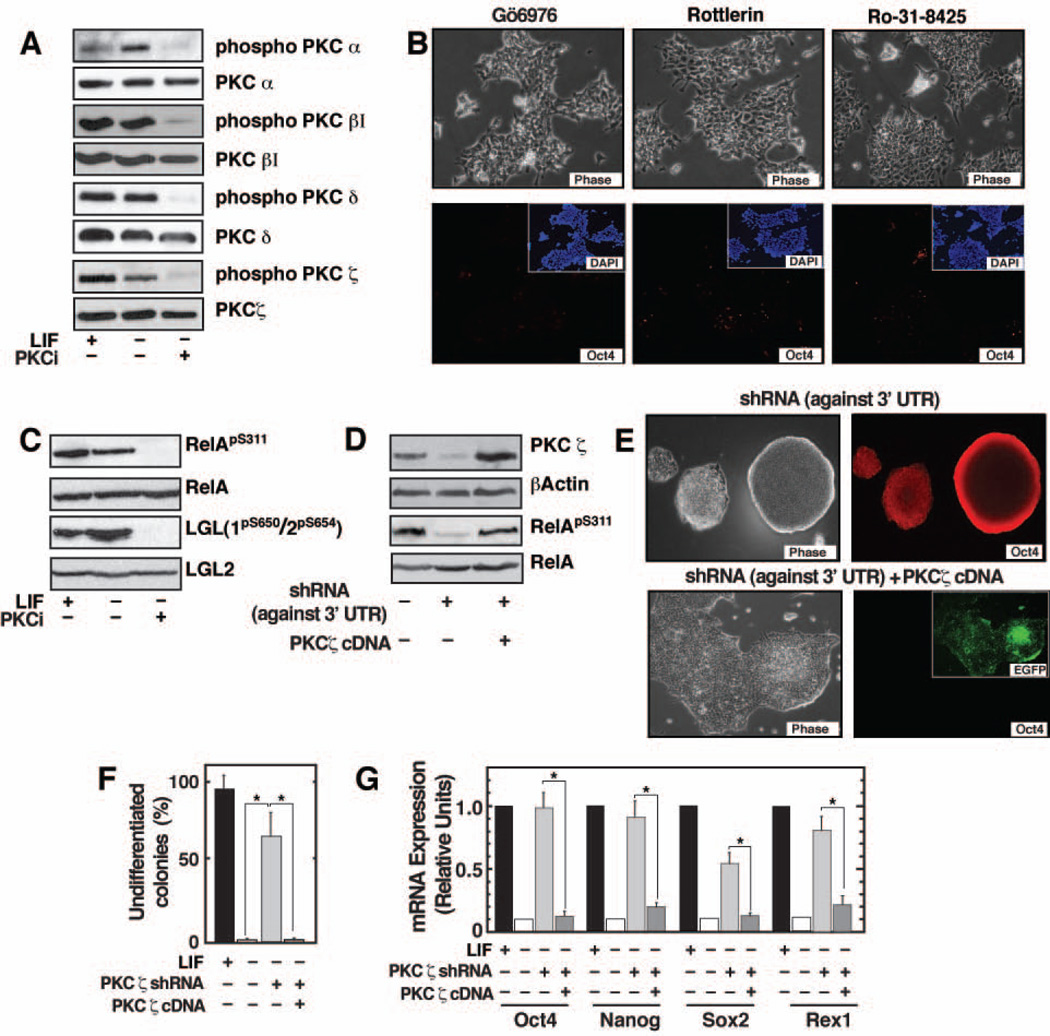
PKCi selectively inhibits 6 different PKC isoforms (α, βI, βII, γ, δ, and ζ) and at a higher concentration (>20 µM) inhibits isoform PKCµ 9 Since 2.5 µM of PKCi inhibits mESC differentiation (Figure S1B), we concluded that PKCμ function is dispensable for ES cell differentiation. Western blot analysis showed that all of the other six PKC isoforms are expressed in mESCs (Figure S4). Western blot analyses further showed that PKC α, βI, δ, and ζ are phosphorylated in mESCs and their phosphorylation were strongly inhibited by PKCi (Figure 4A). Due to our inability to obtain specific antibody, we were unable to definitively determine the phosphorylation state of PKC βII and PKC γ in mESCs. We next used a series of PKC inhibitors possessing different specificities to narrow our search for the PKC isoform responsible for mESC differentiation. However, Gö6976 (inhibits PKC α, βI βII 21 (Figure 4B, left), Rottlerin (inhibits PKCδ, 22) (Figure 4B, middle), and Rö-31-8425 (inhibits PKC α, βI, βII, γ and ε 23) (Figure 4B, right), could not prevent differentiation of mESCs in the absence of LIF. Therefore, we predicted that the atypical PKC, PKCζ, might be important for mESC differentiation. Although western blot analysis showed that PKCζ is phosphorylated in mESCs and phosphorylation is strongly inhibited by PKCi (Figure 4A), to further validate that PKCi impairs PKCζ function, we looked at PKCζ target proteins. PKCζ directly phosphorylates the serine 311 (S311) residue of the RelA subunit of NF-κB 24 and the lethal giant larvae 1 and 2 (LGL1/2) proteins at conserved serine residues 25, 26. We found that PKCi inhibits the phosphorylation of RelA and LGL1/2 (Figure 4C) in mESCs, confirming that activity of PKCζ is disrupted with PKCi treatment.
Figure 4. LIF-independent maintenance of self-renewal of PKCζ-depleted ES cells.
A, Western blots showing inhibition of phosphorylation of different PKC isoforms by PKCi in E14 cells. B, Images showing differentiation of E14 cells in the presence of selective PKC isoform/s inhibitors. C, Western blots showing loss of RelA(S311) and LGL1/2(S650/S654) phosphorylation in PKCi-treated E14 cells. D, Western blots showing knockdown of PKCζ and loss of RelA(S311) phosphorylation in E14 cells with shRNA molecules, targeted against the 3’ UTR region of PKCζ mRNA. The blots also show rescue of PKCζ expression and RelA phosphorylation with ectopic expression PKCζ from an RNAi immune construct. E, (Top) Images showing maintenance of undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) in PKCζkd cells after five passages without LIF. (Bottom) Images showing rescue of differentiation of PKCζkd cells with ectopic expression of RNAi immune PKCζ. (Inset) Ectopic expression of PKCζ in differentiated cells was confirmed from the expression of EGFP (expressed from the same construct). F, The plot shows percentage of undifferentiated E14 ES cell colonies (mean ± standard error; three independent experiments) when PKCζkd cells, with or without ectopic expression of RNAi-immune PKCζ, were cultured at clonal density. The data indicates significant loss (p≤0.01) of undifferentiated colony formation with ectopic expression of RNAi-immune PKCζ. H, qRT-PCR analysis (mean ± standard error; three independent experiments) showing significant (p≤0.01) loss of pluripotency gene expression in PKCζkd cells upon ectopic expression of PKCζ from the RNAi-immune construct.
Next, to test the importance of PKCζ activity during mESC differentiation, we tested differentiation potential of mESCs, in which PKCζ was knocked-down by RNA interference (RNAi). We used the RNAi approach because, the PKCζ (Prkcz)−/− ES cells are not available for our study. For RNAi, we designed a shRNA molecule that specifically targets the 3’ untranslated region (UTR) of PKCζ and efficiently knocks-down its expression in E14 cells (Figure 4D). The loss of PKCζ function in PKCζ-knocked-down (PKCζkd) cells was validated from the loss of RelAS311 phosphorylation (Figure 4D). We found that, when cultured on gelatin-coated plates for multiple passages and without LIF, the PKCζkd cells maintain undifferentiated ES cell colony morphology and expression of pluripotency markers (Figure 4E). Similar results were obtained when PKCζ was specifically knocked-down using a different shRNA construct, which targets the PKCζ coding sequence (data shown in Figure S5). To validate that impaired mESC differentiation is specifically due to the loss of PKCζ function, we ectopically expressed an RNAi-immune PKCζ mRNA (without 3’UTR) in PKCζkd cells using a lentiviral vector. The viral vector also expressed an enhanced green fluorescence protein (EGFP) cDNA (Figure S6) for monitoring ectopic expression of PKCζ. When PKCζ is ectopically expressed from the RNAi-immune construct (right lane of Figure 4D), the PKCζkd cells readily differentiate in the absence of LIF (Figure 4E). To further validate the role of PKCζ in mESC differentiation, we cultured PKCζkd cells at clonal density without LIF and found that PKCζkd cells maintain undifferentiated colony morphology at a >60% efficiency (Figure 4F) with expression of pluripotency markers (Figure 4G). However, they failed to do so when PKCζ was ectopically expressed from the RNAi immune construct. These results confirm that depletion of PKCζ promotes mESC self-renewal and implicate an active role of PKCζ in inducing lineage commitment.
Interestingly, when cultured on collagen IV without LIF, the majority of the PKCζkd cells undergo differentiation along with the presence of some undifferentiated ES cell colonies (Figure S7A). We reasoned that other PKC isoforms might compensate the loss of PKCζ function in the presence of additional differentiation cues on collagen IV. So, we knocked down PKCδ in PKCζkd cells (Fig. S7B). We chose PKCδ because of a recent report27 that implicated PKCδ inhibition in maintaining mESCs self-renewal in vitro. Compared to the PKCζkd cells, the expression of pluripotency genes, Oct4, Nanog and Sox2, were significantly induced when both PKCζ and PKCδ were knocked-down (Figure S7C). However, the expression of pluripotency genes was further induced significantly when double knocked-down cells were cultured on collagen IV with PKCi (Figure S7C). Thus, we concluded that function of PKCζ alone promotes differentiation in mESCs but a combinatorial function of other PKC isoforms along with PKCζ further potentiate lineage commitment of ES cells. However, detailed studies are needed to make definitive conclusions regarding contribution of other PKC isoforms towards mESCs differentiation.
Inhibition of PKC signaling inhibits NF-κB activity in mouse ES cells
Although PKCi is a selective PKC inhibitor, it might regulate other signaling pathways that are implicated in the maintenance of ES cell pluripotency. Activation of JAK-STAT3 and PI(3)K–Akt pathways have been implicated in maintaining mESC pluripotency 2, 28. We found that PKCi does not induce STAT3 phosphorylation in E14 cells (Figure 5A). Furthermore, PKCi efficiently prevents differentiation of Stat3−/− ES cells 5 (Figure 5B) indicating that PKCi-mediated inhibition of ES cell differentiation is independent of JAK-STAT3 signaling pathway. We also tested whether PKCi activates PI(3)K–Akt signaling in mESCs. Similar to an earlier observation 29, we found that LIF induces Akt phosphorylation in mESCs. However, unlike LIF, PKCi-mediated inhibition of mESC differentiation is not associated with Akt phosphorylation (Figure 5C). Moreover, LY294002, a potent inhibitor of PI3 kinase 29, does not prevent the PKCi-mediated maintenance of mESC self-renewal (Figure 5D). Thus, PKCi-mediated maintenance of mESC self-renewal is not associated with the activation of the PI(3)K–Akt pathway.
Figure 5. Effect of PKC isoform inhibition on signaling pathways that are implicated in ES cell pluripotency.
A, Western blots showing lack of STAT3 (Tyrosine (Y)705) phosphorylation in PKCi-treated E14 ES cells in the absence of LIF. B, Images of Stat3−/− ES cells, cultured with PKCi, showing undifferentiated ES cell colony morphology (left) and expression of Nanog (right). C, Western blot analysis of Akt phosphorylation in E14 ES cells. D, Images showing undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) in E14 ES cells, cultured with PKCi and PI3 kinase inhibitor, Ly294002, in the absence of LIF. E, Western blot analysis of GSK-3phosphorylation in E14 cells when cultured with or without LIF and PKCi. F, Western blot analysis of β-catenin in the cytosolic fractions of E14 cells, cultured with or without LIF, and in the presence of PKCi with or without Wnt3A conditioned medium. G, TopFlash luciferase reporter analysis (mean ± standard error; three independent experiments) in E14 cells, cultured with or without LIF, and in the presence of PKCi with or without Wnt3A conditioned medium (* indicates statistically significant (p≤0.05) changes). H, Western blots showing c-Myc phosphorylation in E14 cells, cultured with or without LIF, and in the presence of PKCi with or without GSK-3 inhibitor CHIR99201. I, Western blots showing ERK1/2 and RSK1 phosphorylation in E14 cells, cultured in the presence and absence of LIF, and in the presence of PKCi with or without ERK inhibitor PD0325901. J, qRT-PCR analysis (mean ± standard error; three independent experiments) of NF-κB target genes (Plaur; plasminogen activator, urokinase receptor, Vim; vimentin, and Igfpb2; insulin-like growth factor binding protein 2) expression in E14 ES cells, cultured with or without LIF and PKCi, and in PKCζkdcells, with or without ectopic expression of RNAi-immune PKCζ cDNA (* indicates statistically significant (p≤0.05) changes). K, Analysis (mean ± standard error; three independent experiments) of NFκ5x-Luc reporter activation in cells, analyzed in panel J. * indicates statistically significant (p≤0.05) change.
Using small molecule inhibitors, it has been shown that inhibition of GSK3 and ERK1/2 signaling also promote mESC pluripotency. In addition, GSK-3/Wnt/β-catenin pathway has also been shown to maintain undifferentiated phenotype of both mESCs and hESCs 3. However, PKCi does not change phosphorylation of either GSK-3α or GSK-3β. Figure 5E, does not stabilize β-catenin (Figure 5F), or modulate β-catenin-mediated transcriptional activation of a canonical Wnt reporter (TOPflash reporter, Figure 5G). We also found that PKCi does not inhibit GSK-3β-mediated phosphorylation of c-Myc (Figure 5H) at threonine 58(T58) residue, which induces degradation of c-Myc and contributes to mESC differentiation 30. Rather, in the PKCi culture condition, addition of the GSK-3 inhibitor, CHIR99021 5, abolishes c-Myc phosphorylation (Figure 5H). Next, we tested whether ERK signaling is functional in PKCi-treated ES cells. We found that PKCi does not inhibit phosphorylation of ERK1/2 or p90Rsk1 (Rsk1), a downstream target of ERK1/2 31 (Figure 5I). However, a combination of PKCi and PD0325901, a potent MEK inhibitor5, almost completely inhibited ERK1/2 and Rsk1 phosphorylation (Figure 5I). Therefore, GSK-3 or ERK1/2 inhibition is not involved in maintenance of pluripotency in the PKCi-treated mESCs.
Recently, inhibition of NF-κB activity has been linked to stem cell pluripotency32. Studies in knockout mice indicated that PKCζ function is essential for NF-κB transcriptional activity in response to several signaling pathways 33. Furthermore, it has also been shown that in PKCζ-deficient cells NF-κB is transcriptionally inactive due to impaired phosphorylation at S311 residue of the RelA subunit 24. Since, both PKCi and PKCζ knockdown inhibit RelA phosphorylation in mESCs (Figure 4C, D), and ectopic expression of PKCζ rescues the phosphorylation in PKCζkd cells (Figure 4D), we tested whether PKCi downregulates NF-κB activity in mESCs. mRNA analysis showed that, in the absence of LIF, transcription of NF-κB target genes are activated, and PKCi inhibits their activation (Figure 5J). Analysis with PKCζkd cells in the absence of LIF also showed similar results (Figure 5J). However, ectopic expression of PKCζ rescues activation of NF-κB target genes in PKCζkd cells. To further evaluate impairment of NF-κB transcriptional activity, we used a reporter plasmid, in which Luciferase expression is regulated by five NF-κB binding motifs (NFκ5x-Luc reporter). Interestingly, we detected considerable reporter activity in E14 cells in the presence of LIF and the reporter activity was further induced in the absence of LIF (Figure 5K). However, reporter gene activation is strongly inhibited with PKCi or in PKCζkd cells. Furthermore, similar to NF-κB target gene activation, ectopic expression of PKCζ rescues reporter activity in PKCζkd cells (Figure 5K). These multiple lines of evidence strongly indicate that in mESCs NF-κB is a target downstream of PKCζ and the involvement of a PKCζ-NF-κB signaling axis contributes to the regulation of lineage commitment in mESCs.
Inhibition of PKC signaling facilitates reprogramming of differentiated cells
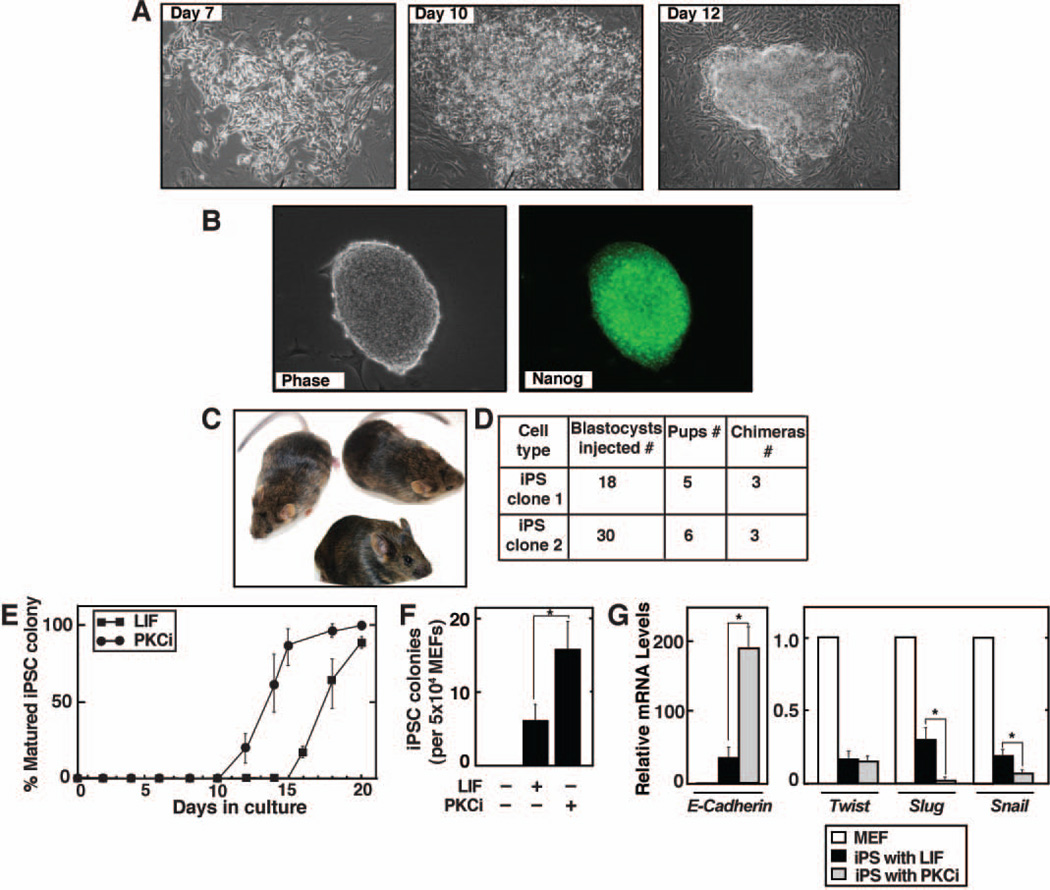
As PKCi condition maintains ES-cell pluripotency, next, we tested whether PKCi facilitates derivation of iPSCs 6. We infected 129/Sv MEFs with lentiviral vectors encoding the four reprogramming factors Oct4, Sox2, Klf4 and c-Myc, and infected MEFs were cultured in the presence or absence of PKCi or LIF. Several ES cell like iPSC colonies appeared beginning 12 days after culturing with PKCi (Figure 6A) and expression of pluripotency marker Nanog was confirmed in forming iPS colonies (Figure S8). Those iPSC colonies were readily propagated at clonal density with PKCi and expression of pluripotency markers were confirmed (Figure 6B). Finally, we injected PKCi-derived iPSCs into blastocysts and generated chimeric mice (Figure 6C, D). Interestingly, compared to LIF, in the presence of PKCi, true iPSC colonies, defined by the expression of Nanog and Rex-1 after the cells are propagated at clonal density, were obtained at a significantly faster rate (Figure 6E). Furthermore, the efficiency of obtaining iPSC colonies was ~three fold higher with PKCi compared to LIF (Figure 6F). As MET is crucial for the formation of iPSC colonies34, we tested expression of genes implicated in MET. We found that, compared to MEFs, mRNA expression of E-Cadherin was induced in both PKCi-and LIF-induced iPSCs (Figure 6G). However, E-Cadherin induction was much higher in PKCi-derived iPSCs compared to LIF-derived iPSCs (Figure 6G). Moreover, the expression of Snail, and Slug were more strongly repressed with PKCi compared to LIF (Figure 6G). These results indicate an increased MET response in PKCi-cultured cells compared to the LIF-cultured cells. Collectively, our results indicate that inhibition of PKC by PKCi provides an efficient culture condition for reprogramming differentiated cells to iPSCs.
Figure 6. PKC inhibition facilitates reprogramming and the derivation of induced pluripotent stem cells.
A, Images of a developing iPSC colony from MEFs in the presence of PKCi. B, Immunostaining showing Nanog expression in iPSCs after replating with PKCi at clonal density. C, Chimeric mice that were generated with iPSCs, which are derived and propagated (passage 4) with PKCi. D, The table shows number of chimeras obtained with different iPSC clones. E, Plot shows comparative time course of iPSC colony formation in the presence of LIF and PKCi. F, Relative numbers of iPSC colonies that were derived in different culture conditions (mean ± standard error; three independent experiments, (* indicates statistically significant (p≤0.05) change). G, qRT-PCR analysis (mean ± standard error; three independent experiments) showing significantly (p≤0.05) higher expression of E-Cadherin mRNA and reduced expression of Snail and Slug mRNAs in PKCi-derived iPSCs compared to LIF-derived iPSCs.
Discussion
In recent years, a major area of research has focused on studying the molecular basis of pluripotency. This study reports for the first time that inhibition of PKC isoform function is sufficient to maintain undifferentiated cultures of mESCs without affecting their multi-differentiation and developmental potency.
Activation of the ERK pathway downstream to FGF4 or other stimuli has been indicated to be a key-signaling component to induce lineage specification in ES cells 5. This is supported by the findings that small molecule inhibitors of this pathway along with inhibition of GSK-3 is sufficient to sustain ES cell pluripotency and to derive new ES cells from multiple species 5, 35. In this study, we have clearly shown that, under different culture conditions, similar to a “neutralized” environment without external stimuli (in N2B27 medium) or in the presence of strong differentiation cues (collagen IV, RA-treatment), a single selective PKC inhibitor maintains ES cell pluripotency without affecting ERK or GSK-3-dependent pathways. Furthermore, we showed in this study that PKC inhibition by PKCi is an efficient strategy for derivation of new ES cells and reprogramming of differentiated cells to iPSCs. Therefore, it is attractive to propose that similar to blockade of ERK activation, inhibition of PKC isoforms also promote ground state of self-renewal in mouse ES cells.
How does blockade of two different signaling pathways independently promote ground state of ES cell self-renewal? As the decision between ES cell self-renewal vs. differentiation to other cell types is executed through epigenetic regulations, we hypothesize that individual signaling pathways like ERK or PKC regulate distinct and/or overlapping cellular components that modulate the epigenetic regulators in stem cells, thereby altering the gene expression program and leading to lineage commitment. Therefore, future studies investigating a role for PKC signaling in relation to regulation of epigenetic components will provide insight into cellular mechanisms that dictate stem cell self renewal vs. differentiation.
A significant finding of this study is the involvement of PKCζ-NF-κB pathway during ES cell differentiation. We showed here that PKCζ function is involved in the activation of NF-κB pathway during ES cell differentiation and inhibition of PKCζ signaling by PKCi or knockdown of PKCζ inhibits NF-κB activity and target gene expression. These results strongly indicate that a PKCζ-NF-κB signaling axis contributes to lineage commitment in mESCs. PKCζ is known to interact with other proteins including prostate apoptosis response (PAR) proteins and is involved in regulation of other cellular functions including cell polarity 36. Other cellular mechanisms, downstream to PKCζ function, might also be involved in inducing ES cell differentiation. In that relation, it is worthwhile to mention that downregulation of both PKCζ and PAR3 in blastomeres of pre-implantation mouse embryos increases their differentiation towards inner cell mass 37, the source of ES cells. We found that PKCζ is phosphorylated in undifferentiated ES cells that are maintained in the presence of LIF. Furthermore, PKCζ overexpression from the RNAi-immune construct was not sufficient to induce mESC differentiation when cultured with LIF (data not shown). These results indicate that LIF-signaling pathway in mESCs overrides the differentiation signals mediated via activated PKCζ. However, the mechanism of PKCζ activation in ES cells and other pathways downstream of PKCζ activation merits further study.
Although PKCζ is involved in ES cell differentiation, depletion of PKCζ is not sufficient to inhibit multilineage differentiation of ES cells in the presence of strong differentiation cues, such as culturing on collagen IV or with RA. Rather, PKCi, which inhibits four additional PKC isoforms, prevents multilineage differentiation in diverse culture conditions. Thus, combinatorial function of other PKC isoforms along with PKCζ might be important to induce multilineage commitment of ES cells. Roles of individual PKC isoforms have been implicated in regulating self-renewal vs. differentiation of lineage-specific stem or progenitor cells 38–41. Thus, it will be interesting to understand whether an individual PKC isoform plays a distinct role in differentiation of pluripotent stem cells towards a specific lineage.
Supplementary Material
Acknowledgements
We thank Dr. Stuart H. Orkin for critical comments. Dr. Austin Smith for Stat3−/− cells and NIH for grant (HL094892, P20 RRO24214, HD062546) supports.
Footnotes
Author Contribution: D Dutta, S Ray, P Home, and M Larson performed experiments. MW Wolfe provided reagents and designed experiments. S Paul designed experiments and wrote the manuscript.
Conflict of Interest
D.D., S.R. and S.P have filed patent applications through University of Kansas Medical Center. The other authors have no financial interest to disclose.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Niwa H, Burdon T, Chambers I, et al. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & development. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 4.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 5.Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Bibikova M, Laurent LC, Ren B, et al. Unraveling epigenetic regulation in embryonic stem cells. Cell stem cell. 2008;2:123–134. doi: 10.1016/j.stem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Spitaler M, Cantrell DA. Protein kinase C and beyond. Nature immunology. 2004;5:785–790. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- 9.Gschwendt M, Dieterich S, Rennecke J, et al. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS letters. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 10.Mor-Vaknin N, Punturieri A, Sitwala K, et al. Vimentin is secreted by activated macrophages. Nature cell biology. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 11.Dutta D, Ray S, Vivian JL, et al. Activation of the VEGFR1 chromatin domain: an angiogenic signal-ETS1/HIF-2alpha regulatory axis. The Journal of biological chemistry. 2008;283:25404–25413. doi: 10.1074/jbc.M804349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Quann E, Gallicano GI. Differentiation of nonbeating embryonic stem cells into beating cardiomyocytes is dependent on downregulation of PKC beta and zeta in concert with upregulation of PKC epsilon. Developmental biology. 2003;255:407–422. doi: 10.1016/s0012-1606(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 13.Heo JS, Han HJ. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem cells (Dayton, Ohio) 2006;24:2637–2648. doi: 10.1634/stemcells.2005-0588. [DOI] [PubMed] [Google Scholar]
- 14.Prudhomme W, Daley GQ, Zandstra P, et al. Multivariate proteomic analysis of murine embryonic stem cell self-renewal versus differentiation signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2900–2905. doi: 10.1073/pnas.0308768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Silva J, Krishnamurthy K, et al. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. The Journal of biological chemistry. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Jeong CH, Cha JH, et al. PKC-delta inhibitors sustain self-renewal of mouse embryonic stem cells under hypoxia in vitro. Exp Mol Med. 2010 doi: 10.3858/emm.2010.42.4.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Home P, Ray S, Dutta D, et al. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. The Journal of biological chemistry. 2009 doi: 10.1074/jbc.M109.016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nature reviews. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa SI, Nishikawa S, Hirashima M, et al. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development (Cambridge, England) 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 20.Lee ER, Murdoch FE, Fritsch MK. High histone acetylation and decreased polycomb repressive complex 2 member levels regulate gene specific transcriptional changes during early embryonic stem cell differentiation induced by retinoic acid. Stem cells (Dayton, Ohio) 2007;25:2191–2199. doi: 10.1634/stemcells.2007-0203. [DOI] [PubMed] [Google Scholar]
- 21.Martiny-Baron G, Kazanietz MG, Mischak H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. The Journal of biological chemistry. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 22.Gschwendt M, Muller HJ, Kielbassa K, et al. Rottlerin, a novel protein kinase inhibitor. Biochemical and biophysical research communications. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. The EMBO journal. 2003;22:3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plant PJ, Fawcett JP, Lin DC, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nature cell biology. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 26.Klezovitch O, Fernandez TE, Tapscott SJ, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes & development. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HJ, Jeong CH, Cha JH, et al. PKC-delta inhibitors sustain self-renewal of mouse embryonic stem cells under hypoxia in vitro. Exp Mol Med. 2010;42:294–301. doi: 10.3858/emm.2010.42.4.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe S, Umehara H, Murayama K, et al. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 29.Paling NR, Wheadon H, Bone HK, et al. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. The Journal of biological chemistry. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 30.Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Molecular and cellular biology. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman E, Frodin M, Gammeltoft S, et al. Activation of p90 Rsk1 is sufficient for differentiation of PC12 cells. Molecular and cellular biology. 2004;24:10573–10583. doi: 10.1128/MCB.24.24.10573-10583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres J, Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nature cell biology. 2008;10:194–201. doi: 10.1038/ncb1680. [DOI] [PubMed] [Google Scholar]
- 33.Leitges M, Sanz L, Martin P, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Molecular cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell stem cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Tong C, Mehrian-Shai R, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D, Edwards AS, Fawcett JP, et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature cell biology. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 37.Plusa B, Frankenberg S, Chalmers A, et al. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. Journal of cell science. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- 38.Koyanagi M, Iwasaki M, Haendeler J, et al. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PloS one. 2009;4:e5765. doi: 10.1371/journal.pone.0005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- 40.Richardson JA, Amantea CM, Kianmahd B, et al. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. Journal of cellular biochemistry. 2007;100:1131–1145. doi: 10.1002/jcb.21112. [DOI] [PubMed] [Google Scholar]
- 41.Steinhart R, Kazimirsky G, Okhrimenko H, et al. PKCepsilon induces astrocytic differentiation of multipotential neural precursor cells. Glia. 2007;55:224–232. doi: 10.1002/glia.20454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.