Abstract
Purpose
Loteprednol etabonate (LE) is approved by the US FDA in a suspension and ointment form (0.5%) for the treatment of postoperative ocular inflammation. This study examined the gel formulation of LE, an improved, nonsettling formulation with a lower preservative level and a more physiologic pH.
Patients and methods
This multicenter, double-masked, parallel-group, vehicle-controlled study randomized patients aged ≥18 years with postoperative anterior chamber cell (ACC) ≥ grade 2 following uncomplicated cataract surgery to either LE gel or vehicle four times a day for 14 days. Primary efficacy end points included the proportion of patients with complete resolution of ACC and grade 0 (no) pain by postoperative day 8. Secondary efficacy end points included complete resolution and change from baseline in ACC and flare (individual and combined), and grade 0 pain at each visit. Safety end points included treatment-emergent adverse events, ocular symptoms, changes in intraocular pressure (IOP) and visual acuity, and biomicroscopy and funduscopy findings.
Results
A total of 407 patients were randomized to treatment (n = 206, LE gel; n = 201, vehicle). At day 8, 31.1% (64) of LE-treated patients and 13.9% (28) of vehicle-treated patients had complete resolution of ACC (P < 0.001), and 75.7% (156) of LE-treated patients and 45.8% (92) of vehicle-treated patients had grade 0 pain (P < 0.001). Secondary efficacy end points also favored LE gel. Fewer patients treated with LE gel required rescue medication (10.7% versus 42.3%) prior to day 15, and fewer had an ocular adverse event (16.0% versus 28.9%, P = 0.002). No drug-related adverse effects were reported more than once in the LE group. Mean IOP decreased in both treatment groups; one patient in the LE group demonstrated a clinically significant increase (≥10 mm Hg) in IOP that was not considered drug-related. Visual acuity and funduscopy findings were similar between treatments.
Conclusion
LE gel 0.5% was efficacious and safe in treating postoperative inflammation and pain in this clinical study.
Keywords: cataract surgery, corticosteroids, gel, loteprednol etabonate, postoperative inflammation, postoperative pain
Introduction
Cataract surgery is a common procedure undergone by millions of patients worldwide.1 Surgical trauma to the eye, however, often results in an inflammatory response. This response is characterized by the release of phospholipids from cell membranes, culminating in the production of chemical inflammatory mediators such as prostaglandins and leukotrienes, and the recruitment of neutrophils and macrophages to the site of trauma. This inflammation usually manifests as perilimbal injection, and inflammatory cells and flare in the anterior chamber along with hyperalgesia.1,2 This inflammation, although usually self-limited, may result in serious sequelae, such as capsular opacification and cystoid macular edema (CME).3,4 To prevent these outcomes, anti-inflammatory agents are administered postsurgically. The most commonly administered class of anti-inflammatory agents is topical corticosteroids.
Corticosteroids have been used to treat ocular inflammation for over 50 years.5 They offer relief from a broad range of signs and symptoms of ocular inflammation and are used in a variety of ocular inflammatory conditions. However, they carry a risk of side effects, particularly an increase in intraocular pressure (IOP).6,7 If left untreated, this increase in IOP could lead to corticosteroid- induced ocular hypertension and eventually glaucoma. Cantrill et al demonstrated that IOP increases are particularly common with older corticosteroids such as dexamethasone.8
Loteprednol etabonate (LE), a unique ester-based corticosteroid, was first approved as an ocular anti-inflammatory agent by the US FDA in 1998. It has since steadily evolved into a commonly prescribed topical ocular corticosteroid, including for inflammation occurring after cataract surgery. LE differs from other ocular corticosteroids in that the ketone group in the carbon-20 position of the traditional prednisolone structure is replaced by an ester. This substitution is the result of retrometabolic drug design, intended to produce a drug that is converted into inactive metabolites after exerting its effects at the glucocorticoid receptor.9,10 This quick transformation to an inactive form results in fewer side effects and smaller increases in IOP, making it an ideal candidate for treating ocular inflammation. LE has been shown to have the lipophilicity required for tissue penetration and a glucocorticoid receptor–binding affinity that is 4.3 times that of dexamethasone, allowing the drug to maintain potent anti-inflammatory activity along with decreased side effects.11 Clinical studies have demonstrated the efficacy and safety of LE in its suspension and ointment forms for the treatment of inflammation and pain after cataract surgery.12–14 It has also been shown to be safe and efficacious in the treatment of numerous other ocular inflammatory conditions, including but not limited to anterior uveitis, giant papillary conjunctivitis, and seasonal allergic conjunctivitis.15–20
The present study was designed to assess the efficacy and safety of a gel formulation of LE versus its vehicle in the treatment of inflammation and pain occurring after cataract surgery. The gel formulation is designed to increase surface retention through the use of polycarbophil and includes the demulcents glycerin and propylene glycol. LE gel is a nonsettling suspension that provides consistent dose uniformity without shaking. The pH range of 6.0–6.5, is closer to physiologic pH than the current LE suspension. Finally, the concentration of the preservative used, benzalkonium chloride (BAK), was reduced from 0.01% to 0.003%.
Methods
Study design
This randomized, double-masked, parallel-group study evaluated the efficacy and safety of the gel formulation of LE against its vehicle. Ethical approval was obtained from Schulman Associates Institutional Review Board in Cincinnati, OH, or Ethik-Kommission in Saarbrucken, Germany. The study was conducted in accordance with good clinical practice, as described in the ICH Harmonised Tripartite Guidelines for Good Clinical Practice; FDA regulation 21 CFR parts 50, 54, 56, and 312, 42 USC 282(j); applicable local regulations; and the Declaration of Helsinki. All patients provided written informed consent.
Participants
The study enrolled patients from 20 investigative sites in the US and two investigative sites in Germany, and included patients ≥18 years planning to undergo routine uncomplicated cataract surgery (phacoemulsification with posterior chamber intraocular lens implantation) not combined with any other surgery. Only patients who in the investigators’ opinion had potential postoperative pinhole Snellen visual acuity (VA) of at least 20/200 in the study eye were enrolled. Female patients of childbearing potential were included only if they had negative urine pregnancy tests at screening. Further inclusion criteria on postoperative day 1 ensured that only patients with postoperative anterior chamber cell (ACC) ≥ grade 2 (6–15 cells) were included.
Patients expected to require concurrent ocular therapy either with nonsteroidal anti-inflammatory drugs (NSAIDs), mast cell stabilizers, antihistamines, or decongestants during the 18 days following cataract surgery or those that had used any of the above within 2 days prior to surgery were excluded from this study. Also excluded were patients expected to require systemic NSAIDs (with the exception of ≤81 mg of acetylsalicylic acid), systemic or ocular (either eye) corticosteroids, or concurrent ocular therapy with immunosuppressants during the 18 days following cataract surgery or who had used ocular immunosuppressants within 30 days prior to surgery; having a history of chronic generalized systemic disease or with severe ocular conditions; monocular patients; and patients with elevated IOP (≥21 mm Hg), uncontrolled glaucoma, known hypersensitivity to the study drug or any of its components; or patients currently undergoing treatment for glaucoma in the study eye.
Study treatments and assessments
The investigational product in this study was LE gel, manufactured by Bausch and Lomb (Tampa, FL), and containing LE 0.5%, and the preservative BAK 0.003%. The vehicle preparation matched the investigational product in concentration, amount, and formulation for all inactive ingredients and preservative, but did not contain LE. The investigators, patients, and other personnel involved in the monitoring or conduct of the study were masked to study treatment. For masking purposes, equal volumes of both the test drug and vehicle were packaged into identical polyethylene bottles and provided in kit boxes (one study kit per patient), each with two dropper bottles.
Eligible patients completed seven visits to the clinic during the 4-week study period. Visit 1 (the screening visit) occurred within 14 days before cataract surgery; patient demographics and medical and ophthalmic history were collected, and patient eligibility was assessed. Eligible patients underwent cataract surgery by phacoemulsification with posterior chamber intraocular lens implantation at visit 2 (day of surgery). Visit 3 (baseline) occurred 18–34 hours postsurgery. At visit 3, patients who had undergone uncomplicated surgery and presented with ACC ≥ grade 2 were randomized in a 1:1 ratio to receive masked treatment of either LE gel or vehicle. Instillation of the first dose of the study medication occurred at the clinic. Subsequently, one or two drops of the study drug were instilled by the patient in the study eye four times a day (at approximately 4-hour intervals) for 14 days. Visits 4 and 5 were on postoperative days 3 and 8, respectively. The fourth dose instilled on the day before visit 6 (postoperative day 15) was the last dose. Visit 7 was a posttreatment follow-up, performed on postoperative day 18. In order to assess compliance of administration, patients recorded the date and time of each study-drug administration in a diary. Patients brought their study drug and diary to the clinic at visits 4–6, at which times the study drug was weighed for accountability and patient diaries reviewed to assess compliance of administration. Patients could be placed on anti-inflammatory rescue medication(s) at the investigators’ discretion any time during the study. Patients requiring rescue medication discontinued study medication; however, they were followed up until the end of the study. The use of topical antibiotics was permitted, provided they were not formulated as a fixed-dose combination with a steroid.
Investigators assessed ocular signs and symptoms, pinhole Snellen VA, and IOP at screening (visit 1) and all postoperative visits (visits 3–7). Ocular signs (cells, flare, ciliary flush, chemosis, eyelid erythema, palpebral conjunctival injection, bulbar conjunctival injection, corneal staining, corneal edema, hyphema, posterior synechiae, and anterior vitreous haze) were evaluated through slit-lamp biomicroscopy. Symptoms (pain, photophobia, itching, tearing, dryness, and discharge) were assessed by the investigator through direct patient inquiry. IOP was measured using a Goldmann applanation tonometer (or an equivalent technique). In addition, study-gel comfort was assessed at visits 4–6. Funduscopy was performed at screening (visit 1) and postoperative day 15 (visit 6) only. Assessments for adverse events (AEs) and concomitant medications were done at each study visit (visits 1–7). Baseline AE assessments were carried out 1 day postoperatively (visit 2) to avoid confounding immediate procedure-related AEs with treatment-emergent (TE) AEs. Therefore, TEAEs were either new events that were not present at baseline or AEs that subsequently worsened from baseline. All TEAEs were summarized by severity and relationship to study treatment.
ACC and anterior chamber flare were both graded on a scale of 0–4 (cells: 0 = no cells, 1 = 1–5 cells, 2 = 6–15 cells, 3 = 16–30 cells, 4 = ≥30 cells; flare: 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) using a 1 × 1-mm high-power-field slit beam. Ciliary flush, hyphema, and posterior synechiae were graded as either absent or present, while chemosis, eyelid erythema, palpebral conjunctival injection, bulbar conjunctival injection, corneal staining, corneal edema, and anterior vitreous haze were graded on a scale of 0–3, with 0 being absent/none and 3 severe. Ocular pain was defined as an unpleasant sensation in the eye including foreign body sensation, throbbing, stabbing, or aching, and was based on a 0–5 scale (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = moderately severe, 5 = severe). Photophobia, itching, tearing, dryness, and discharge were graded on a 0–3 scale, with 0 being absent and 3 severe. Study-gel comfort was also graded on a 0–3 scale, with 0 being none and 3 severe.
Outcome measures
The primary efficacy end points were the proportion of study eyes with complete resolution of ACC and the proportion of study eyes with grade 0 (no) pain at postoperative day 8 (visit 5). The secondary efficacy end points were the proportion of study eyes with complete resolution of ACC and flare, both individually and combined, at each visit, grade 0 pain at each visit, and change from baseline to each follow-up visit in the severity of ACC and flare, both separately and combined.
Tolerability end points included ocular symptoms and study-gel comfort. Safety end points included the incidence of AEs, change in IOP, VA, and biomicroscopy and funduscopy findings.
Statistical methods
Approximately 400 participants were planned to be randomized, with 200 patients per treatment group. A total of 200 patients in the LE gel group would yield approximately 166 LE gel patients treated for the full duration of 14 days, if a 17% rescue medication and discontinuation rate was assumed, estimated from rates reported in previous similar studies.14 Using the asymptotic Pearson chi-squared test, on day 8 a total of 200 patients per treatment group would yield 97% and 99% power for detecting a difference in the rates of complete resolution of ACC at postoperative day 8 and grade 0 pain, respectively, assuming population rates estimated from previous LE postoperative studies.
The intention-to-treat (ITT) population comprised all patients randomly assigned to receive one of the two treatments; the safety population comprised all patients who had received at least one dose of the study drug. The per-protocol (PP) population comprised those patients who remained in the study through day 8 and did not deviate from the protocol in any way likely to seriously affect the primary outcome of the study. End-point analyses were carried out for the ITT population.
The primary efficacy end-point analyses of resolution of ACC and grade 0 pain were carried out by testing both the difference in proportion of study eyes with complete resolution of ACC and the difference in the rates of grade 0 pain between treatments on day 8, using the asymptotic Pearson chi-squared statistic and a two-sided alpha = 0.05 level. Patients with missing data or placed on rescue medication prior to the visit being summarized were considered treatment failures in the analysis. Similar analyses were conducted for complete resolution of ACC and flare combined and for flare separately at day 8, and for resolution of ACC, flare, ACC and flare combined, and grade 0 pain at all other postoperative visits. Both continuous and discrete variables by treatment and by visit were used to assess the change from baseline for ACC, flare, and composite ACC and flare; these were analyzed by carrying the last observation forward for missing data or patients placed on rescue medication.
Tolerability end points of gel-comfort assessment and ocular symptoms were summarized by using discrete summary statistics by visit and by treatment group. The difference in severity of symptoms in the study eye was analyzed, excluding patients placed on rescue medication prior to the visit being summarized.
Safety end points were summarized by visit and treatment group, and were presented separately for data obtained prior to and after receiving rescue medication. Pinhole Snellen VA, biomicroscopy measures, and funduscopy measures were summarized using discrete summary statistics. IOP was summarized using both continuous summaries (including change from baseline scores and change from screening) and discrete summaries (including the proportion of subjects with an increase in IOP ≥ 10 mm Hg from baseline and the proportion of subjects with TE IOP ≥ 30 mm Hg). Nonocular TEAEs were summarized using discrete summaries at the subject and event level by system organ class and preferred term for each treatment group. Ocular TEAEs were summarized for treated eyes and fellow eyes separately. All TEAEs were summarized by severity and relationship separately.
All statistical analyses were conducted using SAS version 9.1 or higher (SAS, Cary, NC).
Results
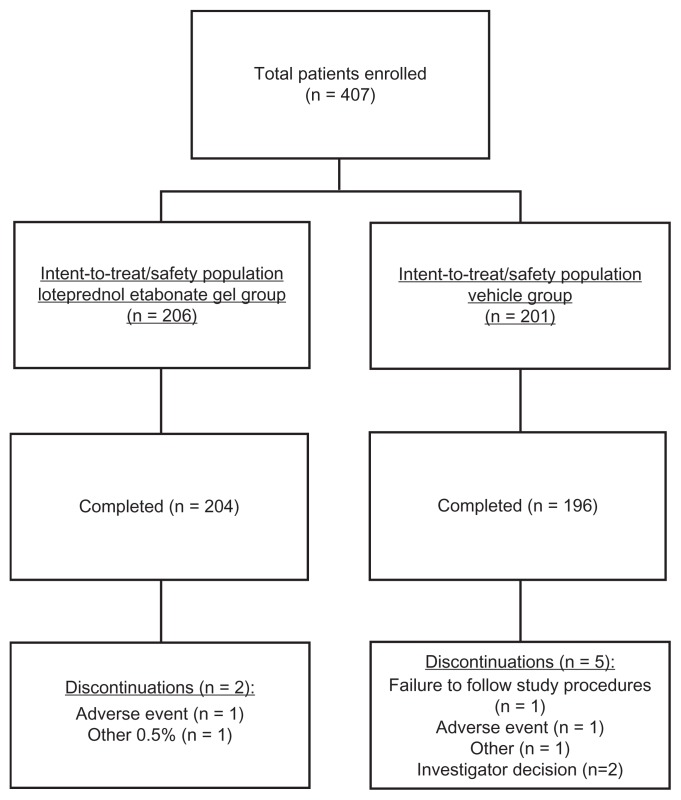
A total of 407 patients enrolled from 22 centers in the US and Germany were randomized (n = 206 in the LE gel group and n = 201 in the vehicle group) and included in both the ITT and safety populations. Of these, 400 patients (n = 204 in the LE gel group and n = 196 in the vehicle groups) completed the study. The primary reasons for discontinuation included AEs (n = 1 for LE gel, diverticulitis; n = 1 for vehicle group, increased IOP), investigator decision (n = 2 for vehicle group), failure to follow study procedures (n = 1 for vehicle group), and other (n = 1 for each group). Figure 1 provides a detailed description of participant flow. Thirty-four (8.4%) patients had major protocol deviations, leaving 373 patients (n = 187 in the LE gel group and n = 186 in the vehicle group) in the PP population.
Figure 1.
Participant flow.
Table 1 provides details on the patient demographics. A total of 73.7% patients were white and 57.2% were female. The mean ± standard deviation (SD) age of the study population was 68.9 ± 9.62 years, with an overall age range of 30–89 years. Similar ocular and nonocular medical histories were recorded between treatment groups in the ITT population. For all the study populations analyzed, demographic and baseline characteristics were similar between treatment groups and across study centers.
Table 1.
Patient demographics for the intention-to-treat population
| Loteprednol etabonate gel (n = 206) | Vehicle (n = 201) | Overall (n = 407) | |
|---|---|---|---|
| Age (years) | |||
| Mean (standard deviation) | 68.3 (9.66) | 69.4 (9.56) | 68.9 (9.62) |
| Median (range) | 69.0 (30, 89) | 71.0 (43, 88) | 70.0 (30, 89) |
| Race, n (%) | |||
| White | 151 (73.3%) | 149 (74.1%) | 300 (73.7%) |
| Black/African American | 22 (10.7%) | 21 (10.4%) | 43 (10.6%) |
| American Indian/Alaskan | 2 (1.0%) | 2 (1.0%) | 4 (1.0%) |
| Asian | 28 (13.6%) | 25 (12.4%) | 53 (13.0%) |
| Other race | 3 (1.5%) | 4 (2.0%) | 7 (1.7%) |
| Gender, n (%) | |||
| Male | 82 (39.8%) | 92 (45.8%) | 174 (42.8%) |
| Female | 124 (60.2%) | 109 (54.2%) | 233 (57.2%) |
| Ethnicity, n (%) | |||
| Not Hispanic and not Latino | 189 (91.7%) | 181 (90.0%) | 370 (90.9%) |
| Hispanic or Latino | 17 (8.3%) | 20 (10.0%) | 37 (9.1%) |
| Country, n (%) | |||
| US | 198 (96.1%) | 193 (96.0%) | 391 (96.1%) |
| Germany | 8 (3.9%) | 8 (4.0%) | 16 (3.9%) |
Mean ± SD baseline (postoperative day 1, visit 3) ACC severity was 2.3 ± 0.49 in the LE gel group and 2.3 ± 0.46 in the vehicle group, with more than 99% of subjects having grade 2 (6–15 cells) or 3 (16–30 cells) ACC at baseline. Mean ± SD flare severity was 1.0 ± 0.62 and 1.1 ± 0.69, while mean ± SD combined ACC and flare were 3.3 ± 0.84 and 3.3 ± 0.89, respectively. At baseline, 50.5% of patients in the LE gel group and 50.2% of patients in the vehicle group reported ≥ grade 1 (minimal) pain.
A total of 185 (45.5%) patients required rescue medication (NSAIDs and/or corticosteroids) during the study, with twice as many patients in the vehicle group compared to the LE gel group requiring rescue medication (61.2% [123] vs 30.1% [62]). The cumulative number of patients requiring rescue medication in the LE gel group and vehicle groups, respectively, was six (2.9%) and 47 (23.4%) by postoperative day 8 (visit 5), and 22 (10.7%) and 85 (42.3%) by postoperative day 15 (visit 6). All other rescue medication was initiated after the 2-week study treatment ended.
Nearly all patients – 203 patients (99.0%) in the LE gel group and 184 patients (99.5%) in the vehicle group – provided complete diary data and reported administering four doses (±20%) per day through postoperative day 8.
Efficacy
Primary efficacy end points
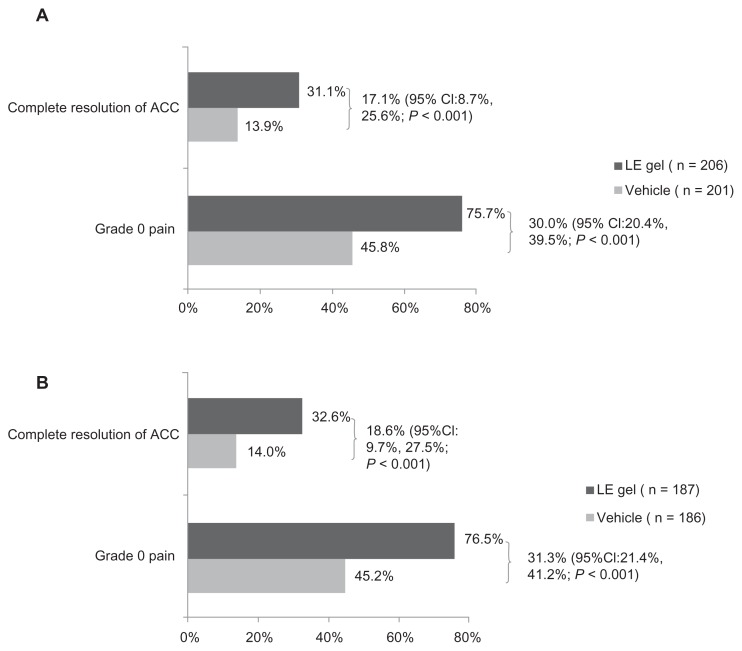
When missing values and data from patients placed on rescue medication were imputed as failures, 31.1% (64) of patients in the LE gel group and 13.9% (28) of patients in the vehicle group had complete resolution of ACC at day 8 (visit 5). This 17.1% treatment difference significantly favored the LE gel group (95% confidence interval: 8.7%, 25.6%; P < 0.001). Grade 0 pain was reported in 75.7% (156) of patients in the LE gel group and 45.8% (92) of patients in the vehicle group. The 30% between-group treatment difference was statistically significant in favor of LE gel (95% confidence interval: 20.4%, 39.5%; P < 0.001). Similar results were obtained for the PP population; 32.6% (61) of patients in the LE gel group and 14.0% (26) of patients in the vehicle group had complete resolution of ACC (P < 0.001); while 76.5% (143) of patients in the LE gel group and 45.2% (84) of patients in the vehicle group had grade 0 pain (P < 0.001). The primary end points and treatment differences observed in the ITT and PP populations are shown in Figure 2.
Figure 2.
Proportion of patients with complete resolution of anterior chamber cell (ACC) and grade 0 pain at day 8 (visit 5) in the intention-to-treat population (A) and per-protocol population (B).
Abbreviations: ACC, Anterior chamber cell; CI, confidence interval; LE, Loteprednol etabonate.
Secondary end points
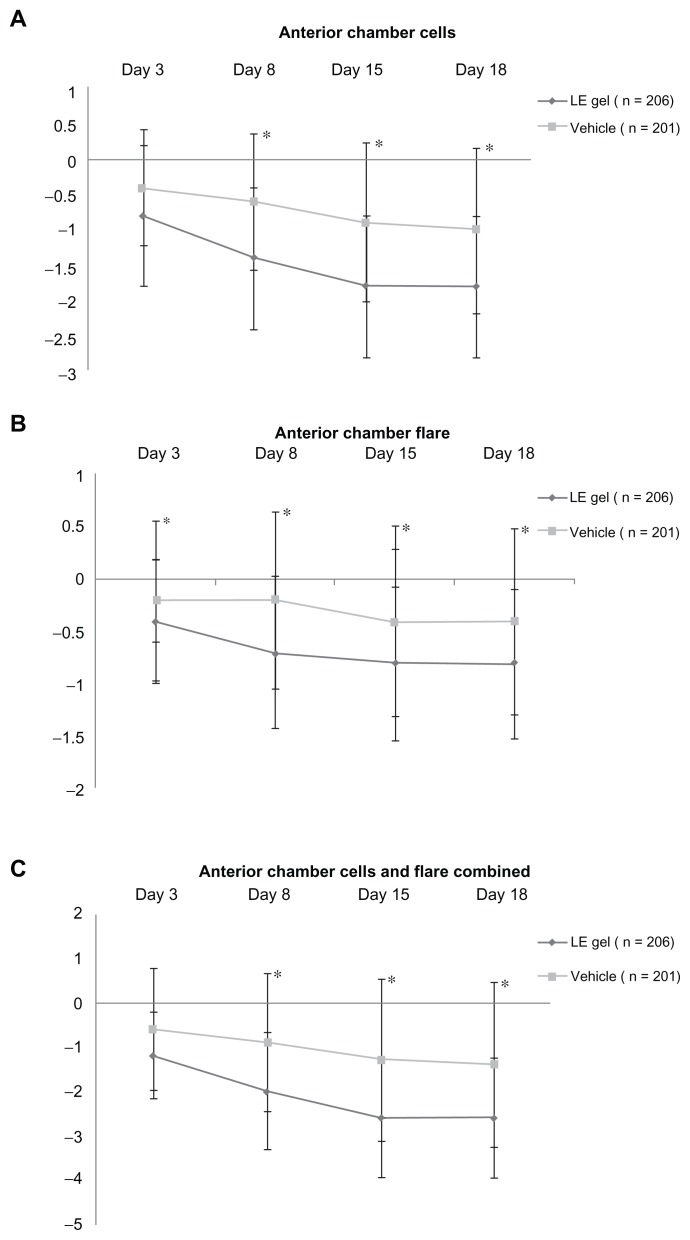
Secondary end points were analyzed for the ITT population only. Table 2 shows the proportion of patients with resolution of ACC, resolution of flare, resolution of ACC and flare combined, and grade 0 pain on postoperative days 3, 8, 15, and 18 (visits 4–7). With the exception of day 3 resolution of ACC and day 3 resolution of ACC and flare combined, all secondary end points significantly favored the LE gel group (P ≤ 0.006). Figure 3 shows the mean change from baseline in ACC and flare, both individually and combined, on postoperative days 3–18 (visits 4–7). Mean change from baseline in ACC, anterior chamber flare, and ACC and flare severity combined were significantly better in the LE gel group compared to the vehicle group at all visits (P ≤ 0.002).
Table 2.
Proportion of patients with complete resolution of ACC, flare, ACC and flare combined, and grade 0 (no) pain on postoperative days 3–18 (ITT population)
| Day (visits) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Day 3 (visit 4) | Day 8 (visit 5) | Day 15 (visit 6) | Day 18 (visit 7) | |||||||||
|
|
|
|
|
|||||||||
| LE gel n = 206 n (%) |
Vehicle n = 201 n (%) |
Difference (95% CI) P-value |
LE gel n = 206 n (%) |
Vehicle n = 201 n (%) |
Difference (95% CI) P-value |
LE gel n = 206 n (%) |
Vehicle n = 201 n (%) |
Difference (95% CI) P-value |
LE gel n = 206 n (%) |
Vehicle n = 201 n (%) |
Difference (95% CI) P-value |
|
| Complete resolution of ACC2 | 8 (3.9%) | 7 (3.5%) | 0.4% (−3.7%, 4.6%) 0.830 |
64 (31.1%) | 28 (13.9%) | 17.1% (8.7%, 25.6%) <0.001 |
116 (56.3%) | 61 (30.3%) | 26.0% (16.2%, 35.7%) <0.001 |
114 (55.3%) | 59 (29.4%) | 26.0% (16.2%, 35.7%) <0.001 |
| Complete resolution of flare | 93 (45.1%) | 64 (31.8%) | 13.3% (3.5%, 23.2%) 0.006 |
134 (65.0%) | 72 (35.8%) | 29.2% (19.4%, 39.0%) <0.001 |
162 (78.6%) | 90 (44.8%) | 33.9% (24.5%, 43.2%) <0.001 |
143 (69.4%) | 75 (37.3%) | 32.1% (22.4%, 41.8%) <0.001 |
| Complete resolution of ACC and flare | 6 (2.9%) | 6 (3.0%) | −0.1% (−3.9%, 3.7%) 0.966 |
63 (30.6%) | 23 (11.4%) | 19.1% (11.0%, 27.3%) ≤0.001 |
115 (55.8%) | 57 (28.4%) | 27.5% (17.8%, 37.2%) <0.001 |
113 (54.9%) | 57 (28.4%) | 26.5% (16.8%, 36.2%) <0.001 |
| Grade 0 (no) pain | 139 (67.5%) | 93 (46.3%) | 21.2% (11.3%, 31.1%) <0.001 |
156 (75.7%) | 92 (45.8%) | 30.0% (20.4%, 39.5%) <0.001 |
160 (77.7%) | 89 (44.3%) | 33.4% (24.0%, 42.8%) <0.001 |
151 (73.3%) | 79 (39.3%) | 34.0% (24.4%, 43.5%) <0.001 |
Note: Primary end points are shaded in gray.
Abbreviations: LE, loteprednol etabonate; ACC, anterior chamber cell; CI, confidence interval; ITT, intention-to-treat.
Figure 3.
Mean +/− standard deviation change from baseline in anterior chamber cell and flare severity, individually and combined, for the loteprednol etabonate (LE) gel and vehicle groups (intention-to-treat population).
Notes: Negative values denote an improvement. *(P ≤ 0.002).
Tolerability
The tolerability of the study medication was judged from assessments of ocular symptoms of discharge, dryness, itching, pain, photophobia, and tearing at each postoperative visit, and as gel comfort at postoperative visits from days 3–15. Ocular pain, photophobia, and tearing – primarily mild – were reported at baseline by 50.4%, 57.2%, and 42.0% of patients, respectively. Excluding patients placed on rescue medication, analysis of these symptoms at postoperative follow-up visits showed that fewer patients treated with LE gel reported pain (P < 0.001 at days 3 and 8, and P = 0.005 at day 15), photophobia (P = 0.006 at day 3 and P = 0.002 at day 8), and tearing (P = 0.001, 0.007, and 0.037 at days 3, 8, and 15 respectively). The majority of patients had no discharge, dryness, or itching at baseline, and these symptoms either improved or did not change from baseline in both treatment groups at follow-up postoperative visits.
Drop comfort was assessed at postoperative visits from days 3–15 as drop sensation (none, mild, moderate, or severe). At each of these visits, more than 85% patients in each group reported drop sensation as “none.” There were no reports of severe sensation at any visit or group, and moderate sensation was reported by less than 2% patients in either group at all visits.
Safety
Mean ± SD treatment exposure was 13.2 ± 2.33 days in the LE gel group and 10.2 ± 4.56 days in the vehicle group. The proportion of patients with at least one ocular TEAE prior to rescue medication use was 16.0% ± 33% for the LE gel group and 28.9% ± 58% for the vehicle group (P = 0.002). As is consistent with cataract surgery, the most common ocular TEAEs were ocular inflammation, eye pain, iritis, foreign body sensations, and dry eye. Table 3 lists ocular TEAEs prior to rescue medication that were considered drug-related. The proportion of patients with at least one ocular TEAE considered drug-related was 2.4% (5) for the LE gel group and 7.5% (15) for the vehicle group. Most drug-related ocular TEAEs were mild to moderate in severity, and no specific drug-related AE was reported more than once in the LE gel group. Blurred vision, a consideration with gel formulations, was reported for only one patient (LE vehicle group). One patient discontinued the study due to a potentially drug-related reaction (increased IOP in the vehicle group).
Table 3.
Drug-related ocular TEAEs prior to rescue medication
| LE gel (n = 206) | Vehicle (n = 201) | |
|---|---|---|
| Patients with at least one related AE | 5 (2.4%) | 15 (7.5%) |
| Total drug-related AEs | 5 | 17 |
| Eye pain | 1 (0.5%) | 3 (1.5%) |
| Foreign body sensation | 1 (0.5%) | 3 (1.5%) |
| Dry eye | 1 (0.5%) | 1 (0.5%) |
| Eye irritation | 1 (0.5%) | 2 (1.0%) |
| Ocular hyperemia | 0 | 2 (1.0%) |
| Photophobia | 0 | 1 (0.5%) |
| Punctate keratitis | 0 | 1 (0.5%) |
| Increased lacrimation | 1 (0.5%) | 0 |
| Ocular discomfort | 0 | 2 (1.0%) |
| Blurred vision | 0 | 1 (0.5%) |
| IOP increased | 0 | 1 (0.5%) |
Abbreviations: LE, loteprednol etabonate; TEAE, treatment-emergent adverse event; IOP, intraocular pressure.
Nonocular TEAEs were reported for 5.8% (12) of patients in the LE gel group and 2.5% (5) of patients in the vehicle group (P = 0.136). The only nonocular TEAEs reported at a rate of at least 1% (n = 2) in any treatment group were headache (LE gel, n = 2; vehicle, n = 1) and rash (LE gel, n = 2). One nonocular TEAE report of rash (facial) in a patient treated with LE gel and one nonocular TEAE report of dry mouth were considered potential drug-related reactions. Five TE nonocular serious AEs were reported by three patients in the LE gel group (diverticulitis, cholecystitis, and myocardial infarction) and one patient in the vehicle group (dehydration and hypokalemia). All TE nonocular serious AEs were considered unrelated or unlikely to be related to the study drug.
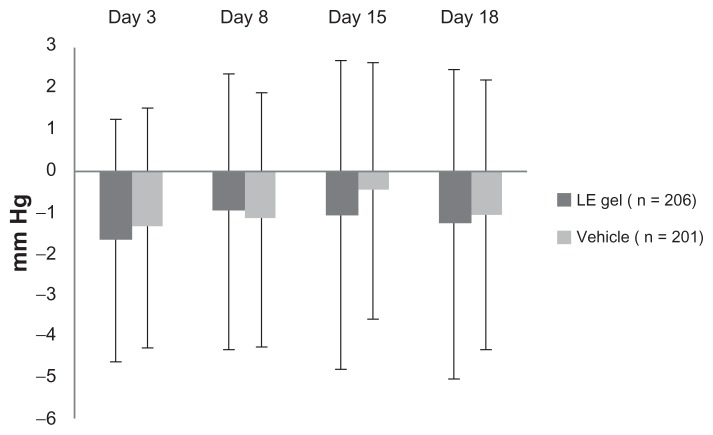
The mean ± SD baseline (postoperative day 1) IOP for study eyes was similar between treatment groups (LE gel group, 14.8 ± 3.37 mm Hg; vehicle group, 14.4 ± 3.48 mm Hg). Figure 4 presents the mean ± SD change in IOP for each group from baseline to each postoperative visit through day 18. Mean IOP was similar between treatment groups and consistently lower than baseline at these visits. One patient in the LE gel group exhibited a clinically significant increase from baseline in IOP (≥10 mm Hg). The increase was first observed at the day 15 visit, and because IOP was also increased in the untreated fellow eye, the investigator did not consider the event treatment-related. One subject in the vehicle treatment group had a 6-mm increase in IOP reported at the day 15 visit that was assessed as potentially drug-related (see Table 3).
Figure 4.
Mean +/− standard deviation change from baseline in intraocular pressure for the loteprednol etabonate (LE) gel and vehicle groups (safety population).
Baseline VA ranged from 20/15 to 20/60 for 94.6% of study eyes in the LE gel group and 93.0% in the vehicle group. As expected after cataract surgery, most patients had an improvement in VA at day 18, with VA ranging from 20/15 to 20/30 for 88.1% of study eyes in the LE gel group and 75.9% in the vehicle group. Few study eyes in either treatment group had a reduction of more than two lines at days 3–18 (LE gel, n = 9; vehicle, n = 11 at worst visit analysis), and there were no between-treatment differences in VA, with the exception of change from baseline at day 8 (significantly worse in the vehicle group, P = 0.032). Dilated funduscopy findings were comparable across treatment groups at screening and day 15. No fundus pathology was reported for the majority of study eyes, and only four study eyes (LE gel, n = 1; vehicle, n = 3) had an increase in retinal abnormalities compared to screening, all judged clinically insignificant.
As expected, biomicroscopy findings showed few patients with worsening ocular signs in the LE gel group. Analysis of ocular signs at postoperative follow-up visits prior to rescue medication showed that fewer patients in the LE gel group had an increased (worsening) of flare, ciliary flush, and bulbar conjunctival injection at days 3–18 (P ≤ 0.048, P ≤ 0.014, and P ≤ 0.010, respectively), of corneal edema and palpebral conjunctival injection at days 3–15 (P ≤ 0.014 and P ≤ 0.011, respectively), of ACC at day 3 and day 8 (P ≤ 0.002), and of chemosis at day 3 (P = 0.002).
Discussion
Results from this multicenter, randomized, double-masked, parallel-group study demonstrate that LE gel administered four times daily for 14 days was significantly more effective than vehicle in resolving inflammation and pain after cataract surgery. Significantly more patients receiving LE gel than those receiving vehicle had complete resolution of ACC and grade 0 pain at day 8 (P < 0.001 for both). In addition, half as many patients in the LE gel group as in the vehicle group required rescue medication. Results from secondary end points, including complete resolution of ACC at days 15 and 18, grade 0 pain at days 3, 15 and 18, and anterior chamber flare at all visits were supportive of the primary efficacy findings, with significantly better resolution of inflammation and pain in patients receiving LE gel compared to vehicle. The finding of significant between-group differences in anterior chamber flare beginning as early as day 3 suggests that the resolution of ocular flare precedes that of ACC and is consistent with observations in previous studies.14
LE gel was shown to be both well tolerated and safe. At most postoperative visits, patient reports of ocular pain, photophobia, and tearing were significantly better in patients treated with LE gel, while symptoms of ocular discharge, dryness, and itching, reported by few patients at baseline, either improved or did not change from baseline, with no significant differences between treatment groups. The majority (>85%) of patients in each group reported no drop sensation. Ocular TEAEs were consistent with signs observed after cataract surgery, and occurred less frequently, overall, in the LE gel group compared to the vehicle group (P = 0.002). Few TEAEs were drug-related, and no drug-related TEAE was reported more than once in the LE gel group. No patients in the LE gel group and only one patient (0.5%) in the vehicle group reported blurred vision.
LE gel did not affect IOP. Mean IOP was lower than baseline at postoperative days 8, 15 and 18, in both treatment groups; further, only one patient in the LE gel group demonstrated a clinically significant increase (≥10 mm Hg) in IOP, and the finding was considered unrelated to study treatment. The lack of significant IOP findings in the LE gel group is attributed to the rapid de-esterification of LE to inactive metabolites.9,21 Finally, biomicroscopy findings showed few LE patients with worsening ocular signs, while dilated funduscopy results and VA measurements were comparable across treatment groups.
The efficacy and safety of LE 0.5% has previously been established in randomized controlled trials; however, these earlier studies assessed alternate formulations of LE, ie, a suspension and an ointment.12–14 Two studies evaluated the efficacy and safety of LE suspension 0.5% versus placebo in the treatment of inflammation following cataract surgery.12,13 Resolution of anterior chamber inflammation (ACI), representing the sum of cell and flare severity, was the primary parameter evaluated. A total of 227 patients in the first study and 203 patients in the second study with ACI ≥ 3 (0–9 scale) on the first postoperative day were randomized to either LE suspension or vehicle administered every 4 hours for 14 days. Using last-observation-carried-forward analyses, resolution of ACI by the final visit (postoperative day 17) occurred in 64% of patients in the LE suspension group, compared with 29% of patients in the vehicle groups in the first study (difference of 35%, P < 0.001), and 55% of patients in the LE suspension group, compared with 28% patients in the vehicle group in the second study (difference of 27%, P < 0.001). No significant difference between groups in the mean change in IOP was observed at any treatment interval. A clinically significant increase (≥10 mm Hg) in IOP was seen in three patients in the LE group in the first study and one patient in the vehicle group in the second study. Comstock et al performed a pooled analysis of data from two randomized controlled trials, each comparing the safety and efficacy of LE ointment 0.5% to vehicle in the treatment of pain and inflammation following cataract surgery.14 Patients (n = 805) with ACI ≥ 3 on the first postoperative day were randomized to either LE ointment or vehicle four times daily for 14 days. The primary efficacy end points included complete resolution of ACI and grade 0 pain at postoperative day 8. Complete resolution of ACI was observed in 28% of patients in the LE ointment group and 13% of patients in the vehicle group. Further, 76% and 43% of patients had grade 0 pain at postoperative day 8. Both end points significantly favored the LE ointment group (P < 0.0001). Clinically significant increases in IOP (≥10 mm Hg) were observed in three patients in the LE ointment group and one patient treated with vehicle.
Despite the use of a less stringent definition for resolution of inflammation in the LE suspension studies (in the LE suspension study, grade 0 ACC was defined as ≤5 cells and grade 0 flare was defined as none-to-trace; while in the LE ointment and LE gel studies, grade 0 acc was defined as no cells and grade 0 flare was defined as none) and the use of different statistical analyses across studies, resolution rates for LE 0.5% appear consistent across studies. By postoperative day 8, similar resolution rates for anterior chamber cells and flare combined were seen across all LE formulations – 43% and 34% in the LE suspension studies, 28% in the LE ointment study, and 31% in this LE gel study.12–14 Further, both the suspension and the ointment formulations demonstrated good safety profiles, with few ocular TEAEs reported in the studies on these formulations.12–14 Taken together with data from earlier trials of LE in suspension and ointment forms, data from the present study reinforce the use of LE as an efficacious and safe ocular anti-inflammatory agent for patients undergoing cataract surgery.
The availability of a gel formulation of LE allows physicians an additional choice in dosage forms when treating patients with ocular inflammation. The LE gel formulation differs from the LE suspension formulation in that it contains a lower level of BAK with a more physiologic pH and is non-settling. A potential advantage of using BAK is the enhanced corneal penetration of some drugs caused by epithelial tight junction separation.22 On the other hand, long-term use of BAK has been reported to impact corneal health or produce symptoms similar to dry eye in some patients.23,24 To minimize any potential negative impact of BAK, the concentration of this preservative in the gel formulation has been reduced relative to the suspension formulation – from 0.01% to 0.003%. This formulation change, along with a pH adjustment to one more similar to that of human tears (6.0–6.5 in the current gel formulation vs 5.3–5.6 in the nonsettling LE suspension), may increase comfort in some patients. Indeed, as indicated earlier, more than 85% of patients reported no discomfort upon instillation in either treatment group. Finally, the nonsettling LE gel formulation does not require shaking to resuspend the drug particles. We believe this may improve ease of use while providing consistent dose uniformity.
In conclusion, the results of this study indicate that LE gel was safe and effective in the treatment of postoperative inflammation and pain following ocular surgery. The availability of a gel formulation of the already well-characterized LE 0.5% concentration allows physicians a choice of dosage forms in treating ocular inflammation following ocular surgery.
Acknowledgments
Medical writing services were provided by Cactus Communications and were funded by Bausch and Lomb. The authors retained full control of the manuscript content.
Footnotes
Disclosure
Raymond Fong has been a speaker for Bausch and Lomb. Raphaele Siou-Mermet and Tara Erb are employees of Bausch and Lomb. Martin Leitritz has no potential conflicts to declare.
References
- 1.El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8. doi: 10.1097/00055735-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rao NA. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- 3.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 4.McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataractsurgery. Curr Opin Ophthalmol. 2000;11(1):3–6. doi: 10.1097/00055735-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Woods AC. Clinical and experimental observation on the use of ACTH and cortisone in ocular inflammatory disease. Am J Ophthalmol. 1950;33(9):1325–1349. doi: 10.1016/0002-9394(50)91827-7. [DOI] [PubMed] [Google Scholar]
- 6.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 7.Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965;4:198–205. [PubMed] [Google Scholar]
- 8.Cantrill HL, Palmberg PF, Zink HA, Waltman SR, Podos SM, Becker B. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. Am J Ophthalmol. 1975;79(6):1012–1017. doi: 10.1016/0002-9394(75)90687-x. [DOI] [PubMed] [Google Scholar]
- 9.Bodor N, Loftsson T, Wu WM. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9(10):1275–1278. doi: 10.1023/a:1015849132396. [DOI] [PubMed] [Google Scholar]
- 10.Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000;20(1):58–101. doi: 10.1002/(sici)1098-1128(200001)20:1<58::aid-med3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Druzgala P, Hochhaus G, Bodor N. Soft drugs – 10. Blanching activity and receptorbinding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991;38(2):149–154. doi: 10.1016/0960-0760(91)90120-t. [DOI] [PubMed] [Google Scholar]
- 12.Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1. J Cataract Refract Surg. 1998;24(11):1480–1489. doi: 10.1016/s0886-3350(98)80170-3. [DOI] [PubMed] [Google Scholar]
- 13.LE Postoperative Study Group 2. A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. Ophthalmology. 1998;105(9):1780–1786. doi: 10.1016/s0161-6420(98)99054-6. [DOI] [PubMed] [Google Scholar]
- 14.Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–186. doi: 10.2147/OPTH.S16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell SJ, Lowry GM, Northcutt JA, Howes J, Novack GD, Hart K. A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol. 1998;102(2):251–255. doi: 10.1016/s0091-6749(98)70094-6. [DOI] [PubMed] [Google Scholar]
- 16.LE Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am J Ophthalmol. 1999;127(5):537–544. doi: 10.1016/s0002-9394(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 17.Shulman DG, Lothringer LL, Rubin JM, et al. A randomized, double-masked, placebo-controlled parallel study of loteprednol etabonate 0.2% in patients with seasonal allergic conjunctivitis. Ophthalmology. 1999;106(2):362–369. doi: 10.1016/S0161-6420(99)90077-5. [DOI] [PubMed] [Google Scholar]
- 18.Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30(1):10–13. doi: 10.1097/01.ICL.0000092071.82938.46. [DOI] [PubMed] [Google Scholar]
- 19.Sheppard JD, Scoper SV, Samudre S. Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther. 2010;27(1):23–27. doi: 10.1089/jop.2010.0085. [DOI] [PubMed] [Google Scholar]
- 20.Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123(4):455–464. doi: 10.1016/s0002-9394(14)70171-0. [DOI] [PubMed] [Google Scholar]
- 21.Druzgala P, Wu WM, Bodor N. Ocular absorption and distribution of loteprednol etabonate, a soft steroid, in rabbit eyes. Curr Eye Res. 1991;10(10):933–937. doi: 10.3109/02713689109020329. [DOI] [PubMed] [Google Scholar]
- 22.Pflugfelder SC. Ophthalmic Preservatives: The Past, Present, and Future. 2008. [Accessed August 26, 2011]. Available from: http://www.candeocsc.com/Preservatives-Final-web.pdf.
- 23.Lin Z, Liu X, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–264. [PMC free article] [PubMed] [Google Scholar]
- 24.Ichijima H, Petroll WM, Jester JV, Cavanagh HD. Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride. Cornea. 1992;11(3):221–225. [PubMed] [Google Scholar]