Abstract
Objective
To evaluate responses by time to non-biologic DMARD initiation in an early seropositive, DMARD naïve, rheumatoid arthritis (RA) cohort.
Patients and Methods
Subjects were categorized by the time from symptom onset to the first DMARD use (median 5.7 months; range 0.6 to 15.9). Subjects who started their first DMARD within 5 months of symptom onset were compared to subjects who started after 5 months. Disease activity scores (DAS-44) and total Sharp Score (TSS) progression rates were analyzed using Wilcoxon rank-sum and Chi-square tests; multiple linear regression analysis adjusted for potential covariates. The slope of the least-squares regression line was calculated to estimate the annualized TSS progression rates.
Results
Of 233 RA patients, 76% were female, mean age was 50 (SD 13).
At DMARD start, DAS-44 was similar in all subsets within the 0.6 to 15 months duration between symptom onset and DMARDS initiation. Erosion scores tended to be higher in those who started DMARD later, but HAQ scores were higher in those who started DMARD earlier During the 2 years after DMARD initiation, improvements in HAQ and DAS-44 were similar in the various duration subsets with about 25% ever achieving DAS remission (<1.6). Radiographic progression tended to be numerically but not statistically more rapid in the earlier subsets.
Conclusion
Following initiation of non-biologic DMARD therapy at various times within 15 months of symptom onset, improvements of DAS-44, HAQ-DI, remission rate and radiographic progression rate were similar, although higher baseline erosion scores were present in those with later DMARD initiation.
Keywords: Rheumatoid arthritis, DMARDS, Window of opportunity, DAS, Radiograph, Remission
INTRODUCTION
Rheumatoid arthritis (RA) is a symmetric inflammatory polyarthritis leading to significant loss of functional mobility and deformity, ultimately resulting in physical disability. Disease-modifying anti-rheumatic drugs (DMARDs) decrease inflammation and slow this destructive process. However, DMARDs are variable in efficacy, and only a small percentage of patients reach long-lasting disease remission (1).
In the 1980’s, the mainstay of treatment was the pyramid approach (step-up therapy approach) until a pivotal article in 1989 proposed that RA patients treated early with DMARDs achieved better long-term outcomes than patients treated later (2). Eventually, the pyramid theory was discarded and new paradigms for RA treatment emerged.
A newer paradigm, the “window of therapeutic opportunity” (3;4), as used in oncology, suggests that early treatment of a smaller mass of cancer cells is more responsive to chemotherapeutics and more likely to result in remission and cure, compared to later treatment of a larger mass of cancer cells. Aggressive treatment of RA close to its onset would equate to treating a smaller mass of destructive inflammatory cells; thus, this might be more effective in achieving remission than treatment later in the disease course when the inflammatory cell burden is increased and a self-perpetuating chronic autoimmune inflammation has developed.
Rheumatologists have had different interpretations for the “window of therapeutic opportunity” (3;4). For this analysis, we define the “window of therapeutic opportunity” to mean that DMARD treatment closer to symptom onset should effect a substantial long-lasting change in the course of the disease, decrease the rate of radiographic progression, improve functional disability, and most importantly, result in high rates of RA remission. To some rheumatologists, however, “window” means that earlier DMARD is necessary before the “window” closes, so that later treatment is less effective than therapy started early, when the window is open. In this interpretation, later therapy will result in more radiographic damage and less remission than early starts (3).
We agree that earlier treatment with DMARDs decreases the cumulative joint damage in RA although few patients achieve remission. However, if the oncologic definition of the “window of opportunity” theory holds true, treatment of RA prior to a specific time of disease duration (i.e. inflammatory threshold) should markedly increase the remission rate by limiting the immune system activation. Previous studies have demonstrated mixed results with regards to the “window of therapeutic opportunity” theory (5-11).
The objective of this analysis is to evaluate whether a “window of therapeutic opportunity” exists in a strictly defined, observational, DMARDs-naïve, seropositive early RA cohort treated by practicing rheumatologists before the availability of anti-TNF and other biologic therapies. Entry criteria selected for RA patients (fulfilling the 1987 ACR criteria (12)) with rheumatoid factor positivity and other characteristics associated with a poorer prognosis. This study’s objective is to examine whether a specific window of opportunity exists between symptom onset and initiation of DMARDS during which induction of durable remission and arrest of radiographic progression is possible and more likely than with DMARD therapy initiated after such a point.
MATERIALS and METHODS
Patients
We studied a subset of early RA patients (within 16 months from symptoms onset to DMARD initiation) participating in a long-term observational study by the Western Consortium of Practicing Rheumatologists, a regional consortium of rheumatology practices in the Western United States and Mexico. The consortium of 36 rheumatology physicians participating in this subset study were from 22 community and 4 university practices in California; Idaho; New Mexico; Oregon; Utah; Colorado; Washington; Wyoming; and Guadalajara, Mexico. Patient entry into this observational cohort began in 1993.
Patients with RA according to the 1987 ACR criteria (12) were entered in this observational study if they satisfied entry criteria. The study entry criteria included: ≤24 months of disease duration since symptom onset, no previous DMARD treatment, rheumatoid factor seropositivity (RF ≥1:80 titer, or ≥40 IU/mL), ≥6 swollen joints (out of 66), and ≥9 tender joints (out of 69 measured joints).
Study Assessments
Assessment at study entry (baseline), 6 months, 1 year, and yearly thereafter included all of the core set measures required to calculate the ACR response criteria (13) and disease activity score (DAS-44) [using the Ritchie index, swollen joint count of 44 joints and erythrocyte sedimentation rate (ESR) in mm/hr] (14,15). Blood specimens were collected for C-reactive protein (CRP) and Westergren ESRs were determined when clinically indicated. Antibodies to cyclic citrullinated peptide (CCP) were also determined from frozen specimens for a subset of patients.
At study entry and every 6 months thereafter, patients were asked to complete a detailed self-report mailed questionnaire that included demographics, health, pain, and detailed medication use, as well as global visual analog scale (VAS) assessments and the Health Assessment Questionnaire-Disability Index (HAQ-DI) (16). Patients were also asked to recall their symptom-onset date (the date of first appearance of joint symptoms that led to the diagnosis of RA) (17).
Study visits included radiographs of the hands, wrists and forefeet. Standard posteroanterior radiographs that included both hands and wrists and anteroposterior radiographs that included both forefeet were obtained at entry, 6 months, 12 months and yearly in the rheumatologists’ offices or by their local radiology facility
Outcomes
Damage due to RA was scored by two experienced readers (JTS, RHG) for erosions (scale 0 to 5) and joint space narrowing (scale 0 to 4); total Sharp score (TSS) is the sum of the erosion scores (ES) and joint space narrowing scores (JSNS) (18,19). Radiographs were read in patient sets, randomized and blinded for sequence. The reader’s independent scores for each radiograph were averaged and the mean was used for the analysis.
Reliability of the readers was assessed by the intra-class correlation coefficient (ICC) and smallest detectable difference (SDD). The ICC and SDD for the average of two readers’ scores were 0.97 and 3.07 units for ES, 0.93 and 7.52 units for JSNS and 0.90 and 12.71 units for TSS, respectively. The progression rate was expressed as change in (total, erosion, or joint space narrowing) score per month; annualized to express progression rate per year (20-26). For each patient, this was calculated by determining the slope of the least-squares linear regression line of all available radiographic observations. Progression rates were assumed to remain relatively constant during the observation interval.
Statistical Analysis
Patients were categorized by the interval from symptom onset to first DMARD use and this analysis included patients from initiation of DMARD to second year assessment. Four separate cut-points for time to first DMARD were examined [3, 4, 5, 6 months]. Each cut-point divided the data into two separate sub-populations which were then compared on the following outcome variables: ACR 20, 50, or 70 response, DAS-44 remission (<1.6), change in DAS-44, HAQ-DI at 1- and 2-years, TSS progression per year, JSNS progression per year and ES progression per year. A very early group (<3 months) was also compared to a very late group (≥10 months). Less than 3 months was selected based on a previous study by Nell et al (9); the very late group included a similar number of our patients with the latest DMARD initiations. For clarity of presentation, only the 5 month cut point groups and the very early and very late group comparisons are included in the publications. The other cut point groups are available on request.
The groups were compared using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables (for baseline outcome variables).
To assess the outcome variables (DAS-44 or radiographic scores) at 1- and 2-years after study entry we used generalized linear regression models in which the outcome is regressed on time to first DMARD and baseline score of the outcome. The first set of these models included terms for time-to-first DMARD and the baseline scores as covariates. The second set included anti-CCP antibody positivity as a covariate to see if adjustment for anti-CCP affected outcomes, as anti-CCP antibody positivity has been associated with higher disease activity and poorer radiographic outcomes (27-29). The third set of models included the first DMARD as indicator variables, whether it was methotrexate, sulfasalazine, hydroxychloroquine, prednisone or a combination. To adjust for imbalances at baseline, our last set of models included variables which were statistically different between the groups at baseline.
All primary outcome analyses were performed using SAS statistical software (Release 9.1, SAS Institute Inc., Cary, NC, USA). Statistical significance was set at the 0.05 level. Because we conducted exploratory analyses for the purpose of generating hypotheses, there was no adjustment for multiple testing.
RESULTS
Of the 233 RA patients, 77% are female and 76% are Caucasian. Mean age of disease onset is 50 years (SD 13) and mean duration of disease is 6.2 months (SD 3.3; range from 0.6 to 14.1 months, one outlier at 20.5 months). At baseline, the patients had active disease with mean DAS-44 of 4.8 (SD 1.2), and mean tender joint count (TJC) and swollen joint count (SJC) of 23 (SD 13) and 20 (SD 11), respectively. Mean HAQ-DI and TSS were 1.22 (SD 0.7) and 6.0 (SD 8.2), respectively.
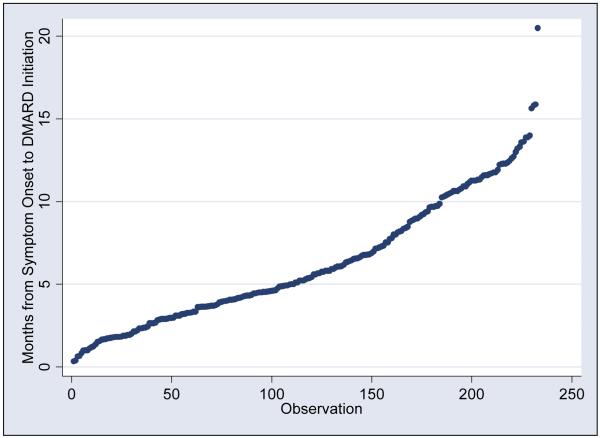
The months between symptom onset and DMARD initiation are distributed relatively evenly among the patients, with median time from symptom onset and start of first DMARDS of 5.7 months (range 0.6 to 15.9, one outlier at 20.5 months). To illustrate the distribution of time between symptom onset and start of first DMARD, a cumulative probability plot is depicted in Figure 1. The patients were ranked from those with the shortest time from symptom onset to DMARD initiation to the longest time. Each dot represents a single patient.
Figure 1.
Cumulative probability plot: Months between symptom onset and start of first DMARD
The patients were ranked from those with the shortest time between symptom onset and DMARDS start to the longest time and the distribution of the patients is depicted in Figure 1. Each dot represents a single patient.
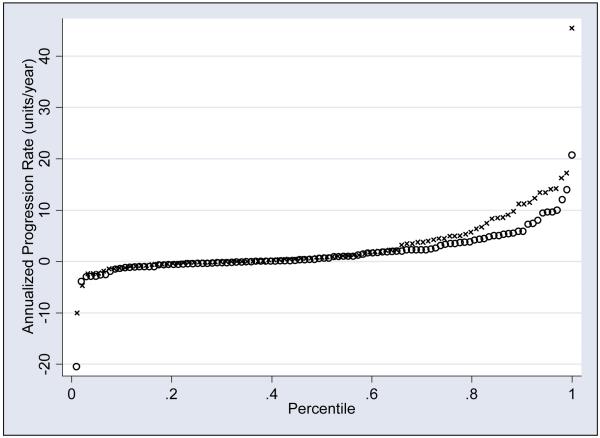
Radiographic progression rates during the first 2 years after DMARD initiation were available for 197 patients. Figure 2 presents TSS progression rate cumulative probability plots of 94 patients who initiated DMARD <5 months (i.e., <150 days), with 103 who initiated DMARD ≥5 months (i.e., ≥150 days) after symptoms onset. In the <5 months group, 28 had negative progression rates, 6 had zero progression rates and 60 had positive progression rates. In the ≥5 months group, 37 had negative progression rates, 5 had zero progression rates and 60 had positive progression rates. The mean TSS progression rates are numerically higher in the <5 months group (3.13±6.49 SD units/year) than in the ≥5 months group (1.69 ± 4.43 SD units/year), but the differences were not statistically significant by the Wilcoxon rank-sum test (p=0.3).
Figure 2.
Cumulative probability plot: Annualized total Sharp score progression rate, by time between symptom onset and DMARD initiation
X: <150 days between symptom onset and DMARD initiation
Hollow circle ○: >150 days between symptom onset and DMARD initiation
The cumulative probability plot of patients who had initiation of DMARD < 5 months after symptoms onset compared to those who had initiation > 5 months demonstrate no significant differences in TSS progressing rates after DMARD initiation.
Table 1 presents the baseline characteristics of subsets of our cohort, using the 5 month cut point of time from symptom onset to first DMARD and comparing <3 months with ≥10 months. No significant differences in percent female or baseline DAS-44 were detected. Patients treated earlier were slightly older if the 3 month cutoff of the very early versus very late comparison was used (Table 1) (p < 0.05). The RA patients treated earlier had significantly higher baseline HAQ-DI scores as compared to patients treated later (Table 1) (p < 0.05). RA patients starting treatment later had significantly higher baseline erosion scores (Table 1) if the very early group (<3 months) was compared to the very late group (≥10 months) (3.59 versus 1.55, p< 0.01). There were no significant baseline differences in ESR or CRP or in DAS-44 scores.
Table 1.
Baseline Characteristics of Subject Subsets Using 150 day, and Very Early versus Very Late Cutoff Times
| Cut Point | 150 Days (5 months) | Very Early | Very Late | |
|---|---|---|---|---|
| Mean (SD) | Early < 150 days |
Late ≥ 150 days |
Early < 90 days (< 3 months) |
Late >= 300 days (≥ 10 months) |
| Number (n=233) | 110 | 123 | 51 | 49 |
| Female, % | 77% | 76% | 76% | 69% |
| Age, years | 51.48 (12.4) | 49.40 (13.1) | 53.31 (11.1) | * 48.14(14.11) |
|
Time to first
DMARDS, months |
3.1 (1.3) | 9.1 (3.0) | 1.9 (0.7) | 12.2 (1.9) |
|
Tender Joint
Count, 0-69 |
23.5 (14.3) | 22.7 (12.0) | 19.6 (12.5) | 23.1 (13.9) |
|
Swollen Joint
Count, 0-66 |
20.0 (11.4) | 19.2 (10.1) | 23.4 (15.5) | 20.9 (11.3) |
|
Physician
Global Assessment, 0- 100 scale |
52.50 (21.03) | 47.69 (20.50) | 52.30(21.16) | 48.3 (20.5) |
|
Patient Global
Assessment, 0- 100 scale |
44.3 (28.3) | 43.3 (27.9) | 46.3 (27.3) | 45.7 (26.9) |
|
Disease Activity
Score (DAS44- ESR) |
4.73 (1.18) | 4.76 (1.16) | 4.60 (1.33) | 4.92 (1.19) |
|
Health
Assessment Questionnaire, 0-3 |
1.35 (0.72) | ** 1.09 (0.68) | 1.41 (0.71) | ** 1.03 (0.56) |
|
Rheumatoid
factor titer, IU/ml |
360.44(405.9) | 389.30(624.0) | 385.29(428.5) | 399.88(724.2) |
|
Anti-CCP
(number positive/total), % Positive |
43/48 90% |
60/69 87% |
19/21 90% |
25/28 89% |
|
C-Reactive
Protein (CRP), mg/dL |
2.95 (3.57) | 2.90 (4.01) | 2.74 (3.36) | 2.9(3.79) |
|
Erythrocyte
Sedimentation Rate (ESR), mm/hr |
41.4 (23.1) | 41.0 (26.3) | 41.3 (24.5) | 48.0 (30.4) |
|
Erosion Score
(ES) |
1.49 (2.18) | 2.60 (4.74) | 1.55 (2.45) | ** 3.59(4.29) |
|
Joint Space
Narrowing Score (JSNS) |
3.78 (4.51) | 4.07 (6.80) | 4.50 (4.73) | 4.94(8.84) |
|
Total Sharp
Score (TSS) |
5.27 (5.69) | 6.67 (9.97) | 6.06 (6.43) | 8.53(12.32) |
significantly different at p<0.05, earlier versus later institution of DMARDS comparison using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables
significantly different at p<0.01, earlier versus later institution of DMARDS comparison using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables
None of the earlier versus later DMARD initiation sub-groups differed in methotrexate dosage, or duration of use during the first 24 months after starting DMARD (Table 2). Patients who were started on hydroxychloroquine later than 10 months took it longer than those who started earlier than 3 months (9.1 versus 15.0 months, p <0.05). With the 5 month cutoff point, those who started hydroxychloroquine later than 5 months were treated with a lower dose than those who started earlier (341 versus 375 mg, p< 0.05). Patients in the very late initiation of DMARDS group (≥10 months) were on prednisone significantly longer compared to those in the very early group (17 versus 13 months, p<0.05), but there was no significant difference in their average daily dose. The proportion of patients started on methotrexate or methotrexate combinations did not differ in early versus later DMARD starts.
Table 2.
Medication Profile during the first 24 months after initiation of DMARD
| Cut Point | 150 days (5 months) | Very early vs Very late | ||
|---|---|---|---|---|
| Mean (SD) | Early < 150 days N=110 |
Late ≥ 150 days N=123 |
Early < 90 days N=51 |
Late ≥ 300 days N=49 |
|
Number of Months
on Methotrexate (MTX) (N=183) |
15.1 (8.1) | 15.7 (7.2) | 13.84 (8.24) | 17.4 (7.4) |
| Mg/wk | 13.2 (8.4) | 12.3 (6.1) | 14.88 (11.78) | 13.5 ( 6.7) |
| n (percent) | 93 (84.5) | 90 (73.2%) | 40 (78.4) | 33 (67.3) |
|
Number of Months
on Hydroxychloroquine (HCQ) (N=182) |
11.0 (8.5) | 14.3 (8.0) | 9.14 (8.05) | * 15.0 (8.4) |
| Mg/day | 374.6 (109.9) | * 340.9 (102.3) | 362.37 (143.0) | 312.8 (87.6) |
| n (percent) | 43 (39.1) | 65 (52.8) | 21 (41.2) | 26 (53.1) |
|
Number of Months
on Prednisone (PRED) (N=147) |
14.1 (8.1) | 14.6 (8.7) | 12.78 (7.33) | * 17.15 (7.2) |
| Mg/day | 6.5 (3.0) | 6.29 (3.7) | 6.62 (2.72) | 5.4 (2.3) |
| n (percent) | 73 (66.4) | 74 (60.2) | 31 (60.8) | 27 (55.1) |
|
Number of Months
on SSZ (N=50) |
13.0 (8.6) | 11.8 (7.9) | 16.36 (7.62) | 9.5 (8.5) |
| Mg/day | 1845.5 (735.3) |
1742.3 (587.7) | 1763.30 (646.0) | 1835.0 (660.3) |
| n (percent) | 26 (23.6) | 24 (19.5) | 14 (27.5) | 10 (20.4) |
|
MTX+HCQ #patients
(percent) |
12 (10.9) | 13 (10.6) | 5 (9.8) | 5 (10.2) |
|
MTX+PRED
#patients (percent) |
25 (22.7) | 21 (17.0) | 10 (19.6) | 8 (16.3) |
significantly different at p<0.05, earlier versus later institution of DMARDS using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables
Outcomes
There were no significant differences in changes of ESR, CRP, HAQ-DI scores or radiographic Sharp score progression rates from baseline in any of the groups (Table 3).
Table 3.
One and Two-year Follow-Up Outcomes
| Cut Point | 150 days | Very Early | Very late | |
|---|---|---|---|---|
| Early < 150 | Late >= 150 | Early < 90 | Late >= 300 | |
|
% meeting
ACR20 1yr (N=165) |
50 | 42 | 42 | 39 |
|
/% ACR20 2yr
(N=125) |
55 | 48 | 48 | 40 |
| % ACR50 1yr | 24 | 27 | 28 | 29 |
| % ACR50 2yr | 36 | 29 | 28 | 23 |
| % ACR70 1yr | 11 | 13 | 6 | 13 |
| % ACR70 2yr | 27 | 14 | 16 | 17 |
|
% 1yr Ever
DAS-44<1.6 |
19 | 14 | 16 | 15 |
|
% 2yr Ever
DAS-44<1.6 |
25 | 25 | 22 | 21 |
|
Mean (SD)
Change from Baseline |
Early < 150 | Late >= 150 | Early < 90 | Late >= 300 |
|
1yr DAS-44
(N=153) |
−1.75 (1.55) | −1.61 (1.52) | −1.83( 1.53) | −1.74 (1.80) |
|
2yr DAS-44
(N=120) |
−1.96 (1.49) | −1.85 (1.45) | −1.90( 1.60) | −1.74 (1.56) |
|
1yr HAQ-DI
(N=179) |
−0.59 (0.66) | −0.45 (0.58) | −0.55(0.77) | −0.41 (0.56) |
|
2yr HAQ-DI
(N=161) |
−0.61 (0.57) | −0.42 (0.68) | −0.63(0.58) | −0.35 (0.61) |
|
1yr CRP
(N=187) |
−0.97 (3.27) | −0.98 (7.00) | −0.91(2.59) | −1.89 (3.74) |
|
2yr CRP
(N=144) |
−1.70 (3.59) | −0.89 (4.53) | −0.90(2.36) | −1.12 (3.62) |
|
1yr ESR
(N=187) |
−13.4 (25.14) |
−12.6 (26.64) | −14.9(26.99) | −18.03 (26.54) |
|
2yr ESR
(N=144) |
−16.4 (26.40) |
−10.96 (28.51) |
−13.3(28.81) | −17.00 (31.05) |
|
2yr ES, units
per yr (N=197) |
1.70 (2.92) | 1.04 (2.78) | 2.36(3.39) | 1.14 (2.05) |
|
2yr JSNS, units
per yr (N=197) |
1.47 (4.65) | 0.72 (2.49) | 2.16(6.42) | 0.63 (1.37) |
|
2yr TSS, units
per yr (N=197) |
3.13 (6.49) | 1.69 (4.43) | 4.62(8.46) | 1.77 (2.75) |
Outcomes are adjusted for time to first DMARD and the baseline scores as covariates.
Progression rates for ES, JSNS and TSS are expressed as units per year.
Comparison of proportions was done with Chi-square tests of association, Fisher’s exact tests
Change scores from baseline were compared using Wilcoxon rank-sum tests
At both 1 and 2 year time points, there were no differences in percent of patients achieving ACR20 or ACR50 in any of the time to DMARD start subsets (Table 3). There were no significant differences in the proportion of patients who achieved DAS-44 remission (< 1.6) in any group during 1 or 2 year follow-up. In the generalized linear models with adjustment for anti-CCP (n=106 because not all patients had anti-CCP done), time from symptom onset to DMARDS initiation did not significantly effect outcomes like DAS-44, ES, JSNS, and TSS.
In the models adjusting time to first DMARD by baseline DAS-44, HAQ-DI, ESR, CRP, ES and JSNS, the only outcome variables significantly associated with time to first DMARD were ESR at 2 years (p=0.002) and DAS-44 at 2 years (p=0.05). Shorter time to first DMARD is associated with less improvement in ESR or DAS-44 scores within the 2 years. In the models adjusting for initial use of MTX, HCQ, SSZ, or prednisone, the time to first DMARD did not significantly influence any of the outcome measures after 1 or 2 years.
DISCUSSION
The goal of this study was to evaluate the relationship of symptom duration to DMARD initiation in an early, seropositive moderately severe RA cohort, and to determine whether very early and varying periods to DMARD treatment of RA can effect a major long-lasting change in functional capacity, radiographic progression rate and DAS-44 remission (3;4). Our observational cohort of 233 rheumatoid factor-positive, DMARD-naïve RA patients were all early in their disease course, with time from symptom onset to DMARD initiation of 0.6 to 15.9 months. They were subdivided into categories based on the time from symptom onset to DMARD initiation using a 5 month cut point as well as a very early group (<3 months) versus a very late group (≥10 months).
In this cohort, earlier institution of DMARD therapy, did not significantly change the DAS-44 remission rate, improvement in functional disability or radiographic progression rate compared to subjects who started DMARD later (up to 15 months after symptom onset).
At study entry, most parameters, e.g. DAS-44, TJC, SJC, ESR, and CRP were not significantly different when compared in subsets created by dividing the cohort at various timepoints from symptom onset to DMARD initiation. However, comparing the very early <3 months with the very late ≥10 months subsets, the very early subset was 5 years older and had poorer physical function (HAQ-DI 1.41 vs 1.03). Indeed all subgroups comparisons demonstrated higher baseline HAQ-DI in the earlier groups, suggesting that those with more functional impairment may have sought a rheumatologist sooner. This may result in confounding-by-indication bias, in which those patients with more symptomatic disease saw a rheumatologist sooner and received earlier and more aggressive treatment. Treatment of more symptomatic patients earlier could lead to similar or improved outcomes as compared to those with more indolent disease who sought care later (30). However, in our cohort the selection of a specific DMARD treatment was not more aggressive for those patients who started on DMARDs earlier compared to those who started later (Table 2). One way to adjust for this bias is to use propensity score analysis (31, 32), but since only a few variables are different at baseline, we elected to adjust for them by adding them to our generalized linear models. Adjusting for these baseline differences did not significantly affect the outcomes.
Of note, patients who started a DMARD later had higher ES at baseline suggesting that they had more time to accumulate damage prior to DMARDs initiation. Following DMARD initiation, at one and two-year follow-up, despite earlier treatment, the earlier treatment groups demonstrated no clear difference in therapeutic response in terms of ACR50 or 70 response or DAS-44 < 1.6 remission or change from baseline in DAS-44, HAQ-DI, CRP, ESR or in Sharp score progression rates.
Strengths of our study include our homogeneous study population: 1) early RA subjects with disease duration assessed from the symptom onset and not from the referral-dependent time of diagnosis by a physician, 2) all subjects had active seropositive RA (with positive rheumatoid factor greater or equal to 40 IU/ml) with a mean of 23 TJC and 20 SJC (minimum 9 TJC and 6 SJC) when they entered the study (note that the findings of this study should not be applied to patients with seronegative RA and undifferentiated polyarthritis, who are more likely to have a spontaneous remission and were not included in our study), and 3) all of the patients were treated by practicing rheumatologists, and the findings are representative of those attained in routine clinical practice.
Limitations include that our study was not designed specifically to answer the proposed question, and the lack of control over choice of DMARDs. Perhaps certain DMARDs, biologic agents or combinations can eliminate the inflammatory cell burden during the postulated window and thus the choice of DMARD could influence whether a window of opportunity is detected. The problem of confounding by indication includes situations where patients may have initiated certain DMARD(s) depending on the status of their RA (30). However, in our cohort, the proportion of patients started on MTX or MTX combinations did not differ in early vs later DMARD starts.
We cannot rule out that patients who started DMARD therapy later than 15 months from symptoms onset would have had markedly poorer responses; however, we could not detect major duration-dependent differences in outcomes within the range of times that we tested. The cohort only included one patient who started DMARDs 20 months after symptom onset, but there were no other subjects after 15 months and inferences beyond 15 months are not possible. We have not tested the possibility that DMARD treatment during a window of opportunity in “pre-RA” (prior to fulfilling RA ACR criteria) or asymptomatic genetically predisposed persons with rheumatoid factor and/or anti-CCP antibodies could prevent the eventual expression of clinical RA.
There may be a complex interaction between choice of DMARDs and their early initiation that is optimal for the treatment of RA but we have not demonstrated a “window of therapeutic opportunity” within which initiation of DMARDs in active seropositive early RA patients induces durable and complete remission, with normalization of laboratory parameters, as well as clinical and radiographic outcomes used in this study. A well-designed controlled clinical trial randomizing patients with early RA to either early or late treatment is the ideal way to answer this question, but it is no longer ethical to randomly withhold treatment in order to prove that more joint damage occurs in one group. We believe that early initiation of DMARDs is of proven clinical benefit to RA patients to prevent or decrease the accumulation of irreversible joint damage regardless of duration of symptoms (33-35).
In terms of clinical implications, an inability to demonstrate an oncologic (curative) window of opportunity in our cohort does not mean that DMARD initiation can be delayed without consequences. Substantial joint damage was present at baseline in all of the disease duration subgroups; even those with less than 3 months disease duration had a mean total Sharp score of 6.06. Presumably irreversible joint damage accumulates during the interim before initiation of DMARDs, and the subset of our cohort who initiated DMARD very late after symptom onset had significantly higher baseline erosion scores than those starting DMARD very early (Table 1). Biologic agents (i.e. TNF inhibitors) were not available when this cohort was treated but several large clinical trials in patients with a wide range of disease durations demonstrate that anti-TNF agents in combination with methotrexate are more likely to arrest radiographic progression than methotrexate alone (33-35). If the currently available anti-TNF/MTX combinations are started early enough, this pre-DMARD joint damage might be avoidable.
However, during the 2 years after starting DMARD (up to 15 months after symptom onset), the rate of progression of joint damage was not less in those with a shorter interval between symptom onset and DMARD initiation. Indeed, ES and TSS progression rates were numerically higher in the subsets who started DMARD earlier, supporting the assumption that channeling bias caused patients with more severe early RA to seek earlier rheumatologic care. This finding is contrary to what one would expect if a limited early window of therapeutic opportunity was associated with markedly better disease control.
Thus, in our observational cohort, we could not detect a “window” of therapeutic opportunity that could achieve the sought after goal of curative ablation of the inflammatory cell mass. However, despite the absence of a “window”, we advocate that effective DMARD therapy should be initiated as soon as possible to minimize cumulative damage in patients with early aggressive RA.
Acknowledgments
Grant Support:
NIH/NIAMS P60 AR 26834; Southern California Chapter of the Arthritis Foundation; Specialty Laboratories; Oregon Arthritis Foundation
Dr. Khanna received support from the UCLA Older Americans Independence Center, NIH/NIA Grant P30-AG028748, and the content does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Dr. Ranganath received support from the ACR/REF CIFA and UCLA Older Americans Independence Center, NIH/NIA Grant P30-AG028748, and the content does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
This is a pre-copy-editing, author-produced PDF of an article accepted for publication in The Journal of Rheumatology following peer review. The definitive publisher-authenticated version (Weng HH, Ranganath VK, Khanna D, Oh M, Furst DE, Park GS, Elashoff DA, Sharp JT, Gold RH, Peter JB, Paulus HE; Western Consortium of Practicing Rheumatologists. Equivalent responses to disease modifying antirheumatic drugs initiated at any time during the first 15 months after symptom onset in patients with seropositive rheumatoid arthritis. J Rheumatol. 2010 Mar;37(3):550-7. Epub 2010 Jan 28) is available online at: http://www.jrheum.org/cgi/pmidlookup?view=long&pmid=20110517
Reference List
- (1).Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–3773. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- (2).Wilske KR, Healey LA. Remodeling the pyramid--a concept whose time has come. J Rheumatol. 1989;16(5):565–567. [PubMed] [Google Scholar]
- (3).Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–1774. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- (4).’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46(2):283–285. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- (5).Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum. 2003;48(1):46–53. doi: 10.1002/art.10727. [DOI] [PubMed] [Google Scholar]
- (6).Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum. 2004;50(7):2072–2081. doi: 10.1002/art.20351. [DOI] [PubMed] [Google Scholar]
- (7).Landewe RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46(2):347–356. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- (8).van Aken J, Lard LR, le Cessie S, Hazes JM, Breedveld FC, Huizinga TW. Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63(3):274–279. doi: 10.1136/ard.2003.010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43(7):906–914. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- (10).Verstappen SM, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, Borg EJ, et al. Five-year followup of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum. 2003;48(7):1797–1807. doi: 10.1002/art.11170. [DOI] [PubMed] [Google Scholar]
- (11).Lard LR, Boers M, Verhoeven A, Vos K, Visser H, Hazes JM, et al. Early and aggressive treatment of rheumatoid arthritis patients affects the association of HLA class II antigens with progression of joint damage. Arthritis Rheum. 2002;46(4):899–905. doi: 10.1002/art.10151. [DOI] [PubMed] [Google Scholar]
- (12).Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- (13).Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- (14).van der Heijde DM, van ’t Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916–920. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).van der Heijde DM, van’t Hof MA, van Riel PL, van Leeuwen MA, van Rijswijk MH, van de Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):177–181. doi: 10.1136/ard.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- (17).Amjadi-Begvand S, Khanna D, Park GS, Bulpitt KJ, Wong WK, Paulus HE. Dating the “window of therapeutic opportunity” in early rheumatoid arthritis: accuracy of patient recall of arthritis symptom onset. J Rheumatol. 2004;31(9):1686–1692. [PubMed] [Google Scholar]
- (18).Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 1971;14(6):706–720. doi: 10.1002/art.1780140605. [DOI] [PubMed] [Google Scholar]
- (19).Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum. 1985;28(12):1326–1335. doi: 10.1002/art.1780281203. [DOI] [PubMed] [Google Scholar]
- (20).Dawes PT, Fowler PD, Clarke S, Fisher J, Lawton A, Shadforth MF. Rheumatoid arthritis: treatment which controls the C-reactive protein and erythrocyte sedimentation rate reduces radiological progression. Br J Rheumatol. 1986;25(1):44–49. doi: 10.1093/rheumatology/25.1.44. [DOI] [PubMed] [Google Scholar]
- (21).Ory PA. Interpreting radiographic data in rheumatoid arthritis. Ann Rheum Dis. 2003;62(7):597–604. doi: 10.1136/ard.62.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Paulus HE, Oh M, Sharp JT, Gold RH, Wong WK, Park GS, et al. Correlation of single time-point damage scores with observed progression of radiographic damage during the first 6 years of rheumatoid arthritis. J Rheumatol. 2003;30(4):705–713. [PubMed] [Google Scholar]
- (23).Paulus HE, Oh M, Sharp JT, Gold RH, Wong WK, Park GS, et al. Classifying structural joint damage in rheumatoid arthritis as progressive or nonprogressive using a composite definition of joint radiographic change: a preliminary proposal. Arthritis Rheum. 2004;50(4):1083–1096. doi: 10.1002/art.20270. [DOI] [PubMed] [Google Scholar]
- (24).Paulus HE, Di Premio D, Sharp JT, Genant HK, Weissman BN, Weisman MH, et al. Patient retention and hand-wrist radiograph progression of rheumatoid arthritis during a 3-year prospective study that prohibited disease modifying antirheumatic drugs. J Rheumatol. 2004;31(3):470–481. [PubMed] [Google Scholar]
- (25).Sharp JT. Radiologic assessment as an outcome measure in rheumatoid 5arthritis. Arthritis Rheum. 1989;32(2):221–229. doi: 10.1002/anr.1780320218. [DOI] [PubMed] [Google Scholar]
- (26).Sharp JT, Wolfe F, Lassere M, Boers M, van der Heijde D, Larsen A, et al. Variability of precision in scoring radiographic abnormalities in rheumatoid arthritis by experienced readers. J Rheumatology. 2004;31:1062–1072. [PubMed] [Google Scholar]
- (27).del Val del Amo N, Ibanez BR, Fito MC, Gutierrez PR, Loza CE. Anti-cyclic citrullinated peptide antibody in rheumatoid arthritis: relation with disease aggressiveness. Clin Exp Rheumatol. 2006;24(3):281–286. [PubMed] [Google Scholar]
- (28).Kaltenhauser S, Pierer M, Arnold S, Kamprad M, Baerwald C, Hantzschel H, et al. Antibodies against cyclic citrullinated peptide are associated with the DRB1 shared epitope and predict joint erosion in rheumatoid arthritis. Rheumatology (Oxford) 2007;46(1):100–104. doi: 10.1093/rheumatology/kel052. [DOI] [PubMed] [Google Scholar]
- (29).Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, et al. Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford) 2007;46(2):342–349. doi: 10.1093/rheumatology/kel237. [DOI] [PubMed] [Google Scholar]
- (30).Landewe RB. The benefits of early treatment in rheumatoid arthritis: confounding by indication, and the issue of timing. Arthritis Rheum. 2003;48(1):1–5. doi: 10.1002/art.10732. [DOI] [PubMed] [Google Scholar]
- (31).Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- (32).Park GS, Wong WK, Oh M, Khanna D, Gold RH, Sharp J, et al. Classifying radiographic progression status in early rheumatoid arthritis patients using propensity scores to adjust for baseline differences. Statistical Methods in Medical Research. 2007;16:13–29. doi: 10.1177/0962280207070623. [DOI] [PubMed] [Google Scholar]
- (33).Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- (34).Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- (35).van der Heijde DM, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54(4):1063–1074. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]