Abstract
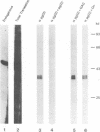
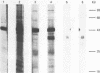
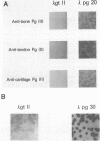
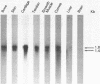
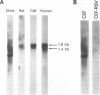
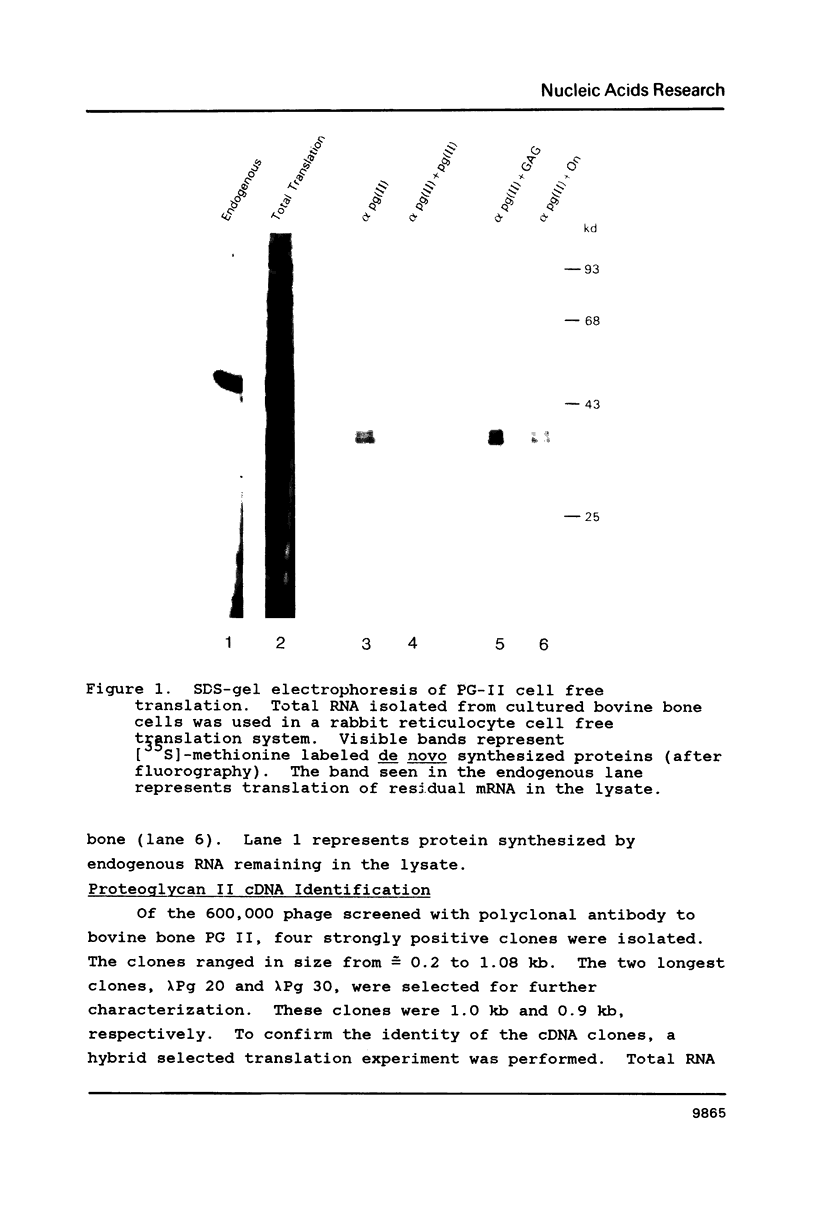
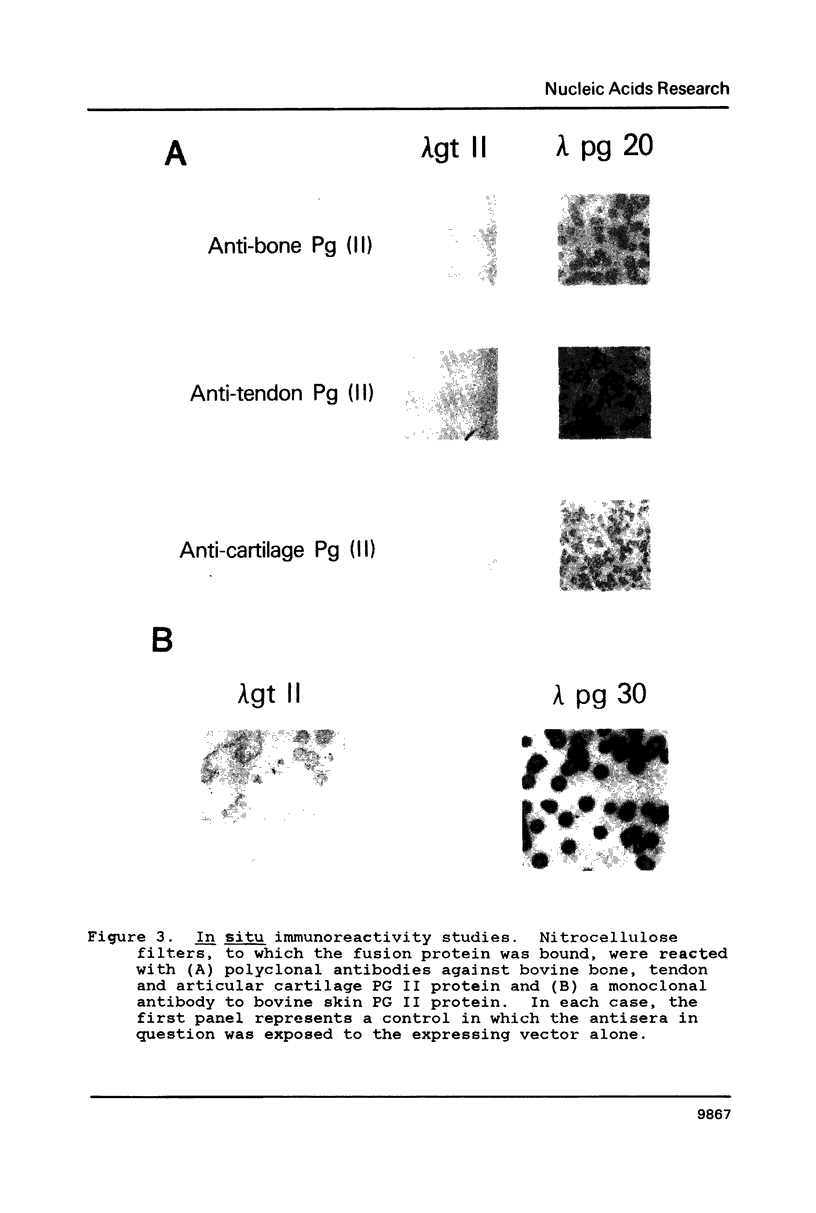
Two cDNA clones encoding the small proteoglycan II (PG II) of bone were isolated from a lambda gt11 expression library. These clones expressed recombinant protein which was cross-reactive with polyclonal and monoclonal antisera to PG II molecules from several connective tissues. The longest clone, lambda Pg 20 was studied in detail. The clone was shown to encode PG II by hybrid selected translation and immunoprecipitation. Northern analysis showed two species of the PG II message of approximately 1.4 and 1.8 kb. Substantial amounts of PG II message were found in bone, tendon, articular cartilage, skin, smooth muscle and cornea. Trace amounts of message were also detected in liver and brain. Radiolabeled bovine PG II cDNA hybridized to RNA from several other species including the human, rat and chicken. The level of PG II mRNA in chick embryonic fibroblasts was sensitive to transformation by Rous sarcoma virus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann D. K., Carlson D. M. The structure and organization of a proline-rich protein gene of a mouse multigene family. J Biol Chem. 1985 Dec 15;260(29):15863–15872. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Kleinman H. K., Hassell J. R., Martin G. R., Termine J. D., Trelstad R. L. Regulation of type I collagen fibril assembly by link protein and proteoglycans. Coll Relat Res. 1984 Oct;4(5):323–337. doi: 10.1016/s0174-173x(84)80001-1. [DOI] [PubMed] [Google Scholar]
- Fagan J. B., Sobel M. E., Yamada K. M., de Crombrugghe B., Pastan I. Effects of transformation on fibronectin gene expression using cloned fibronectin cDNA. J Biol Chem. 1981 Jan 10;256(1):520–525. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Dejter S. W., Jr, Whitson S. W., Yanagishita M., Kimura J. H., Hascall V. C., Kleinman H. K., Hassell J. R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983 May 25;258(10):6588–6594. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D. Noncollagenous proteins influencing the local mechanisms of calcification. Clin Orthop Relat Res. 1985 Nov;(200):362–385. [PubMed] [Google Scholar]
- Gregory J. D., Cöster L., Damle S. P. Proteoglycans of rabbit corneal stroma. Isolation and partial characterization. J Biol Chem. 1982 Jun 25;257(12):6965–6970. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Pearson C. H., Winterbottom N., Fackre D. S., Scott P. G., Carpenter M. R. The NH2-terminal amino acid sequence of bovine skin proteodermatan sulfate. J Biol Chem. 1983 Dec 25;258(24):15101–15104. [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle G. A., Dodd C. M., Osborn J. W., Pearson C. H., Mosmann T. R. Production and characterization of monoclonal antibodies to bovine skin proteodermatan sulfate. Coll Relat Res. 1985 Jan;5(1):23–39. doi: 10.1016/s0174-173x(85)80045-5. [DOI] [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Sobel M. R., Yamamoto T., de Crombrugghe B., Pastan I. Regulation or procollagen messenger ribonucleic acid levels in Rous sarcoma virus transformed chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2678–2684. doi: 10.1021/bi00512a049. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Heinegård D. Characterization of proteoglycans from adult bovine tendon. J Biol Chem. 1985 Aug 5;260(16):9298–9306. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. F., Bolander M. E., Day A. A., Ramis C. I., Robey P. G., Yamada Y., Termine J. D. Osteonectin mRNA: distribution in normal and transformed cells. Nucleic Acids Res. 1986 Jun 11;14(11):4483–4497. doi: 10.1093/nar/14.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]