Abstract
Background and Purpose
For toxicities occurring during the course of radiotherapy, it is conceptually inaccurate to perform normal-tissue complication probability analyses using the complete dose-volume histogram. The goal of this study was to analyze acute rectal toxicity using a novel approach in which the fit of the Lyman-Kutcher-Burman (LKB) model is based on the fractional rectal dose-volume histogram (DVH).
Materials and Methods
Grade ≥2 acute rectal toxicity was analyzed in 509 patients treated on Radiation Therapy Oncology Group (RTOG) protocol 94-06. These patients had no field reductions or treatment-plan revisions during therapy, allowing the fractional rectal DVH to be estimated from the complete rectal DVH based on the total number of dose fractions delivered.
Results
The majority of patients experiencing Grade ≥2 acute rectal toxicity did so before completion of radiotherapy (70/80=88%). Acute rectal toxicity depends on fractional mean rectal dose, with no significant improvement in the LKB model fit when the volume parameter differs from n=1. The incidence of toxicity was significantly lower for patients who received hormone therapy (P=0.024).
Conclusions
Variations in fractional mean dose explain the differences in incidence of acute rectal toxicity, with no detectable effect seen here for differences in numbers of dose fractions delivered.
Keywords: prostate cancer, acute rectal toxicity, dose-volume histogram, normal-tissue complication probability, Lyman model
INTRODUCTION
Rectum is one of the major dose-limiting organs for radiotherapy (RT) of prostate cancer. Many studies have investigated dose-volume factors associated with increased risk of late rectal toxicity, as summarized in the recent QUANTEC (QUantitative Analysis of Normal Tissue Effects in the Clinic) review (1). Fewer studies have analyzed dosimetric factors associated with acute rectal toxicity (2–12).
To the best of our knowledge, published dose-volume analyses of acute rectal toxicity have, with one exception (8), utilized the full dose-volume histogram (DVH) representing rectal exposure during the entire course of radiotherapy. The exception is a report on patients treated with 78 Gy versus 68 Gy (8). In that study, Peeters et al. analyzed DVHs corresponding to the first 68 Gy only, since they noted that nearly all cases of acute toxicity occurred before delivery of the 10-Gy boost in the 78-Gy group (8).
For endpoints such as acute rectal toxicity that often occur during the course of radiotherapy, it is conceptually inaccurate to perform normal-tissue complication probability (NTCP) analyses using the complete DVH. Instead, it is preferable to use a parameter representing the rate or intensity of dose delivery to tissue. Therefore, the goal of the present study was to analyze acute rectal toxicity based on rectal exposure per dose fraction. The impact of the total number of fractions was assessed separately, as were the effects of several nondosimetric factors, including neoadjuvant hormone therapy, patient age, and comorbidities.
MATERIALS AND METHODS
Patient data
The study cohort comprises a subset of the patients treated on Radiation Therapy Oncology Group (RTOG) protocol 94-06, a multi-institutional dose-escalation trial of 3D-conformal radiotherapy of prostate cancer that accrued >1000 patients from 1994 to 2000 (13). The trial included 5 dose levels: 68.4 Gy, 73.8 Gy or 79.2 Gy (levels I–III) delivered to the target in 1.8-Gy fractions, and 74.0 Gy or 78.0 Gy (levels IV-–V) delivered in 2-Gy fractions (13). Depending on the estimated risk of seminal vesicle (SV) involvement, patients were treated to the prostate only (treatment group 1), to prostate plus bilateral SVs (group 3), or to prostate and SVs for the initial part of treatment, followed by a field reduction to prostate alone (group 2). Neoadjuvant androgen suppression was permitted if it began 2 to 6 months before study registration; further details of the hormone therapy are presented elsewhere (14). Rectal toxicity was scored prospectively using RTOG criteria, with acute toxicity defined as toxicity occurring within the first 120 days after start of RT. The DVH was computed for rectum as a solid structure, as described previously (15).
The present analysis includes patients treated in groups 1 and 3 who had no revisions to their treatment plan during radiotherapy for any reason. For these patients, the fractional DVH could be estimated by dividing the dose in each dose bin of the full DVH by the number of dose fractions delivered. These secondary analyses of the data from RTOG 94-06 were approved by the RTOG Publications Committee and by the Institutional Review Boards of The University of Texas MD Anderson Cancer Center, Washington University Medical Center, and the American College of Radiology.
Statistical methods
The endpoint for analysis was occurrence of Grade ≥2 acute rectal toxicity (yes/no). Data were analyzed using a novel version of the LKB model based on fractional treatment, as follows. In the standard LKB model (16–17), the effective dose to rectum, Deff, is computed from the DVH representing the complete course of RT by
| (1) |
where n is the volume parameter, Di is the dose to relative subvolume vi of rectum, and the sum extends over all dose bins in the DVH. When n=1, Deff is equal to the mean rectal dose. The complication probability is modeled as a probit function of Deff using two additional model parameters, TD50 and m:
| (2) |
where
| (3) |
The present analysis differs from a standard LKB analysis in that the doses Di represent fractional doses to rectum instead of total doses over the entire course of RT. Therefore, here Deff represents the fractional effective dose. To emphasize this point, we refer to TD50 as the fractional TD50. Covariates were added to the model by introducing multiplicative factors to modify the value of the fractional TD50 (15,18). Covariates considered were: number of treatment fractions, prescribed dose per fraction (2 Gy versus 1.8 Gy), use of neoadjuvant hormonal therapy, rectal volume, rectal length, patient age, cardiovascular disease, diabetes, and hypertension. The statistical significance of adding covariates to the model was assessed using the likelihood ratio test.
RESULTS
Patient cohort
Of the 1084 patients enrolled on RTOG 94-06, 74 were excluded from analyses of rectal toxicity for reasons detailed elsewhere (15). In the current study, 501 additional patients were excluded because they had planned field reductions during therapy (treatment group 2) or had unplanned treatment-plan revisions for clinical reasons. The resulting cohort (N=509) is the same one studied previously for the effects of dose fractionation on late rectal toxicity (19). Patient and treatment characteristics are listed in Table 1.
Table 1.
Patient and treatment characteristics
| Factor | Number of patients (%) | |
|---|---|---|
| Age (years) | ||
| Median 69 (range 41–84) | ||
| Cardiovascular disease | ||
| Yes | 146 (29) | |
| No | 319 (33) | |
| Unknown | 44 (9) | |
| Diabetes | ||
| Yes | 58 (11) | |
| No | 407 (80) | |
| Unknown | 44 (9) | |
| Hypertension | ||
| Yes | 203 (40) | |
| No | 261 (51) | |
| Unknown | 45 (9) | |
| Rectal length (cm) | ||
| Median 10.5 (range 4.5–16) | ||
| Rectal volume (mL) | ||
| Median 88 (range 29–446) | ||
| Hormone therapy | ||
| Yes | 146 (29) | |
| No | 363 (71) | |
| Dose level (see text) | ||
| I | 43 (8) | |
| II | 171 (34) | |
| III | 92 (18) | |
| IV | 108 (21) | |
| V | 95 (19) |
Occurrence and timing of acute rectal toxicity
All but one patient had at least 120 days of follow-up after start of RT and could therefore be scored for occurrence of acute toxicity. The remaining patient was followed for 95 days without toxicity and is considered here to have had Grade 0 acute rectal toxicity, as did 281 of the other patients. There were 147 patients with Grade 1, 79 patients with Grade 2, 1 patient with Grade 3, and no patients with Grade 4 or 5 acute rectal toxicity.
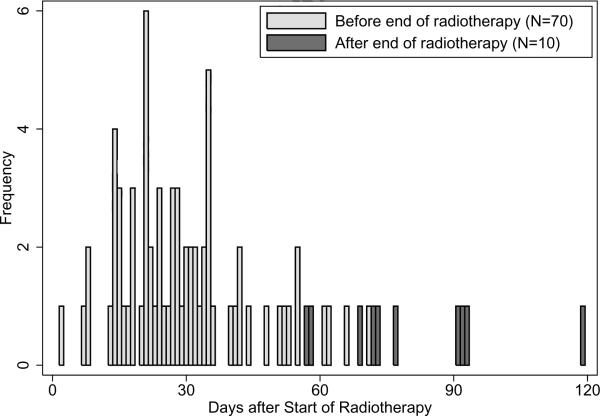
Figure 1 shows the times at which Grade ≥2 acute rectal toxicity occurred. All but one of the 80 patients experiencing the endpoint (99%) did so within 93 days after start of RT, supporting the decision to score the patient followed for 95 days without toxicity as Grade 0. The median time to Grade ≥2 acute rectal toxicity was 30 days, compared to a median RT duration of 57 days (range 49–81 days); the majority of patients experiencing toxicity (70/80=88%) did so before the end of treatment.
Figure 1.
Histogram of times at which Grade ≥2 acute rectal toxicity occurred.
Incidence of toxicity versus fractional mean dose
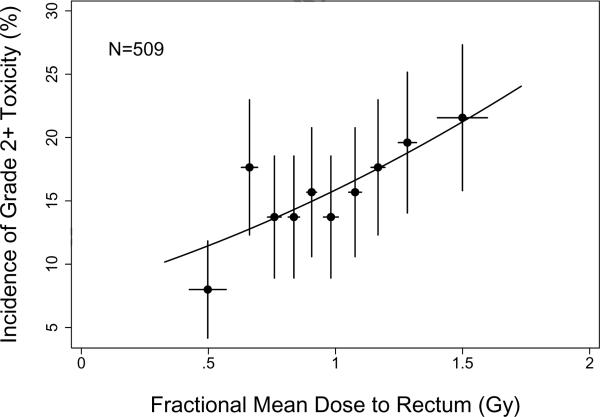
Figure 2 shows the incidence of Grade ≥2 acute rectal toxicity as function of the fractional mean rectal dose. The curve represents the fit of the LKB model based on fractional rectal DVHs, with volume parameter fixed at n=1. The parameter estimates from this model fit are fractional TD50=3.47 Gy (68% confidence interval [CI] 2.30–14.5 Gy) and m=0.712 (68% CI 0.572–0.938 Gy). There was no significant improvement in model fit when the volume parameter, n, was included as a parameter to be estimated from the data (P=0.622, likelihood ratio test).
Figure 2.
Incidence of Grade ≥2 acute rectal toxicity in each of 10 subgroups of 50–51 patients. Points are plotted at the average (mean) value of fractional mean dose per subgroup. Horizontal error bars show ±1 standard deviation; vertical error bars show ±1 standard error computed using binomial statistics. The solid curve illustrates the fit of the Lyman-Kutcher-Burman model using fractional rectal DVHs.
Patients receiving higher fractional rectal doses did not experience Grade ≥2 acute rectal toxicity significantly earlier than those exposed to lower doses, although there was a slight trend in that direction. Events occurred at a median of 28 days in the half of patients with higher fractional mean doses, compared to 31.5 days among patients exposed to lower fractional mean doses (P=0.410, Mann-Whitney test).
Impact of hormone therapy
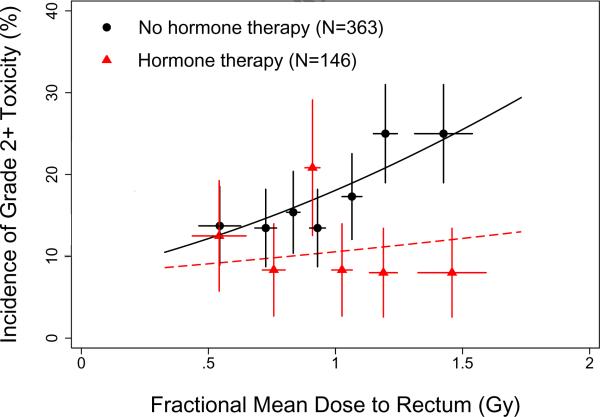
Of the non-dosimetric factors investigated, the only one found to improve the fit of the daily LKB model was neoadjuvant hormone therapy, which had a significant protective effect (P=0.024). The dose-modifying factor was 2.97 (68% CI 1.38 to infinite), indicating that hormone treatment increased the fractional TD50 value by a factor of nearly 3; fractional TD50 is 8.37 Gy for patients receiving hormone therapy versus 2.80 Gy for patients treated with RT alone.
Figure 3 shows the incidence of Grade ≥2 acute rectal toxicity as a function of fractional mean dose to rectum for patients treated with or without hormone therapy. Although the model predicts that the risk of Grade ≥2 acute rectal toxicity increases with increasing fractional mean dose, the data suggest that this might be true only for the patients treated without hormone therapy. Among patients receiving hormone therapy, the incidence of toxicity appears to be essentially independent of fractional mean dose over this dose range.
Figure 3.
Incidence of Grade ≥2 acute rectal toxicity as a function of fractional mean dose in subgroups of patients treated with (6 groups, 24–25 patients each) or without (7 groups, 51–52 patients each) neoadjuvant hormone therapy. Points and error bars are as in Figure 2. Solid curves show the fit of the Lyman-Kutcher-Burman model with hormone therapy included as a dose-modifying factor on the fractional TD50 value.
Lack of effect of prescribed dose per fraction
As noted above, no significant effect (P=0.838) was found for the effect on acute rectal toxicity of size of prescribed dose per fraction, which was 1.8 Gy for dose levels I–III and 2 Gy for dose levels IV–V. Although this may be due in part to the relatively small difference in prescribed dose per fraction, a lack of effect of fraction size is expected in general for acute radiation endpoints. In fact, when a method described previously for correcting for differences in dose per fraction was applied to these data (19), the estimated α/β ratio was effectively infinite, indicating a lack of fractionation effect for the Grade ≥2 acute rectal endpoint, and supporting use of the physical dose rectal DVHs in the present analysis.
Early events
Four of the acute rectal events in the present cohort occurred very early during the course of RT, at 2–9 days, while the remaining events occurred at 13 days or later (Figure 1). It is unusual for radiation-induced acute proctitis to be expressed much before the 2-week time point, so the symptoms for these patients would more likely be due to comorbidity or unrelated events.
Interestingly, Figure 3 also suggests that there might be a “background” level of Grade ≥2 acute rectal toxicity independent of radiation exposure. To test this, the model was refitted to the data with an additional parameter b0 included to represent a background level of toxicity. Specifically, the quantity NTCP in equation (2) was replaced by b0 + (1 − b0) NTCP, as described elsewhere (20). The estimated value of b0 was 10.4%, although inclusion of the background parameter did not significantly improve the model fit (P=0.487). This lack of significance might result from the fact that, even without background toxicity explicitly included, the model itself already predicts a 7.8% incidence of toxicity when extrapolated to zero fractional mean dose; it is a feature of the LKB model that it does not predict NTCP=0% at 0 Gy.
Comparison between fractional and total mean rectal doses
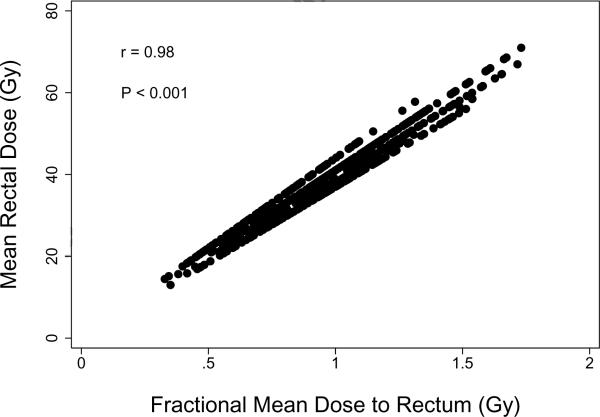
Figure 4 illustrates the strong correlation in the present cohort between fractional and total mean doses to rectum. In particular, most of the patient-to-patient variation in total rectal exposure over the course of RT resulted from differences occurring during each fractional dose, with differences in fraction number playing a smaller role. This likely contributes to the lack of significance of fraction number in the present analysis.
Figure 4.
Correlation between overall mean rectal dose and fractional mean dose to rectum in the study cohort. The Pearson correlation coefficient (r) and significance level (P) are shown.
DISCUSSION
The conventional rectal DVH representing the complete course of radiotherapy reflects two separate aspects of treatment: dose delivery to rectum during each treatment fraction, and the total number of dose fractions delivered. A major goal of the present study was to separate the impact of these two effects in the analysis of acute rectal toxicity.
The rationale for basing NTCP analyses on the fractional DVH instead of on the complete rectal DVH is that most patients who experience acute rectal toxicity do so before radiotherapy is complete. However, the difference between the two approaches may be relatively minor in practice. In the present cohort, for example, the overall mean dose to rectum was highly correlated with the fractional mean dose, as shown in Figure 4. A similar finding was reported by Vavassori et al., who found a strong correlation between the overall mean dose and the mean dose from the first 5–6 weeks of treatment, when most acute rectal toxicities are observed (12). For that reason, they noted that the mean rectal dose corresponding to the full DVH should be regarded as a surrogate of the true dose-volume relationship. Use of fractional dose-volume metrics would likely be much more important in studies in which patients were treated with a wider range of fraction numbers than was the case in RTOG 94-06 or in the study of Vavassori et al.
It is important to note that the fractional rectal DVH, as used here, represents only an estimate of the actual rectal exposure per dose fraction. The fractional DVH is computed from the overall rectal DVH, and is therefore based on the treatment-planning CT scan. Set-up variations, changes in prostate volume over the course of therapy, and organ motion resulting from daily variations in bladder and rectal filling all contribute to discrepancies between the planned and delivered doses. Imaging and registration techniques are increasingly being used to ensure that the daily dose to the target and surrounding normal tissues, including rectum, are known more precisely. In the future, actual daily rectal dose distributions will be available, and more detailed risk modeling will be required to take into account known daily variations in rectal exposure.
An alternative modeling approach to the one used here would be to describe acute complication risk as a function of accumulated dose-volume exposure to rectum as treatment progresses. This approach would be complicated, however, by the fact that there is a time lag between radiation exposure and subsequent manifestation of tissue injury that would have to be taken into account. In addition, the biologically effective dose would likely diverge from the physical accumulated dose as exposure continued, because of increased cell proliferation and other recovery processes initiated in response to tissue injury during the early part of therapy. Such processes would limit the biological impact of dose fractions delivered during the latter part of treatment. Support for this possibility comes from the study of Peeters et al., who found few additional cases of acute toxicity among patients receiving a 10-Gy boost after 68 Gy (8). Proliferation during the latter part of therapy might also contribute to the lack of effect of total fraction number in the present study, once differences in fractional mean rectal dose were taken into account. Detailed modeling taking time lags and recovery processes into account for acutely reacting tissues would require enormous data sets for development and validation.
Our finding that patients receiving hormone therapy had a significantly lower incidence of Grade ≥2 acute rectal toxicity is consistent with several reports (7,8,11). Other studies, however, have not found such an association. Among these was the study of Valicenti et al. regarding patients from dose levels I–III of RTOG 94-06 (14), which included data from many of the same patients analyzed here. The difference in results between the two studies is likely due in part to the fact that the effects of hormone therapy are most apparent for patients with daily mean rectal doses greater than about 1.2 Gy (Figure 3). In RTOG 94-06, patients with daily mean doses in this range are more likely to have been on treated on dose level V, where significantly larger margins were employed than for the other dose levels (13,15). Another possible explanation is that dose differences to rectum were not taken into account in the earlier study. We also find that the effect of hormones does not reach statistical significance in the present cohort when considered as a univariate factor, without adjusting for differences in daily dose (P=0.061, chi-squared test).
It has been suggested that the protective effect of neoadjuvant hormone therapy on acute gastrointestinal symptoms is a consequence of prostate shrinkage, which results in reduced radiation exposure to rectum. Prostate volumes were available in the present study for the patients treated in group 1 (because GTV=prostate in this group). For those patients, it is true that the median prostate volume was significantly smaller among patients who received hormone therapy (median 45 mL, range 16–210 mL) than among those who did not (median 56 mL, range 15–201 mL): P=0.002, Mann-Whitney test. However, there also appears to be a beneficial effect of hormone treatment that is not explained by dose-volume effects alone (Figure 3). Additional studies are warranted in which the impact of timing, duration, and choice of agents used as anti-androgen therapy on dose-volume relationships for acute rectal toxicity are investigated in more detail.
Finally, we note that the occurrence of 4 early acute rectal events, unrelated to the effects of radiotherapy, is consistent with the predictions of the LKB model incorporating a baseline level of toxicity, described in connection with Figure 3. Specifically, if unrelated events occur in 10.4% of untreated patients followed over a 120-day time period (corresponding to the defined time interval for acute toxicity), and assuming that the hazard for these events is constant over time, then 4–5 events are expected to occur during the first 9–10 days. Some of the later events, during and after the course of radiotherapy, would also be attributable to causes other than acute toxicity, but unlike the very early events, it would more difficult to determine which of these were truly background events and which were due to toxicity.
CONCLUSION
In summary, it is proposed that risk analyses of acute endpoints occurring before the end of radiotherapy be performed using dosimetric information reflecting the rate at which dose is delivered to tissue, such as the fractional DVH, with the effect of total number of dose fractions considered separately.
Acknowledgements
Supported in part by grants R01 CA104342, U24 CA81647, U10 CA21661, U10 CA37422 and U10 CA32115 from the National Cancer Institute, the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- 1.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;3:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng JC, Schultheiss TE, Nguyen KH, Wong JYC. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71:351–357. doi: 10.1016/j.ijrobp.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Jereczek-Fossa BA, Zerini D, Fodor C, et al. Correlation between acute and late toxicity in 973 prostate cancer patients treated with three-dimensional conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:26–34. doi: 10.1016/j.ijrobp.2009.07.1742. [DOI] [PubMed] [Google Scholar]
- 4.Karlsdottir A, Johannessen DC, Muren LP, Wentzel-Larsen T, Dahl O. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol. 2004;71:43–53. doi: 10.1016/j.radonc.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Leborgne F, Fowler J. Acute toxicity after hypofractionated conformal radiotherapy for localized prostate cancer: nonrandomized contemporary comparison with standard fractionation. Int J Radiat Oncol Biol Phys. 2008;72:770–776. doi: 10.1016/j.ijrobp.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Nuyttens JJ, Milito S, Rust PF, Turrisi AT. Dose-volume relationship for acute side effects during high dose conformal radiotherapy for prostate cancer. Radiother Oncol. 2002;64:209–214. doi: 10.1016/s0167-8140(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 7.Peeters STH, Heemsbergen WD, Putten WLJ, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2006;61:1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 8.Peeters STH, Hoogeman MS, Heemsbergen WD, et al. Volume and hormonal effects for acute side effects of rectum and bladder during conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1142–1152. doi: 10.1016/j.ijrobp.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 9.Strigari L, Arcangeli G, Arcangeli S, Benassi M. Mathematical model for evaluating incidence of acute rectal toxicity during conventional or hypofractionated radiotherapy courses for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:1454–1460. doi: 10.1016/j.ijrobp.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Teh BS, Mai WY, Uhl BM, et al. Intensity-modulated radiation therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: acute toxicity and dose-volume analysis. Int J Radiat Oncol Biol Phys. 2001;49:705–712. doi: 10.1016/s0360-3016(00)01428-0. [DOI] [PubMed] [Google Scholar]
- 11.Valdagni R, Magli A, Baccolini M, et al. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D-CRT. Int J Radiat Oncol Biol Phys. 2008;71:1065–1073. doi: 10.1016/j.ijrobp.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Vavassori V, Fiorino C, Rancati T, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: Results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007;67:1401–1410. doi: 10.1016/j.ijrobp.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76:14–22. doi: 10.1016/j.ijrobp.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valicenti RK, Winter K, Cox JD, et al. RTOG 94-06: Is the addition of neoadjuvant hormonal therapy to dose-escalated 3D conformal radiation therapy for prostate cancer associated with treatment toxicity? Int J Radiat Oncol Biol Phys. 2003;57:614–620. doi: 10.1016/s0360-3016(03)00640-0. [DOI] [PubMed] [Google Scholar]
- 15.Tucker SL, Dong L, Bosch WR, et al. Late rectal toxicity on RTOG 94-06: Analysis using a mixture Lyman model. Int J Radiat Oncol Biol Phys. 2010;78:1253–1260. doi: 10.1016/j.ijrobp.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–19. [PubMed] [Google Scholar]
- 17.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 18.Peeters STH, Hoogeman MS, Heemsbergen WD, Hart AAM, Koper PCM, Lebesque JV. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11–19. doi: 10.1016/j.ijrobp.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81:600–605. doi: 10.1016/j.ijrobp.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]