Abstract
The proper function of the craniofacial skeleton requires the proper shaping of many individual skeletal elements. Neural crest cells generate much of the craniofacial skeleton and morphogenesis of skeletal elements occurs in transient, reiterated structures termed pharyngeal arches. The shape of individual elements depends upon intrinsic patterning within the neural crest as well as extrinsic signals to the neural crest from adjacent tissues within the arches. Hedgehog (Hh) signaling is known to play roles in craniofacial development, yet its involvement in intrinsic and extrinsic patterning of the craniofacial skeleton is still not well understood. Here, we show that morphogenetic movements of the pharyngeal arches and patterning of the neural crest require Hh signaling. Loss of Hh signaling, in smoothened (smo) mutants, disrupts the expression of some Dlx genes as well as other markers of dorsal/ventral patterning of the neural crest. Transplantation of wild-type neural crest cells into smo mutants rescues this defect, demonstrating that the neural crest requires reception of Hh signals for proper patterning. Despite the rescue, morphogenesis of the facial skeleton is not fully recovered. Through transplant analyses, we find two additional requirements for Hh signaling. The endoderm requires the reception of Hh signals for proper morphogenetic movements of the pharyngeal arches and the neural crest require the reception of Hh signaling for the activity of a reverse signal that maintains sonic hedgehog expression in the endoderm. Collectively, these results demonstrate that Hh signaling is essential to establish intrinsic and extrinsic patterning information for the craniofacial skeleton.
Keywords: Hedgehog signaling, neural crest, endoderm, zebrafish, morphogenesis
Introduction
The craniofacial skeleton is a montage of individual skeletal elements that support diverse functions, such as biting and protection of cranial sensory organs. The functionality of the craniofacial skeleton depends upon the proper shaping of each individual skeletal element. Changes in the shape of craniofacial skeletal elements have important implications in both human craniofacial disease and vertebrate evolution (He et al., 2009). However, the precise mechanisms that shape the craniofacial skeleton still remain highly elusive.
In all vertebrate species the majority of the craniofacial skeleton is composed of cranial neural crest cells. Cranial neural crest cells migrate from their site of origin, the dorsal neural tube, into the developing face to form skeletal condensations within transient reiterated structures known as pharyngeal arches. Within the pharyngeal arches, cranial neural crest cells interact with facial epithelia: ectoderm and pharyngeal endoderm (Trainor and Krumlauf, 2001).
It is widely agreed that factors intrinsic to the neural crest and extrinsic factors, from sources such as the adjacent epithelia, are responsible for shaping the facial skeleton. A number of transcription factors, including gsc, barx1 and hand2 have specific dorsal/ventral expression domains and are important in craniofacial development (Firulli et al., 2005; Miller et al., 2003; Rivera-Perez et al., 1995; Rivera-Perez et al., 1999; Sperber and Dawid, 2008; Thomas et al., 1998; Yamada et al., 1995; Yanagisawa et al., 2003). Of particular importance for intrinsic patterning within individual pharyngeal arches is the Dlx family of transcription factors. The Dlx family displays a nested pattern of gene expression along the dorsal/ventral axis of individual arches (Depew et al., 2002). The most dorsal neural crest cells express only Dlx1 and Dlx2, while more ventral neural crest cells express the greatest number of Dlx genes (6 in mammals). This nested pattern of gene expression then provides dorsal/ventral identity to the neural crest-derived skeleton. Loss of function of these ventrally nested Dlx genes causes ventral skeletal elements to adopt dorsal identities in both mouse and fish (Beverdam et al., 2002; Depew et al., 2002; Talbot et al., 2010). Therefore, the proper establishment of this intrinsic patterning information is necessary for proper craniofacial morphogenesis
Proper neural crest condensation within the pharyngeal arches and subsequent morphogenesis also requires neural crest/epithelial interactions. For instance, zebrafish mutants lacking endoderm lose nearly the entire neural crest-derived skeleton (David et al., 2002). Neural crest/endoderm interactions are likely to be local. Evidence for this is provided by zebrafish itga5 mutants, in which the first pharyngeal endoderm pouch is lost causing the loss of the adjacent anterior half of the hyomandibular cartilage (Crump et al., 2004b). These studies demonstrate that endoderm is necessary for development of most of the craniofacial skeleton. In addition to being necessary, the endoderm provides patterning information to the neural crest-derived skeletal elements. In avian species, rotation of pharyngeal endoderm causes reorientation of the craniofacial skeleton with regard to the orientation of the endoderm (Couly et al., 2002). Thus, morphogenesis of the facial skeleton depends upon appropriate endodermal signals.
Numerous signaling pathways are known to be involved in morphogenesis of the facial skeleton. Hh signaling is known to play important roles in craniofacial development. Disruption of Hh signaling underlies the genesis of holoprosencephaly (Solomon et al., 2010) and causes defects in palatogenesis and tooth development (Bush and Jiang, 2012; Eberhart et al., 2006; Wada et al., 2005; Zhang et al., 2005). During zebrafish palatogenesis, Hh signaling is a crucial component regulating reciprocal signaling between neural crest cells and the oral ectoderm (Eberhart et al., 2008; Eberhart et al., 2006). Hh signaling is also important in cartilage and bone development (Barresi et al., 2000; Eberhart et al., 2006; Komori, 2011; Schwend and Ahlgren, 2009; Wada et al., 2005). Additionally, reception of Hh signaling by the neural crest is essential to the establishment of a Fox gene code in mouse (Jeong et al., 2004). However, the role for Hh signaling during patterning and shaping of the pharyngeal arches is not well understood.
We have found that Hh signaling regulates both intrinsic and extrinsic patterning of the craniofacial skeleton. The pharyngeal endoderm must receive Hh signaling for the proper morphogenetic movements of the pharyngeal arches. Neural crest cells require the reception of Hh-signaling for proper dorsal/ventral patterning within the pharyngeal arch. Neural crest cells also require the reception of Hh signaling in order to signal back to the endoderm and maintain appropriate gene expression in the endoderm. We propose that these separate signaling events may have important clinical and evolutionary significance.
Materials & Methods
Zebrafish embryology
Zebrafish embryos were raised and cared for as previously described (Westerfield, 1993) under IACUC approved protocols (AUP 08080601). We preformed all of our analyses in the crest labeling tg(fli1a:EGFP)y1 transgenic line (termed fli1:EGFP for clarity) and used the smob577 and sox32ta56 alleles (Eberhart et al., 2006; Kikuchi et al., 2001; Lawson and Weinstein, 2002; Varga et al., 2001). Cyclopamine treatments were performed on fli1:EGFP transgenics as previously described (Eberhart et al., 2006; Hirsinger et al., 2004). The shha and shhb morpholinos have been characterized (Nasevicius and Ekker, 2000).
Transplantation analyses
To target neural crest cells, we injected donor embryos with Alexa 546 dextran (Molecular Probes) at the one-cell stage. We then transplanted donor cells into the crest progenitor domain at shield stage (Eberhart et al., 2008; Eberhart et al., 2006; Woo and Fraser, 1995). To target endoderm, we co-injected one-cell stage donors with Alexa 546 dextran and sox32 mRNA (Chung and Stainier, 2008). We transplanted donor cells into the margin of the host embryos at sphere stage (Crump et al., 2004b). We analyzed all transplants at 30 hpf for donor contribution and only those embryos with substantial donor contribution to the appropriate tissue were analyzed further.
Tissue labeling
We used standard techniques for in situ hybridization (Miller et al., 2000). All probes have been previously described: dlx2a, dlx3b, gsc and hand2 (Miller et al., 2000); dlx5a, dlx6a and barx1 (Walker et al., 2006); dlx4b (Ellies et al., 1997); bapx1 (Miller et al., 2003) and shha (Krauss et al., 1993). Cartilage and bone staining via Alcian Blue and Alizarin Red, respectively, was performed according to a modified double staining protocol (Walker and Kimmel, 2007). Flat mounting of zebrafish skeletal elements (Kimmel et al., 1998) and anti-anti-Caspase and anti-phospho-Histone antibody staining has been described (Eberhart et al., 2008; Eberhart et al., 2006).
Imaging
Confocal analyses were performed on a Zeiss 710 using Zen software. All other images were captured on a Zeiss Axioimager. Images were processed using Photoshop CS.
Results
Shaping of the facial skeleton fails in the absence of Hh signaling
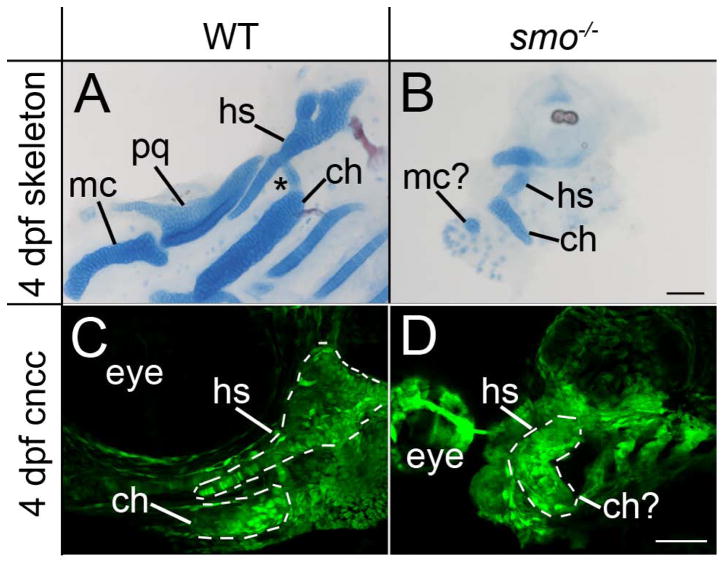
To determine the involvement of Hh signaling in shaping the craniofacial skeleton, we initially examined facial cartilage formation in smo mutant embryos, which lack all Hh signaling. We found that chondrogenesis was variably disrupted, with some mutant embryos lacking nearly all craniofacial cartilage elements (n=22/42; data not shown). No mutant embryos produced cartilage in pharyngeal arches 3–7, consistent with reports of another Hh pathway mutant, disp1 (Schwend and Ahlgren, 2009). In those mutants that did produce cartilage in the first and second arch, these elements were hypoplastic and greatly malformed (n=20/42; Fig. 1A & B).
Fig. 1.
Proper craniofacial morphogenesis requires Hh signaling. (A & B) Flat mounted, Alcian Blue/Alizarin Red stained pharyngeal skeleton from 4 dpf (A) wild-type and (B) smo mutant embryos. (A) The palatoquadrate (pq) and Meckel’s cartilages (mc) are the dorsal and ventral cartilage elements, respectively, generated in the first pharyngeal arch. In the second arch the dorsal hyosymplectic (hs) articulates with the ventral ceratohyal (ch) through the interhyal cartilage (asterisk). (B) In smo mutants the pharyngeal skeleton is hypoplastic, with only a small cartilage nodule residing in the first pharyngeal arch. In the second arch, the hyosymplectic and ceratohyal cartilages are recognizable only by position and appear fused to one another. (C & D) Confocal images of 4 dpf fli1:EGFP transgenic (C) wild types and (D) smo mutants. (C) Skeletal morphology is clearly evident in wild-type embryos. The hyosymplectic and proximal ceratohyal are outlined. (D) In smo mutants, the second arch has developed the shape of a bent rod. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
In the first arch, a small cartilage nodule in the position of Meckel’s cartilage remained while there was no apparent dorsal element. We previously characterized a neural crest cell condensation defect in the maxillary domain of the first pharyngeal arch (Eberhart et al., 2006), which could be partially responsible for this apparent loss of the dorsal first arch element. In the second pharyngeal arch, there appeared to be a dorsal and a ventral element that were fused together (Fig. 1A & B). In the second pharyngeal arch of wild-type embryos, the dorsal hyosymplectic has a distinctive morphology with a plate-like hyomandibular cartilage and the symplectic cartilage, a rod-like extension from the plate. In smo mutants that generate cartilage, this dorsal element is highly disrupted. The symplectic fails to form and the hyomandibular is misshaped (Fig. 1B), making the skeletal element recognizable only on the basis of position. In wild-type embryos, the hyosymplectic and ceratohyal cartilages articulate with one another through the intermediately located interhyal cartilage (asterisk in Fig. 1A). This intermediate skeletal element appears to be missing or fused into the remaining second arch skeleton in smo mutants, as is the case for disp1 mutants (Schwend and Ahlgren, 2009). In the ventral second arch of wild-type embryos, the ceratohyal is a rod shaped cartilage with the distal tip pointing towards the anterior (Fig. 1A). In the ventral second arch of smo mutants, a small rod shaped cartilage is present in the appropriate position for the ceratohyal, although the distal tip points to the posterior of the embryo (Fig. 1B). Collectively, these analyses demonstrate a clear defect in the morphology of craniofacial cartilage in embryos lacking Hh signaling.
Hh signaling is necessary for proper chondrogenesis (Barresi et al., 2000; Eberhart et al., 2006; Schwend and Ahlgren, 2009; Wada et al., 2005), which could cause defects in cartilage morphology that are not present in the precartilage condensations. We examined 4 dpf fli1:EGFP transgenic embryos to determine if neural crest cells were properly distributed in smo mutants, as this model would predict. We find that the morphology of the second arch skeleton is readily apparent in embryos wild type at the smo locus (Fig. 1C). In smo mutants, neural crest cells are present in the pharyngeal arches (Fig. 1D). In all smo mutants analyzed, the second arch is shaped like a bent cylinder and no extension of symplectic precursors is present (Fig. 1D). These data suggest that the morphological defects present in the facial skeleton of smo mutants could be due to a failure of morphogenesis of the precartilage condensations.
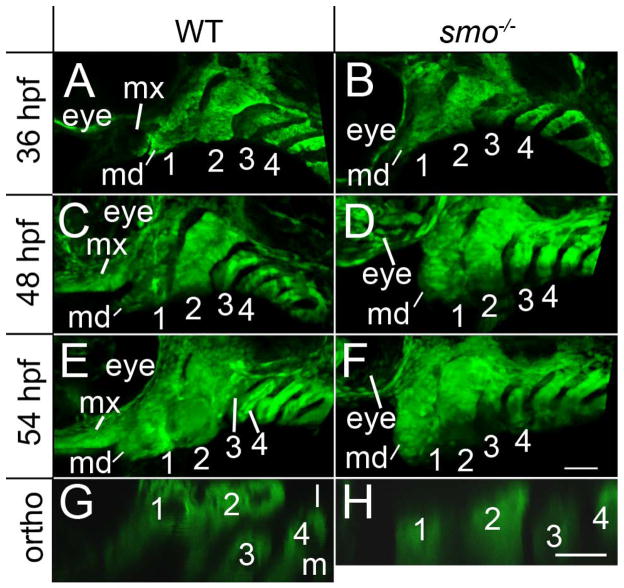
We analyzed precartilage condensations within the pharyngeal arches during morphogenesis to determine if Hh signaling was required for this process. At 36 hpf, when the condensed neural crest have an established dorsal/ventral pattern (Walker et al., 2006), crest condensations in wild-type embryos and smo mutants closely resemble one another (Fig. 2A & B), with the exception of the loss of the maxillary domain in smo mutants (Eberhart et al., 2006). By 48 hpf, the pharyngeal arches in both wild-type and smo mutant embryos have elongated along their dorsal/ventral axis (Fig 2. C & D), although smo mutants may have mandibular defects. It is because of early defects to the first arch that we focus our analysis on the second, and more posterior, arches. At 54 hpf, when the cell rearrangements that sculpt the shape of the facial skeleton are underway (Crump et al., 2004b), differences in the morphogenesis of posterior arches in wild-type embryos and smo mutants become apparent. In wild types the third arch has moved medial to the second arch (Fig. 2E & G), while the third arch remains posterior to the second arch in smo mutants (Fig. 2F & H). This failure to reposition the third arch is not a developmental delay because even at 4 dpf, the third arch remains posterior to the second arch in smo mutants (see Fig. 1D and Fig. 9). Additionally in wild-type embryos, the second arch becomes subtly wider in the anterior-posterior axis, relative to 48 hpf, while the 54 and 48 hpf smo mutant closely resemble one another (Fig. 2E). These findings suggest that Hh signaling is necessary for the proper orchestration of morphogenetic movements of the pharyngeal arches between 36 and 54 hpf (see Supplemental Movies 1 and 2 for time lapse analysis).
Fig. 2.
Morphogenetic movement of the pharyngeal arches fail in smo mutants. (A–H) Confocal images of fli1:EGFP transgenic embryos that are (A, C, E & G) wild type and (B, D, F & H) mutant at the smo locus. (A & B) In fixed and anti-EGFP stained 36 hpf embryos, aside from the loss of the maxillary domain (mx), the pharyngeal arches (numbered) of wild types and smo mutants closely resemble one another. (C, D) At 48 hpf, the pharyngeal arches elongate similarly in both wild type and smo mutant embryos, although morphogenesis of the mandibular (md) region of the first arch may be disrupted. (E) By 54 hpf, the second arch of wild-type embryos has widened, along the anterior/posterior axis, and the third pharyngeal arch has moved medial to the second arch. (F) 54 hpf smo mutants have not undergone these morphological changes and resemble 48 hpf mutants. (G & H) Orthogonal views showing the relative position of the 3rd arch in 54 hpf wild-type and smo mutant embryos. The first four pharyngeal arches are numbered. Anterior is to the left in all images; dorsal is up in A-F; lateral is up in G & H. Scale bar=50μm.
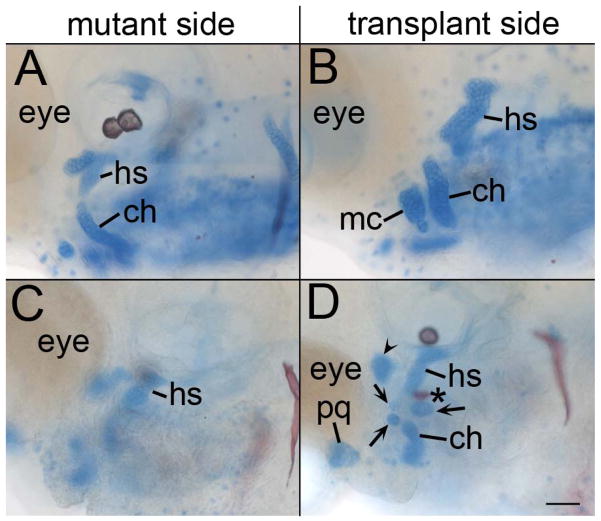
Fig. 9.
Reception of Hh signaling by the endoderm can reorganize the facial skeleton. (A & C) Mutant and (B & D) transplant side of smo mutant embryos that have received wild-type endoderm transplants and were stained with Alcian Blue/Alizarin Red at 4 dpf. (A & B) In embryos that produce cartilage, these transplants result in differently shaped cartilage elements. (C & D) In the most extensive reorganization of the pharyngeal skeleton, (C) the mutant side of the embryo has a single cartilage nodule in the appropriate location for the hyosymplectic (hs). (D) The transplanted side of the same embryo has a nodule in the correct location for the hyosymplectic with an attached opercle bone (asterisk). A cartilage nodule is also in the appropriate location for the ceratohyal cartilage (ch) and there are three nodules (arrows) in the location for the interhyal or symplectic rod. Adjacent to the eye there is a nodule in the appropriate location for the palatoquadrate (pq) and an ectopic nodule is located just posterior to the eye (arrowhead). Anterior is to the left in all images. Scale bar=50μm.
Proper craniofacial development requires continued Hh signaling during pharyngeal arch patterning and morphogenesis
Throughout this time window of Hh-dependent morphogenesis, the developing head, particularly the pharyngeal endoderm, expresses shha and shhb, the zebrafish Shh duplicates (Balczerski et al., 2011; Eberhart et al., 2006; Strahle et al., 1996; Teraoka et al., 2006). We find that injection of morpholinos directed against the two zebrafish Shh duplicates phenocopies the craniofacial defects present in smo mutants (Supplemental Fig. 1). While injection of either shha or shhb morpholino alone causes craniofacial defects, they do not fully recapitulate the smo mutant phenotype (Eberhart et al., 2006; Nasevicius and Ekker, 2000), data not shown). These findings show that Shh signaling is necessary for the morphogenetic events sculpting the zebrafish face.
Because the Shh duplicates are expressed for a prolonged period, we sought to determine when Hh signaling was necessary for facial morphogenesis. We applied cyclopamine, a potent Hh pathway inhibitor, to zebrafish embryos for 12-hour periods and examined the craniofacial skeleton at 5 dpf. We previously demonstrated the oral ectoderm must receive Hh signaling for proper development of dorsal skeletal elements within the first arch skeleton (Eberhart et al., 2006), because of this requirement and the potential defects to the mandibular region of the first arch (Fig. 2), we focus here on the effects of cyclopamine on the second pharyngeal arch (Fig. 3).
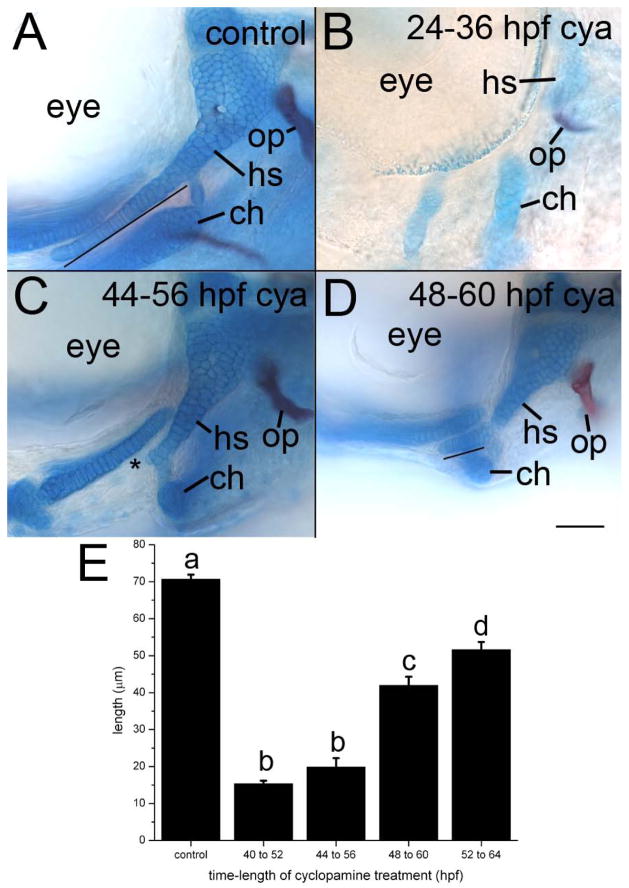
Fig. 3.
Morphogenesis of the pharyngeal skeleton requires continued Hh signaling. (A–D) Whole mount images of (A) control (ethanol treated) and (B–D) cyclopamine (cya)-treated 4 dpf fli1:EGFP embryos. (A) Regardless of the timing of the control treatment, the symplectic rod (line) extends beyond the interhyal in control embryos. The opercle (op) bone attaches to the proximal hyosymplectic. (B) All skeletal elements are severely hypoplastic following cyclopamine treatment from 24–36 hpf. (C) The symplectic rod is completely lost in treatments as late as 44–56 hpf (asterisk). In treatments that initiate at 48 hpf, the symplectic rod (line) forms but is shortened. Anterior is to the left; dorsal is up in all images. Scale bar=50μm. (E) Average (+/− 2 standard errors of the mean) symplectic length across treatments. Different letters over the bars denote statistically significant differences (p<0.5).
Cyclopamine treatments that initiated at or before 24 hpf result in severe craniofacial defects (Fig. 3B, compare to the control in Fig. 3A). These embryos had reduced cartilage staining and greatly disrupted morphology of the second arch skeletal elements (n=25/25). In the dorsal second arch, only the posterior portion of the hyomandibular cartilage, which articulates with the opercle bone, remained and the symplectic cartilage was absent. In the ventral second arch, the ceratohyal appeared smaller and its distal tip did not project anteriorly, as it did in control embryos. Thus, cyclopamine treatments occurring before 24 hpf appear to most closely resemble the defects observed in smo mutants.
Later cyclopamine treatments caused more subtle defects to the craniofacial skeleton. Treatments that initiated between 30 hpf to 36 hpf invariably resulted in the complete loss of the symplectic cartilage and the inappropriate projection of the distal tip of the ceratohyal towards the posterior of the embryo (n=25/25). The majority of embryos treated with cyclopamine beginning at 40 hpf or 44 hpf also had a complete loss of the symplectic (n=19/25 in both treatment groups, Fig. 3C and data not shown). The remaining embryos produced a shortened symplectic (data not shown). In all cases at these two time points the ceratohyal failed to project anteriorly. The symplectic formed and the ceratohyal projected anteriorly in all embryos that received treatments initiating at either 48 hpf or 52 hpf (n=25/25 in each treatment). However, the symplectic was still shorter than in controls (Fig. 3D and see quantification, below). Collectively, our inhibitor studies suggest that Hh signaling is required over an extended period of time, at least from 24 until 52 hpf, for proper morphogenesis of the facial skeleton and that the symplectic cartilage is particularly susceptible to the loss of Hh signaling.
To quantify the effect of the loss of Hh signaling on development of the symplectic we measured this cartilage in control embryos and those cyclopamine embryos that produced a symplectic. The average symplectic length in control embryos was 70.7 μm. The average symplectic length for the six embryos that produced this cartilage in treatments that initiated at 40 hpf and 44 hpf was 15.3 μm and 19.9 μm, respectively. While all embryos that were treated starting at 48 and 52 hpf produced symplectic cartilages, these cartilages were shorter than controls, 40.0 μm and 51.7 μm, respectively. Analysis of Variance (ANOVA, F=72.60217) followed by means comparison using a Tukey test demonstrates that these differences in symplectic length were statistically significant (p<0.5) across all groups with the exception of between the 40 to 52 hpf and 44 to 56 hpf cyclopamine treatment (Fig. 3E). Collectively, our cyclopamine data show that craniofacial morphogenesis requires continued Hh signaling throughout the time when arch elongation and reorganization is occurring.
Neural crest cells require the reception of Hh-signaling for the proper expression of dorsal/ventral markers within the pharyngeal arches
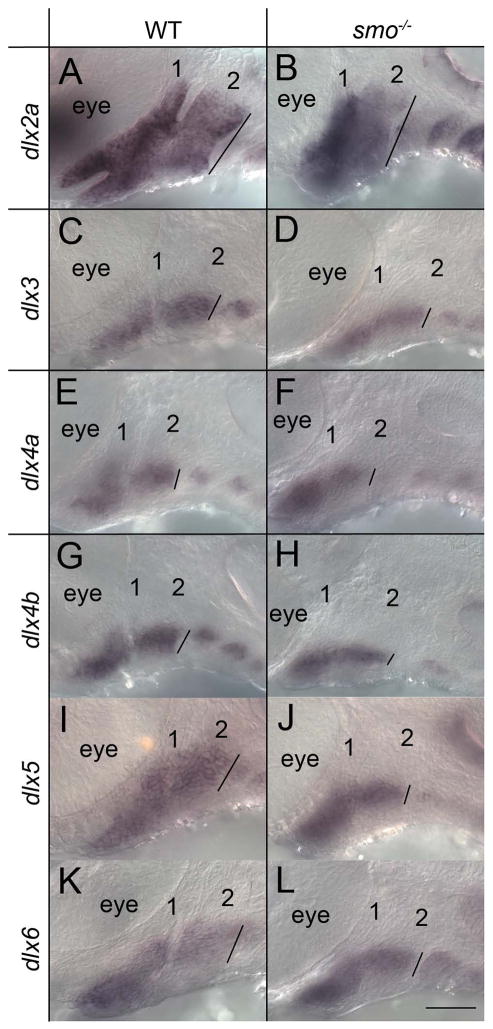
Many studies have suggested that dorsal/ventral patterning and morphogenesis of the facial skeleton go hand in hand (for review see (Kimmel et al., 2001), suggesting a potential mechanism for the morphogenetic defect in smo mutants. One primary source of dorsal/ventral patterning information in the pharyngeal arches involves the nested expression of Dlx family members (Beverdam et al., 2002; Depew et al., 2002; Talbot et al., 2010). We analyzed the expression of the Dlx gene family in wild-type embryos and smo mutants (Fig. 4). In both wild-type embryos and smo mutants, dlx2a expression labeled the dorsal/ventral extent of the pharyngeal arches at 36 hpf. The expression of dlx2a, as well as the extent of fli1:EGFP (see Fig. 2), also showed that the overall dorsal/ventral length of the pharyngeal arch at 36 hpf was similar in smo mutants compared to wild-type embryos, 77.5 and 81.3 μm, respectively (n=3 for each genotype). Likewise the mean length of dlx6 expression was within 5% of that observed in wild-type embryos (31.1 and 32.6 μm, respectively; n=3 for each genotype). The dorsal/ventral extent of dlx4a expression was not greatly altered (average= 33 μm and 36.3 μm for smo mutants and wild-type embryos, respectively; n=3 for each genotype,). However, the remainder of the Dlx genes that we examined, dlx3b, dlx4b and dlx5a had an approximate 25% reduction in the dorsal/ventral expression domains in smo mutants versus wild-type embryos (dlx3b: 29.6 μm vs. 38.35 μm; dlx4b: 25.6 μm vs. 34.9 μm; dlx5a: 29.7 μm vs. 40.6 μm; n=3 in each group). In no instance was the expression of any Dlx gene completely lost and the expression of these Dlx genes in the intermediate region of the pharyngeal arches appeared most resistant to loss of Hh signaling. These data show that Hh signaling is necessary for the proper dorsal/ventral expression pattern of a subset of Dlx genes.
Fig. 4.
Proper Dlx gene expression requires Hh signaling. (A-L) 36 hpf embryos stained via in situ hybridizations for Dlx family members (A, B) dlx2a, (C, D) dlx3b, (E, F) dlx4a, (G, H) dlx4b, (I, J) dlx5a and (K, L) dlx6. (A, C, E, G, I, & K) wild-type embryos were compared to (B, D, F, H, J, & L) smo mutants. The overall dorsal/ventral extent of staining in the second pharyngeal arch (shown by the black line in each panel) for each gene was measured in 3 embryos of each genotype to calculate the mean. Anterior is to the left; dorsal is up in all images. The first two pharyngeal arches are numbered. Scale bar=50μm.
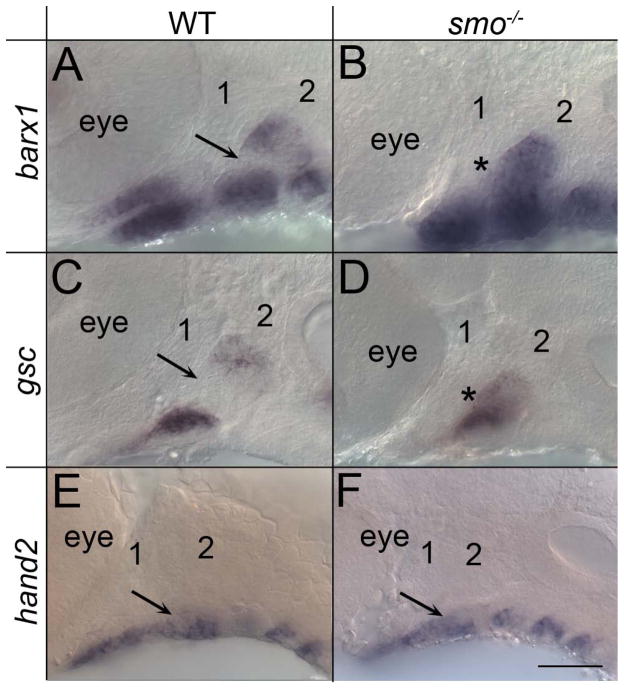
Proper expression of other markers of dorsal/ventral patterning within the arch also required Hh-signaling. Within the pharyngeal arches both barx1 and gsc had a dorsal and a ventral expression domain, with an intermediate region free of expression of either marker (Fig. 5A & C). In smo mutant embryos this intermediate region expressed both barx1 and gsc, resulting in the fusion of what would normally be a dorsal and ventral domain of expression (Fig. 5B & D). On the other hand, the expression domain of hand2 in the ventral pharyngeal arch appeared normal in smo mutants (Fig. 5E & F). We observed no clear differences in the endoderm or levels of neural crest cell proliferation or apoptosis between wild types and smo mutants at 36 hpf (Supplemental Fig. 2). In contrast, apoptosis, in the neural crest and endoderm, did appear elevated by 52 hpf, after the morphogenetic defect was readily evident (Supplemental Fig. 3). Therefore, while later loss of neural crest cells or the endoderm could explain some of the skeletal defects in smo mutants, such loss is unlikely to underlie the alteration in expression of dorsal/ventral markers in smo mutants.
Fig. 5.
Dorsal/ventral patterning of the pharyngeal arches requires Hh signaling. (A-F) 36 hpf embryos hybridized with probes for other markers of dorsal/ventral pattern in the pharyngeal arches. (A-D) In wild-type embryos, both (A) barx1 and (C) gsc have a dorsal and a ventral expression domain, with an intermediate domain free of expression (arrow). (B, D) In smo mutants, this intermediate region is lost (asterisk). (E & F) Expression of hand2 is similar between wild types and smo mutants (arrow). The first two arches are numbered. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Because the endoderm expresses both zebrafish shh duplicates (Balczerski et al., 2011; Eberhart et al., 2006; Strahle et al., 1996) and is in intimate contact with neural crest, the endoderm is a likely source for the Hh signal. However, a recent report suggested that endoderm was only involved in growth, not dorsal/ventral patterning, of facial skeletal elements (Balczerski et al., 2011). We examined the expression of a subset of our dorsal/ventral markers in sox32 mutants, that lack endoderm. We found that, particularly in the second and more posterior arches, dorsal/ventral patterning was highly disrupted in sox32 mutants (Supplemental Figs. 4 & 5). The exception to this is the expression of hand2, in which we saw no evidence for an alteration in expression pattern in the neural crest. While these defects tended to be much more severe than we found in smo mutants, the expression of gsc in sox32 mutants was very reminiscent to that found in smo mutants (Supplemental Fig. 5 D). These results are consistent with the strong expression of the shh duplicates by the endoderm (Balczerski et al., 2011; Eberhart et al., 2006; Strahle et al., 1996) being involved in proper dorsal/ventral patterning, although other sources, such as the oral ectoderm, are likely to also play a role, particularly for the first pharyngeal arch (Eberhart et al., 2006).
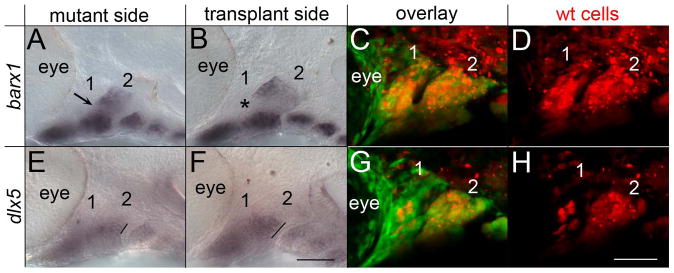
Proper dorsal/ventral patterning in the pharyngeal arches requires the reception of signaling factors, such as Edn1, Bmp and Jag2, by neural crest cells (Alexander et al., 2011; Clouthier and Schilling, 2004; Zuniga et al., 2011; Zuniga et al., 2010). To test if neural crest cells also require reception of Hh signaling for the proper expression of dorsal/ventral markers within the pharyngeal arches, we generated genetic mosaics by transplanting smo+/+ neural crest cells into smo mutant embryos. In these genetic mosaics, we analyzed a subset of markers, barx1, gsc and dlx5a, which we found to be disrupted in smo mutants. In smo mutant embryos with pharyngeal arches populated by smo+/+ neural crest cells (Fig. 6B-D), the barx1 (n=6/6; Fig. 6B) and gsc (n=2/2; data not shown) negative region of the intermediate arch was recovered. While on the non-transplanted side, these markers were expressed in the intermediate region of the arch (Fig. 6A and data not shown). In mosaic pharyngeal arches, the domain of dlx5a was larger along the dorsal/ventral axis as compared to the control side of the embryo (n=5/5; Fig. 6E-H). Although the transplantation technique can damage cells, because we observe a rescue of expression, it is unlikely that the cellular debris itself had any effect. These results clearly show that neural crest cells must receive Hh-signaling for the appropriate expression of dorsal/ventral markers within the pharyngeal arches.
Fig. 6.
Neural crest cells require the reception of Hh signaling for proper dorsal/ventral patterning. (AH) 36 hpf fli1:EGFP;smo-/- embryos imaged following the transplantation of neural crest cells from fli1:EGFP;smo+/+ donors. The fli1:EGFP;smo+/+ donors were injected with Alexa 568 dextran to visualize the transplanted cells (in red). The mutant side, not receiving the transplant, shows dorsal/ventral patterning defects, with the fusion of the dorsal and ventral domains of barx1 (A, arrow) and a reduced extent of dlx5a expression (E, line). (B-D) Neural crest cells wild type for smo restore the intermediate barx1 free expression domain (B, arrowhead). (F-H) Transplanted neural crest cells also increase the extent of dlx5a expression (E, line). (C & G) Show the overlay of fli1:EGFP and Alexa 568 while (G & H) are the same embryos showing just the transplanted cells. Note that in this embryo there is poor contribution of neural crest cells to the first pharyngeal arch, although the second arch is highly populated with transplanted cells. The first two pharyngeal arches are labeled. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
A partially neural crest non-autonomous requirement for smo during morphogenesis of the facial skeleton
Because disruption of dorsal/ventral patterning causes defects in morphogenesis (Kimmel et al., 2001), it would seem to follow that transplantation of wild-type neural crest cells would also completely rescue morphogenesis in smo mutants. Surprisingly, even in mutant embryos with a nearly complete contribution of wild-type neural crest cells to the pharyngeal arches, we detected variable and always incomplete rescue of morphogenesis of facial skeletal elements derived from those neural crest populations (n=6/6; Fig. 7). In most of these transplants, wild-type crest transplanted into smo mutants generated slightly larger cartilages with a clear dorsal and ventral element, compared to the control side of the embryo, however, the cartilages lacked any clear morphology (n=4/6; Fig. 7A & B). In the most extensive rescues, the dorsal and anterior portions of the hyomandibular cartilage were reduced and the symplectic cartilage failed to extend properly (n=2/6; Fig. 7D). In these instances, the dorsal cartilage was discernable as a hyosymplectic due to the flattened morphology, slightly formed symplectic and the presence of a commissure in the cartilage (Supplemental Fig. 6, compare to Fig. 1). Analysis of transplanted embryos at 54 hpf revealed that morphogenetic movements positioning the 3rd arch medial to the 2nd arch had failed (n=6/6; Supplemental Fig. 7) These results suggest that Smo function in neural crest cells is not sufficient to completely drive morphogenesis of these skeletal elements.
Fig. 7.
Morphology of the pharyngeal skeleton is still disrupted in smo mutants receiving wild-type neural crest transplants. (A & C) Mutant and (B & D) transplant side of 4 dpf Alcian Blue/Alizarin Red stained smo mutants receiving wild-type neural crest transplants. The dashed lines outline the second pharyngeal arch elements for each respective side. (A & B) Compared to the mutant side, the side of the embryo populated with wild-type neural crest cells generates larger pharyngeal skeleton elements. (C & D) Even in the most complete rescue, the skeletal elements generated by wild-type neural crest cells are still misshaped. The overall shape of the hyosymplectic (hs) is recognizable. However, the symplectic rod is nearly absent (arrow) and there is a notch out of the proximal most portion of the element (arrowhead). ch, ceratohyal. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Our transplants from smo mutants into wild-type embryos provided further evidence that morphogenesis of skeletal precursors does not solely require Smo function in the neural crest. As has been previously demonstrated (Eberhart et al., 2006; Jeong et al., 2004; Wada et al., 2005), smo mutant crest cells contribute readily to the pharyngeal arches (n=4/4; Supplemental Fig. 8 A & B). At 4dpf, we find that smo mutant neural crest cells are present throughout the region of the hyosymplectic cartilage (Supplemental Fig. 8 C & D). Some of these mutant cells are contributing to the symplectic cartilage stack (Supplemental Fig. 8 C, arrow), which is consistently absent in smo mutants. Collectively, our transplantation analyses suggest that Hh signaling to the neural crest is sufficient for dorsal/ventral patterning but is not completely sufficient for morphogenesis of the crest-derived skeleton. Therefore, the reception of Hh by additional tissues is likely to play a role in morphogenesis.
Development of the facial skeleton requires Hh-signaling to the endoderm
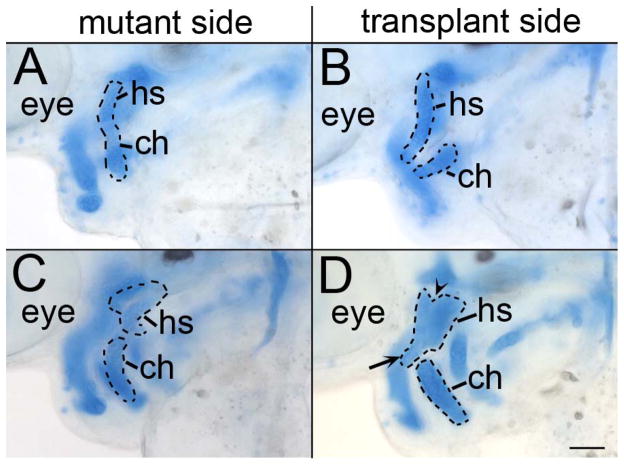
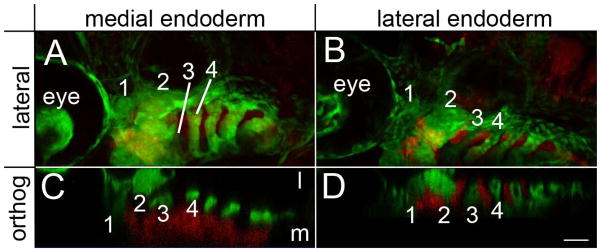
The pharyngeal endoderm is necessary for proper craniofacial morphogenesis (David et al., 2002) and Hh signaling is necessary for proper patterning of pharyngeal endoderm derivatives (Grevellec et al., 2011; Moore-Scott and Manley, 2005). Therefore, we examined if Hh signaling to the endoderm was also involved in craniofacial morphogenesis. We transplanted sox32 mRNA injected cells into smo mutant embryos and generated genetic mosaics capable of receiving Hh signaling only in the endoderm. In these embryos, the defects in expression of dorsal/ventral markers in the pharyngeal arches were not rescued (Supplemental Fig. 9). Occasionally, these transplants only populate the lateral or medial endoderm. In these instances, we find that transplants populating the medial endoderm (n=3/3), but not the lateral endoderm (n=4/4), were capable of rescuing this morphogenetic movement (Fig. 8). These results show that early morphogenetic movements of craniofacial condensations require the reception of Hh signaling by the endoderm.
Fig. 8.
Morphogenetic movements of the pharyngeal arches require the reception of Hh signaling by the medial endoderm. (A & B) Lateral and (C & D) orthogonal views of 54 hpf smo mutants that received transplants of wild-type endoderm (in red) with the first 4 pharyngeal arches numbered. (A & C) In embryos with contribution of wild-type cells to the medial endoderm, the second arch thickens along the anterior/posterior axis and the third arch moves medially to the second arch. (B & D) These movements are not rescued if the transplanted cells are restricted to lateral (pouch) endoderm. l, lateral; m, medial. Anterior is to the left in all images. Scale bar=50μm.
To determine if rescuing early morphogenetic movements affected the morphology of the craniofacial skeleton, we grew mosaic embryos to 4 dpf and analyzed the craniofacial skeleton (n=11). In those smo embryos with a discernable craniofacial skeleton, wild-type endoderm altered the size and shape of the cartilage elements that were present (n=4; Fig. 9). As with our neural crest transplants, the results were variable in nature. In the most extensive reorganization of the craniofacial skeleton the non-transplanted side of the embryo had a single cartilage nodule, in the appropriate location for the hyosymplectic. The transplanted side of the embryo had cartilage nodules in the appropriate position for the hyosymplectic, ceratohyal, palatoquadrate, interhyal and symplectic rod as well as an opercle bone (which is always absent in smo mutants) and an ectopic cartilage nodule (Fig. 9D).
Hh-signaling mediates cross talk between the neural crest and the endoderm
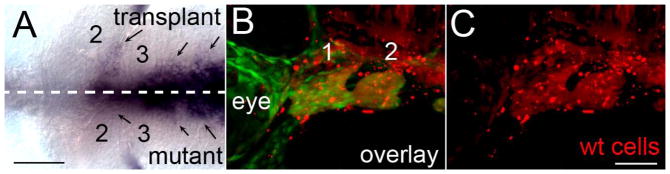
Our transplant analyses strongly suggest that proper craniofacial development requires Hh signaling to both the neural crest and the endoderm. The expression of Shh in the oral ectoderm is itself Hh-dependent as well as dependent upon cues from the neural crest (Cordero et al., 2004; Eberhart et al., 2008; Eberhart et al., 2006; Hu and Marcucio, 2009). We reasoned that this relationship might also be the case between the crest and the endoderm, which would help explain some of the variation in our manipulations. Similar to our previous analyses (Eberhart et al., 2006), we found that shha was expressed in the endoderm of smo mutants. However, we found that the appropriate distribution of shha in the endoderm is Hh dependent and that the disruption of shha expression in the endoderm is variable across smo mutants (Supplemental Fig. 10). To test if this expression was dependent upon crest signals, we transplanted smo+/+ neural crest into smo mutants and assayed the expression of shha in the endoderm. We compared the lateral extent of shha expression across transplant and mutant sides of the embryo (Fig. 10; dashed line indicates the midline as determined by the ventral neural tube and notochord). As our model predicted, transplantation of smo+/+ neural crest cells into smo mutants expanded the distribution of shha expression in the smo mutant endoderm on the side of the transplant (n=3/3; Fig. 10, arrows indicate the lateral-most extent of the shha-expressing endoderm in the first 3 arches). This finding suggests that a Hh-dependent signal from the neural crest to the endoderm is necessary to maintain appropriate gene expression in the endoderm. Because Hh signaling continues to be important through craniofacial morphogenesis, this feedback from neural crest to endoderm has important implications in the shaping of facial skeletal elements.
Fig. 10.
A Hh-dependent relay from neural crest to the endoderm regulates shha expression. fli1:EGFP;smo−/− embryos received transplants of neural crest cells from fli1:EGFP;smo+/+ donors. The fli1:EGFP;smo+/+ donors were injected with Alexa 568 dextran to visualize the transplanted cells (in red). (A) Ventral view of a 36 hpf smo mutant labeled with a riboprobes against shha showing more extensive shh expression on the side receiving the transplant (arrows). The dashed line marks the midline of the embryo as determined by the position of the notochord and ventral diencephalon. (B & C) Lateral view of the same embryo in A, imaged previously at 30 hpf, demonstrating contribution of wild-type neural crest cells to the pharyngeal arches. (B) shows the overlay of fli1:EGFP expression and Alexa 568 dextran fluorescence while (C) shows just the dextran fluorescence. The first two pharyngeal arches are numbered, anterior is to the left in all images. Scale bar=50μm.
Discussion
Collectively, our data show that Hh signaling plays 3 important roles in craniofacial development (Fig. 11). First, our endoderm transplants show that signaling directly to the endoderm is essential for early morphogenetic movements of the pharyngeal arches. Our neural crest transplants demonstrate the next two roles. Second, signaling directly to the neural crest is necessary for the proper expression of dorsal/ventral markers in the pharyngeal arches. Third, this signaling to the neural crest establishes a feedback signal to the endoderm to maintain proper gene expression, notably of shha itself. Because the endoderm is mutant for smo in these crest transplants, this feedback signal is extremely unlikely to be Shh.
Fig. 11.
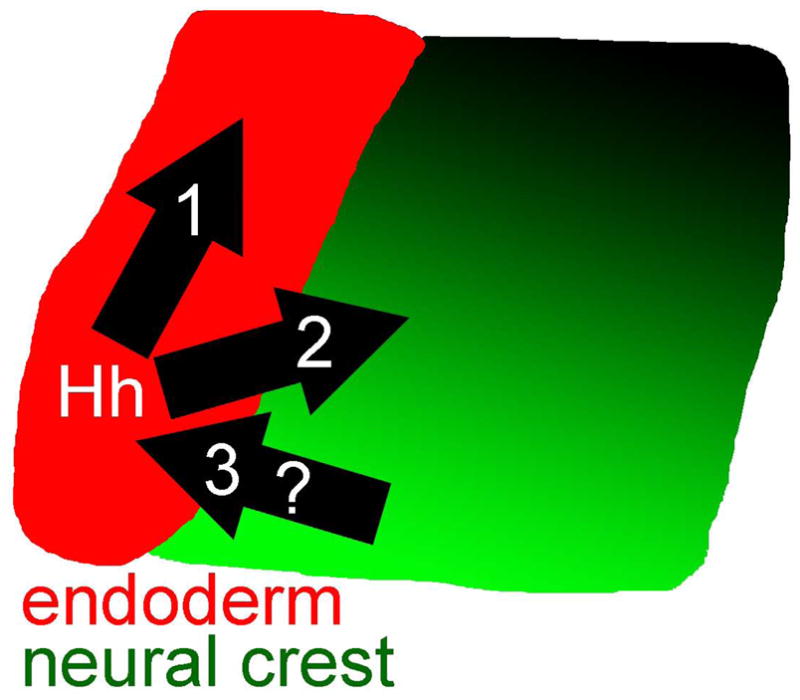
Model. Hh signals to both the endoderm (red) and neural crest (graded shades of green, with darker shades being dorsal). Signaling to the endoderm is essential for morphogenetic movements of the pharyngeal arches and likely aids in sculpting the shape of skeletal elements. Signaling to the neural crest is required for the proper dorsal/ventral patterning of the pharyngeal arches. The reception of Hh signaling by the neural crest is required for a neural crest-derived signal that maintains Hh expression in the endoderm.
These three functions help explain the variation in our transplant analyses. Since the expression of shha in the endoderm is variably disrupted, in some smo mutants receiving wild-type neural crest cells, there would not be a strong source of Shh. Therefore, morphogenesis might not be rescued to a large extent, due to an inadequate Shh signal. In those smo mutants receiving wild-type crest transplants that had stronger Shh signaling, we predict a more extensive rescue. Even in these instances though, the endoderm requires the reception of Shh signaling, therefore, the rescue will still not be complete. This, at least partly, explains why dorsal/ventral patterning can be rescued without a substantial rescue of morphogenesis.
Because shh in the endoderm requires a Hh-dependent signal from the neural crest, smo mutants with wild-type endoderm transplants are also predicted not to be fully rescued. Because this reverse signal will be lost in these transplanted embryos, we would predict that the Hh-mediated reciprocal signaling would break down. In these embryos then, it may be possible to rescue the earliest morphogenetic defect, movement of the 3rd arch, but not later elaboration of craniofacial morphology. Our model suggests that combined activation or inactivation of the Hh-signaling pathway in the endoderm and neural crest will have the most dramatic effect on craniofacial morphogenesis. More complex genetic approaches will be needed to test this model for the role of Hh signaling in patterning the craniofacial skeleton.
One likely source of Hh ligand for this patterning is the pharyngeal endoderm because endoderm expresses the zebrafish shh duplicates and is in close proximity to the neural crest. Consistent with this model, we find a severe disruption of dorsal/ventral patterning in the pharyngeal arches in mutants lacking endoderm. This would seem to be contradictory to recent results in zebrafish suggesting that endoderm is dispensable for dorsal/ventral patterning (Balczerski et al., 2011). We note though that in our analyses dorsal/ventral patterning within the first pharyngeal arch is much more intact and it is first arch skeletal elements that Balczerski et al. focus on. So while other sources, such as the ectoderm (Balczerski et al., 2011; Eberhart et al., 2006), may play important roles in dorsal/ventral patterning in the first arch, more posterior arches do require endoderm-derived signals. The dorsal/ventral patterning marker we found most resistant to loss of endoderm was hand2 which would seem to be in contrast to recent reports (Balczerski et al., 2011). We note though that Balczerski et al. state that hand2 is ectopically expressed in the ectoderm of sox32 mutants. While we did not observe any obvious expression of hand2 in the ectoderm, this may simply be due to the different in situ hybridization techniques used between these two reports and that the ectoderm was not specifically analyzed here. Importantly, we share Balczerski et al.’s conclusion that hand2 expression remains restricted to ventral arch cranial neural crest cells in sox32 mutants.
Our model does not rule out other Shh sources, such as the brain, being involved in the morphogenetic processes that we’ve examined. Indeed the brain is known to play an important role in craniofacial morphogenesis (Eberhart et al., 2006; Hu and Marcucio, 2009; Marcucio et al., 2005; Wada et al., 2005). Our model also does not rule out the possible involvement of other Hh ligands, although the Shh duplicates would seem to play the most important role. Collectively our data support the model where endoderm-derived Shh maintains or refines the dorsal/ventral patterning the pharyngeal arches, although mosaic analysis in Shh loss of function embryos will be needed to fully understand the source of the ligand.
Hh signaling is involved in proper dorsal/ventral gene expression within the pharyngeal arches
We have found that appropriate dorsal/ventral patterning information within the pharyngeal arches requires Hh signaling received by the neural crest. The expression of many, but not all, dorsal/ventral markers is altered in smo mutants. Consistent with our results, crest specific deletion of Smo does not appear to alter the expression of Hand2 in mouse (Jeong et al., 2004). On the other hand, deletion of Smo within the crest appears to cause distal expansion of Dlx5 expression within the arch (Jeong et al., 2004). This result would seem to stand in contrast to our finding that dlx5a expression is reduced in zebrafish smo mutants. It is unclear whether this apparent difference is due to species differences or differences between conditional and non-conditional Smo loss of function. To our knowledge, a more comprehensive analysis of Dlx gene expression following Hh loss of function has not been performed in mouse. These analyses will be necessary to understand the potential conservation of Hh-dependent Dlx gene expression between zebrafish and mammals.
Proper dorsal/ventral patterning within the pharyngeal arches is dependent upon a complex series of signaling interactions. These interactions impinge upon the expression of numerous transcription factors such as Dlx genes, which are known mediators of dorsal/ventral patterning (Beverdam et al., 2002; Depew et al., 2002; Talbot et al., 2010). The Endothelin 1 (Edn1) pathway is critically important for this patterning (Clouthier et al., 1998; Miller et al., 2000; Nair et al., 2007). Edn1 signaling to the neural crest is responsible for the expression of ventral pharyngeal arch markers and Edn1 pathway mutants have homeotic transformations of ventral to dorsal identity (Clouthier and Schilling, 2004; Kimmel et al., 2001). Neural crest cells express jag2, which through Notch signaling, sets up an opposing dorsal gradient (Zuniga et al., 2010). Consequently, mutation of jag2 causes homeotic transformation of dorsal to ventral identity (Zuniga et al., 2010). Bmp signaling feeds into this patterning network by both inducing edn1 expression and through a direct effect on neural crest cells, inducing ventral identity (Alexander et al., 2011; Zuniga et al., 2011).
The alteration in proper dorsal/ventral gene expression patterns that we observe in smo mutants are consistent with a model in which Hh signaling helps to maintain or refine this patterning information. Because the zebrafish Shh duplicates are expressed in the endoderm after dorsal/ventral patterning is initiated (Balczerski et al., 2011), it seems unlikely that Shh participates in the initiation of this patterning. A later role is consistent with the more moderate effects on dorsal/ventral gene expression that we observe in smo mutants as compared to Bmp or Edn1 mutants (Alexander et al., 2011; Miller et al., 2000; Nair et al., 2007; Walker et al., 2006; Walker et al., 2007; Zuniga et al., 2011; Zuniga et al., 2010). In the developing limb, Shh signaling directly regulates the expression of the Bmp antagonist, Gremlin (Vokes et al., 2008), providing a potential mechanism for how Hh signaling may interact with other dorsal/ventral patterning signals in the pharyngeal arches. While Bmp coated beads inhibit Barx1 expression in the chicken maxillary prominence (Barlow et al., 1999), similar treatments induce barx1 in the intermediate region of the zebrafish pharyngeal arch (Sperber and Dawid, 2008), similar to the effect of loss of Hh signaling. Therefore, negative interactions between Shh and Bmp signaling may be involved in the pharyngeal arches as well. Given the importance of barx1 in zebrafish craniofacial development (Sperber and Dawid, 2008), this alteration may play a significant role in the etiology of the craniofacial defects in smo mutants. Additionally, a Fox gene code in the developing mouse skull is dependent upon Hh signaling (Jeong et al., 2004) and, should a similar code exist in zebrafish, disruption of this code could cause some of the facial defects that we observe in zebrafish smo mutants. Foxa2 has been shown to physically interact with homeodomain transcription factors, including Gsc and this interaction between Fox genes and homeodomain containing transcription factors, which includes Dlx genes, is likely to be widespread (Foucher et al., 2003). Thus, it is possible that dual regulation of Fox genes and homeodomain transcription factors may play a role in the events downstream of Hh signaling that are critical for the maintenance/refinement of this dorsal/ventral patterning information. Therefore, it is likely that complex interactions between these various signaling pathways underlie our somewhat paradoxical finding that Dlx gene expression in the intermediate arch is most resistant to the loss of Hh signaling while gsc or barx1 expression expands into the same region in smo mutants.
Hh-mediated reciprocal signaling between the neural crest and the pharyngeal endoderm
Reciprocal signals between neural crest and their adjacent epithelia have been demonstrated in several contexts, particularly the developing maxillary prominence which gives rise to the palatal skeleton (Bush and Jiang, 2012). We’ve previously shown that, during maxillary development in zebrafish, Hh-mediated reciprocal signals are essential for craniofacial development. The expression of shha in the oral ectoderm is lost in smo mutants (Eberhart et al., 2006) as well as pdgfra mutants (Eberhart et al., 2008), in which neural crest cells fail to migrate to the oral ectoderm. These two results demonstrate that epithelial shha expression is dependent upon Hh-signaling and, currently, unidentified signals from the neural crest.
Here, we have shown that a very similar circumstance exists between neural crest and the pharyngeal endoderm. While the expression of shha is never lost in the pharyngeal endoderm, the pattern of shha expression is variably disrupted in smo mutants. Our neural crest transplants demonstrate that the presence of neural crest cells that can receive Hh signaling rescues the expression of shha in the adjacent endoderm, which cannot respond to Hh. Therefore, the expression of shha in the endoderm is dependent both upon Hh signaling as well as reciprocal signals from the neural crest. While, the identity of these reciprocal signals are unknown, based on expression patterns and known interactions with the Hh pathway, promising candidates include members of the Bmp, Fgf and Tgf signaling pathways (Lan and Jiang, 2009; Oka et al., 2008; Swartz et al., 2011). The significance of this reciprocal signal is unclear, but it may help promote continued outgrowth of the pharyngeal skeleton and maintain signaling to the endoderm itself.
Our results demonstrate that the endoderm requires Hh signaling for both proper patterning and morphogenetic movements of the pharyngeal arches. While the endoderm clearly plays a role in shaping the craniofacial skeleton (Couly et al., 2002), how it accomplishes this task is largely unknown. Work in zebrafish would suggest that the interactions between neural crest cells and the endoderm are likely to be local in nature. For instance, loss of the first pharyngeal pouch associates with the loss of the symplectic (Crump et al., 2004a). Furthermore, the symplectic, of the hyosymplectic, is stacking out (Crump et al., 2004a) during the time window in which we have found the movements of the pharyngeal arches to be disrupted in smo mutants. Therefore, the reorganization of the pharyngeal arches that we have shown to be Hh-dependent could potentially explain the defects to the hyosymplectic and ceratohyal that are present in smo mutants. However, our interpretation is limited by the lack of information regarding normal endoderm morphogenesis during these later stages of craniofacial development examined here. Future experiments detailing the coordinated morphology of endoderm and crest-derived skeleton will aid in our understanding of how Hh signaling mediates morphogenesis.
The partial separation of dorsal/ventral patterning and morphogenesis may be evolutionarily adaptive
There are many examples demonstrating that dorsal/ventral patterning clearly influences morphogenesis (Clouthier and Schilling, 2004; Depew et al., 2005). However, any disruption of dorsal/ventral pharyngeal arch patterning, be it through Edn1, Jag2 or Dlx loss of function, causes deleterious morphological transformations that frequently include fusions of dorsal and ventral skeletal elements (Alexander et al., 2011; Miller et al., 2000; Miller et al., 2007; Nair et al., 2007; Talbot et al., 2010; Walker et al., 2006; Walker et al., 2007; Zuniga et al., 2011; Zuniga et al., 2010). So while patterning and morphogenesis do tend to go hand in hand, altering neural crest patterning may not be the most adaptive way to alter craniofacial form evolutionarily. Because the endoderm directs the outgrowth of most of the craniofacial skeleton (Couly et al., 2002), alterations of endoderm morphogenesis may be another way to alter craniofacial form.
In both zebrafish (our results) and mouse (Moore-Scott and Manley, 2005), Hh signaling is necessary for patterning of the pharyngeal endoderm. Our work shows that endoderm capable of receiving Hh signaling can cause the morphogenetic movements of the pharyngeal arches to occur in embryos otherwise mutant for smo. The restoration of these movements correlates with an altered morphology of smo mutant cartilages, although further work is necessary to show if these morphogenetic movements of the arches are directly responsible for the morphology of the cartilage from the arches. Therefore, our results suggest that by specifically altering the response to Hh signaling in the pharyngeal endoderm, and not the neural crest, it is conceivable that the shapes of individual pharyngeal skeletal elements can be altered without changing their identity.
The shape of the hyomandibular and symplectic cartilages, collectively referred to as the hyosymplectic, in fishes has undergone extensive evolutionary modification (DeBeer, 1937). We note that the symplectic cartilage in particular appears to be most sensitive to alteration of Hh signaling; even relatively late cyclopamine treatments reduce the extension of the symplectic. It will be of great interest to examine Hh response genes in the endoderm of fishes with morphologically diverse pharyngeal skeletal elements, particularly the symplectic.
Supplementary Material
Supplemental Fig. 1. Combined loss of shha and shhb recapitulates the phenotypes observed in smo mutants. (A–D) Embryos were injected with shha and shhb morpholinos. (A) At 4 dpf, the facial skeleton is severely hypoplastic, (B) the third arch fails to move medial to the second arch in 54 hpf fli:1EGFP embryos (see orthogonal view in bottom part of the panel) and (C & D) the 36 hpf expression of dorsal/ventral markers of the arches are disrupted in embryos injected with shha and shhb morpholinos. Pharyngeal arches are numbered. hs=hyosymplectic; ch=ceratohyal; mc=Meckel’s cartilage. Anterior is to the left in all panels. Scale bar=50μm.
Supplemental Fig. 2. The endoderm as well as levels of cell death and proliferation appears normal in smo mutants at 36 hpf. fli1:EGFP transgenic embryos were stained with antibodies as listed in the left column. No clear differences are present between wild types and smo mutants with any antibody. Anterior is to the left; dorsal is up in all images. The first two pharyngeal arches are numbered. Scale bar=50μm.
Supplemental Fig. 3. 54 hpf smo mutants have elevated levels of cell death. (A & B) fli1:EGFP embryos were labeled with anti-active Caspase to detect cell death. (A) While the levels of cell death are low in wild-type embryos, (B) extensive regions of cell death (arrows) are present in smo mutants at this time-point. Anterior is to the left; dorsal is up in all images. The first two pharyngeal arches are numbered. Scale bar=50μm.
Supplemental Fig. 4. Proper Dlx gene expression requires the endoderm. Riboprobes used are listed in the left column. The expression pattern of dlx2, dlx4b and dlx5a is greatly disrupted in 36 hpf sox32 mutants. The expression pattern of dlx3b appears more normal, but the overall expression level may be reduced. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 5. Proper dorsal/ventral patterning of the pharyngeal arches at 36 hpf requires endoderm. Riboprobes used are listed in the left column. (A–D) Loss of endoderm causes disruption to the expression pattern of barx1 and gsc. (E & F) While the expression domain of hand2 appears normal, the level of expression might be reduced. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 6. Flat mounted cartilage from Fig 7C & D. (A) The hyosymplectic (hs) and ceratohyal (ch) are greatly reduced on the side of the embryo that did not receive wild-type neural crest cells. (B) Wild-type neural crest cells have improved the overall morphology of the hyosymplectic. A short symplectic rod is evident (arrow). Anterior is to the left, dorsal is up. Scale bar=50μm.
Supplemental Fig. 7. Wild type neural crest cells do not rescue the morphogenetic movement of the pharyngeal arches. 54 hpf smo mutant that had received a transplant of wild-type neural crest cells is shown. Top panels show a z-projection (dorsal is up, anterior to left), while the bottom panels show an orthogonal view (lateral is up). The first four arches are numbered and the 3rd arch has not moved medial to the 2nd arch. Scale bar=50μm.
Supplemental Fig. 8. Neural crest cells do not require reception of Hh signaling to undergo morphogenetic movements. (A & B) smo mutant neural crest cells (in red) have populated the first and second pharyngeal arch (numbered) of a fli1:EGFP host embryo at 30 hpf. (C & D) In the same embryo at 4 dpf, mutant neural crest cells have populated much of the morphologically recognizable hyosymplectic. Some smo mutant cells have populated the distal symplectic rod (arrows), which is not rescued in smo mutants receiving wild-type crest transplants. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 9. Wild type endoderm does not rescue dorsal/ventral patterning in the pharyngeal arches. (A–D) 36 hpf smo mutants that had received a transplant of wild-type endoderm. (A & B) The extent of expression of dlx5a along the dorsal/ventral axis is similar on the mutant and transplanted sides of the embryos (line). (C & D) On both sides of the embryo, barx1 is expressed in the intermediate region of the pharyngeal arch (arrow). The first two pharyngeal arches are numbered. Dorsal is up, anterior to left in all panels. Scale bar=50μm.
Supplemental Fig. 10. The proper expression of shh in the endoderm at 36 hpf is Hh-dependent. (A) In wild-type embryos the oral ectoderm (oe) and pharyngeal endoderm (pe) express shha. (B, C) In smo mutants, the expression of shha in the oral ectoderm is lost (Eberhart et al., 2006) and the expression in the pharyngeal endoderm is variably disrupted. The first two pharyngeal arches are numbered. Anterior is to the left and dorsal is up in all panels. Scale bar=50μm.
Highlights.
Hh signaling is necessary for proper dorsal/ventral gene expression in the pharyngeal arches.
Dorsal/ventral patterning of the pharyngeal arches requires endoderm.
Hh signaling to the endoderm is necessary for pharyngeal arch morphogenesis.
Neural crest signals to the endoderm to maintain proper shh expression.
Acknowledgments
We thank members of the Eberhart lab for insights and critical feedback on the manuscript. This work was supported by NIH/NIDCR R00DE018088 to J.K.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander C, Zuniga E, Blitz IL, Wada N, Le Pabic P, Javidan Y, Zhang T, Cho KW, Crump JG, Schilling TF. Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development. 2011;138:5135–46. doi: 10.1242/dev.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczerski B, Matsutani M, Castillo P, Osborne N, Stainier DY, Crump JG. Analysis of Sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AJ, Bogardi JP, Ladher R, Francis-West PH. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev Dyn. 1999;214:291–302. doi: 10.1002/(SICI)1097-0177(199904)214:4<291::AID-AJA2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–99. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, Barbieri O, Janvier P, Levi G. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis. 2002;34:221–7. doi: 10.1002/gene.10156. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–43. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Stainier DY. Intra-endodermal interactions are required for pancreatic beta cell induction. Dev Cell. 2008;14:582–93. doi: 10.1016/j.devcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–24. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Schilling TF. Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res C Embryo Today. 2004;72:190–9. doi: 10.1002/bdrc.20007. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu B, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. Journal of Clinical Investigation. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–73. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004a;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biology. 2004b;2:1432–1445. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirements for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- DeBeer GR. The Development of the Vertebrate Skull. University Press; Oxford: 1937. [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–5. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–61. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan Y-L, Song H, Bolin TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nature Genetics. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Stock DW, Hatch G, Giroux G, Weiss KM, Ekker M. Relationship between the genomic organization and the overlapping embryonic expression patterns of the zebrafish dlx genes. Genomics. 1997;45:580–90. doi: 10.1006/geno.1997.4978. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–81. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher I, Montesinos ML, Volovitch M, Prochiantz A, Trembleau A. Joint regulation of the MAP1B promoter by HNF3beta/Foxa2 and Engrailed is the result of a highly conserved mechanism for direct interaction of homeoproteins and Fox transcription factors. Development. 2003;130:1867–76. doi: 10.1242/dev.00414. [DOI] [PubMed] [Google Scholar]
- Grevellec A, Graham A, Tucker AS. Shh signalling restricts the expression of Gcm2 and controls the position of the developing parathyroids. Dev Biol. 2011;353:194–205. doi: 10.1016/j.ydbio.2011.02.012. [DOI] [PubMed] [Google Scholar]
- He X, Eberhart JK, Postlethwait JH. MicroRNAs and micromanaging the skeleton in disease, development and evolution. J Cell Mol Med. 2009;13:606–18. doi: 10.1111/j.1582-4934.2009.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsinger E, Stellabotte F, Devoto SH, Westerfield M. Hedgehog signaling is required for commitment but not initial induction of slow muscle precursors. Developmental Biology. 2004;275:143–157. doi: 10.1016/j.ydbio.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009;136:107–16. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes and Development. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, Snyder HC. The shaping of pharyngeal cartilages during early development of the zebrafish. Developmental Biology. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Moens CB. Specification and morphogenesis of the zebrafish larval head skeleton. Developmental Biology. 2001;233:239–257. doi: 10.1006/dbio.2001.0201. [DOI] [PubMed] [Google Scholar]
- Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–5. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lan Y, Jiang R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development. 2009;136:1387–96. doi: 10.1242/dev.028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N, Weinstein BM. In vivo imiagining of embryonic vascular development using transgenic zebrafish. Developmental Biology. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K-H, Parker J, Kimmel CB. sucker encodes a zebrafish endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–3828. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Miller CT, Swartz ME, Khuu PA, Walker MB, Eberhart JK, Kimmel CB. mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev Biol. 2007;308:144–57. doi: 10.1016/j.ydbio.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Yelon D, Stainier DY, Kimmel CB. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130:1353–65. doi: 10.1242/dev.00339. [DOI] [PubMed] [Google Scholar]
- Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005;278:323–35. doi: 10.1016/j.ydbio.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Nair S, Li W, Cornell R, Schilling TF. Requirements for Endothelin type-A receptors and Endothelin-1 signaling in the facial ectoderm for the patterning of skeletogenic neural crest cells in zebrafish. Development. 2007;134:335–45. doi: 10.1242/dev.02704. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Oka K, Oka S, Hosokawa R, Bringas P, Jr, Brockhoff HC, 2nd, Nonaka K, Chai Y. TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev Biol. 2008;321:303–9. doi: 10.1016/j.ydbio.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez JA, Mallo M, Gendron-Maguire M, Gridley T, Behringer RR. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995;121:3005–12. doi: 10.1242/dev.121.9.3005. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Wakamiya M, Behringer RR. Goosecoid acts cell autonomously in mesenchyme-derived tissues during craniofacial development. Development. 1999;126:3811–21. doi: 10.1242/dev.126.17.3811. [DOI] [PubMed] [Google Scholar]
- Schwend T, Ahlgren SC. Zebrafish con/disp1 reveals multiple spatiotemporal requirements for Hedgehog-signaling in craniofacial development. BMC Dev Biol. 2009;9:59. doi: 10.1186/1471-213X-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Mercier S, Velez JI, Pineda-Alvarez DE, Wyllie A, Zhou N, Dubourg C, David V, Odent S, Roessler E, Muenke M. Analysis of genotype-phenotype correlations in human holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:133–41. doi: 10.1002/ajmg.c.30240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber SM, Dawid IB. barx1 is necessary for ectomesenchyme proliferation and osteochondroprogenitor condensation in the zebrafish pharyngeal arches. Dev Biol. 2008;321:101–10. doi: 10.1016/j.ydbio.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle U, Blader P, Ingham PW. Expression of axial and sonic hedgehog in wildtype and midline defective zebrafish embryos. Int J Dev Biol. 1996;40:929–40. [PubMed] [Google Scholar]
- Swartz ME, Kelly Sheehan-Rooney Dixon MJ, Eberhart JK. Examination of a palatogenic gene program in zebrafish. Developmental Dynamics. 2011;240:2204–2220. doi: 10.1002/dvdy.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JC, Johnson SL, Kimmel CB. hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development. 2010;137:2507–17. doi: 10.1242/dev.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Okuhara Y, Iwasa H, Shindo A, Hill AJ, Kawakami A, Hiraga T. Impairment of lower jaw growth in developing zebrafish exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and reduced hedgehog expression. Aquat Toxicol. 2006;78:103–13. doi: 10.1016/j.aquatox.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Thomas T, Kurihara H, Yamagishi H, Kurihara Y, Yazaki Y, Olson EN, Srivastava D. A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development. 1998;125:3005–14. doi: 10.1242/dev.125.16.3005. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Current Opinion in Cell Biology. 2001;13:698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–63. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–8. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Coffin Talbot J, Stock DW, Kimmel CB. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol. 2006;295:194–205. doi: 10.1016/j.ydbio.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Swartz ME, Eberhart JK, Kimmel CB. phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish. Dev Biol. 2007;304:194–207. doi: 10.1016/j.ydbio.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book; A guide for the laboratory use of zebrafish (Brachydanio rerio) 1993. [Google Scholar]
- Woo K, Fraser SE. Order and coherence in the fate map of the zebrafish nervous system. Development. 1995;121:2595–2609. doi: 10.1242/dev.121.8.2595. [DOI] [PubMed] [Google Scholar]
- Yamada G, Mansouri A, Torres M, Stuart ET, Blum M, Schultz M, De Robertis EM, Gruss P. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121:2917–22. doi: 10.1242/dev.121.9.2917. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Clouthier DE, Richardson JA, Charite J, Olson EN. Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development. 2003;130:1069–78. doi: 10.1242/dev.00337. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: growth factors, transcription factors, and stem cells. Cell Res. 2005;15:301–16. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- Zuniga E, Rippen M, Alexander C, Schilling TF, Crump JG. Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development. 2011;138:5147–56. doi: 10.1242/dev.067785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E, Stellabotte F, Crump JG. Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development. 2010;137:1843–52. doi: 10.1242/dev.049056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Combined loss of shha and shhb recapitulates the phenotypes observed in smo mutants. (A–D) Embryos were injected with shha and shhb morpholinos. (A) At 4 dpf, the facial skeleton is severely hypoplastic, (B) the third arch fails to move medial to the second arch in 54 hpf fli:1EGFP embryos (see orthogonal view in bottom part of the panel) and (C & D) the 36 hpf expression of dorsal/ventral markers of the arches are disrupted in embryos injected with shha and shhb morpholinos. Pharyngeal arches are numbered. hs=hyosymplectic; ch=ceratohyal; mc=Meckel’s cartilage. Anterior is to the left in all panels. Scale bar=50μm.
Supplemental Fig. 2. The endoderm as well as levels of cell death and proliferation appears normal in smo mutants at 36 hpf. fli1:EGFP transgenic embryos were stained with antibodies as listed in the left column. No clear differences are present between wild types and smo mutants with any antibody. Anterior is to the left; dorsal is up in all images. The first two pharyngeal arches are numbered. Scale bar=50μm.
Supplemental Fig. 3. 54 hpf smo mutants have elevated levels of cell death. (A & B) fli1:EGFP embryos were labeled with anti-active Caspase to detect cell death. (A) While the levels of cell death are low in wild-type embryos, (B) extensive regions of cell death (arrows) are present in smo mutants at this time-point. Anterior is to the left; dorsal is up in all images. The first two pharyngeal arches are numbered. Scale bar=50μm.
Supplemental Fig. 4. Proper Dlx gene expression requires the endoderm. Riboprobes used are listed in the left column. The expression pattern of dlx2, dlx4b and dlx5a is greatly disrupted in 36 hpf sox32 mutants. The expression pattern of dlx3b appears more normal, but the overall expression level may be reduced. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 5. Proper dorsal/ventral patterning of the pharyngeal arches at 36 hpf requires endoderm. Riboprobes used are listed in the left column. (A–D) Loss of endoderm causes disruption to the expression pattern of barx1 and gsc. (E & F) While the expression domain of hand2 appears normal, the level of expression might be reduced. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 6. Flat mounted cartilage from Fig 7C & D. (A) The hyosymplectic (hs) and ceratohyal (ch) are greatly reduced on the side of the embryo that did not receive wild-type neural crest cells. (B) Wild-type neural crest cells have improved the overall morphology of the hyosymplectic. A short symplectic rod is evident (arrow). Anterior is to the left, dorsal is up. Scale bar=50μm.
Supplemental Fig. 7. Wild type neural crest cells do not rescue the morphogenetic movement of the pharyngeal arches. 54 hpf smo mutant that had received a transplant of wild-type neural crest cells is shown. Top panels show a z-projection (dorsal is up, anterior to left), while the bottom panels show an orthogonal view (lateral is up). The first four arches are numbered and the 3rd arch has not moved medial to the 2nd arch. Scale bar=50μm.
Supplemental Fig. 8. Neural crest cells do not require reception of Hh signaling to undergo morphogenetic movements. (A & B) smo mutant neural crest cells (in red) have populated the first and second pharyngeal arch (numbered) of a fli1:EGFP host embryo at 30 hpf. (C & D) In the same embryo at 4 dpf, mutant neural crest cells have populated much of the morphologically recognizable hyosymplectic. Some smo mutant cells have populated the distal symplectic rod (arrows), which is not rescued in smo mutants receiving wild-type crest transplants. Anterior is to the left; dorsal is up in all images. Scale bar=50μm.
Supplemental Fig. 9. Wild type endoderm does not rescue dorsal/ventral patterning in the pharyngeal arches. (A–D) 36 hpf smo mutants that had received a transplant of wild-type endoderm. (A & B) The extent of expression of dlx5a along the dorsal/ventral axis is similar on the mutant and transplanted sides of the embryos (line). (C & D) On both sides of the embryo, barx1 is expressed in the intermediate region of the pharyngeal arch (arrow). The first two pharyngeal arches are numbered. Dorsal is up, anterior to left in all panels. Scale bar=50μm.
Supplemental Fig. 10. The proper expression of shh in the endoderm at 36 hpf is Hh-dependent. (A) In wild-type embryos the oral ectoderm (oe) and pharyngeal endoderm (pe) express shha. (B, C) In smo mutants, the expression of shha in the oral ectoderm is lost (Eberhart et al., 2006) and the expression in the pharyngeal endoderm is variably disrupted. The first two pharyngeal arches are numbered. Anterior is to the left and dorsal is up in all panels. Scale bar=50μm.