Abstract
Learning involving interoceptive stimuli likely plays an important role in many diseases and psychopathologies. Within this area, there has been extensive research investigating the interoceptive stimulus effects of abused drugs. In this pursuit, behavioral pharmacologists have taken advantage of what is known about learning processes and adapted the techniques to investigate the behavioral and receptor mechanisms of drug stimuli. Of particular interest is the nicotine stimulus and the use of the two-lever operant drug discrimination task and the Pavlovian drug discriminated goal-tracking task. There is strong concordance between the two methods when using “standard” testing protocols that minimize learning on test days. For example, ABT-418, nornicotine, and varenicline all fully evoked nicotine-appropriate responding. Notably, research from our laboratory with the discriminated goal-tracking task has used an alternative testing protocol. This protocol assesses stimulus substitution based on how well extinction learning using a non-nicotine ligand transfers back to the nicotine stimulus. These findings challenge conclusions based on more “standard” testing procedures (e.g., ABT-418 is not nicotine-like). As a starting point, we propose Thurstone scaling as a quantitative method for more precisely comparing transfer of extinction across doses, experiments, and investigators. We close with a discussion of future research directions and potential implications of the research for understanding interoceptive stimuli.
Keywords: Drug discrimination, Extinction learning, Nornicotine, Pavlovian conditioning, Smoking, Tobacco, Varenicline
1. Introduction
Human behavior is guided by stimuli outside the body (exteroceptive), as well as those within the body (interoceptive). Take as an example a morning cup of hot coffee. The sight of the mug on the table, the smoothness and warmth of the mug, and the aroma of the coffee are considered exteroceptive stimuli. Such stimuli may make the person walk across the room, sniff the coffee more deeply as she shifts to holding the mug by its handle before taking a sip. Ingestion of this coffee then has interoceptive stimulus effects that can affect behavior. These may include the warmth in the throat, the change in stomach acidity, and the increased alertness induced by caffeine’s effects in the central nervous system. Like the exteroceptive stimuli, these interoceptive stimuli are available to serve as a stimulus that can enter in association with other stimuli in the environment. Such a learning history can change subsequent behavior evoked by these interoceptive stimuli (cf. Alessi et al., 2002; Bevins and Murray, 2011).
The challenge for scientists is to develop methods for assessing learning involving these interoceptive stimuli and to accurately characterize the nature of these stimuli. This mini-review will describe a subset of methods that have been used to study the interoceptive effects of drugs. We will focus on nicotine, a key addictive constituent of tobacco products, and its interoceptive stimulus effect using rats. In the doing so, we will discuss some recent disparate results that prompt us to think more deeply about the issues around how we measure interoceptive stimuli and infer stimulus similarity and underlying receptor mechanisms.
2. Interoceptive drug stimuli: training methods
Understanding how interoceptive stimuli guide an organism’s behavior has been a focus of conditioning research since the pioneering studies of Ivan Pavlov (Pavlov and Anrep, 1927). In fact, Pavlov coined the term “conditioned reflex”, currently adopted as “conditioned response”, by observing and measuring the flow of gastric and intestinal fluids in dogs anticipating reward (Pavlov and Anrep, 1927). Early interoceptive conditioning studies used internal stimuli ranging from stomach irrigation, electric brain stimulation, and injected ligands (Bykov, 1957; Doty, 1961; Cook et al., 1960; Pavlov and Anrep, 1927). Take as an example Bykov’s work with water irrigation of the stomach as the interoceptive conditioned stimulus (CS). Using dogs, this water irrigation CS was repeatedly paired with a bread or meat powder unconditioned stimulus (US). Eventually, this interoceptive stimulation of the stomach evoked an increase in salivation, as well as orientation and licking directed toward the source of the US (Bykov, 1957).
A variety of methods to study interoceptive drug stimuli in animal models have evolved from this early research. The most widely used is the two-lever operant drug discrimination procedure. In this task, there are intermixed drug and no drug sessions. On a drug session [e.g., 0.4 mg (base)/kg nicotine], rats are reinforced with food for pressing one of the levers (say the right lever) under some schedule of reinforcement; left lever presses are not reinforced on nicotine sessions. Conversely, on a no drug (saline) session, the opposite contingency is in force; left lever presses reinforced and right lever presses under extinction. Control of behavior by the interoceptive stimulus is demonstrated by greater than 80% accuracy of lever pressing on the drug-appropriate lever under test conditions where the reinforcer cannot serve as a signal for correct lever choice. For recent reviews of the nicotine operant drug discrimination literature and the underlying neural processes mediating the discriminative stimulus (SD) effects of nicotine, we refer the reader to Smith and Stolerman (2009) and Wooters et al. (2009).
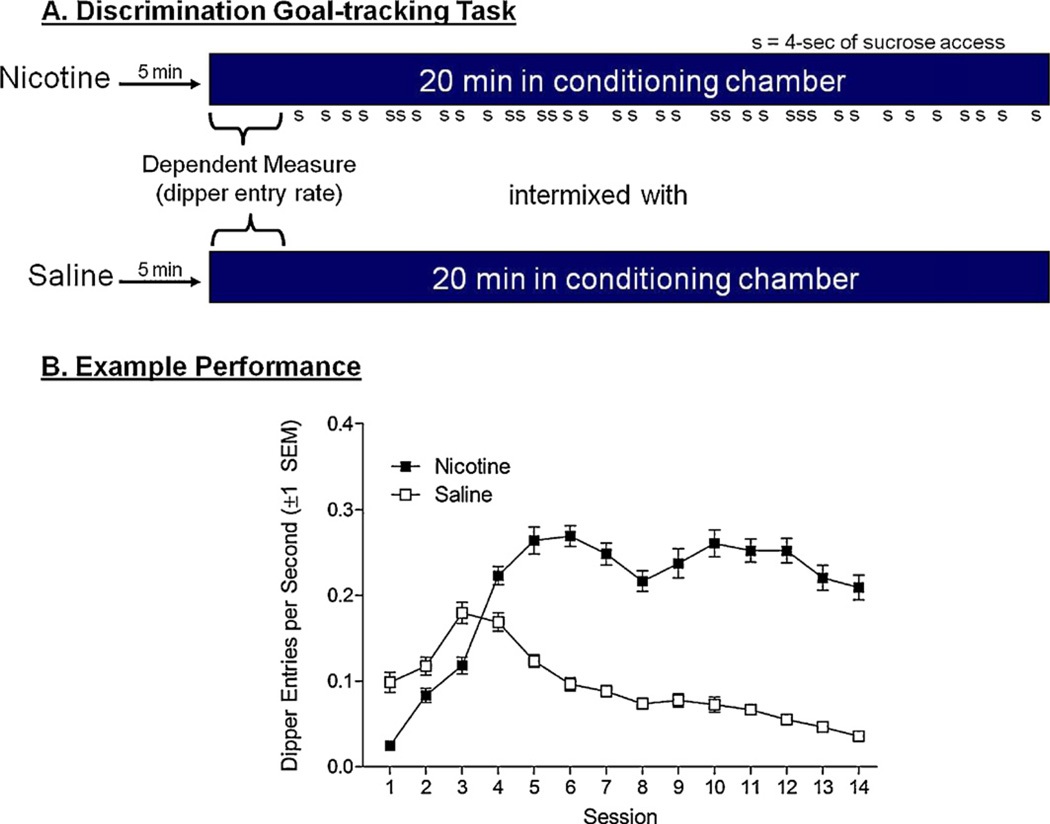
In our laboratory, we have used an alternative procedure to study the nicotine stimulus (e.g., Besheer et al., 2004; Murray et al., 2011; Wilkinson et al., 2006). In this particular task, we use a Pavlovian drug discrimination procedure that differentially pairs the nicotine stimulus (i.e., the CS) with an appetitive event such as sucrose delivery (i.e., the US). More specifically, there are intermixed nicotine and saline sessions (see Fig. 1A). On nicotine sessions, rats receive intermittent access to 4 s of sucrose (26%, w/v) in a 20-min session. On saline sessions, no sucrose is available. Control of behavior by the nicotine CS is evidenced by a differential increase in dipper entries [i.e., goal-tracking (Farwell and Ayres, 1979)] on nicotine sessions. Fig. 1B shows acquisition in rats trained under this protocol (see caption for more detail). As can be seen, the discrimination is acquired quickly with a robust goal-tracking conditioned response (CR) being maintained from sessions 5 to 14. For a summary of the neuropharmacological and behavioral research using this Pavlovian drug discriminated goal-tracking task with a nicotine CS, we refer the reader to Bevins (2009) and Wooters et al. (2009).
Fig. 1.
(A) A cartooned version of the standard discriminated goal-tracking protocol used in the laboratory with 0.4 mg (base)/kg nicotine as the CS. As indicated in this graphic, the measure of conditioning comes from the time before the first sucrose delivery on nicotine sessions or an equivalent time in saline session. Given that this time varies across sessions, we use rate of dipper entries per second as the primary dependent measure. (B) Acquisition data from rats in Experiment 5 (transfer of extinction with varenicline) from Reichel et al. (2010); these data were not reported in that paper.
3. Inferring stimulus similarity from substitution tests
Pigeons reinforced to peck a yellowish-orange (580 nm) key will respond at a comparable rate to wavelengths close to the training value. As the deviation increases, key pecking decreases accordingly (Guttman and Kalish, 1956). A common inference from these stimulus generalization (substitution) studies is that wavelength is a relevant stimulus dimension controlling behavior, and that the extent of the difference in responding between two wavelengths reflects their perceived similarity or difference of the stimuli being tested. A similar approach is used in drug discrimination research. Once stimulus control of behavior by the nicotine stimulus (SD or CS) is gained, then other ligands, often with at least partially known receptor mechanisms, can be given in place of nicotine on test days. There are several different methods for conducting substitution testing. One popular approach is to make the test sessions much shorter (ca. 4 min) than the training sessions (ca. 20 min) and to withhold the reinforcer. Another common method is to keep the test session similar to the training sessions, except that responding on either lever is reinforced according to the training schedule of reinforcement. The extent to which the test ligand evokes nicotine-appropriate responding serves as the index of stimulus similarity. Both approaches are successful. That is, they generate orderly and lawful dose–effect curves, they do not seem to disrupt discrimination performance on intervening training session, and they can be employed repeatedly over extended time periods (more than a year in rats). This suggests that these approaches minimize what is learned during testing, thus not affecting the nature of the drug stimulus under investigation.
Take as an example of substitution testing a recent study from our lab assessing a variety of nicotinic acetylcholine agonists or partial agonist for their similarity to the nicotine CS (Reichel et al., 2010). Once performance stabilized (cf. Fig. 1B), substitution testing began in which rats were tested every fifth day given that the discrimination was maintained on the intervening four training days. For each substitution test, the rat was pretreated with the test ligand (ABT-418, varenicline, or nornicotine) at the prescribed time before being placed in the chamber for a 4-min extinction test (no access to sucrose). Of particular interest for the present report was the finding that the higher test doses of varenicline (Chantix®), ABT-418, and nornicotine all evoked a goal-tracking CR statistically comparable to the nicotine CS (Reichel et al., 2010). According to this method of assessing stimulus similarity, these ligands, which differ in their binding at nicotinic acetylcholine receptor subtypes, are all nicotine-like as they fully substitute for the nicotine CS. There is good concordance of these findings with the operant drug discrimination task. ABT-418 (Damaj et al., 1995), nornicotine (Goldberg et al., 1989), and varenicline (Jutkiewicz et al., 2011; Paterson et al., 2010) fully substitute for the nicotine SD [see LeSage et al. (2009) and Smith et al. (2007) for partial substitution with varenicline]. The inference of stimulus similarity from the results of these substitution tests also comes with an inference of receptor mechanism. Take as an example ABT-418 and varenicline. ABT-418 receptor specificity for nicotinic acetylcholine receptor subunits is α4β2 > α3β4 > α3β2 > α7 (Hahan et al., 2003). For varenicline it is α4β2 > α3β4 > α7 (Smith et al., 2007). The overlap suggests at least involvement of receptors containing α4β2 and α3β4 subunits. The α7 is not included in this list given that the α7 antagonist MLA does not block the goal-tracking CR evoked by the nicotine CS (Struthers et al., 2009).
4. Testing the inference of stimulus similarity
The known differences in receptor binding profile of nicotine versus ABT-418, nornicotine, and varenicline made us wonder whether these differences were irrelevant to what is ultimately perceived as the nicotine stimulus (Hahan et al., 2003; Middleton et al., 2007; Papke et al., 2007; Smith et al., 2007). If this conclusion regarding irrelevance was true, then we should be able to demonstrate stimulus similarity among the ligands in other situations besides the brief substitution test (cf. Reichel et al., 2010). Namely, we were interested in prolonged and repeated extinction sessions (see following paragraph). To test this idea, we trained nicotine as a CS in the drug discriminated goal-tracking task described earlier. Following acquisition of the discrimination, rats were treated with nicotine, saline, ABT 418, nornicotine, or varenicline for 6 consecutive 20-min extinction sessions (NB. each non-nicotine ligand was actually assessed in a separate study with its own controls). If these non-nicotine ligands are nicotine-like, then the extinction of the goal-tracking CR should follow the same course for all but the saline-treated rats. The results, however, revealed incongruities between the ‘typical’ substitution tests versus the repeated prolonged extinction sessions. Recall that in the ‘typical’ substitution tests, nornicotine (3 mg/kg) and varenicline (1 mg/kg) fully substituted for the nicotine CS. In contrast, these two ligands only partially substituted for nicotine over repeated extinction sessions. That is, goal-tracking was significantly lower than nicotine, but higher than saline across extinction. This finding in the repeated extinction tests even held for a dose of nornicotine (1 mg/kg) and varenicline (0.1 mg/kg) that only partially substituted for the nicotine CS in the ‘typical’ substitution tests. Surprisingly, pretreatment with ABT-418 evoked a partial CR only on the first extinction session. On the remaining 5 extinction sessions, responding did not differ from saline controls (Reichel et al., 2010). Thus, the conclusion regarding how nicotine-like a ligand may be, and hence the inferred receptor mechanisms, shifts with the test used. As soon as rats had a short history with non-reinforcement, ABT-418 no longer substituted (full or partial) for the nicotine stimulus. Varenicline and nornicotine continued to evoke a partial CR after this history of non-reinforcement.
After the last extinction test, we conducted a transfer test to assess whether extinction learning with any of these non-nicotine ligands would generalize back to the nicotine CS (Reichel et al., 2010). The day following extinction, all rats were tested with the training dose of nicotine in a 20-min extinction session. Rats that received nicotine during extinction showed significantly lower levels of conditioned responding during the transfer test than rats who had received saline (i.e., no extinction, the CR was intact on this nicotine test). ABT-418 (0.3 or 0.6 mg/kg) treatment during extinction had no effect on dipper entry rates during the subsequent nicotine transfer test. Thus, the lack of responding in the extinction phase predicted the lack of transfer of extinction learning back to the nicotine CS. However, we observed partial transfer of extinction at both doses of varenicline (0.1 and 1.0 mg/kg) and nornicotine (3.0 mg/kg). Nicotine-evoked conditioned responding was higher than rats that had nicotine in the extinction phase, but lower than saline controls that did not receive any extinction training. As with the repeated and prolonged extinction tests, the conclusions regarding stimulus similarity with nicotine and its receptor mechanism differ from the ‘typical’ substitution tests used in the field. Although the results in the repeated extinction tests parallel those in the transfer of extinction test in our published research (Reichel et al., 2010), unpublished data with bupropion (Zyban®), an inhibitor of dopamine and norepinephrine reuptake, as well as nicotinic antagonist (Dwoskin et al., 2006), suggest that this relation does not always hold.
5. A ruler for comparing ligands: Thurstone scaling
As briefly noted earlier, each non-nicotine ligand was tested in a separate experiment with its own controls. Further, we have both planned and ongoing experiments using this transfer of extinction test of stimulus similarity with other ligands. In order to better compare the transfer of extinction results across experiments and between substitution ligands, we employed Thurstone scaling to the data (Thurstone, 1927). Such scaling allows us to examine how a history of non-reinforcement with the various ligands impacted the CR, via comparison to the training nicotine CS along a common continuum with saline and the training dose of nicotine as the endpoints. To calculate the endpoints for the scale, we used the average data from the nicotine and saline conditions across all three transfer of extinction experiments in order to generate their most reliable estimates of this continuum across time (see Table 1). The scale was constructed using the following calculation of effect size:
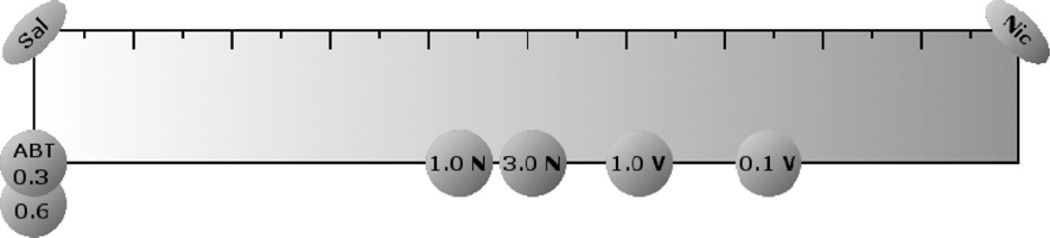
where X represents average observed dipper entries under the condition of interest (nicotine, saline, or substitution ligand), S represents the average observed dipper entries under the saline condition, and σ represents the pooled standard deviation between nicotine and saline conditions. This approach allows for comparison between substitution ligands using comparative effect size with the pooled standard deviation between nicotine and saline conditions as the unit. Note that this transformation places the value assigned to saline at the origin and the value now assigned to nicotine as the scaled discriminability of these two standards from one another (Peter Killeen, personal communication). Fig. 2 shows the scaled dipper entries (conditioned responding) for the non-nicotine ligands examined in the transfer of extinction tests by Reichel et al. (2010). We are excited about the possibilities afforded by this and related approaches (see next section). At least for the transfer of extinction test data, we can move away from the ambiguous language that classifies a ligand into one of 3 categories—full, partial, or no substitution—to a quantitative measure based on a common and empirically established ruler.
Table 1.
Dipper entries and Thurstone scaling values.
| Test ligand | Dipper entries | Scaled entries |
|---|---|---|
| Saline | 138 ± 6.09 | 0.00 ± 0.00 |
| 0.4 Nicotine | 79.9 ± 3.62 | −3.65 ± 0.522 |
| 0.3 ABT-418 | 145 ± 16.7 | 0.451 ± 1.20 |
| 0.6 ABT-418 | 146 ± 10.7 | 0.495 ± 0.798 |
| 0.1 Varenicline | 94.6 ± 10.4 | −2.72 ± 0.663 |
| 1.0 Varenicline | 102 ± 7.57 | −2.24 ± 0.553 |
| 1.0 Nornicotine | 113 ± 8.71 | −1.58 ± 0.626 |
| 3.0 Nornicotine | 108 ± 9.43 | −1.85 ± 0.646 |
Fig. 2.
Graphical representation of comparative effect sizes between substitution ligands along a Thurstone scale for the transfer of extinction tests. The range of the scale represents the scaled discriminability between saline and 0.4 mg/kg nicotine on the transfer of extinction tests. Therefore, points on the leftmost end of scale may be characterized as “less nicotine-like” and points on the rightmost end of the scale may be characterized as “more nicotine-like”. Points on the scale represent the group means for each substitution ligand. Each tick denotes 0.2 units, ABT denotes ABT-418, N denotes nornicotine, and V denotes varenicline.
A percent substitution measure such as percentage of CR in the discriminated goal-tracking task or percent drug-appropriate responding in the two-lever task (see earlier) could also provide this ruler. However, the Thurstone scaling method more precisely defines the endpoints of this ruler than a percent substitution value. For example, one common way to calculate percent value is to declare the absolute rates of response under nicotine conditions as 100%, and no responding as 0%. This approach is problematic because it is rare in drug discrimination research to observe no responding under saline conditions (cf. Fig. 1B). Alternatively, a second method to calculate a percent value is to declare the difference between nicotine and saline conditions as the upper endpoint (100%), and then compare substitution ligands by calculating a difference from saline conditions and dividing that by the nicotine difference score. Although this method is similar to Thurstone scaling, only the latter approach scales these difference scores by the pooled standard deviation for the endpoints. This scaling addresses issues that might arise from differences in variability across testing conditions within or between experiments by normalizing the scale into units of relative effect size.
6. Considerations and future directions
Morrison and Stephenson (1969) were the first to establish that nicotine could serve as a stimulus that could come to control behavior. Since this seminal paper, many investigators have adopted the two-lever operant drug discrimination task (see earlier) to assess receptor mechanisms and potential pharmacotherapies [see Smith and Stolerman (2009) and Wooters et al. (2009) for reviews]. Our recent research on transfer of extinction learning prompts several questions regarding what different approaches might tell us about the nicotine stimulus. In the discriminated goal-tracking task with the nicotine CS, a standard 4-min generalization test with some other ligand does not necessarily reflect performance in the face of continued non-reinforcement (repeated extinction tests), nor does it inform us as to how this non-reinforcement will subsequently affect behavior evoked by the nicotine CS. At present, there has been no test of whether similar dissociations would occur in the two-lever drug discrimination task. For example, ABT-418 prompts full substitution for nicotine-appropriate responding in brief generalization tests (Damaj et al., 1995); a result similar to the nicotine CS (Reichel et al., 2010). Would ABT-418 continue to prompt discriminated lever pressing at a level comparable to the training dose of nicotine over repeated extinction sessions or, would nicotine-appropriate responding more quickly decrease and/or switch quickly to the saline (no-drug) lever? The latter result would be consistent with our finding that ABT-418 is not nicotine-like once the rat has a brief history of non-reinforcement (Reichel et al., 2010). If ABT-418 was similar to nicotine in extinction for the two-lever task, then the question becomes whether that extinction learning influences stimulus control by the nicotine SD. Of course, any attempt at an answer to these questions would be pure speculation on our part.
The generality of these findings to other variants of the drug discriminated goal-tracking task would also be of interest. For example, in a feature positive occasion setting version of the task, there are intermixed saline and nicotine sessions. On nicotine sessions, a discrete stimulus (e.g., 15-s illumination of a cue light) is followed by 4-s access to sucrose. On saline sessions, the same 15-s light is presented, but no sucrose is delivered. Thus, nicotine occasions when the light presentation will be reinforced. To test transfer of extinction in this situation, varenicline, as an example, would replace nicotine and there would be several sessions of presenting the lights without sucrose before retesting nicotine. Would the partial substitution of varenicline seen in the transfer test for the nicotine CS hold for the nicotine feature positive modulator? Given that the nature of the associative learning differs for CSs versus occasion setters, including drug stimuli in these roles (cf. Palmatier and Bevins, 2007, 2008), there may well be differences in the substitution pattern of different ligands in the repeated extinction versus the transfer of extinction learning tests. As with the two-lever procedure, answers to these questions and hence implications for understanding the nature of the nicotine stimulus will have to await further research.
As noted earlier, the two-lever operant drug discrimination task has been widely used by researchers with the goal of understanding the nature of the nicotine stimulus and potential targets for medication development. As a result of these efforts, we know quite a bit about the nicotine SD and its receptor mechanisms (Smith and Stolerman, 2009; Wooters et al., 2009). Our recent findings regarding repeated extinction and transfer of extinction, along with questions regarding potential generality of such findings across different drug discrimination tasks, prompt us to reflect on the relation between how stimulus substitution is measured and how these findings will inform treatment and drug development. Does the widely used brief generalization test tell us about a mechanism that is engaged in the addiction process that differs from the transfer of extinction tests? Intuitively, the answer would seem to be yes. The former test is explicitly designed to test for nicotine-like responding in a situation that minimizes the opportunity for new learning. The latter test does just the opposite. The assessment of stimulus similarity in the transfer of extinction test is based on how well learning regarding non-reinforcement generalizes back to the training stimulus.
Such reflection also makes us wonder which, if any, of the methods for assessing the nature of the nicotine stimulus and its receptor mechanisms would more readily translate to addiction treatment or better predict targets for medication development. Alternatively, perhaps an approach that measures stimulus substitution across a range of protocols, including approaches not mentioned here, would be better than only one assay. This, of course, would be a large undertaking given the need to train separate animals in each protocol. To this end, a standardized metric that combines these scores in a manner that allows comparison of composite scores across experiments and investigators will be invaluable. In this report, we have made the first step toward this end by using Thur-stone scaling to compare the transfer of extinction findings across the experiments evaluating ABT-418, nornicotine, and varenicline. Up to now, we merely referred to their substitution for nicotine in the transfer test using the imprecise terms of partial (nornicotine, varenicline) to none (ABT-418). We now have a value that allows us to more precisely compare these ligands at various doses within and across experiments (see Fig. 2 and Table 1). Future efforts will obviously need to build toward a scaled metric for each method/measure of stimulus substitution, and then determine the utility of developing a composite score.
In an earlier paper, we used the expression “associative substitution” (vs. stimulus substitution) to refer to the approach of using learning processes to assess the overlap in the stimulus effects of a ligand with nicotine (Wilkinson et al., 2010, p. 826). Extinction learning, of course, is just one form of associative learning that could be used to this end. The list is long, interesting, and completely unexplored. Take as an example the CS preexposure effect [latent inhibition; e.g., Ayres et al., 1992; Lubow, 1973]. Procedurally, in a CS preexposure experiment, the animal first receives repeated nonreinforced exposure to CS. Subsequently, this CS is then paired with the US. The CS preexposure effect is evidenced by slowed acquisition of a CR relative to controls. Translating this description into an experiment examining associative substitution seems straightforward. The first phase would involve repeated administration of the ligand of interest (e.g., nornicotine) in the conditioning chamber without access to the sucrose US. In the second phase, the primary group of interest would be one that then received nicotine trained as a CS. That is, intermixed nicotine and saline sessions with nicotine sessions including intermittent reinforcement. The extent to which the expression of the goal-tracking CR was delayed relative to controls would serve as the measure of stimulus similarity between the preexposed ligand and nicotine.
Although this hypothetical CS preexposure experiment is simple in description, one of several challenges of working with drug stimuli (whether as a US or a CS) is the dynamic nature of these complex stimuli (see Bevins and Murray, 2011). Take as an example nornicotine. Preexposure to nornicotine alters the behavioral and neurobiological impact of nicotine (e.g., Dwoskin et al., 1999; Papke et al., 2007). Thus, in the conditioning phase of the hypothetical preexposure experiment, the nicotine CS may not be the same stimulus as that in control conditions that did not receive nornicotine preexposure. This alternation may mean that diminished acquisition occurred for a reason different from that hypothesized for the CS preexposure effect involving exteroceptive stimuli (Lubow and Weiner, 2010). For this matter, acquisition may be quickened if the neurobiological effects of nornicotine preexposure enhanced the salience or perceptibility of the nicotine stimulus. Either result would be quite interesting and would point to particular individual histories that slow or accelerate processes related, in this case, to chronic tobacco use and addiction.
7. Closing reflections
In general, we hope this brief review has generated an increased appreciation for the study of interoceptive stimuli and related behavioral and neural processes. More specifically, we also hope the reader leaves with an understanding that disentangling the nature of the nicotine stimulus (or any drug stimulus for that matter) may not be as simple as implied by the vast literature on drug discrimination [cf. Fig. 7.2 in Bevins (2009)]. This point is not meant to diminish the importance of the nicotine drug discrimination research over the past 30+ years. In fact, our point is just the opposite. We believe it is critically important to understand the nature of interoceptive stimuli. As such, the research community should bring to bear all of the tools that behavioral pharmacology and learning theory has to provide. By doing so, the field will acquire a deeper understanding of basic processes that could provide new molecular targets for drug development and/or improved behavioral approaches for many of the important public health problems. The present report had focused on drug addiction, with an emphasis on nicotine [see Bevins and Murray (2011) for a more detailed review of interoceptive stimuli and drug abuse]. However, interoceptive stimuli have been implicated in such health issues as treatment of cancer (Meagher, 2010), cardiovascular disease (Koroboki et al., 2010), chronic pain (De Peuter et al., 2011), schizophrenia (Wylie and Tregellas, 2010), impaired cognition in aging (Fadel and Burk, 2010), anxiety disorders (Domschke et al., 2010), depression (Paulus and Stein, 2010), eating disorders (Oldershaw et al., 2011; Davidson, 1993), and obesity (Davidson, 1993).
Acknowledgements
We thank Peter Killeen for his help with the Thurstone scaling used for the transfer of extinction data, and Terry Davidson for suggesting the CS preexposure experiment that we use as a hypothetical study in the present paper. The time to develop the ideas expressed in this report and the research described here was supported by USPHS grants DA018114 and DA023951.
Footnotes
The research and R.A. Bevins were supported by United States Public Health Service Grants DA018114 and DA023951.
References
- Alessi SM, Roll JM, Reilly MP, Johanson C-E. Establishment of a diazepam preference in human volunteers following differential-conditioning history of placebo versus diazepam choice. Experimental and Clinical Psychopharmacology. 2002;10:77–83. [PubMed] [Google Scholar]
- Ayres JJB, Philbin D, Cassidy S, Bellino L, Redlinger E. Some parameters of latent inhibition. Learning and Motivation. 1992;23:268–287. [Google Scholar]
- Besheer J, Palmatier MI, Metschke D, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Altering the motivational function of nicotine through conditioning processes. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use: The 55th Nebraska Symposium on Motivation. New York: Springer; 2009. pp. 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Murray JE. Internal stimuli generated by abused substances: role of Pavlovian conditioning and its implications for drug addiction. In: Schachtman TR, Reilly S, editors. Associative Learning and Conditioning: Human and Non-Human Applications. New York: Oxford University Press; 2011. pp. 270–289. [Google Scholar]
- Bykov KM. The Cerebral Cortex and the Internal Organs. New York: Chemical Publishing Company; 1957. [Google Scholar]
- Cook L, Davidson A, Davis DJ, Kelleher RG. Epinephrine, norepinephrine, and acetylcholine as conditioned stimuli for avoidance behavior. Science. 1960;131:990–991. doi: 10.1126/science.131.3405.990. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Creasy KR, Welch SP, Rosecrans JA, Aceto MD, Martin BR. Comparative pharmacology of nicotine and ABT-418, a new nicotinic agonist. Psychopharmacology. 1995;120:483–490. doi: 10.1007/BF02245822. [DOI] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: toward integration of physiological and learning perspectives. Psychological Review. 1993;100:640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Van Diest I, Vansteenwegen D, Van den Bergh O, Vlaeyen JWS. Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. European Journal of Pain. 2011;15:889–894. doi: 10.1016/j.ejpain.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Doty RW. Conditioned reflexes formed and evoked by brain stimulation. In: Sheer DE, editor. Electrical Stimulation of the Brain: An Interdisciplinary Survey of Neurobehavioral Integrative Systems. Austin, TX: University of Texas Press; 1961. pp. 397–412. [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Reviews. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology. 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: role in attention. Brain Research. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘goal tracking’) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology. 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Guttman N, Kalish HI. Discriminability and stimulus generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Hahan B, Sharples CGV, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks E, Kynaston A, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. Journal of Pharmacology and Experimental Therapeutics. 2011;339:194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroboki E, Zakopoulos N, Manios E, Rotas V, Papadimitriou G, Papageorgiou C. Interoceptive awareness in essential hypertension. International Journal of Psychophysiology. 2010;78:158–162. doi: 10.1016/j.ijpsycho.2010.07.003. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacology Biochemistry and Behavior. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition. Psychological Bulletin. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- Lubow R, Weiner I. Latent Inhibition: Cognition, Neuroscience, and Applications to Schizophrenia. NY: Cambridge University Press; 2010. [Google Scholar]
- Meagher MW. Developing translational animal models of cancer-related fatigue. In: Cleeland C, Fisch M, Dunn A, editors. Cancer Symptom Science: Measurement, Mechanisms, and Management. Cambridge University Press; 2010. pp. 124–141. [Google Scholar]
- Middleton LS, Crooks PA, Wedlund PJ, Cass WA, Dwoskin LP. Nor-nicotine inhibition of dopamine transporter function in striatum via nicotinic receptor activation. Synapse. 2007;61:157–165. doi: 10.1002/syn.20351. [DOI] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- Murray JE, Walker AW, Polewan RJ, Bevins RA. An examination of NMDA receptor contribution to conditioned responding evoked by the conditional stimulus effects of nicotine. Psychopharmacology. 2011;213:131–141. doi: 10.1007/s00213-010-2022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, Schmidt U. The socio-environmental processing stream in Anorexia Nervosa. Neuroscience and Biobehavioral Reviews. 2011;35:970–988. doi: 10.1016/j.neubiorev.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behavioural Brain Research. 2007;176:292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Occasion-setting by drug states: functional equivalence following similar training history. Behavioural Brain Research. 2008;195:260–270. doi: 10.1016/j.bbr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. Journal of Neurochemistry. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone B, Ghavami A. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1455–1464. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP, Anrep GV. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with vareni-cline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58:1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Trickelbank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalize to the nicotine discriminative stimulus in the rat. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Nicotine Psychopharmacology: Handbook of Experimental Pharmacology. 2009;192:295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-β-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacology, Biochemistry and Behavior. 2009;94:319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LL. A law of comparative judgments. Psychological Review. 1927;34:273–286. [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of number of conditioning trials and unpaired sucrose deliveries. Behavioural Pharmacology. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Carroll FI, Bevins RA. An investigation of bupropion substitution for the interoceptive conditional stimulus effects of nicotine. Journal of Psychopharmacology. 2010;24:817–828. doi: 10.1177/0269881109102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT. Neuropharmacology of the interoceptive stimulus properties of nicotine. Current Drug Abuse Reviews. 2009;2:243–255. doi: 10.2174/1874473710902030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia Research. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]