Abstract
Recent studies on the restoration of locomotion after spinal cord injury have employed robotic means of positioning rats above a treadmill such that the animals are held in an upright posture and engage in bipedal locomotor activity. However, the impact of the upright posture alone, which alters hindlimb loading, an important variable in locomotor control, has not been examined. Here we compared the locomotor capabilities of chronic spinal rats when placed in the horizontal and upright postures. Hindlimb locomotor movements induced by exteroceptive stimulation (tail pinching) were monitored with video and EMG recordings. We found that the upright posture alone significantly improved plantar stepping. Locomotor trials using anaesthesia of the paws and air stepping demonstrated that the cutaneous receptors of the paws are responsible for the improved plantar stepping observed when the animals are placed in the upright posture. We also tested the effectiveness of serotonergic drugs that facilitate locomotor activity in spinal rats in both the horizontal and upright postures. Quipazine and (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT) improved locomotion in the horizontal posture but in the upright posture either interfered with or had no effect on plantar walking. Combined treatment with quipazine and 8-OH-DPAT at lower doses dramatically improved locomotor activity in both postures and mitigated the need to activate the locomotor CPG with exteroceptive stimulation. Our results suggest that afferent input from the paw facilitates the spinal CPG for locomotion. These potent effects of afferent input from the paw should be taken into account when interpreting the results obtained with rats in an upright posture and when designing interventions for restoration of locomotion after spinal cord injury.
Key points
Locomotor training of rats held in an upright posture has been used recently to restore locomotion after spinal cord injury. Our results show that the upright posture alone improves locomotor recovery in spinal rats.
This improvement is reversed by the removal of cutaneous afferent feedback from the paw, showing that sensory feedback from the foot facilitates the spinal central pattern generator (CPG) for locomotion.
5-HT2 and 5-HT1A/7 agonists improve locomotion in the horizontal posture but can impair locomotion in the upright posture, suggesting that a proper balance of afferent feedback from the foot and 5-HT receptor activation is necessary for optimal locomotor recovery.
Our results provide new insights into the organization of the CPG for locomotion and the evolution of hominid bipedalism. The potent effects of cutaneous afferents from the paw revealed here must be taken into account in the design of strategies to restore locomotion after spinal cord injury.
Introduction
Locomotor recovery after spinal cord injury in animals and humans depends upon the presence of a central pattern generator (CPG) for locomotion in the spinal cord, since the injury interrupts the descending pathways normally responsible for the control of locomotion. Locomotor training is a powerful means of activating the CPG and promoting locomotor recovery after spinal cord injury. Loading of extensor muscles is a major factor contributing to the success of locomotor training in humans (Harkema et al. 1997; Dietz, 1998) and in spinal animals (de Leon et al. 2002; Timoszyk et al. 2002, 2005). The physiological basis for the effect of loading, based on current knowledge of the central actions of proprioceptors, has been reviewed in detail (McCrea & Rybak, 2008; Pearson, 2008). Cutaneous input from the plantar surface of the paw also constitutes afferent input related to loading of the limb, and it is also known to have a potent influence on locomotion in spinal animals (Bouyer & Rossignol, 2003a,b).
Recent studies on recovery of locomotor function after spinal cord injury have employed a training method with rats held in a bipedal upright posture. When subjected to a combination of training, epidural stimulation and drug application, such animals recover weight bearing locomotion on a treadmill that can be sustained for long periods (Courtine et al. 2009; Musienko et al. 2011). However, the impact of the upright posture has not been explored, despite the fact that this change in posture, because of its impact on hindlimb loading, has the potential to alter locomotor activity on its own. Therefore, we designed experiments to test the effects of the upright posture alone on locomotor ability of chronic spinal rats.
Key elements in recovery of locomotor function drawn from work using rats in an upright posture were the actions of quipazine, a 5-HT agonist acting at 5-HT2A, 5-HT2B and 5-HT2C receptors, and (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT), a 5-HT agonist acting at 5-HT1A/7 receptors (Fong et al. 2005; Ichiyama et al. 2008; Courtine et al. 2009, 2011; Musienko et al. 2011). Previous demonstrations of the efficacy of 5-HT agonists and grafts of 5-HT neurons for the restoration of locomotion in the absence of any other intervention have been carried out in spinal rodents (Feraboli-Lohnherr et al. 1999; Gimenez y Ribotta et al. 2000; Schmidt & Jordan, 2000; Sławińska et al. 2000; Hochman et al. 2001; Antri et al. 2002, 2003, 2005; Landry & Guertin, 2004; Majczyński et al. 2005; Landry et al. 2006) and spinal cats (Barbeau & Rossignol, 1990; Brustein & Rossignol, 1999) walking in the normal horizontal posture. Any changes in the response to 5-HT that might occur when the animals are held in the upright posture have not been assessed.
We report here that the upright posture alone dramatically improves locomotion in spinal rats. Reducing afferent feedback derived from the hindlimbs during air-stepping or after anaesthesia of the hindpaw showed that the facilitation of plantar stepping that occurs in rats in the upright posture is due largely to afferent feedback from the plantar surface of the paw. Moreover, in contrast to the horizontal posture, the ability of 5-HT receptor activation to improve locomotor capability in animals placed in the upright posture was altered.
Methods
Experiments were performed on female Wistar Albino Glaxo (WAG) rats (Charles River) (10 spinal and 5 intact). In the case of spinal animals, the experiments were commenced 10 weeks after spinal cord injury. All surgical and experimental procedures were conducted with care to minimize pain and discomfort of animals with the approval of the First Local Ethics Committee in Poland, according to the principles of experimental conditions and laboratory animal care of the European Union and the Polish Law on Animal Protection, and of the University of Manitoba Animal Care Committee, in accordance with the guidelines of the Canadian Council on Animal Care. All the procedures used in this study conform to the principles of UK regulations, as described in Drummond (2009).
Complete spinal transection procedure
We performed complete transection of the spinal cord using surgical procedures described in detail in previous papers (Sławińska et al. 2000; Majczyński et al. 2005). Briefly, in ten 3-month-old WAG rats under deep anaesthesia (isofluorane, 2% plus Butomidor, 0.05 mg (kg body weight (b.w.))−1), and under sterile conditions, a laminectomy of the Th8 vertebrae was performed. The spinal cord was completely transected at the thoracic level Th9/10. To prevent the possibility of axonal regrowth through the cavity of the lesion, 2–3 mm of spinal cord tissue was aspirated using a glass pipette. Then the muscles and fascia overlying the paravertebral muscles were closed in layers using sterile sutures, and the skin was closed with stainless-steel surgical clips. After surgery, the animals received a non-steroidal anti-inflammatory and analgesic treatment (s.c., Tolfedine, 0.4 mg (100 g b.w.)−1), and during the following 8 days, the animals were given antibiotics (s.c. Baytril, 0.5 mg (100 g b.w.)−1 and gentamycin, 0.2 mg (100 g b.w.)−1). During this postoperative period, the bladder was emptied manually twice a day until the voiding reflex was re-established. When the data collection was completed the animals were killed by i.p. injection of an overdose of pentobarbital (150 mg kg−1). Then the spinal cords were inspected to confirm that the transection was complete.
Implantation of EMG recording electrodes
Ten weeks after spinal cord transection the animals were anaesthetized with Equithesin (i.p. 0.35 ml (100 g b.w.)−1)) and bipolar EMG recording electrodes were implanted in the soleus (Sol) and tibialis anterior (TA) muscles of both hindlimbs. The electrodes were made of Teflon-coated stainless-steel wire (0.24 mm in diameter; AS633, Cooner Wire, Chastworth, CA, USA). The tips of the electrodes with 1–1.5 mm of the insulation removed were pulled through a cutaneous incision at the back of the animal, and each of the hook electrodes was inserted into the appropriate muscle, where it was secured by a suture. The distance between the tips of electrodes was 1–2 mm. The ground electrode was placed under the skin on the back of the animal at some distance from hindlimb muscles. A custom-made connector with the other ends of the wires fixed to it, covered with dental cement (SpofaDental) and silicone (3140 RTV, Dow Corning), was secured to the back of the animal. A wire loop left under the skin on the back of the animal prevented the electrodes from being pulled out from the muscles during movements. The incisions on the hindlimbs were closed. To prevent infection animals were treated by single dose of antibiotic (s.c. Baytril, 0.5 mg (100 g b.w.)−1). EMG recordings started 3–5 days after electrode implantation. Electrode position in the muscles was verified visually after the animals were killed.
Video and electromyographic recordings
The hindlimb locomotor pattern was investigated 3–4 months after spinal cord transection with the animals placed on a moving treadmill (treadmill speed 5–10 cm s−1) in either a horizontal or an upright posture (Fig. 2A). For the horizontal posture the rats were manually positioned with their forelimbs on a platform above the treadmill and their hindlimbs touching the moving belt. For the upright posture, the rat was manually held above the treadmill belt in a vertical position with the hindlimbs touching the moving belt. In intact rats the EMG activity of hindlimb muscles was investigated during spontaneous locomotor movement on a treadmill. In order to obtain sustained locomotion with horizontal posture without pauses in the intact animals, the treadmill speed was kept at 20 cm s−1. In the upright posture the intact animals were unwilling to sustain locomotor activity at speeds above 10 cm s−1, and so these trials were done at 5–10 cm s−1. Manual stimulation of the tail was used to elicit locomotor-like hindlimb movements in the spinal rats. Stimulation of tail or perineal area afferents is routinely used for eliciting locomotion in cases of complete spinal cord transection (Meisel & Rakerd, 1982; Pearson & Rossignol, 1991; Sławińska et al. 2000; Majczyński et al. 2005; Rossignol et al. 2006; Lev-Tov et al. 2010). We adopted it as a suitable method for comparing locomotion in the horizontal and upright postures. The tail was stimulated in an attempt to induce weight support and stepping movements as previously described in spinal rats in the horizontal posture (Sławińska et al. 2000; Majczyński et al. 2005). In the upright posture the spinal rats were held above the treadmill by an experimenter who adjusted body weight support and tail stimulation to obtain the best hindlimb walking pattern. The EMG activity of two muscles, the extensor soleus (Sol) and the flexor tibialis anterior (TA), of both hindlimbs was simultaneously digitized and stored on a computer (3 kHz sampling frequency) as previously described (Sławińska et al. 2000; Liu et al. 2009). The EMG activity was simultaneously recorded with synchronized video recordings, thus enabling the further off-line analysis of precisely verified portions of EMG activity related to the best locomotor performance in every animal. EMG amplitude was determined from filtered (band pass 0.1–1 kHz), integrated (20 Hz) and rectified EMG records of 10–30 consecutive steps. Peak amplitude, burst duration and burst area were determined using Winnipeg Spinal Cord Research Centre custom analysis programs (http://www.scrc.umanitoba.ca/doc/). The locomotor pattern was analysed using polar plots to determine the coordination between flexor and extensor muscles on the two sides, as well as left–right coordination among the muscles of the left and right sides, as previously described (Liu et al. 2009). The analysis of the relationships between the EMG burst duration and step cycle duration was performed using the regression line method. The correlation coefficients from the polar plots and the slopes from regression line analysis from EMG recordings in pre-drug conditions were then compared to those obtained after the drug applications using Student's paired t test. The normal distribution of the data was confirmed using a Shapiro–Wilk test. For comparison of the EMG cycle or burst duration as well as the peak amplitude and area expressed as a ratio of the control values (Fig. 3) the non-parametric Wilcoxon's test was used.
Figure 2. Spinal rats placed in the upright posture (n = 6) display near-normal locomotor patterns.
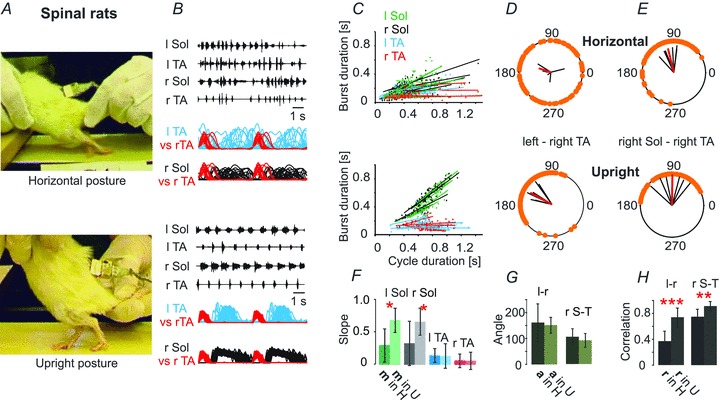
A, the same rat in the horizontal posture (upper panel) and the upright posture (lower panel) is depicted. C, the relationships between the step cycle durations and burst durations for the left and right TA and Sol muscles. F, bar graph showing that the slopes of this relationship differ significantly between the horizontal and upright posture trials (Student's paired t test). D and E, polar plots illustrating the interlimb (D) and intralimb (E) coordination changes that occur when the animals is placed in the upright posture (lower panel) compared to the horizontal posture (upper panel). G, bar graph showing the mean angle (a, ±SD) of polar plots for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships. H, bar graph demonstrating the mean correlation coefficients (r, ±SD) for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships, indicating a highly significant increase in r for inter- and intralimb coordination in the upright posture trials. Student’t test: *P < 0.05, **P < 0.005, ***P < 0.0005.
Figure 3. Changes in mean ± SD cycle duration, peak amplitude, burst duration and burst area established on the basis of EMG recordings from soleus (Sol) and tibialis anterior (TA) muscles of both hindlimbs during locomotion in the horizontal and upright postures in different experimental conditions.
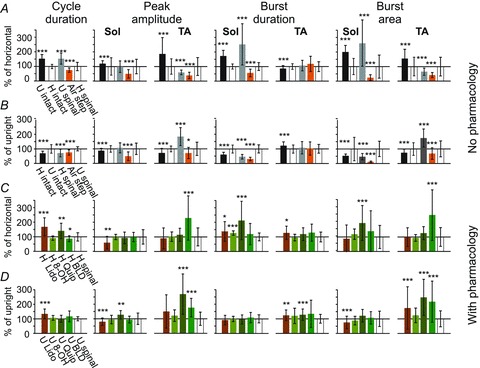
Each bar is the mean of 4–6 spinal rats and 5 experiments from intact rats taken from 10–30 consecutive steps in both hindlimbs during rhythmic movements in a particular experimental condition. The means in A are expressed as the percentage of that obtained during locomotor trials in the horizontal posture. The means in B are expressed as the percentage of the values obtained during trials in the upright posture. 100% represents the mean value obtained in the control situation for each analysed index (white bar). The black bar on the left represents results obtained during locomotor trials of intact rats in the upright posture in comparison to the horizontal posture (A) and in the horizontal posture in comparison to the upright posture (B). For example, in the case of the cycle duration index, the mean cycle duration of intact rats in the upright posture is longer than that obtained during locomotor trials in the horizontal posture. Similarly, the grey bars represent the means obtained in spinal rats, showing that the cycle duration increases significantly when going from the horizontal to the upright posture (A), and decreases when going from upright to the horizontal posture (B). The orange bar represents the means of each index for air-stepping. C and D, pharmacological treatments induce different effects on locomotor movements recorded in the horizontal and upright postures. The values after treatment are expressed as a percentage of the values measured from locomotor trials taken before the pharmacological treatment. Abbreviations: U, upright posture; H, horizontal posture; air step, air-stepping; Quip, quipazine (0.1–0.25 mg kg−1i.p.); 8-OH, 8-OH-DPAT (0.1–0.4 mg kg−1i.p.); Lido, lidocaine injections into the hindpaw bilaterally (0.05 ml, one medial and one lateral in each paw); BLD, both Quip and 8-OH-DPAT, low dose (0.1 mg kg−1). Wilcoxon's non-parametric test: *P < 0.05, **P < 0.02, ***P < 0.01.
Evaluation of hindlimb locomotor performance
Three months after spinal cord transection, treadmill locomotion was tested approximately twice per week, as previously described (Majczyński et al. 2005). The testing periods were kept as short as possible (less than 5 min on the treadmill on any given test) to minimize training effects. We established the quality of plantar stepping based upon EMG analysis. The features that we used to characterize plantar stepping were (1) sustained soleus activity throughout the stance phase, (2) soleus burst duration related to step cycle duration, (3) brief TA activity of consistent duration, (4) consistent intra- and interlimb coordination with highly significant correlation coefficients, and (5) weight support detected by the experimenter. Tests were conducted with the experimenter manually placing the animal in the horizontal or the upright posture (see videos). Hindlimb movement was induced by tail pinching. Tests were conducted at treadmill speeds of 5 and 10 cm s−1 for all cases involving spinal rats.
Drug tests
To determine the role of afferent input from the hindlimb on their locomotor ability, the spinal rats were tested on a treadmill before and after the hindpaw anaesthesia using bilateral injections of lidocaine (2%, Polfa). Two injections (0.05 ml) were made into the foot pad of each hindpaw, one medial and one lateral. This served to temporarily block cutaneous feedback from the plantar surface of the paw. In order to reduce the afferent feedback from loading the hindlimb, locomotor trials evoked by tail stimulation were conducted during air-stepping. In this case, the animal was held in the upright posture with its hindpaws pendent.
To determine the effects of serotonergic agonists on the hindlimb locomotor abilities on a treadmill we started from evaluation of the pre-drug baseline performance. Then the evaluation of hindlimb movements was carried out at 15–30 min intervals after drug injection (i.e. when the maximal effect of the drugs administration was usually observed). The effects were assessed at all time intervals after drug application with the animals held for 60 s in the horizontal posture, then in the upright posture at two treadmill speeds (5 and 10 cm s−1). Experiments using different drug applications performed on a single animal were separated by at least 72 h to prevent drug interactions. In order to prevent any possible effect of training on the time course of recovery, locomotor performance was tested no more frequently than twice a week, and each test was limited to 5 min. duration. The following agonists of 5-HT2 and 5-HT7 receptors were used with i.p. administration in the volume 0.1–0.2 ml per 100 g of body weight: quipazine (5-HT2 agonist; Sigma-Aldrich) 0.25 mg kg−1 (dissolved in saline with 10% of propylene glycol); 8-OH-DPAT (5-HT7 and 5-HT1A agonist; Sigma-Aldrich) 0.2–0.4 mg kg−1 (dissolved in saline). During the combined treatment, both drugs were applied with the dose 0.1 mg kg−1 with a 20 min delay in between (the quipazine i.p. injection was followed by the 8-OH-DPAT i.p. applications).
Results
Intact rats in the horizontal and upright postures
First we determined the effects of the two postures on the locomotor capability of untrained intact rats. Figure 1 shows the very similar pattern of EMG activity recorded in both postures from hindlimb muscles of intact rats during voluntary locomotion on the treadmill, and provides baseline data for comparison with the locomotor patterns obtained in spinal rats in this study. The presence of plantar paw placement and weight supported stepping is evident in the images taken from videos of the same animal in the two postures (Fig. 1A). Regular and well-coordinated EMG patterns in the soleus (Sol) and tibialis anterior (TA) muscles bilaterally are illustrated for the two postures (Fig. 1B). Rectified and filtered EMG records taken from the raw EMGs illustrated in Fig. 1B were normalized to the step cycle, taking the onset of activity in the right (r) TA as the onset of the cycle, then overlaid, with the first cycle repeated to emphasize the repetitive activity of muscles during locomotion. These plots show left–right and flexor–extensor coordination over the same step cycles displayed in the raw EMG records. The stance phase was characterized by sustained activity of the soleus muscles throughout the period when the TA muscles were inactive, so that weight support persisted throughout the stance phase (Fig. 1B). The relationships between the step cycle durations and burst durations for the left and right TA and Sol muscles established for different animals are illustrated in Fig. 1C. In both the horizontal posture (upper panel) and the upright posture (lower panel) the extensor EMG systematically increased in duration as the step cycle duration increased. The slopes of this relationship did not differ significantly between the horizontal and upright posture trials (Fig. 1F; Student's paired t test; P > 0.05). Polar plots show intra- and interlimb coordination in the two postures. Interlimb coordination (Fig. 1D and H) was decreased in the upright posture, so that stance was initiated earlier (Fig. 1E), and the swing phase was slightly shortened (Fig. 1B and G). There were significant changes in cycle duration, soleus EMG burst duration, TA burst duration and peak EMG amplitude (see Fig. 3A and B). In the upright posture the animals used longer steps with prolonged bursts in extensor muscles and shortened bursts in flexor muscles bilaterally, thereby minimizing the amount of time the limb was off the treadmill surface.
Figure 1. EMG analysis of locomotor patterns of intact rats (n = 5) in horizontal and upright postures.
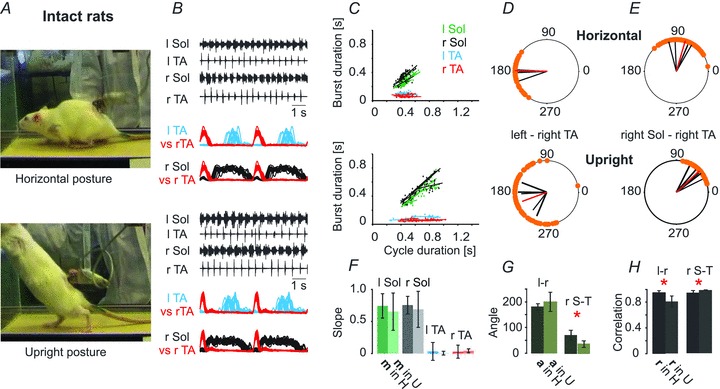
A, plantar paw placement and weight supported stepping is evident in the video frames of the same animal in the two postures. B, well-coordinated EMG patterns in the soleus (Sol) and tibialis anterior (TA) muscles bilaterally are illustrated for the two postures. Rectified and filtered EMG records are normalized to the step cycle, taking the onset of activity in the right TA as the onset of the cycle and show left–right and flexor–extensor coordination over the same step cycles displayed in the raw EMG records. l, left; r, right. C, the relationships between the step cycle durations and burst durations for the left and right TA and Sol muscles. F, bar graph showing the slopes (±SD) of this relationship. The darker green bar is the mean slope from the horizontal posture trials in left soleus muscle (l Sol), while the lighter green bar represents the trials in the upright posture. Dark (horizontal) and light (upright) grey represent the slopes of right soleus muscle (r Sol), and dark and light blue and red represent left tibilalis anterior muscle (l TA) and right tibialis anterior muscle (r TA), respectively. D and E, polar plots showing the relationships between the onset of r TA activity and either the contralateral TA or the ipsilateral extensor (r Sol). The 0 position on the polar plot corresponds to the onset of activity in the right TA muscle, and the positions of the filled circles (orange) indicate the times of onset of activity in the left TA (interlimb coordination, D) or the onset of activity in the right Sol (intralimb coordination, E). The black bars in D demonstrate the average time of onset (angle, a) of activity in the contralateral l TA for each animal, and the length of each bar is a measure of the strength of the relationship (correlation, r) between the right TA and left TA times of onset. The positions of the red bars represent the mean angle and their lengths represent correlation. The polar plots in E show the same relationships for intralimb coordination, with the times of onset of ipsilateral Sol activity plotted in relation to the times of onset of the ipsilateral r TA. The bar graph in G demonstrates the means (±SD) of the angle of the left–right TA (l-r) and of the right Sol – right TA (r S-T) relationships (relative timing of the onsets for the two muscles represented in each polar plot in the two postures, a). The dark green bars represent the angle in the horizontal posture (a in H) and the light green represent the angles in the upright posture (a in U). The bar graph in H shows the means (±SD) of the correlation coefficient (r) between the times of onset of activity in both left–right and flexor–extensor EMG comparisons for the two postures. The black bars represent r in the horizontal posture (r in H) and the grey bars represent r in the upright posture (r in U). Student’t test: *P < 0.05, **P < 0.005, P < 0.0005.
The upright posture alone improves locomotion in spinal rats
There is ample reason to suppose the upright posture should have an impact on the locomotor capacity of animals with spinal cord transection. Sensory feedback from load receptors in muscles of the hindlimb (Timoszyk et al. 2005; Pearson, 2008) and cutaneous receptors from the plantar surface of the hindpaw (Duysens & Pearson, 1976; Bouyer & Rossignol, 2003a,b) can facilitate locomotion, and even lead to the initiation of stepping (Giszter et al. 2007). A comparison of locomotion in the horizontal posture (Video 1) with the same animal in the upright posture (Video 2) demonstrates that there is a dramatic effect of loading the hindlimbs. Rats with complete spinal cord transections (Th9/10), when placed in the horizontal posture, responded to tail pinch with rhythmic EMG activity that was poorly coordinated, resulting in failure of plantar foot placement, prolonged extension and failure to produce a normal swing phase of the locomotor cycle (Video 1, Fig. 2B, upper panel). When the same animal was placed in the upright posture, tail pinch led to sustained bouts of locomotion with successful plantar foot placement, weight support, and near-normal swing and stance phases of locomotion (Fig. 2B, lower panel). The EMG pattern was dramatically improved in the upright posture (Fig. 2B, lower panel), with near-normal intra- and interlimb coordination. The timing of bursts of EMG activity (Fig. 2B) in the extensor Sol changed from a brief burst of activity that occurred irregularly after the flexor TA burst, and sometimes overlaps with it when the rats were in the horizontal position, to sustained activity throughout the period between the bursts of activity in TA (stance phase), with no co-active periods. The relationships between the step cycle durations and burst durations for the left and right TA and Sol muscles is illustrated in Fig. 2C. In the horizontal position (Fig. 2C, upper panel) the extensor EMG did not systematically increase in duration as the step cycle duration increased, whereas the relationship between extensor burst duration and step cycle duration became more normal (see Fig. 1C) when the same animals were placed in the upright posture (Fig. 2C, lower panel). The bar graph in Fig. 2F shows that the slopes of this relationship differed significantly between the horizontal and upright posture trials. Moreover, interlimb and intralimb coordination improved significantly in the upright posture (Fig. 2H). Polar plots illustrate the interlimb (Fig. 2D) and intralimb (Fig. 2E) coordination changes that occurred when the animals were placed in the upright posture compared to the horizontal posture. The bar graph in Fig. 2H shows correlation coefficients (r) for the left–right TA and for the right Sol–right TA relationships, which were significantly increased in the upright posture.
As shown in Fig. 3A and B the mean cycle duration was significantly prolonged in the upright posture, due to a marked increase in soleus burst duration. Peak amplitude was unchanged in Sol but significantly reduced in TA. Comparable changes occurred in burst area for both muscles. Thus the activation of hindlimb load receptors in the upright posture facilitates and prolongs Sol activity while decreasing TA activity in spinal rats. Taken together, these results demonstrate that the upright posture alone dramatically improves coordination and weight support to restore plantar stepping in chronic spinal animals without any interventions other than tail stimulation.
Removal of cutaneous foot pad afferent input reverses the locomotion-promoting effect of the upright posture
The improved locomotion of spinal rats in the upright posture might be attributable to the influence of load receptors, including cutaneous receptors on the foot pads and proprioceptors in joints, muscles and tendons. Load receptors in muscles of the rat (Timoszyk et al. 2005) and cat (Duysens & Pearson, 1980; Conway et al. 1987; Pearson et al. 1992; Guertin et al. 1995) hindlimb or cutaneous receptors from the plantar surface of the hindpaw in spinal cats (Duysens & Pearson, 1976; Bouyer & Rossignol, 2003a,b) can facilitate locomotion. Denervation of cutaneous nerves of the hindpaw of spinal cats eliminated the recovery of locomotion after spinal cord injury that could not be overcome by extensive training (Bouyer & Rossignol, 2003b). We used two strategies to test the contribution from these afferents to the upright posture: first we tested air-stepping induced by tail stimulation of a rat held in the upright posture with its hindpaws pendent, a procedure that removes force feedback from both proprioceptors and cutaneous receptors on the hindpaw (Fig. 4). In order to target the plantar cutaneous receptors only and maintain the contributions of the proprioceptors, we tested the effects of local anaesthesia of the cutaneous receptors of the foot pads (Fig. 5).
Figure 4. Removal of load receptor inputs during air stepping disorganizes the locomotor rhythm induced by tail pinching.
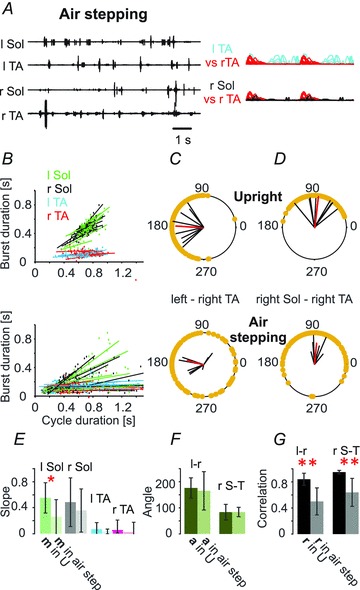
A–D, hindlimb movement during air stepping (n = 6, lower panels) significantly differed from the hindlimb movement obtained during walking in the upright posture as illustrated in the EMG recordings (A) and in the relationship between the soleus EMG burst and cycle duration (B and E) as well as in the inter- and intralimb coordination (upper panels in C and D). F and G, bar graphs showing the mean angle of polar plots (a, ±SD) for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships and the mean of correlation coefficients (r, ±SD) for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships, indicating a highly significant decrease in r for inter- and intralimb coordination in air stepping trials. The remainder of this figure is as described in the legend to Fig. 1.
Figure 5. Anaesthesia of plantar surface of the paw eliminates plantar stepping produced by tail pinching in the upright posture.
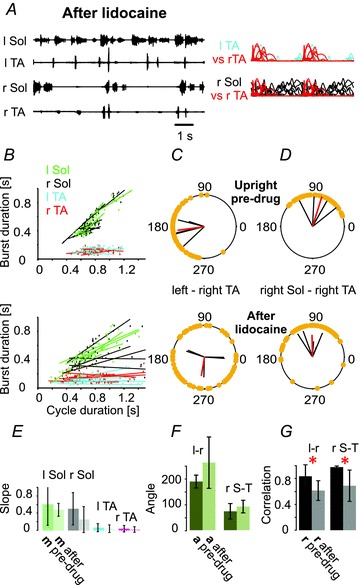
A–D, lidocaine (n = 4) disrupts locomotion in the upright posture (A–C), as shown in EMG recordings (A) and by the alteration in the burst duration–cycle duration relationship (B and E), as well as the disruption of inter- (C) and intra-limb (D) coordination (upper panels, pre-drug; lower panels, after lidocaine application). F and G, bar graphs showing the mean angle of polar plots (a, ±SD) for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships and the mean of correlation coefficients (r, ±SD) for the left–right TA (l-r) and for the right Sol–right TA (r S-T) relationships, indicating a highly significant decrease in r for inter- and intralimb coordination in the upright posture trials after lidocaine application. The remainder of this figure is as described in the Fig. 1 legend.
When the animals in the upright posture were elevated so that the limbs were pendent, the well-developed rhythmic activity observed during foot contact with the treadmill belt in the upright posture disappeared, to be replaced by sustained but irregular rhythmic activity, with very short EMG bursts in soleus (Fig. 4A) that were no longer consistently correlated with the cycle duration (Fig. 4B). Both inter- and intralimb coordination were disrupted (Fig. 4C and D), with correlation coefficients significantly reduced (Fig. 4G). Cycle duration, peak amplitude, burst duration (except TA) and burst area were reduced significantly when compared to hindlimb movement produced by tail pinching in both the horizontal and upright posture (Fig. 3B). These results demonstrate that the improved locomotor capability provided by the upright posture depends upon afferent feedback associated with loading of the hindlimbs.
We tested the importance of the cutaneous receptors in the foot pad by two injections of lidocaine (one medial and one lateral) into each foot pad. Figure 5A shows that removal of cutaneous input from the paws alters plantar walking in the upright posture and produces irregular EMG activity. Inter- and intralimb coordination was decreased significantly (Fig. 5C and D). Paw anaesthesia resulted in increased cycle duration, decreased soleus EMG peak amplitude, and increased TA burst duration in the upright posture; burst area of soleus EMG decreased, while it increased in TA EMG (Fig. 3D). Bilateral lidocaine anaesthesia of the plantar surface of the paws altered plantar stepping in the upright posture, showing that the cutaneous afferents were necessary for the improvement in plantar stepping. Under these conditions, muscle and joint receptors were insufficient to maintain coordinated plantar stepping. We conclude that cutaneous afferents from the plantar surface of the foot are necessary and sufficient for the effects of the upright posture on restoration of locomotion in chronic spinal animals.
Quipazine improves locomotion in the horizontal posture but can impair upright locomotion
Quipazine, a potent means of restoring locomotion in the horizontal posture (Video 3), was often ineffective or even impaired locomotion in the upright posture (Video 4), in contrast to a previous report (Musienko et al. 2011). The differences in the effects imparted by quipazine on locomotor-like activity in the horizontal and upright posture are shown in Figs 6 and 3C and D. The pre-drug trials were the same as those illustrated in Fig. 2A–H. Prior to quipazine administration in the horizontal posture (Fig. 2A, top panel), tail pinching produced limited hindlimb movement, accompanied by irregular bursts of EMG activity (Fig. 2B) characterized by impaired inter- and intralimb coordination (Fig. 2D and E). There were frequent cases of overlap of ipsilateral flexor and extensor muscle activity, and brief bursts of extensor muscle activity that did not span the period between successive flexor bursts, with the two limbs active at different rates such that for one cycle on the right side there were two or three cycles on the left. The soleus muscle activity was brief and was highly variable in relation to the termination of the TA burst (Fig. 2B). After drug administration, however, the soleus bursts began just after termination of the TA burst and continued throughout the stance phase (Fig. 6B). Quipazine (Fig. 6A) improved locomotor-like activity in the horizontal posture so that tail pinch induced regular plantar walking with body weight support and improved hindlimb extension, characterized by doubling of soleus burst duration and burst area and restoration of the normal relationship between soleus muscle burst duration and step cycle duration (Fig. 3C, Fig. 6B, C). The slopes of the regression lines for soleus muscle burst duration vs. step cycle duration were significantly greater after quipazine (Fig. 6F). There was a dramatic improvement in the regularity of the EMG bursts (Fig. 6B) and improved coordination (Fig. 6D and E). The effects of quipazine application on the locomotor pattern were strikingly similar to placing the animal in the upright posture.
Figure 6. Quipazine improves hindlimb movement induced by tail pinching of the rats in the horizontal posture.
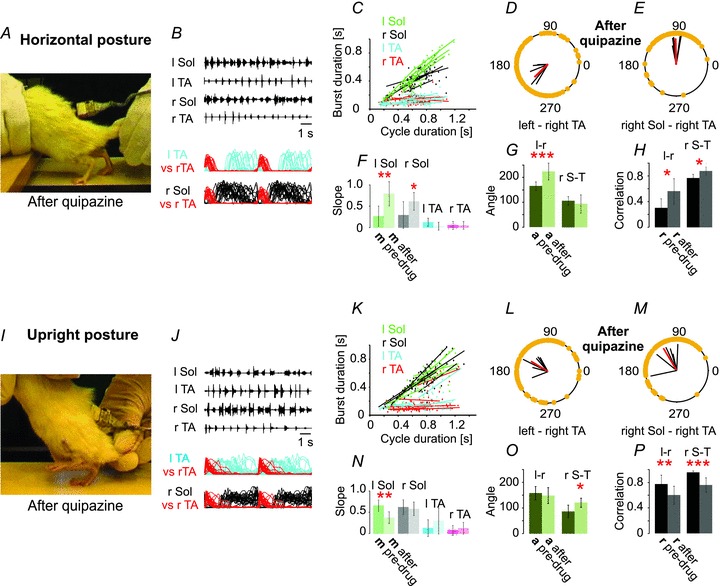
The individual frame in A was taken from the rhythmic movements observed with the animal in the horizontal posture after administration of quipazine (0.25 mg kg−1i.p.). The pre-drug condition for this same animal is shown in Fig. 2A–E (upper panels in C, D and E). I–P, quipazine disrupts locomotion in the upright posture. The photograph in I shows a single frame taken from the rhythmic movements after administration of quipazine (0.25 mg kg−1i.p.). The pre-drug situation for this same animal is shown in Fig. 2A–E (lower panels in G, D and E). The remainder of this figure is as described in the Fig. 1 legend.
The upright posture produced sustained bouts of weight-supported plantar walking (Fig. 2A–H) which were disrupted 15–30 min after i.p. injections of quipazine (Fig. 6I–P). This disruption was characterized by prolongation of the swing phase of locomotion (Fig. 6M) accompanied by increased mean TA peak amplitude, mean TA burst duration and mean burst area (Fig. 3D). There was also an increased soleus EMG activity at the end of the stance phase, which partially overlapped with the onset of TA EMG activity (Fig. 6J), and an increase in soleus peak amplitude (Fig. 3D). In the left soleus muscle the slope was significantly reduced by quipazine, while the slopes of the burst duration–step cycle duration plots for the other muscles were unchanged (Fig. 6N). There was a significant degradation of both interlimb and intralimb coordination after quipazine (Fig. 6P).
A dramatic effect of quipazine when applied to animals in the upright posture was the appearance of a much more abrupt transition from swing to stance than under any other condition (Video 4; Fig. 6J). The soleus EMG, often at its highest amplitude at the end of stance under these conditions, terminated abruptly, simultaneous with or just after the onset of TA EMG. This might be interpreted as a powerful facilitation of the activity of neurons involved in the switch from stance to swing produced by the combined effects of quipazine and the afferent input provided by the upright posture. The swing phase of the locomotor cycle was exaggerated, giving the locomotion observed under these conditions a machine-like appearance (compare Videos 2 and 4).
8-OH-DPAT improves horizontal but not upright locomotion
Enhancement of locomotor recovery in spinal rodents after 8-OH-DPAT (Antri et al. 2003; Landry et al. 2006) alone or combined treatment with quipazine (Antri et al. 2005) has been previously described. 8-OH-DPAT has recently been used in combination with quipazine, locomotor training and epidural stimulation to produce weight-supported stepping in chronic spinal rats (Courtine et al. 2009). We asked if the upright posture might have an impact on the response to the drug, as it did with quipazine. We used i.p. injections of 8-OH-DPAT and compared pre-drug trials with drug-induced locomotor-like activity with the animals held in both the horizontal and the upright posture. Our results confirm that 8-OH-DPAT improves locomotion in the horizontal posture (Fig. 7A–H), but we found that when the animals were in the upright posture there was no significant change produced by the drug (Fig. 7I–P). In the horizontal posture trials, 8-OH-DPAT facilitated well-coordinated plantar walking, with very regular and sustained EMG bursts with characteristic alternating patterns (improved inter- and intralimb coordination). The soleus EMG bursts more reliably commenced at the termination of the TA bursts and were sustained throughout much of the stance phase (Fig. 7B and C). The slope of the regression line derived from soleus burst duration plotted against step cycle duration (Fig. 7C) was significantly greater after 8-OH-DPAT. Peak amplitude was not changed for either the soleus or the TA EMG (Fig. 3C). The polar plots show significantly improved interlimb (Fig. 7D) and intralimb (Fig. 7E) coordination. In the upright posture (Fig. 7I and J), the addition of 8-OH-DPAT did not significantly improve the burst duration–cycle duration relationship (Fig. 7K and N), nor did it alter interlimb or intralimb (Fig. 7L, M, O and P) coordination. Cycle duration, peak amplitude and burst duration were unchanged after drug treatment (Fig. 3D).
Figure 7. 8-OH-DPAT improves hindlimb movement of the rats in the horizontal posture.
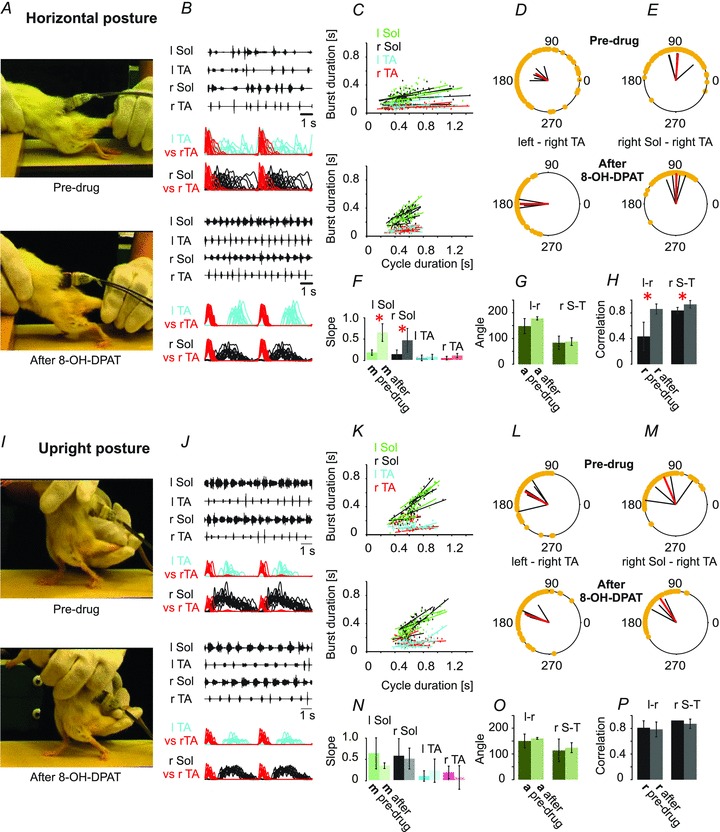
8-OH-DPAT, a 5-HT7/5-HT1A agonist (0.4 mg kg−1i.p.), improves locomotor coordination in chronic spinal rats (n = 5) induced to walk on a treadmill with tail pinching. A and B, the drug improves plantar paw placement (A), and produces a much more regular and well-coordinated EMG pattern in the soleus (Sol) and tibialis anterior (TA) muscles bilaterally (B). In B, rectified and filtered records are overlaid, based upon the onset of activity in r TA, showing the improved left–right and flexor–extensor coordination after the drug. C and F, the relationship between burst duration and step cycle duration (C) is also improved to be more like locomotion in intact animals, with significant changes in the slopes of the extensor muscle records (F). D and E, polar plots showing the relationships between the onset of r TA activity and either the contralateral TA or the ipsilateral extensor (r Sol). H, bar graph demonstrating significant increases in the correlation (r) between the times of onset of activity in both left–right and flexor–extensor comparisons. G, the angles (a) representing the relative timing of the onsets for the two muscles represented in each polar plot were not significantly changed. I–P, 8-OH-DPAT does not significantly alter locomotion induced by tail pinching when the rat is in the upright posture. Further details are as described in Fig. 1 legend.
Combined treatment with quipazine and 8-OH-DPAT
We also determined the effects of the two postures on the response to a combination of quipazine and 8-OH-DPAT at lower doses than used with either drug alone. As shown in Fig. 8A, quipazine alone prolonged the extensor bursts in the horizontal posture; however, the locomotor pattern produced by this low dose was very irregular. Addition of 8-OH-DPAT improved coordination significantly. The need for tail pinching was greatly reduced or eliminated in such cases. There was a significant increase in TA peak amplitude and burst area but not burst duration. Such an increase in TA EMG amplitude was not observed when either drug was administered separately at a higher dose (see Fig. 3C and D).
Figure 8. Combined treatment with low doses of quipazine and 8-OH-DPAT optimizes spinal stepping and mitigates the need for exteroceptive stimulation.
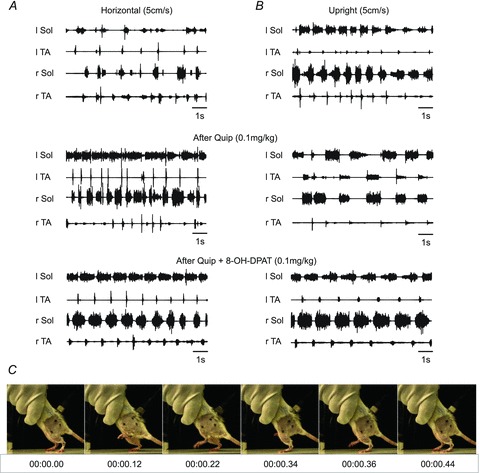
A and B, EMG activity during hindlimb movement induced by tail pinching in the same spinal rat is shown in the horizontal posture (A) and the upright posture (B) pre-drug (upper panel), after quipazine (Quip, middle panel) and after a subsequent dose of 8-OH-DPAT (Quip + 8-OH-DPAT, lower panel). C, consecutive frames of a video recording from a rat walking in the upright posture after quipazine (0.1 mg kg−1, i.p.) and 8-OH-DPAT (0.1 mg kg−1, i.p.) treatment, showing well-coordinated plantar stepping without tail stimulation.
In the upright posture, low-dose quipazine slowed the locomotor rhythm and produced abnormally prolonged TA EMG bursts associated with abrupt transitions between the end of extension and the onset of flexion (Fig. 8B; see also Fig. 6J). After 8-OH-DPAT the EMG bursts approached those of the intact rat (Fig. 7B), and there was no requirement for tail stimulation. As shown in Fig. 7D, coordinated locomotion without tail stimulation occurred 15–30 min after the combined treatment in response to the movement of the treadmill belt alone.
We conclude that the combination of quipazine and 8-OH-DPAT that activates both 5-HT2 and 5-HT1A/7 receptors provides a more robust enhancement of locomotion after spinal cord injury than either drug alone. During such combined treatment, with low doses of the two drugs, recovery was enhanced in both the horizontal posture and the upright posture.
Discussion
As shown previously, limited hindlimb movement is observed when spinal rats are tested in the horizontal posture (Feraboli-Lohnherr et al. 1999; Gimenez y Ribotta et al. 2000; Sławińska et al. 2000), even with exteroceptive stimulation such as tail pinching. To our knowledge this is the first comparison of locomotion in the horizontal and upright posture of intact rats walking voluntarily on the treadmill. Our investigation of locomotor ability of chronic spinal rats demonstrates for the first time that the upright posture alone dramatically improves plantar stepping without any interventions other than tail stimulation. In addition, upright posture effects were shown to be mediated to a large extent by load receptors in the foot pads of the paw. Moreover, we found that a serotonergic 5-HT2 agonist, which facilitates plantar stepping in the horizontal posture, impaired locomotion in the upright posture, while at the same time a 5-HT1A/7 agonist improved plantar stepping in the horizontal posture, but was without effect in the upright posture.
The possible contributors to recovery of plantar stepping in the upright posture are hip joint afferents, muscle proprioceptors, and cutaneous receptors on the plantar surface of the paw. Proprioceptors responding to hip extension were proposed by Sherrington (Sherrington, 1910) to be responsible for the initiation of the swing phase of locomotion. Grillner & Rossignol (1978) showed that hip extension beyond a particular point is necessary for the initiation of the swing phase of locomotion, and hip movements can reset and entrain the locomotor rhythm (Andersson & Grillner, 1981, 1983; Kriellaars et al. 1994), with hip extension leading to flexor bursts. Hip joint capsule denervation was without effect, and low threshold stretch sensitive muscle afferents appear to be responsible for the influence of hip movement to prolong extension and initiate flexion (Kriellaars et al. 1994). In our experiments, the degree of hip extension was even greater when the rats were in the horizontal posture than in the upright posture, yet this was not normally sufficient to induce the swing phase of locomotion. When the rats were held with legs suspended during air-stepping trials, the passive extension of the hip, knee and ankle joints did not induce spontaneous stepping. Other afferents of the hindlimb besides those encoding hip joint angle are likely to be more important to explain the potent effects of the upright posture.
Muscle stretch or vibration of hip flexor muscles during the stance phase of locomotion leads to early onset of the swing phase (Hiebert et al. 1996), implicating muscle spindle afferents in flexor muscles as a possible source of afferent input that could account for the restoration of plantar stepping. Activation of Golgi tendon organs is sufficient (Duysens & Pearson, 1980; Conway et al. 1987; Pearson et al. 1992) for positive force feedback effects to prolong the stance phase of locomotion. Muscle spindle afferents are also effective for controlling the timing of the phases of the locomotor cycle (Guertin et al. 1995; Perreault et al. 1995). The loading of the hindlimb that occurs when the plantar surface of the foot is placed on the treadmill belt would obviously activate Golgi tendon organs in active extensor muscles, providing positive force feedback and consequent weight support during the stance phase of stepping. Muscle length changes also occur, resulting in activation of group I and group II afferents, and these inputs are known to facilitate motoneuron output. Nevertheless, our results with lidocaine anaesthesia of the foot pad show that proprioceptive feedback alone cannot account for the improved locomotion provided by the upright posture.
In contrast, our results with lidocaine anaesthesia suggest that foot pad afferents are the major contributors to the improved plantar walking in the upright posture. This is consistent with findings in chronic spinal cats, where inputs from the plantar surface of the foot are essential for recovery of stepping (Bouyer & Rossignol, 2003b; Rossignol et al. 2006). Stimulation of the plantar surface of the foot in thalamic cats increased extensor activity when applied during the stance phase of locomotion, and prolonged ongoing flexion and shortened the next extensor burst when applied during the swing phase (Duysens & Pearson, 1976). Thus cutaneous inputs from the paw appear to be necessary and sufficient for the production of the improved plantar stepping in chronic spinal rats when in the upright posture.
In our experiments the 5-HT2 agonist quipazine facilitated locomotion in the horizontal posture, but not in the upright posture, unless used in combination with 8-OH-DPAT. Quipazine has been shown to improve locomotion in spinal cats (Barbeau & Rossignol, 1990; Brustein & Rossignol, 1999) and rodents (Feraboli-Lohnherr et al. 1999; Schmidt & Jordan, 2000; Antri et al. 2003; Landry & Guertin, 2004; Fong et al. 2005; Courtine et al. 2009). In a previous paper by Courtine and co-workers using spinal animals placed in an upright posture, the importance of quipazine in the restoration of normal locomotor function was emphasized (Courtine et al. 2009). When quipazine was administered to spinal animals in the upright posture in our study, however, using the same dosage and route of administration as in Courtine et al.'s study, there was a significant degradation of intra- and interlimb coordination. This suggests that the upright posture provides sufficient recruitment of motoneurons and/or CPG interneurons to produce well-coordinated locomotion, and additional 5-HT2 receptor activation produces an apparent unpatterned activation and/or over-excitation of certain of these neurons, leading to disrupted locomotion. In a more recent study (Musienko et al. 2011) it was shown that quipazine facilitates stepping in the upright posture when applied in conjunction with epidural electrical stimulation. In their study, however, body weight support was limited to 25%, a degree of hindlimb loading that may be insufficient to provide the powerful facilitation of plantar stepping observed in our study.
An important observation that emerged from our results was that quipazine prolonged the swing phase of locomotion and increased the burst duration and amplitude of the TA EMG during upright walking (Figs 2 and Fig. 5). This was accompanied by the appearance of a much more abrupt transition from swing to stance than observed in normal locomotion. The soleus EMG terminated abruptly simultaneously with or slightly after the onset of TA EMG activity, which was also abrupt. This can be explained if quipazine specifically facilitates the activity of neurons involved in the switch from stance to swing (Kriellaars et al. 1994; Pearson, 2008).
Both quipazine and 8-OH-DPAT improved horizontal posture locomotor-like activity, with coordinated plantar walking resulting from treatment with either drug. However, interlimb coordination was significantly better after 8-OH-DPAT than after quipazine. This is consistent with our previous suggestion that the activation of 5-HT7 receptors facilitates the rhythm-producing components of the spinal system underlying locomotion, while activation of 5-HT2 receptors directly influences excitability of motoneurons and output elements of the CPG (Jordan & Schmidt, 2002; Liu & Jordan, 2005). This confirms the observation by Antri et al. (2005) that these two drugs in combination facilitate locomotor recovery in spinal rats. Similar observations on the effect of combined treatment (Courtine et al. 2009; Musienko et al. 2011) with these two drugs have been made in animals also subjected to epidural stimulation (equivalent to the tail stimulation used in our experiments) and locomotor training, as well as the combined drug treatment. It is noteworthy that in the experiments reported here, neither training nor any form of exteroceptive stimulation was necessary for plantar stepping after the combined drug treatment.
Our results with the combined treatment and air stepping suggest that appropriate activation of crucial components of the CPG is sufficient to produce the basic locomotor rhythm, and under these conditions can actually interfere with the locomotor activity promoted by afferent feedback. Our results with quipazine suggest that when certain neurons of the CPG are maximally activated by 5-HT receptors, the hindlimb load receptor capacity to promote plantar stepping is disrupted. This shows that controlling the balance between afferent input and activation of receptors on the relevant neurons is crucial. In support of this, we have preliminary data showing that plantar stepping in the upright posture can actually be improved by the 5-HT2 receptor antagonist cyproheptadine.
The results of this study are important for the design of future experiments to study recovery of locomotion after spinal cord injury. First, they demonstrate the powerful effects of cutaneous afferents from the plantar surface of the foot in spinal rats, and they suggest new avenues of research for the discovery of interventions to promote the restoration of locomotion. Locomotor interneurons with powerful input from cutaneous afferents from the plantar surface of the foot and with appropriate 5-HT receptors must exist in the lumbo-sacral spinal cord. Controlling their activity can be expected to provide a powerful means of promoting locomotor recovery after spinal cord injury or disease. Our results also show that the interneurons involved in the transition from swing to stance are activated by both load receptors in the foot and by quipazine, providing important new clues that might aid in their identification. In addition, our results show that the potent effects of afferent input from the paw should be taken into account when the upright posture model is used, and when testing the actions of 5-HT agonists or other similar interventions on the recovery of locomotion. We have found, for example, that in spinal animals with transplants of fetal 5-HT neurons, the upright posture disrupted the improved locomotion provided by the grafted neurons that could be observed in the horizontal posture. This brings into question the utility of the upright posture model for studies on certain types of interventions for providing locomotor recovery after spinal cord injury. Our results also suggest that the potent effects of afferent input from the sole of the foot should be considered when designing interventions for the restoration of locomotion after spinal cord injury in humans.
These experiments also provide a new insight into the evolution of hominid bipedalism, namely that the upright posture can provide a strong stimulus for the activation of the locomotor CPG. The CPG for locomotion appears to have been predisposed to the acquisition of locomotion in the upright posture at an early stage in phylogeny, so that when the afferents of the sole of the foot were recruited during the increased load provided by the bipedal upright posture, the CPG for locomotion would have been strongly facilitated to produce a well-coordinated locomotor pattern. Our study provides the first clear evidence that the evolution of afferent control of the CPG might have contributed to the acquisition of bipedal locomotion.
Acknowledgments
The authors thank Maria Setterbom, Gilles Detillieux, Zhichen Zhou, Anna Cabaj and Krzysztof Miazga for their contributions to these experiments. This work was supported by grants from the Polish Ministry of Science and High Education (N N404 134439) and by the European Union within the European Regional Development Fund POIG 01.01.02-00-109/09-00 to U.S. and grants from the Canadian Institutes of Health Research (CIHR) and the Manitoba Spinal Cord Injury Research Committee (MSCIRC) to L.J.
Glossary
Abbreviations
- CPG
central pattern generator
- l
left
- 8-OH-DPAT
(±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide
- r
right
- Sol
soleus
- TA
tibialis anterior
Author contributions
U.S. and L.M.J. conceived and designed the experiments. U.S. supervised and participated in experiments conducted in Warsaw; L.M.J. supervised and participated in experiments conducted in Winnipeg. Spinal cord injury, implantation of EMG electrodes and locomotor and drug trials were performed in Warsaw by U.S. and H.M., and in Canada by U.S., L.M.J. and Y.D. U.S. and L.M.J. analysed the data and wrote the paper. All authors approved the final version for publication.
References
- Andersson O, Grillner S. Peripheral control of the cat's step cycle. I. Phase dependent effects of ramp-movements of the hip during ‘fictive locomotion’. Acta Physiol Scand. 1981;113:89–101. doi: 10.1111/j.1748-1716.1981.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during ‘fictive locomotion’. Acta Physiol Scand. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol. 2003a;90:3625–3639. doi: 10.1152/jn.00496.2003. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol. 2003b;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. J Neurophysiol. 1999;81:1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioecptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci. 2011;31:9264–9278. doi: 10.1523/JNEUROSCI.5796-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002;40:267–273. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- Dietz V. Evidence for a load receptor contribution to the control of posture and locomotion. Neurosci Biobehav Rev. 1998;22:495–499. doi: 10.1016/s0149-7634(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Barthe J-Y, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res. 1999;55:87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez y Ribotta M, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Davies MR, Graziani V. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J Neurophysiol. 2007;97:2663–2675. doi: 10.1152/jn.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel M, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR-evoked fictive locomotion in the cat. J Physiol. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodualatory control of spinal cord function. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. New York: CRC Press; 2001. pp. 48–87. [Google Scholar]
- Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci Lett. 2008;438:281–285. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Landry ES, Guertin PA. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1053–1060. doi: 10.1016/j.pnpbp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Etlin A, Blivis D. Sensory-induced activation of pattern generators in the absence of supraspinal control. Ann N Y Acad Sci. 2010:1198, 54–62. doi: 10.1111/j.1749-6632.2009.05424.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol. 2009;102:337–348. doi: 10.1152/jn.91239.2008. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Majczyński H, Maleszak K, Cabaj A, Sławińska U. Serotonin-related enhancement of recovery of hind limb motor functions in spinal rats after grafting of embryonic raphe nuclei. J Neurotrauma. 2005;22:590–604. doi: 10.1089/neu.2005.22.590. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Rakerd B. Induction of hindlimb stepping movments in rats spinally transected as adults or as neonates. Brain Res. 1982;240:353–356. doi: 10.1016/0006-8993(82)90235-9. [DOI] [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci. 2011;31:9264–9278. doi: 10.1523/JNEUROSCI.5796-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev. 2008;57:222–227. doi: 10.1016/j.brainresrev.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Ramirez JM, Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res. 1992;90:557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II muscle afferents during fictive locomotion. J Physiol. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension reflex, and reflex stepping and standing. J Physiol. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska U, Majczyński H, Djavadian R. Recovery of hindlimb motor functions after spinal cord transection is enhanced by grafts of the embryonic raphe nuclei. Exp Brain Res. 2000;132:27–38. doi: 10.1007/s002219900323. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol. 2002;88:3108–3117. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]


