Abstract
The parafacial respiratory group (pFRG) in the rostral ventrolateral medulla of the newborn rat is predominantly composed of pre-inspiratory (Pre-I) neurons and is involved in respiratory rhythm generation. The subgroup located close to the ventral surface (at least partially overlapping the retrotrapezoid nucleus, RTN) expresses the Phox2b transcription factor and responds to hypercapnic stimulation with strong depolarization, which suggests it has a role in central chemoreception. Although a CO2 response of pFRG/RTN neurons has been confirmed in the presence of tetrodotoxin (TTX), it is unknown whether the depolarization involved in this response is induced by a direct postsynaptic response of pFRG/RTN neurons or by any presynaptic components mediated by Ca2+-dependent mechanisms. In this study, we examined the effects of ATP or substance P receptor antagonists on hypercapnic responses of rostral pFRG/RTN neurons. We tested effects of Cd2+ and low Ca2+–high Mg2+ in the presence of TTX. The experiments were performed in in vitro brainstem–spinal cord preparations from newborn rats in which Pre-I neurons reflect the discharge pattern of the pFRG. We found that ATP receptor and substance P receptor antagonists do not block membrane potential responses to hypercapnic stimulation (2%→ 8%) of pFRG/RTN neurons in the rostral parafacial region. Moreover, rostral pFRG/RTN neurons were depolarized by hypercapnia under conditions where the contribution of presynaptic components was inhibited in the presence of TTX and Cd2+ or in a low Ca2+–high Mg2+ solution containing TTX and Cd2+. All cases (except some cases in a low Ca2+–high Mg2+ solution) of membrane depolarization by hypercapnic stimulation were accompanied with an increase in input resistance. These neurons were predominantly Phox2b immunoreactive. Our findings suggest that the response of pFRG/RTN neurons to hypercapnia is induced by direct action on the postsynaptic membrane via closing of K+ channels.
Key points
The central chemoreceptors for respiratory control in the medulla sense changes in CO2 concentration and regulate respiratory activity.
Neurons that express a transcription factor, Phox2b, in the parafacial region of the rostral and ventrolateral medulla are excited by hypercapnic stimulation and are proposed to play an important role in central chemoreception.
In this study, we show evidence that Phox2b-expressing parafacial neurons in neonatal rats were sensitive to hypercapnia via direct action on the postsynaptic membrane without contribution of putative presynaptic or other calcium-dependent components.
Since these parafacial neurons are also a part of the respiratory rhythm generator in neonates, they are essential for postnatal survival, which is probably due to their contribution to central chemoreception as well as respiratory rhythm generation.
Introduction
The parafacial respiratory group (pFRG) in the rostral ventrolateral medulla of the neonatal rat is predominantly composed of pre-inspiratory (Pre-I) neurons and is involved in respiratory rhythm generation (Onimaru & Homma, 2003). Of particular interest is the subgroup located close to the ventral surface, which at least partially overlaps the retrotrapezoid nucleus (RTN) in adult rats (Stornetta et al. 2006). This subgroup of the pFRG as well as adult RTN neurons (hereinafter referred to as pFRG/RTN neurons) express a transcription factor, Phox2b, and respond to high CO2 stimulation with strong depolarization, which is suggestive of a role in central chemoreception (Mulkey et al. 2004; Stornetta et al. 2006; Onimaru et al. 2008, 2009). Although the CO2 response of pFRG/RTN neurons has previously been confirmed in the presence of tetrodotoxin (TTX), it remains to be elucidated whether the depolarization was induced by a direct postsynaptic response of pFRG/RTN neurons or by any presynaptic components mediated by a Ca2+-dependent mechanism. Gourine et al. (2010) showed that a decreased pH in the brainstem chemoreceptor area induced an increase in intracellular Ca2+ concentration and release of ATP. Thus, ATP is one of the important presynaptic components that affect chemosensitivity of neurons in the ventral medulla (Guyenet et al. 2010a). It was reported that the response of Phox2b-positive neurons in the RTN induced by lowering the pH was inhibited by application of ATP receptor blockers (Gourine et al. 2010). Alternatively, Mulkey et al. (2004) reported that pH sensitivity of RTN neurons was not affected by ATP receptor blockers. Another candidate presynaptic component of chemosensitivity is substance P, because neurokinin-1 (NK1) receptor-expressing neurons in the ventral medulla are essential for normal central chemoreception in the conscious rat (Nattie & Li, 2006). Phox2b-positive cells in the RTN express NK1 receptors (Onimaru et al. 2008; Takakura et al. 2008), and substance P was still able to activate the chemosensitive RTN neurons in the presence of blockers of excitatory and inhibitory transmitters (Mulkey et al. 2007). Moreover, substance P can induce a depolarizing response in the pFRG/RTN neurons similar to the response to hypercapnic stimulation (Onimaru et al. 2009). Thus, hypercapnic stimulation may cause the effect by releasing substance P from presynaptic sites by a calcium-dependent mechanism. Therefore, in the present study, we examined the effects of ATP receptor antagonists (MRS2179 and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (Gourine et al. 2010), and substance P antagonists (L-703606 and spantide) on the CO2/H+ response. Furthermore, we tested the effects of Cd2+ and low Ca2+–high Mg2+ in the presence of TTX.
Methods
Experimental protocols were approved by the Animal Research Committee of Showa University, which operates in accordance with Law No. 105 of the Japanese Government for the care and use of laboratory animals and conformed to the principles of UK regulations, as described in Drummond (2009). All efforts were made to minimize the number of animals used and their suffering.
Preparations
Experiments were performed with brainstem–spinal cord preparations from 0 to 4-day-old Wistar rats. Newborn rats were deeply anaesthetized with ether. The brainstem and spinal cord were isolated and superfused at a rate of 3.0 ml min−1 with the following artificial cerebrospinal fluid (ACSF) (in mm): 124 NaCl, 5.0 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3 and 30 glucose, equilibrated with 95% O2 and 5% CO2, pH 7.4, at 26–27°C. The preparations were cut transversely at a level just rostral to the anterior inferior cerebellar artery (AICA) with a custom-made vibratome (Fig. 1). This cut was equivalent to a position approximately 0.5 mm rostral to the caudal end of the facial nucleus that corresponds to the level of the Xth cranial nerve root (Ruangkittisakul et al. 2006; Ballanyi et al. 2009).
Figure 1. Preparation and positioning of a transverse section.
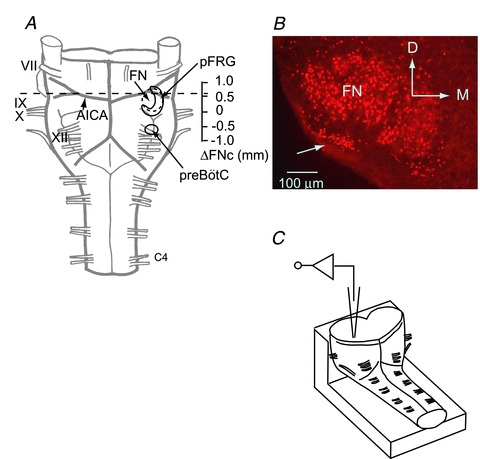
A, ventral view of a brainstem–spinal cord preparation from a neonatal rat. The preparation was cut at the level of the dotted line. B, rostral cut surface view of Phox2b-immunoreactive (-ir) cells at a level 0.5 mm rostral to the caudal end of the facial nucleus (ΔFNc). Phox2b-ir cells (red) are located in the ventral parafacial region (arrow) and in the facial nucleus. C, schematic representation of the preparation in the recording chamber. The preparation was fixed onto a rubber block by pins with the cut surface facing up for whole-cell recordings of the parafacial region via the cut surface of the cross section. AICA, the anterior inferior cerebellar artery; FN, facial nucleus; pFRG, parafacial respiratory group; preBötC, preBötzinger complex; VII–XII, cranial nerves; C4, the fourth cervical ventral root; D, dorsal; M, medial.
Electrophysiology
Inspiratory activity correlating with phrenic nerve activity was monitored from the fourth cervical ventral root (C4) (Suzue, 1984). Membrane potentials and input resistances of neurons in the parafacial region of the rostral medulla were recorded by a blind whole-cell patch-clamp method (Onimaru & Homma, 1992) (Fig. 1). Respiratory-related bursting neurons in the rostral pFRG were approached from the rostral cut surface by monitoring extracellular action potential discharges. Most of the bursting neurons in this region were characterized by pre- and post-inspiratory discharges of variable length, and thus they were considered to be in the same category as Pre-I neurons in previous reports (e.g. Onimaru & Homma, 1992). They received heterogeneous synaptic inputs during the inspiratory phase and more than 70% of them in this region showed only weak inspiratory inhibition (Onimaru et al. 2008; Onimaru & Dutschmann, 2012). The electrodes (inner tip diameter, 1.2–2.0 μm; resistance, 4–8 MΩ) were filled with the following pipette solution (in mm): 130 potassium gluconate, 10 EGTA, 10 Hepes, 2 Na2-ATP, 1 CaCl2 and 1 MgCl2, with pH 7.2–7.3 adjusted with KOH. Membrane potentials were recorded with a single-electrode voltage-clamp amplifier (CEZ-3100; Nihon Kohden Corp., Tokyo, Japan) after compensation for series resistance (20–50 MΩ) and capacitance. After establishment of the whole-cell recordings, the standard solution (5% CO2, pH 7.4) was replaced by an ACSF containing 0.5 μm TTX equilibrated with 2% CO2 (pH 7.8). Effects of antagonists were examined by the two following steps. (1) Control hypercapnic stimulation: After a 15 min incubation with the 2% CO2 solution, the superfusate was replaced by a hypercapnic acidic ACSF equilibrated with 8% CO2 (pH 7.2). After a 5–6 min test of membrane potential responses in the 8% CO2 solution, the superfusate was returned to a 2% CO2 solution (Onimaru et al. 2008). (2) Effects of antagonists: The same procedure as the above control CO2 test was performed in the presence of antagonists, i.e. 15 min pre-treatment in 2% CO2+ antagonists. To examine effects of blockade of Ca2+ channel activation on hypercapnic responses, 0.5 mm Cd2+ or 0.5 mm Cd2+ plus low Ca2+ (0.2 mm) and high Mg2+ (5 mm) in the presence of 0.5 μm TTX (2% CO2) were superfused for more than 15 min before hypercapnic stimulation (8% CO2). This test was performed without the control CO2 test in normal ACSF, because we found it was difficult to maintain whole-cell recordings for long periods in these conditions due to unknown reasons. In all experiments using TTX, a square current pulse (500 ms, 0.1 Hz, 20–40 pA) was applied to monitor changes in input resistance. Values are presented as mean ± SD. Significance values (P < 0.05) were determined with a Student's t test.
For histological analysis of the recorded cells, the electrode tips were filled with 0.2% Lucifer yellow (lithium salt, Sigma-Aldrich, Japan). After the experiments, preparations were fixed for 2–3 h at 4°C in 4% paraformaldehyde in 0.1 m phosphate buffer solution (PBS), transferred into 18% sucrose–PBS (overnight) and cut into 50-μm-thick transverse sections for immunofluorescent detection.
Immunofluorescence
The primary antibodies used for immunofluorescence were rabbit anti-Lucifer yellow (1:400 dilution, Molecular Probes/Invitrogen, Carlsbad, CA, USA) and guinea pig anti-Phox2b (1:1000 dilution) as described previously (Onimaru et al. 2008). The secondary antibodies for fluorescence staining (1:1000 dilutions) were Alexa Fluor 488 anti-rabbit IgG or Alexa Fluor 546 anti-rabbit IgG, and Alexa Fluor 633 anti-guinea pig IgG or Alexa Fluor 546 anti-guinea pig IgG (Molecular Probes/Invitrogen). Images of the immunofluorescent samples were obtained with 10× or 20× objectives on an Olympus FV1000 confocal microscope (Olympus Optical, Tokyo, Japan) or Olympus BX60 conventional fluorescence microscope (Olympus Optical).
Results
Effects of ATP receptor antagonists on the CO2 response of rostral pFRG/RTN neurons
We examined two kinds of ATP receptor antagonists (MRS2179 and PPADS) on the CO2 response of rostral pFRG/RTN neurons. In response to hypercapnia (2%→ 8% CO2), the rostral pFRG/RTN neurons (n = 5) were depolarized by 10.2 ± 4.9 mV in control solution (0.5 μm TTX) and by 10.4 ± 5.5 mV in TTX plus an ATP receptor antagonist, 20 μm MRS2179 (Fig. 2). The input resistance during hypercapnia increased to 154% in control TTX solution and 140% in TTX plus MRS2179 (Table 1). In another set of experiments, similarly, hypercapnic stimulation depolarized the rostral pFRG/RTN neurons (n = 5) by 9.5 ± 4.4 mV in 0.5 μm TTX (control) and by 8.6 ± 5.2 mV in TTX plus an ATP receptor antagonist, 0.1 mm PPADS, (Fig. 3). The input resistance increased to 137% in control and 140% with PPADS (Table 1). Thus, the CO2 responses of rostral pFRG/RTN neurons were preserved in the presence of ATP receptor antagonists (MRS2179 or PPADS). Eight neurons out of the ten examined were successfully stained by Lucifer yellow and were confirmed to be Phox2b immunoreactive.
Figure 2. Effects of an ATP receptor antagonist (MRS2179) on the CO2/H+ response of a rostral pFRG/RTN neuron.
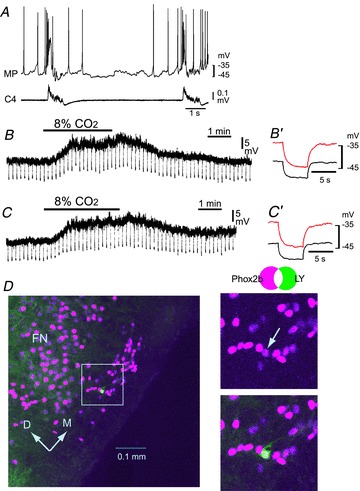
A, burst pattern in control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX. Negative deflections of the baseline membrane potential are proportional to input resistance. B′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that application of 8% CO2 induced membrane depolarization and an increase in input resistance. C, membrane potential responses to hypercapnic stimulation in the presence of TTX plus 20 μm MRS2179. C′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that the depolarizing response to 8% CO2 was preserved. D, location of the recorded neuron stained by Lucifer yellow (LY, green). This neuron is Phox2b positive (magenta). Right panels, higher magnification of the highlighted square in the left image. FN, facial nucleus; D, dorsal; M, medial.
Table 1.
Effects of receptor antagonists on hypercapnic responses of rostral pFRG/RTN neurons
| Control | 20 μm MRS2179 (n = 5) | |||
|---|---|---|---|---|
| 2% | 8% | 2% | 8% | |
| Vm (mV) | −43.2 ± 1.3 | −33.6 ± 3.6** | −41.6 ± 1.1 | −33.0 ± 4.5* |
| Rm (MΩ) | 324 ± 48.7 | 444 ± 84.7* | 350 ± 70.7 | 490 ± 124* |
| Control | 0.1 mm PPADS (n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2% | 8% | 2% | 8% | |||||
| Vm (mV) | −48.6 ± 3.9 | −38.4 ± 4.2** | −48.2 ± 3.8 | −37.8 ± 5.9* | ||||
| Rm (MΩ) | 466 ± 321 | 719 ± 399* | 458 ± 252 | 642 ± 328* | ||||
| Control | 0.4 μm spantide (n = 4) | |||
|---|---|---|---|---|
| 2% | 8% | 2% | 8% | |
| Vm (mV) | −46.3 ± 3.0 | −32.8 ± 7.2* | −45.0 ± 3.9 | −32.0 ± 5.7* |
| Rm (MΩ) | 412 ± 131 | 775 ± 126* | 295 ± 90.0 | 505 ± 204* |
Figure 3. Effects of an ATP receptor antagonist (PPADS) on the CO2/H+ response of a rostral pFRG/RTN neuron.
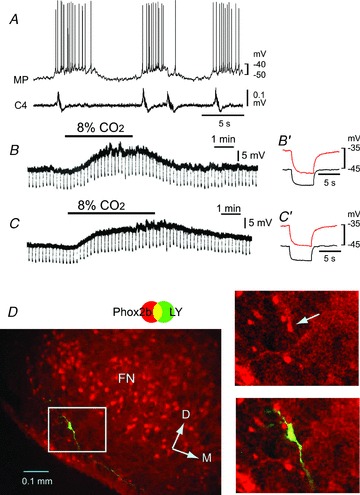
A, burst pattern in control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX. Negative deflections of the baseline membrane potential are proportional to input resistance. B′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that application of 8% CO2 induced membrane depolarization and an increase in input resistance. C, membrane potential responses to hypercapnic stimulation in the presence of TTX plus 0.1 mm PPADS. C′, faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that the depolarizing response to 8% CO2 was preserved. D, location of the recorded neuron stained by Lucifer yellow (LY, green). This neuron is Phox2b positive (red). Right panels, higher magnification of the highlighted square in the left image. FN, facial nucleus; D, dorsal; M, medial.
Effects of NK1 receptor antagonists on the CO2 response of rostral pFRG/RTN neurons
Substance P (50 nm), in the presence of TTX, induced membrane depolarization of rostral pFRG/RTN neurons (15 ± 1.2 mV, n = 4, Fig. 4C), which was accompanied by an increase in the input resistance to 153%. We examined effects of substance P antagonists on the hypercapnic response of pFRG/RTN neurons. The depolarizing response to hypercapnic stimulation (2%→ 8% CO2) of the rostral pFRG/RTN neurons (13.5 ± 7.3 mV with 188% increase in input resistance, n = 4, in TTX) was preserved (13.0 ± 4.9 mV with 167% increase in input resistance) after the effect of substance P was inhibited by treatment with 0.4 μm spantide in TTX (Fig. 4D, Table 1). Similarly, the hypercapnic response of the rostral pFRG/RTN neurons was also preserved after treatment with 2 μm L-703606 (depolarization of 7.7 ± 2.1 mV; input resistance increase to 144%; n = 3) (Table 1). Six out of seven neurons were stained successfully by Lucifer yellow and confirmed as Phox2b immunoreactive, whereas one neuron was not Phox2b immunoreactive.
Figure 4. Effects of the substance P receptor antagonist (spantide) on the CO2/H+ response of a rostral pFRG/RTN neuron.
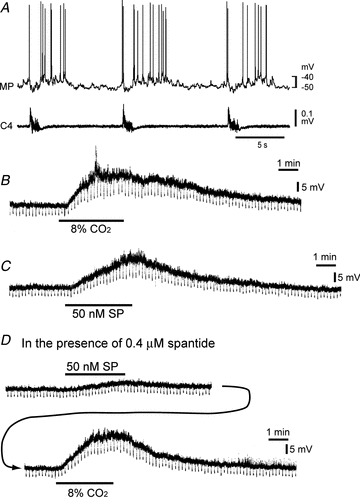
A, burst pattern in control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX. Negative deflections of the baseline membrane potential are proportional to input resistance. Note that application of 8% CO2 induced membrane depolarization and an increase in input resistance. C, membrane potential responses to bath application of 50 nm substance P (SP) in the presence of TTX. Note that the membrane potential response is similar to hypercapnia. D, in the presence of 0.4 μm spantide (20 min), membrane depolarization by 50 nm substance P was significantly reduced, but hypercapnia (2% CO2→ 8% CO2) still induced membrane depolarization.
CO2 response of rostral pFRG/RTN neurons in the presence of TTX and Cd2+
In the presence of 0.5 μm TTX and 0.1 mm Cd2+, hypercapnic stimulation (2%→ 8% CO2) depolarized the rostral pFRG/RTN neurons (n = 9) by 8.8 ± 2.7 mV with a 146% increase in the input resistance (377 ± 115 MΩ in 2% CO2 and 551 ± 186 MΩ in 8% CO2,P < 0.01) (Fig. 5). All but one of these neurons were Phox2b immunoreactive. Furthermore, we examined the CO2 response of eight rostral pFRG/RTN neurons after treatment with low Ca2+ and high Mg2+ in the presence of TTX and Cd2+ (Fig. 6). These neurons were depolarized by 7.8 ± 3.7 mV in response to hypercapnic stimulation (2%→ 8% CO2). The input resistance was increased in four neurons (to 127% of control) but not in another four neurons (93% of control) (Fig. 7). The average change in the input resistance in eight neurons was not significant (356 ± 105 MΩ in 2% CO2versus 389 ± 146 MΩ in 8% CO2). These neurons were confirmed to be Phox2b immunoreactive.
Figure 5. Effects of Cd2+ on the CO2/H+ response of a rostral pFRG/RTN neuron.
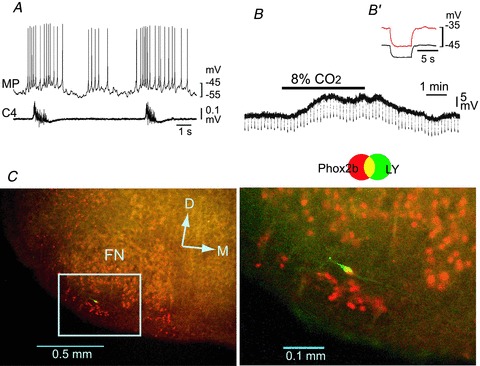
A, burst pattern in control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX + 0.1 mm Cd2+. Negative deflections of the baseline membrane potential are proportional to input resistance. B′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that application of 8% CO2 induced membrane depolarization and an increase in input resistance. C, location of a recorded neuron stained by Lucifer yellow (LY, green). This neuron is Phox2b positive (red). Right panel, higher magnification of the highlighted square in the left image. FN, facial nucleus; D, dorsal; M, medial.
Figure 6. Effects of low Ca2+–high Mg2++ Cd2+ on the CO2/H+ response of a rostral pFRG/RTN neuron.
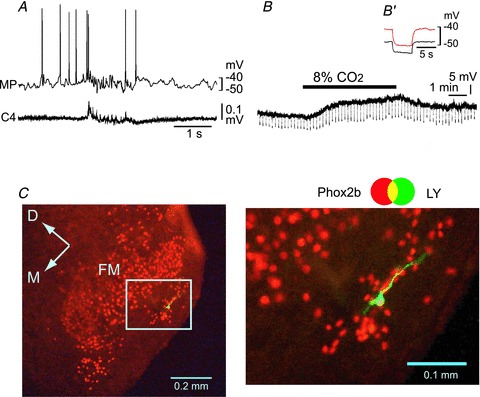
A, burst pattern in the control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX + 0.1 mm Cd2+ in low Ca2+–high Mg2+ solution. Negative deflections of the baseline membrane potential are proportional to input resistance. B′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that application of 8% CO2 induced membrane depolarization and an increase in input resistance. C, location of the recorded neuron stained by Lucifer yellow (LY, green). This neuron is Phox2b positive (red). Right panel, higher magnification of the highlighted square in the left image. FN, facial nucleus; D, dorsal; M, medial.
Figure 7. Effects of low Ca2+–high Mg2++ Cd2+ on the CO2/H+ response of a rostral pFRG/RTN neuron that did not show an increase in the input resistance.
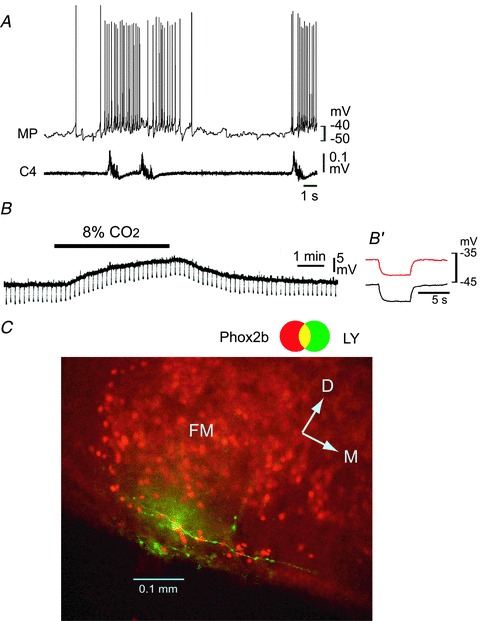
A, burst pattern in control solution. MP, membrane potential trace; C4, fourth cervical ventral root activity. B, membrane potential response to hypercapnic stimulation (2% CO2→ 8% CO2) in the presence of 0.5 μm TTX + 0.1 mm Cd2+ in low Ca2+–high Mg2+ solution. Negative deflections of the baseline membrane potential are proportional to input resistance. B′, a faster sweep representation (average of 5 traces) of the membrane potential response to application of 20 pA hyperpolarizing square current pulses in 2% CO2 (black trace) and 8% CO2 (red trace). Note that application of 8% CO2 induced membrane depolarization but the input resistance was not increased. C, location of the recorded neuron stained by Lucifer yellow (LY, green). This neuron is Phox2b positive (red). FN, facial nucleus; D, dorsal; M, medial.
Discussion
ATP receptor and substance P receptor antagonists could not block the CO2 response of bursting neurons in the rostral pFRG/RTN. Moreover, rostral pFRG/RTN neurons were depolarized by hypercapnia under conditions whereby the contribution of presynaptic components were inhibited in the presence of TTX and Cd2+ or in low Ca2+–high Mg2+ solution containing TTX and Cd2+. Most of these neurons were Phox2b immunoreactive. The main limitations of the present study were: (1) the use of neonates (P0–P4) in in vitro preparations in which glial components in the central chemoreceptor region of the medulla may be immature in comparison to adults; and (2) the fact that the temperature of the superfusate was low (26°C) (Guyenet et al. 2010b).
A recent study by Gourine et al. (2010) showed strong evidence that ATP released from astrocytes in the chemoreceptor area of the ventral medulla acts as an essential factor for central chemosensory transduction. It was shown that the pH response of Phox2b-positive neurons in the RTN of organotypic cultured slices from rat pups (8–10 days old) was blocked by ATP receptor antagonists (MRS2179 and PPADS). Moreover, Wenker et al. (2010) suggested that astrocytes in the RTN sense CO2/H+ in part by inhibition of a Kir4.1–Kir5.1-like current in slice preparations from 7- to 12-day-old rats. They also found that ATP receptor antagonists (PPADS and suramin) decreased CO2 sensitivity of RTN neurons and suggested that RTN astrocytes may provide an excitatory purinergic drive to pH-sensitive RTN neurons. In contrast, Mulkey et al. (2004) previously reported that the pH sensitivity of the RTN neurons in slices from 7- to 12-day-old rats was not blocked by PPADS. Regarding this apparent discrepancy, Wenker et al. (2010) suggested that effects of ATP receptor antagonists in the studies above depend on the buffers used (i.e. less effective in Hepes buffer than bicarbonate buffer). Although we used bicarbonate-buffered media and preparations from 0- to 4-day-old rats, our results demonstrated that ATP receptor antagonists could not block membrane depolarization of pFRG/RTN neurons induced by hypercapnia. The discrepancy may be due to differences among recording sites and experimental conditions as well as the different ages of animals used.
Phox2b-positive cells in the RTN express NK1 receptors and substance P is thought to have a significant role in central chemoreception (Nattie & Li, 2006; Takakura et al. 2008). It has been suggested that substance P is released by mainly serotonergic neurons in the vicinity of the RTN neurons (Mulkey et al. 2007). In slice preparations from 7- to 10-day-old rats, substance P induced an increase in the discharge rate of RTN chemosensitive neurons in the presence of antagonists of glutamate, glycine, GABA and purinergic receptors, suggesting that a postsynaptic mechanism is primarily responsible for activation of RTN neurons by this neuropeptide (Mulkey et al. 2007). Indeed, similar to hypercapnic stimulation, substance P induced a depolarizing response in the pFRG/RTN neurons accompanied by an increase in input resistance (Fig. 4). However, the membrane depolarization induced by hypercapnia was still preserved after the depolarizing response by substance P was depressed. Thus, the NK1 receptor is not necessary for the CO2 response of pFRG/RTN neurons.
The depolarization was accompanied by an increase in input resistance of parafacial neurons, consistent with an involvement of potassium channels in response to hypercapnia as suggested by previous studies (Kawai et al. 2006; Guyenet et al. 2008). However, it is unknown which type of potassium channels are involved in the hypercapnic-induced depolarization (Putnam et al. 2004; Jiang et al. 2005; Guyenet et al. 2010b). In low Ca2+–high Mg2+ (+Cd2+) solution, membrane depolarization was not accompanied by an increase in the input resistance in half of the tested neurons despite the membrane depolarization. Kawai et al. (2006) reported that 20% of tested neurons showed membrane depolarization that was resistant to K+ channel blockers and was not associated with an increase in the input resistance in response to hypercapnic stimulation (Kawai et al. 2006). Thus, it is possible that mechanisms other than ionic channels are involved in the hypercapnic-induced membrane depolarization in some neurons (Putnam, 2010). It is not clear whether the neurons that showed loss of the hypercapnia-induced conductance change in the present study were equivalent to examples of 20% neurons reported by Kawai (2006) or whether unknown effects were exerted on neurons by treatment with low Ca2+–high Mg2+ (+Cd2+). Although treatment with low Ca2+–high Mg2+ may diminish an involvement of K+ channels in membrane depolarization and unmask ionic channel-independent mechanisms, future studies are required to clarify the detailed mechanism.
In conclusion, hypercapnic stimulation depolarized the rostral pFRG/RTN neurons expressing Phox2b in neonatal rats, even after blockade of ATP or NK1 receptors as well as blockade of Ca2+ channels in the presence of TTX. Thus, this group of neurons was postsynaptically CO2 sensitive without a contribution from calcium-dependent presynaptic or glial components. The CO2 sensitivity is linked mainly to closure of the potassium channels of the postsynaptic membrane and it is important for future studies to clarify which type of potassium channels are involved in this response.
Acknowledgments
This work was supported by Grants-in Aid for Scientific Research (KAKENHI: 19500277, 22500296).
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AICA
anterior inferior cerebellar artery
- C4
fourth cervical ventral root
- NK1
neurokinin-1
- PBS
phosphate buffer solution
- pFRG
parafacial respiratory group
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- Pre-I
pre-inspiratory
- RTN
retrotrapezoid nucleus
- TTX
tetrodotoxin
Author contributions
H.O. designed and performed the in vitro electrophysiological recordings, immunohistochemistry, analysed the data and wrote the manuscript. K.I. and K.K. contributed to immunohistochemistry and edited the manuscript. All authors approved the final version.
References
- Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBotC) network bursting in newborn rat brainstems. Pflugers Arch. 2009;458:571–587. doi: 10.1007/s00424-009-0645-3. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010a;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010b;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Dutschmann M. Calcium imaging of neuronal activity in the most rostral parafacial respiratory group of the newborn rat. J Physiol Sci. 2012;62:71–77. doi: 10.1007/s12576-011-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Arch. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Putnam RW. CO2 chemoreception in cardiorespiratory control. J Appl Physiol. 2010;108:1796–1802. doi: 10.1152/japplphysiol.01169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1–Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


