Abstract
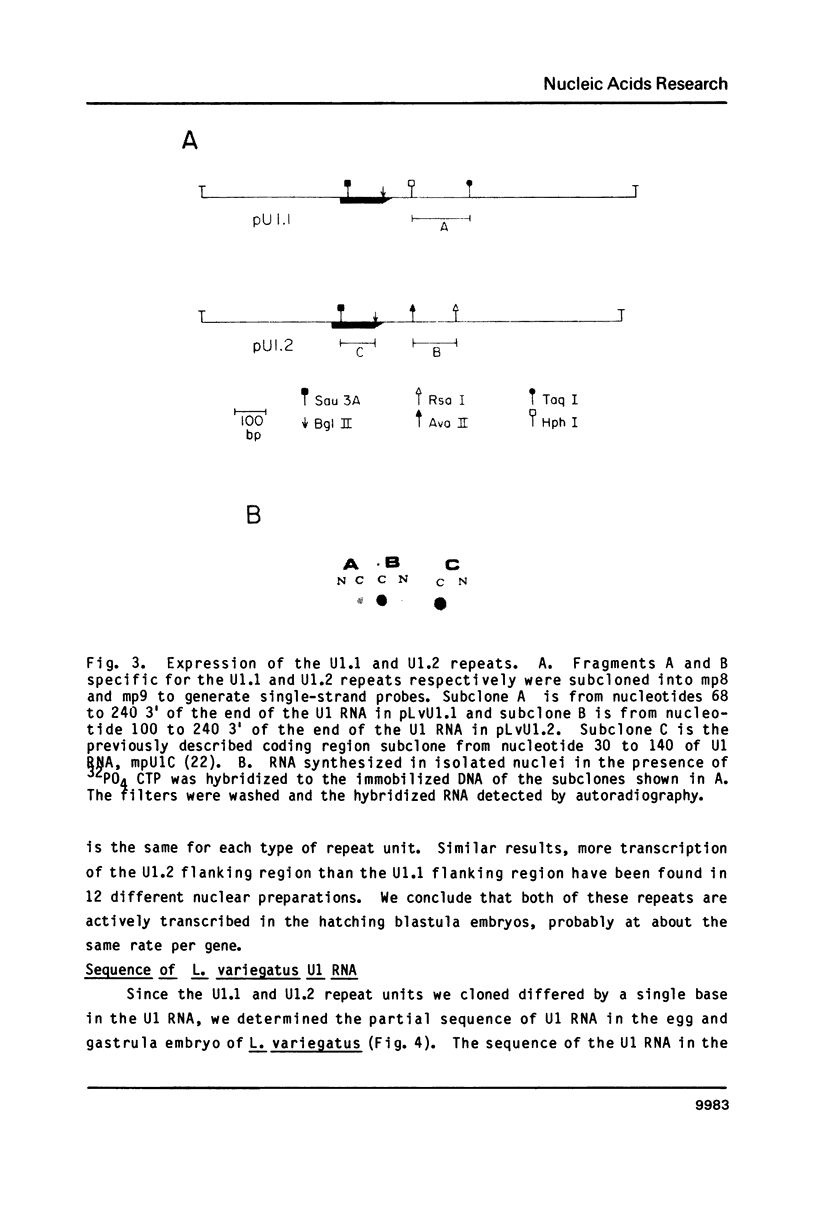
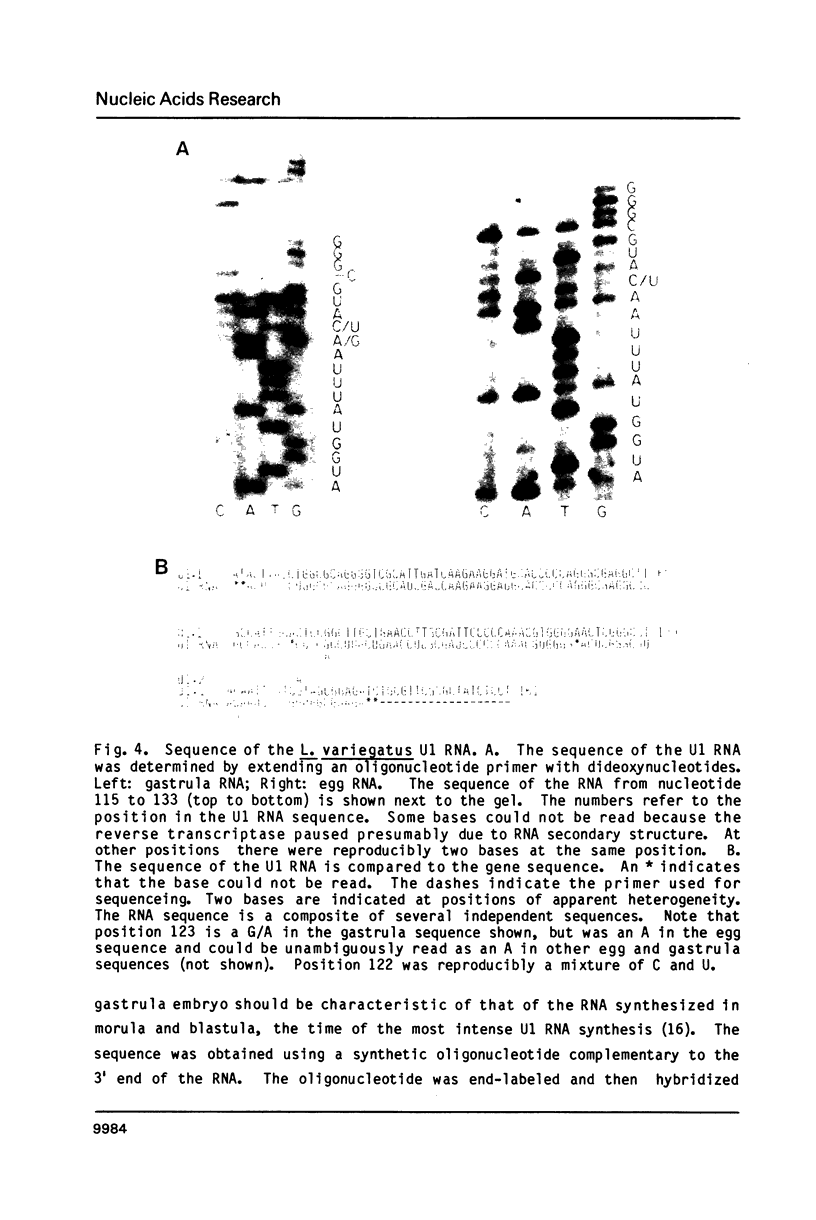
There are two tandemly repeated sets of U1 RNA genes in the sea urchin L. variegatus. Each of these genes is present in a 1.4 kb repeat defined by a HindIII site about 450 bases 5' to the gene. The sequences of a member of both repeating units (U1.1 and U1.2) has been determined. The repeats are nearly identical for 550 nucleotides 5' to the gene but show great divergence starting 30 nucleotides 3' to the gene, just after the CAAAGAAAGAAAA sequence thought to be required for 3' end formation. The other boundary between the conserved and non-conserved sequences is a polypyrimidine sequence (on the strand which codes for U1 RNA). Both of these repeats are expressed in blastula stage embryos, as judged by transcription of unique sequences 3' to the gene in nuclei isolated from blastula stage embryos. At least some of the two types of repeats are interspersed, since representatives of both repeat types on a single gamma phage isolated from a gene library. The sequence of the U1 RNA in L. variegatus eggs and embryos corresponds to the sequence of the U1 repeat.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L. B., Manser T., Weiner A. M. Human U1 small nuclear RNA genes: extensive conservation of flanking sequences suggests cycles of gene amplification and transposition. Mol Cell Biol. 1985 Sep;5(9):2159–2171. doi: 10.1128/mcb.5.9.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Morris G. F., Chodchoy N., Sprecher C., Marzluff W. F. Structure of the sea urchin U1 RNA repeat. Nucleic Acids Res. 1985 Jan 25;13(2):537–556. doi: 10.1093/nar/13.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. H., Kedes L. H. Nonallelic histone gene clusters of individual sea urchins (Lytechinus pictus): polarity and gene organization. Cell. 1979 Nov;18(3):843–853. doi: 10.1016/0092-8674(79)90136-3. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Hernandez N., Weiner A. M. Formation of the 3' end of U1 snRNA requires compatible snRNA promoter elements. Cell. 1986 Oct 24;47(2):249–258. doi: 10.1016/0092-8674(86)90447-2. [DOI] [PubMed] [Google Scholar]
- Holt C. A., Childs G. A new family of tandem repetitive early histone genes in the sea urchin Lytechinus pictus: evidence for concerted evolution within tandem arrays. Nucleic Acids Res. 1984 Aug 24;12(16):6455–6471. doi: 10.1093/nar/12.16.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Forbes D. J. The two embryonic U1 small nuclear RNAs of Xenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol. 1984 Dec;4(12):2580–2586. doi: 10.1128/mcb.4.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Brown D. T., Lobo S., Wang S. S. Isolation and characterization of two linked mouse U1b small nuclear RNA genes. Nucleic Acids Res. 1983 Sep 24;11(18):6255–6270. doi: 10.1093/nar/11.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. Synthesis of U1 RNA in isolated nuclei from sea urchin embryos: U1 RNA is initiated at the first nucleotide of the RNA. Mol Cell Biol. 1985 May;5(5):1143–1150. doi: 10.1128/mcb.5.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. F., Price D. H., Marzluff W. F. Synthesis of U1 RNA in a DNA-dependent system from sea urchin embryos. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3674–3678. doi: 10.1073/pnas.83.11.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa N. M., Lobo S. M., Marzluff W. F. Expression of a mouse U1b gene in mouse L cells. Gene. 1985;36(3):311–319. doi: 10.1016/0378-1119(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Newrock K. M., Cohen L. H., Hendricks M. B., Donnelly R. J., Weinberg E. S. Stage-specific mRNAs coding for subtypes of H2A and H2B histones in the sea urchin embryo. Cell. 1978 Jun;14(2):327–336. doi: 10.1016/0092-8674(78)90118-6. [DOI] [PubMed] [Google Scholar]
- Nijhawan P., Marzluff W. F. Metabolism of low molecular weight ribonucleic acids in early sea urchin embryos. Biochemistry. 1979 Apr 3;18(7):1353–1360. doi: 10.1021/bi00574a035. [DOI] [PubMed] [Google Scholar]
- Roberts S. B., Weisser K. E., Childs G. Sequence comparisons of non-allelic late histone genes and their early stage counterparts. Evidence for gene conversion within the sea urchin late stage gene family. J Mol Biol. 1984 Apr 25;174(4):647–662. doi: 10.1016/0022-2836(84)90088-3. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Kristo P., Stumph W. E., Tsai M. J., O'Malley B. W. Structure and expression of a chicken gene coding for U1 RNA. Cell. 1981 Mar;23(3):671–680. doi: 10.1016/0092-8674(81)90430-x. [DOI] [PubMed] [Google Scholar]
- Schatten G., Maul G. G., Schatten H., Chaly N., Simerly C., Balczon R., Brown D. L. Nuclear lamins and peripheral nuclear antigens during fertilization and embryogenesis in mice and sea urchins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4727–4731. doi: 10.1073/pnas.82.14.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Nagasu N., Itoh Y., Tani T., Okano K., Koga N., Okada N., Ohshima Y. Structural analysis of gene loci for rat U1 small nuclear RNA. Nucleic Acids Res. 1983 Mar 25;11(6):1791–1801. doi: 10.1093/nar/11.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]





