Abstract
The opioid-related receptor, ORL1, is activated by the neuropeptide nociceptin/orphanin FQ (N/OFQ) and inhibits high-voltage-activated (HVA) calcium channel currents (ICa) via a G-protein-coupled mechanism. Endocytosis of ORL1 receptor during prolonged N/OFQ exposure was proposed to cause N-type voltage-gated calcium channel (VGCC) internalization via physical interaction between ORL1 and the N-type channel. However, there is no direct electrophysiological evidence for this mechanism in dorsal root ganglion (DRG) neurons or their central nerve terminals. The present study tested this using whole-cell patch-clamp recordings of HVA ICa in rat DRG neurons and primary afferent excitatory synaptic currents (eEPSCs) in spinal cord slices. DRG neurons were classified on the basis of diameter, isolectin-B4 (IB4) binding and responses to capsaicin, N/OFQ and a μ-opioid agonist, DAMGO. IB4-negative neurons less than 20 μm diameter were selectively responsive to N/OFQ as well as DAMGO. In these neurons, ORL1 desensitization by a supramaximal concentration of N/OFQ was not followed by a decrease in HVA ICa current density or proportion of whole-cell HVA ICa contributed by N-type VGCC as determined using the N-type channel selective blocker, ω-conotoxin CVID. There was also no decrease in the proportion of N-type ICa when neurons were incubated at 37°C with N/OFQ for 30 min prior to recording. In spinal cord slices, N/OFQ consistently inhibited eEPSCs onto dorsal horn neurons. As observed in DRG neurons, preincubation of slices in N/OFQ for 30 min produced no decrease in the proportion of eEPSCs inhibited by CVID. In conclusion, no internalization of the N-type VGCC occurs in either the soma or central nerve terminals of DRG neurons following prolonged exposure to high, desensitizing concentrations of N/OFQ.
Key points
The nociceptin/ORL1 receptor neuropeptide system is related to opioid systems and thought to be involved in pain modulation.
A major mechanism of action of this system is inhibition of calcium channels that control excitability of sensory nerves.
To understand the potential for drugs acting on this system to modulate pain it is important to identify the types of sensory nerve cells functionally expressing the ORL1 receptor and how they are modulated.
Here we identified a subpopulation of small, presumably pain sensing sensory nerves that are highly responsive to this neuropeptide both in their cell bodies and nerve terminals.
We then established that nociceptin/ORL1 stimulation inhibits calcium channels only while the peptide is present on the cells or their nerve terminals but does not produce long-term down-regulation of calcium channel function as had been previously proposed.
Introduction
The opioid-like receptor, ORL1, and its endogenous ligand, nociceptin/orphanin FQ (N/OFQ) comprise a signalling system closely related to, but distinct from, opioid systems (Meunier et al. 1995; Reinscheid et al. 1995). Activation of the ORL1 receptor (ORL1R) couples to G-protein-related signalling mechanisms including inhibition of VGCCs and activation of K+ channels (Connor et al. 1996a,b; Larsson et al. 2000; Borgland et al. 2001). Drugs that act on ORL1Rs have been implicated as potential therapeutics for several conditions, including pain (Reiss et al. 2008; Hayashi et al. 2010). The involvement of N/OFQ and the ORL1R in pain modulation is complex and activation of the ORL1R has been implicated in both analgesia and hyperalgesia (Courteix et al. 2004; Okuda-Ashitaka et al. 2006; Chen & Sommer, 2007). This is dependent on the distribution of the receptor; for example, intrathecal administration of N/OFQ is antinociceptive (Yamamoto et al. 1997) whilst intracerebroventricular injection appears to be pronociceptive (Meunier et al. 1995; Reinscheid et al. 1995).
The ORL1R is expressed by sensory neurons but little is known about the expression patterns and selective actions on specific, potentially nociceptive subpopulations. Studies in rat using in situ hybridisation have reported that ORL1R mRNA is expressed mainly in medium- to large-sized rat dorsal root ganglion (DRG) neurons (Pettersson et al. 2002). However, in rat DRG neurons, N/OFQ selectively inhibited high voltage-activated (HVA) calcium channel currents (ICa) in the majority of small- to medium-sized neurons (<25 μm diameter) but only in a small proportion of larger neurons (Beedle et al. 2004). Similarly, in mouse trigeminal ganglion neurons both N/OFQ and the μ-opioid receptor (MOR) agonist DAMGO selectively inhibited HVA ICa (predominantly Cav2.2) in a large proportion (>80%) of small nociceptive neurons that did not express low-voltage-activated ICa (Borgland et al. 2001). To study mechanisms of antinociceptive actions of N/OFQ in rat spinal cord, we first sought to identify whether functional expression of ORL1Rs was enriched in a subpopulation of rat DRG neurons as well as their terminals in the dorsal horn of spinal cord using patch-clamp electrophysiology in acutely isolated neurons and brain slices. We found that ORL1R coupling to inhibition of HVA ICa was very selective for a subpopulation of small (<20 μm diameter) rat DRG neurons that are isolectin-B4 (IB4) -negative and, therefore, presumably peptidergic (Wang et al. 1994). Most of these neurons were also sensitive to DAMGO. Activation of the ORL1R also produced greater inhibition of primary afferent electrically evoked post-synaptic currents (eEPSCs) in lamina I of the superficial dorsal horn of the spinal cord, where small peptidergic fibres selectively terminate than in lamina IIinner where nociceptive, IB4-positive fibres predominantly terminate (Braz et al. 2005). These findings provide a functional anatomical basis for antinociceptive actions of N/OFQ in spinal cord.
Prolonged agonist stimulation has been reported to desensitize (Connor et al. 1996a; Spampinato et al. 2002) and internalize the ORL1R (Spampinato et al. 2001, 2002; Corbani et al. 2004). A novel mechanism of modulation of ORL1R signalling was more recently proposed that involves co-internalisation of the ORL1R and the VGCC α1-subunit, Cav2.2 (Altier et al. 2006). Altier et al. (2006) reported profound co-internalisation of ORL1Rs and Cav2.2 after exposure to N/OFQ for up to 30 min when these were heterologously expressed in cell lines. Due to a physical interaction between the C-termini of the ORL1R and Cav2.2 (Beedle et al. 2004), it was reported that co-internalisation leads to trafficking and degradation of Cav2.2 in lysosomes, thereby decreasing the membrane expression of Cav2.2 (Beedle et al. 2004; Altier et al. 2006). If present in sensory neurons, this mechanism would be expected to reduce excitability and transmitter release, thereby potentially producing antinociception. However, these studies (Altier et al. 2006) were performed mostly in cultured cells heterologously expressing ORL1R and Cav2.2 constructs. Only indirect, calcium imaging evidence for modulation in sensory neurons was provided to suggest that this phenomenon may occur in vivo (Altier et al. 2006). A more recent study further suggested that hetero-oligomers of ORL1 and MOR form when heterologously expressed in cell lines, and that activation of either receptor could drive internalization of Cav2.2 (Evans et al. 2010).
Having identified a subpopulation of sensory neurons that very selectively express strong coupling of the ORL1R to Cav2.2, we sought to determine whether prolonged exposure of sensory neurons to a high desensitising concentration of N/OFQ could reduce Cav2.2 currents in small IB4-negative neurons or their nerve terminals. Although exposure to N/OFQ reliably desensitized ORL1R coupling to VGCC currents, prolonged incubation of DRGs in a supramaximal concentration of N/OFQ, or ORL1 plus MOR agonists, had no effect on HVA ICa density or the proportion of HVA ICa sensitive to the highly selective Cav2.2 blocker, ω-conotoxin CVID (Lewis et al. 2000, 2012). Consistent with the results from DRG neurons, prolonged exposure to N/OFQ had no effect on the sensitivity of primary afferent eEPSCs onto lamina I or lamina IIinner dorsal horn neurons to inhibition by CVID. Together, these results suggest that sustained activation of the ORL1R does not down-regulate Cav2.2 expression on the soma or nerve terminals of primary afferent neurons.
Methods
Sprague–Dawley rats (3- to 7-week-old males (n = 48) for DRG recordings and 16- to 31-day-old males and females for spinal cord recordings (n = 41)) were used in this study. Animals were housed in groups of two to four with environmental enrichment on a 12 h/12h light–dark cycle at 22 ± 2°C, with ad libitum access to food and water. All experiments were conducted according to protocols approved by the Animals Ethics Committee of the University of Sydney, Sydney, NSW, Australia, which complies with the National Health and Medical Research Council ‘Australian code of practice for the care and use of animals for scientific purposes’.
Isolated DRG neuron preparation
Rats were anaesthetized with isofluorane (4% in air) and decapitated. Dorsal root ganglia (spinal levels L3–L5) were removed and placed in ice-cold Hepes-buffered saline (HBS) containing (mm): NaCl, 154; KCl, 2.5; CaCl2, 2.5; MgCl2, 1.5; Hepes, 10; glucose, 10; pH 7.2 (NaOH), 330 ± 5 mosmol l−1. Cells were prepared using the methods outlined in Borgland et al. (2001). Briefly, ganglia were cut up with iridectomy scissors and incubated at 37°C for 15 min in oxygenated HBS containing 3 mg ml−1 collagenase and for 25 min in oxygenated HBS containing 1 mg ml−1 papain. The digestion was terminated with addition of HBS containing 1 mg ml−1 bovine serum albumin (BSA) and 1 mg ml−1 trypsin inhibitor. Ganglia were washed free of enzyme and enzyme inhibitors with room-temperature HBS. Cells were dispersed by gentle trituration through decreasing bore, silanized Pasteur pipettes with fire-polished tips. The cells were plated onto plastic culture dishes and kept at room temperature in HBS. Cells remained viable for up to 10 h after dissociation.
Spinal cord slice preparation
Rats were anaesthetized with isofluorane (4% in air), decapitated and the lumbar region of the spinal cord was removed as previously described (Rycroft et al. 2007; Vikman et al. 2008). Transverse spinal cord slices (350 μm) were cut on a vibratome (Leica, VT1000) in an ice-cold solution of the following composition (mm): 100 sucrose, 63 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 25 glucose and 2.5 NaHCO3. Slices were maintained at 32°C in a submerged chamber containing physiological saline solution (ACSF) consisting of (mm): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 25 glucose and 2.5 NaHCO3 (pH 7.4, osmolarity 305–310 mosmol l−1), which was equilibrated with 95% O2 and 5% CO2. The slices were then transferred to a recording chamber and superfused continuously (2 ml min−1) with ACSF. Experiments were performed at 32°C.
Visualization of IB4 binding
Isolated DRG cells were pre-treated with 1 μg ml−1 Alexa Fluor 488-conjugated Bandeiraea simplicifolia IB4 (Invitrogen) for 10 min at 22–24°C as previously described (Borgland et al. 2001). Neurons were washed with HBS for 5 min before fluorescence was examined on the inverted microscope (Olympus, IX50) used for patch-clamp recordings.
Electrophysiological recording from DRG neurons
Ionic currents from rat DRG neurons were recorded in the whole-cell configuration of the patch-clamp method at room temperature (22–24°C). Dishes were continually perfused with HBS. For isolating ICa, the extracellular solution contained (mm): 140 tetraethylammonium chloride (TEACl), 2.5 CsCl, 2.5 CaCl2, 10 Hepes, 1 MgCl2, 10 glucose; pH 7.2 (CsOH), 330 ± 5 mosmol l−1. The intracellular pipette solution contained (mm): 120 CsCl, 10 Hepes, 10 EGTA, 2 CaCl2, 5 MgATP, 0.2 Na2GTP, 5 NaCl; pH 7.3 (CsOH), 285 ± 5 mosmol l−1. Recordings were made using an EPC-9 patch-clamp amplifier and corresponding PULSE software from HEKA Electronik (Lambrecht/Pfalz, Germany). Currents were sampled at 20–50 kHz and recorded on hard disk for later analysis. Patch pipettes were pulled from borosilicate glass (AM Systems, Everett, WA, USA). The pipette input resistance ranged between 1.5 and 2.5 MΩ. The capacitance of individual cells was estimated by PULSE software by fitting an exponential to current responses to small rectangular voltage pulses and ranged between 10 and 50 pF. Series resistance was between 3 and 10 MΩ. Series resistance compensation of between 70% and 80% was used in all experiments. Capacitance transients were compensated automatically using a built-in procedure of the HEKA amplifier. Leak current was subtracted online using a P/8 protocol. Liquid junction potential of 4 mV was not corrected for.
Cell diameter was estimated from membrane capacitance measurement derived by PULSE software from the voltage-clamp charging curve. As previously reported (Borgland et al. 2001), directly visualized diameter (using a micrometer graticule) was highly correlated with diameter calculated from cell capacitance using the relationship in Borgland et al. (2001) assuming a specific membrane capacitance of 0.9 μF cm−2. All cell diameter data are reported from the latter.
Peak HVA ICa in each cell was determined by stepping the membrane potential from a holding potential of –90 mV to between –60 and 30 mV, for 10 ms, in 10 mV increments. Following this procedure, the test current was evoked (–80 to 0 mV) every 30 s and monitored for current stability before drugs were applied.
Cells were exposed to drugs via a series of flow pipes positioned above the cells. The inhibition by drugs was quantified by measuring the current isochronically from the peak of the control current in the presence and absence of the drug.
Electrophysiological recording from spinal cord slices
Lamina I was visualised using infra-red Nomarski optics and presumed NK1-positive neurons were selected for experiments based on size and shape of the cell soma (Rycroft et al. 2007). Lamina II was identified as the translucent area adjacent to lamina I. Dorsal cells in this region were considered in lamina IIo and those ventral and adjacent to the less translucent lamina III considered lamina IIi. Whole-cell voltage-clamp recordings (holding potential –74 mV) of synaptic currents were made using a Digidata 1322A and a Multiclamp 700B amplifier (both from Molecular Devices, CA, USA). Patch electrodes with a resistance of 2–4 MΩ were filled with a solution consisting of the following (mm): 113 caesium gluconate, 10 EGTA, 10 Hepes, 17.5 CsCl, 8 NaCl2, 3 QX-314, 2 MgATP and 0.3 NaGTP, plus 0.2% (w/v) biocytin; pH 7.2; osmolarity, 280–290 mosmol l−1). Series resistance (≤15 MΩ) was compensated by 80% and continuously monitored during experiments. Liquid junction potentials of –14 mV were not corrected for. Recordings were low-pass filtered at 4–6 kHz, sampled at 10 kHz and analysed off-line using Axograph X (Axograph Scientific, Australia). A bipolar tungsten electrode (FHC, ME, USA) was carefully placed in the dorsal root entry zone to ensure stimulation of primary afferent terminals and electrically evoked excitatory postsynaptic currents (eEPSCs) were recorded (0.01 Hz, 2–30 V, 100 μs). To isolate eEPSCs, all experiments were performed in the presence of strychnine (5 μm) and picrotoxin (100 μm) to block glycine- and GABAA-mediated synaptic currents. Unless otherwise stated, eEPSCs were quantified by averaging a minimum of 20 consecutive responses for each condition. Only evoked currents with a clear first peak and no latency jitter were measured and included in the analysis to avoid contamination from polysynaptic events.
Data analysis
All data are expressed as the mean ± SEM unless otherwise indicated. Concentration–response data were pooled for each group and fitted to a logistic equation with a minimum constrained to zero using the software package GraphPad Prism v. 3. Where noted, significant differences between means were tested using paired or unpaired two-tailed Student's t tests, or when more than two groups were included, one-factor ANOVA, followed by Dunnett's test. Differences between frequency data were tested using χ2 tests.
Drugs and chemicals
DAMGO ([Tyr-D-Ala-Gly-MePhe-Gly-ol]enkephalin), [Met5]-enkephalin, N/OFQ (Phe-Gly-Gly-Phe-Thr-Gly- Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln), bovine serum albumin, trypsin inhibitor (chicken egg white ovomucoid, Type II-O) and CdCl2 were from Sigma-Aldrich. ω-Conotoxin CVID was provided by R. J. Lewis, Institute of Molecular Biology, University of Queensland. Capsaicin was from Tocris Cookson (Bristol, UK). Papain and collagenase were from Worthington Biochemical Corporation (Freehold, NJ, USA). Alexa Fluor 488-conjugated IB4 was from Invitrogen.
Results
Categorization of rat DRG neurons
Rat DRG neurons were categorised on the basis of several criteria in order to identify whether or not a defined subpopulation of DRG neurons selectively responds to N/OFQ (Fig. 1). The criteria correlated with responses to a supramaximal concentration (see below) of N/OFQ (300 nm, Fig. 1C, E, F and G) included soma diameter (Fig. 1F and G), whether or not they were IB4-negative (presumed peptidergic) or IB4-positive (presumed non-peptidergic) using pre-incubation with Alexa Fluor 488-conjugated IB4 (Fig. 1A), responsiveness to TRPV1 receptor agonist capsaicin (500 nm; Fig. 1B and G), as well as the MOR agonist, DAMGO (1 μm, Fig. 1D and E). Complete current–voltage relationships were also determined in the absence or presence of N/OFQ (data not shown). As previously reported, N/OFQ did not affect the voltage dependence of HVA ICa (Borgland et al. 2001).
Figure 1. Characterisation of DRG neuron phenotypes.
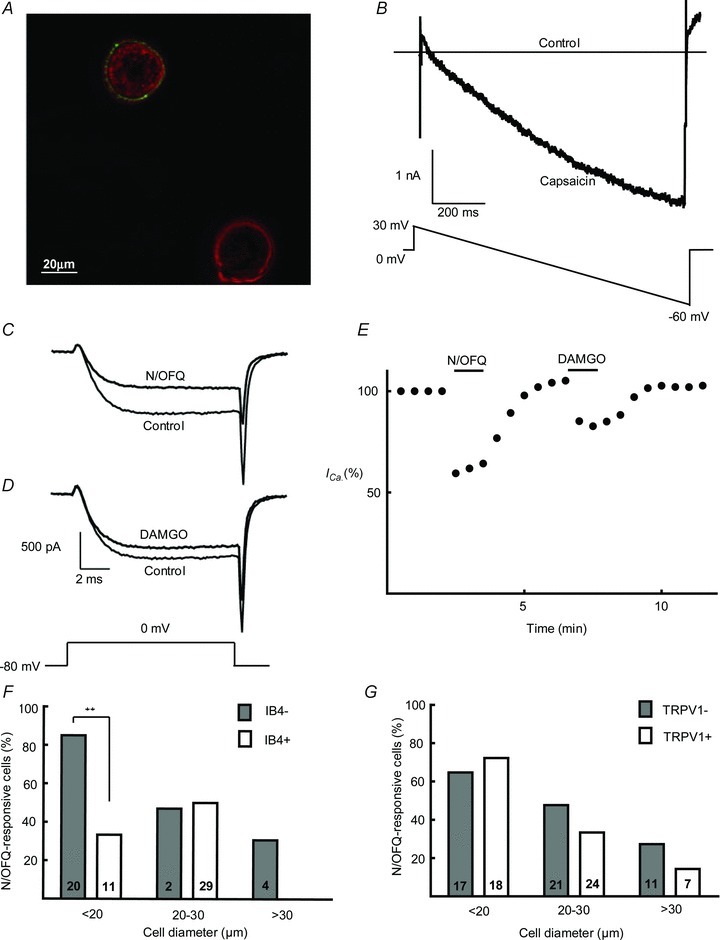
A, representative example of living DRG neurons exhibiting IB4 binding. The neuron at the top left is IB4+ (non-peptidergic) while the other is IB4- (peptidergic). B, representative current trace from a TRPV1+ neuron in the presence and absence of capsaicin (500 nm) during a continuous ramp from +30 mV to –60 mV of 1 s in duration. C and D, representative high-voltage-activated ICa before and after application of N/OFQ (300 nm) and DAMGO (1 μm). E, ICa amplitude as a percentage of total HVA ICa indicating inhibition and recovery from N/OFQ (300 nm) and DAMGO (1 μm). F and G, percentage of neurons responsive to N/OFQ when categorised on the basis of size, IB4 binding and responsiveness to capsaicin (TRPV1+ or -). Note that 85% of IB4- neurons with soma diameter smaller than 20 μm are responsive to N/OFQ (P = 0.003, χ2= 9.08). The total numbers of neurons tested in each category are shown within bars.
In order to demonstrate the voltage dependence of G-protein inhibition of VGCCs by N/OFQ, we used a pre-pulse protocol (data not shown). In these experiments, HVA ICa was evoked by two test steps to 0 mV, separated by 190 ms. The second test step was preceded by a conditioning step to +120 mV for 70 ms. In the presence of 300 nm N/OFQ, the amplitude evoked by the first test step was less than the amplitude evoked by the second test step that followed the conditioning step, due to the voltage-dependent relief of G-protein inhibition. This voltage-dependent relief was also previously observed in trigeminal ganglion neurons (Borgland et al. 2001). In all subsequent experiments, peak HVA ICa was elicited by a single depolarising step from –80 mV to 0 mV. A subpopulation of DRG neurons not labelled by IB4 was found to be highly responsive to both N/OFQ and DAMGO. This was correlated strongly with the soma diameter as discussed in detail below.
N/OFQ selectively inhibits HVA ICa in a subpopulation of small IB4-negative (peptidergic) DRG neurons
Neurons were defined as N/OFQ sensitive if they showed a reversible inhibition of HVA ICa of greater than 10% when exposed to maximally effective concentrations of N/OFQ (300 nm) (Fig. 1C and E). Since size is a good indicator of function in DRG neurons, cells were categorised into three groups based on soma diameter as follows: <20 μm (small), 20–30 μm (medium) and >30 μm (large). In all three groups, neither TRPV1-positive nor -negative neurons emerged as N/OFQ-responsive populations (Fig. 1G). Responsiveness to N/OFQ was greatest among small neurons, with 71% of neurons (27 out of 38) displaying inhibition of ICa greater than 10%, followed by 39% of medium-sized (17 out of 44) then 24% of large (4 out of 17) neurons (χ2= 13.70, P = 0.001). Most neurons that responded to N/OFQ also responded to the MOR agonist, DAMGO (1 μm, Fig. 1D and E).
Responsiveness to N/OFQ in the three neuronal size categories was then examined on the basis of IB4 binding (IB4+ or IB4-; Fig. 1F), responsiveness to capsaicin (TRPV1+ or TRPV1-; Fig. 1G) and DAMGO. Responsiveness to N/OFQ was enriched in small IB4-negative neurons. N/OFQ (300 nm) produced 51 ± 3% inhibition of HVA ICa in 85% (17 out of 20) of small-diameter (<20 μm) IB4 neurons (Fig. 2B). Furthermore, 88% (15 out of 17) of small neurons that responded to N/OFQ were also responsive to DAMGO although DAMGO usually produced less inhibition of HVA ICa than N/OFQ (34 ± 4%, n = 15) in this population of neurons (Fig. 2B). The native peptide agonist [Met5]-enkephalin (10 μm) produced similar inhibition to that produced by DAMGO (data not shown). This group of small, presumed peptidergic neurons was thus identified as a distinct population of DRG neurons highly responsive to both N/OFQ and DAMGO. In contrast to the N/OFQ-responsive population of small, presumed peptidergic neurons, N/OFQ inhibited HVA ICa in only 30% (3 out of 10) of small IB4+ neurons (Fig. 2). All three of these responsive small IB4+ neurons also responded to DAMGO. In neurons >20 μm diameter, there was no distinct correlation between IB4 binding and response to N/OFQ, perhaps because very few medium- to large-sized neurons were IB4+ (6 out of 47), as reported elsewhere (Gerke & Plenderleith, 2001). Responses to capsaicin did not distinguish responses to N/OFQ for any size DRG neurons. Similar proportions of N/OFQ-responsive neurons were found among TRPV1+ and TRPV1- neurons of all sizes (Fig. 1G).
Figure 2. Inhibition of HVA ICa by N/OFQ and DAMGO in IB4-positive and negative DRG neurons.
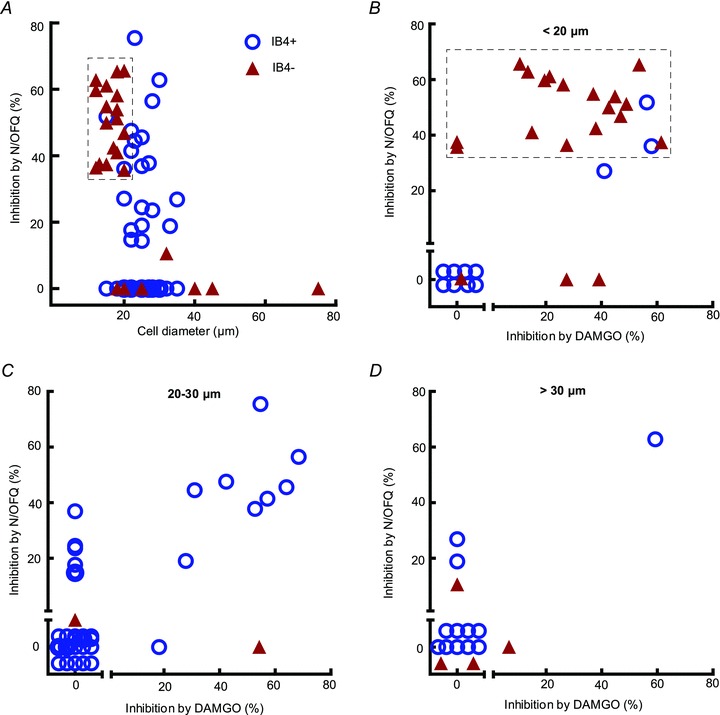
A, scatter-plot of soma size versus percentage of inhibition of HVA ICa by N/OFQ. Most N/OFQ-responsive neurons were smaller than 30 μm. Both IB4+ and IB4- cells showed a response to N/OFQ overall. As highlighted by the box, responsiveness to N/OFQ was concentrated in small IB4- neurons. B, scatter-plot showing percentage of HVA ICa inhibition by DAMGO versus N/OFQ for neurons smaller than 20 μm. As highlighted by the box, almost all IB4- neurons responded to both N/OFQ and DAMGO compared with IB4+ neurons. Subsequent experiments in this study were performed on this population. C and D, scatter-plot showing percentage of HVA ICa inhibition by DAMGO versus N/OFQ for neurons between 20 to 30 μm diameter and greater than 30 μm, respectively. No distinct N/OFQ-responsive subpopulation was seen in these groups.
ORL1R desensitization is not correlated with a decrease in N-type ICa
Experiments to investigate whether or not prolonged application of a supramaximal concentration of N/OFQ reduced surface expression of the N-type VGCCs were carried out in DRG neurons that were less than 20 μm diameter and did not exhibit IB4 binding. Application of increasing concentrations of N/OFQ to this subpopulation of DRG neurons produced a maximal effect at concentrations between 30 and 100 nm (Fig. 3A); 300 nm N/OFQ was therefore considered supramaximal. Ω-Conotoxin CVID (CVID) is a highly selective antagonist of N-type VGCCs (Lewis et al. 2012). Internalization of Cav2.2 channels, if it occurs during desensitization of the ORL1R, should be reflected as a reduction in the proportion of HVA ICa sensitive to inhibition by a maximal concentration of CVID. As shown in Fig. 3B, a concentration of 300 nm of CVID was sufficient to produce maximal inhibition.
Figure 3. Concentration–response curves.
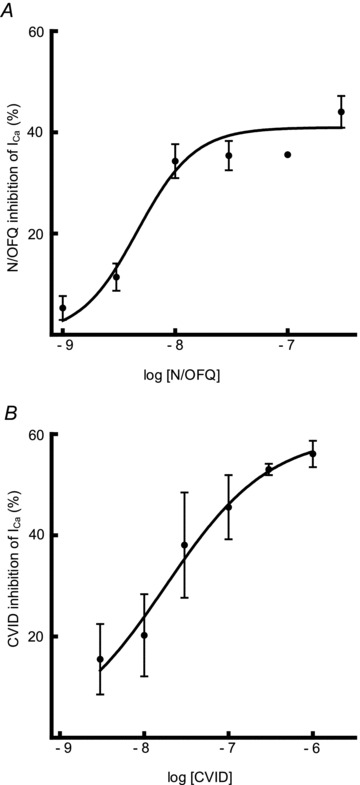
Concentration–response curves for A, N/OFQ and B, CVID in IB4- neurons smaller than 20 μm (n = 3–10 cells per data point). Maximal effects were observed at 300 nm for both N/OFQ and CVID, which was the concentration used in the subsequent experiments.
ORL1R desensitisation was determined in small, IB4-negative DRG neurons during prolonged exposure (10 min) to a supramaximal concentration of N/OFQ (300 nm). Desensitization was measured both by the decline in response to the supramaximal concentration as well as the response to a submaximal, probe concentration of N/OFQ before and after the 10 min application of 300 nm N/OFQ. As previously discussed for desensitization of MOR in neurons, the decline in response to a submaximal concentration of agonist provides a sensitive index of desensitization (Connor et al. 2004).
Pronounced ORL1 desensitization occurred in all neurons tested (Fig. 4A), when measured either as the decline in response to the supramaximal, or the submaximal, probe concentration of N/OFQ. The amplitude of inhibition by 300 nm N/OFQ decreased significantly from 44 ± 3% initially to 25 ± 2% after 10 min (n = 5, P = 0.0004, paired t test). Inhibition of HVA ICa by 10 nm N/OFQ also decreased significantly from 28 ± 6% to 7 ± 2% (P = 0.006). These results suggest that exposure to a supramaximal concentration of N/OFQ for 10 min produces regulation (desensitization) of ORL1R function under our recording conditions.
Figure 4. Effects of prolonged exposure to N/OFQ on N-type HVA ICa in IB4-negative DRG neurons.
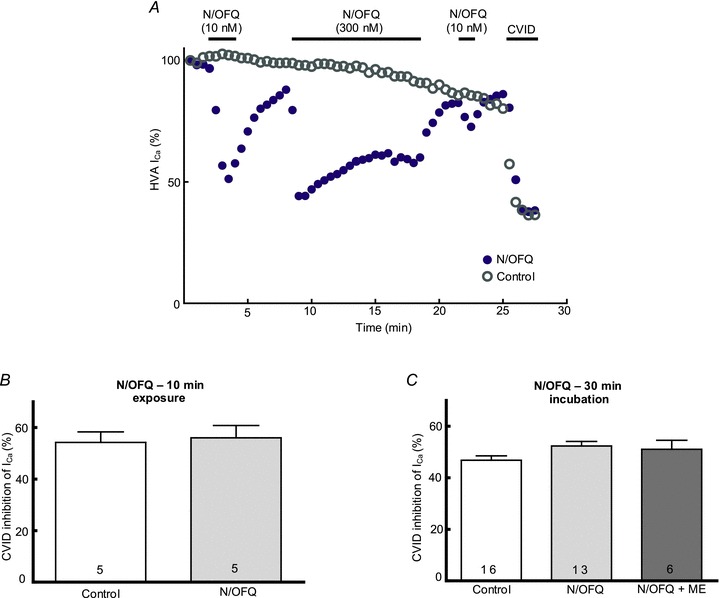
A, representative time plot of ORL1R desensitisation in IB4- DRG neurons smaller than 20 μm. 10 nm N/OFQ was used as a probe concentration and 300 nm N/OFQ as a supramaximal desensitising concentration. Note the attenuated response to the probe concentration following a 10 min exposure to 300 nm N/OFQ. Following recovery of ICa after washout of N/OFQ, 300 nm CVID was applied to measure the proportion of N-type current. A representative time plot from a neuron not exposed to N/OFQ is also shown. Note that the rundown in ICa is comparable in both cells. N-type ICa proportions measured using 300 nm CVID are also very similar in both cells. B, no change was observed in the proportion of N-type current in IB4- neurons smaller than 20 μm following desensitisation of ORL1R in response to 10 min agonist exposure. C, no change was observed in the proportion of N-type current in IB4- neurons smaller than 20 μm following incubation at 37°C for 30 min with vehicle, N/OFQ (300 nm) or both N/OFQ and [Met5]-enkephalin (ME, 10 μm).
Although ORL1R desensitization was readily observed, exposure of small, IB4-negative DRG neurons to a supramaximal concentration of N/OFQ (300 nm) for 10 min affected neither overall HVA ICa density nor the proportion of N-type channels susceptible to inhibition by CVID. To control for rundown of HVA ICa, recordings were made for a similar duration in control neurons (20–30 min) before applying 300 nm CVID. Over the total duration of recording prior to application of CVID, similar rundown was observed in N/OFQ-exposed (87 ± 5.5% of initial ICa, n = 5) and control (88 ± 7% of initial ICa, n = 5) neurons. The duration of recordings over which rundown was determined did not differ between groups (25.1 ± 1.6 min, n = 5 for control, 25.7 ± 2 min, n = 5 for N/OFQ-exposed). In the same group of neurons, HVA ICa density was not significantly affected after washout from 10 min exposure to N/OFQ (127 ± 24 pA pF-1 in treated versus 172 ± 39 pA pF-1 in control neurons; P = 0.36). There was also no significant difference in the proportion of HVA ICa susceptible to inhibition by CVID (Fig. 4B).
Prolonged incubation with N/OFQ or N/OFQ plus met-enkephalin did not decrease N-type HVA ICa
The failure to observe a reduction in N-type HVA ICa during whole-cell patch-clamp recordings might have been due to incubation temperature (22–24°C), duration of N/OFQ exposure (10 min) or the whole-cell procedure per se. To test this, DRG neurons were incubated at 37°C with either N/OFQ (300 nm) or N/OFQ (300 nm) plus met-enkephalin (ME, 10 μm) for 30 min immediately prior to patch-clamp recording. Following incubation, the culture dishes containing the neurons were perfused with HBS at room temperature for 5 min before recordings were made. HVA ICa density and susceptibility to inhibition by CVID was then used to determine the proportion of N-type currents. Control neurons were incubated at 37°C for 30 min in the absence of either agonist. Incubation in N/OFQ or N/OFQ plus ME did not reduce HVA ICa density, which was 112 ± 11 (n = 16), 133 ± 9 (n = 13) and 174 ± 27 (n = 6) pA pF-1 in control, N/OFQ- and N/OFQ plus ME-exposed neurons, respectively. If anything there was a small increase in HVA ICa density in cells exposed to N/OFQ plus ME (P = 0.02, Dunnett's post hoc test). There was also no decrease in the proportion of HVA ICa susceptible to inhibition by CVID (Fig. 4C). Therefore, prolonged incubation with ORL1R or MOR agonists did not produce any decrease in the N-type HVA ICa in rat DRG neurons.
Incubation with N/OFQ does not reduce the contribution of N-type HVA ICa to primary afferent synapses in spinal dorsal horn
Calcium influx through N-type VGCCs contributes substantially to neurotransmitter release at primary afferent synapses in the spinal cord dorsal horn (Heinke et al. 2004; Rycroft et al. 2007) and N/OFQ directly inhibits neurotransmitter release at the same synapses, at least partly via inhibition of N-type HVA ICa (Liebel et al. 1997). The possibility that N/OFQ down-regulates Cav2.2 on primary afferent synapses was therefore examined in spinal cord slices. As reported elsewhere (Liebel et al. 1997), N/OFQ (10 μm) reduced the primary afferent eEPSC amplitude in all neurons tested (Fig. 5A). Higher concentrations of both N/OFQ and CVID were used in the slice experiments in order to compensate for peptidase activity, as previously reported in spinal tissue (Suder et al. 1999). Interestingly, the inhibitory effect of N/OFQ was significantly larger in lamina I neurons where peptidergic afferents (IB4-negative) predominantly terminate (Braz et al. 2005) compared with lamina IIinner neurons where IB4-positive fibres terminate (P = 0.025).
Figure 5. Effects of prolonged exposure to N/OFQ on primary afferent eEPSCs in dorsal horn of the spinal cord.
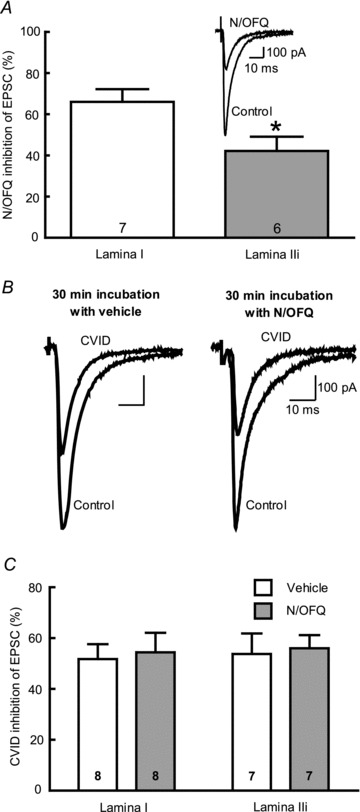
A, percentage of inhibition of evoked EPSCs by N/OFQ (1 μm). A significantly higher inhibition was observed in lamina I neurons compared with lamina IIinner (P = 0.025). Inset, representative trace showing inhibition of an evoked EPSC in the dorsal horn by N/OFQ (1 μm). B, representative traces showing inhibition of evoked EPSCs by CVID (1 μm) following a 30 min incubation with vehicle or N/OFQ (10 μm). C, percentage of inhibition of evoked EPSCs by CVID (1 μm). No significant difference was seen between EPSCs recorded from lamina I and lamina IIinner. Further, incubating the slices with N/OFQ (10 μm) for 30 min prior to recording did not affect the inhibition of EPSC amplitude by CVID.
Spinal cord slices were then incubated at 37°C with N/OFQ (10 μm) or ACSF for 30 min to determine whether activation of ORL1Rs in primary afferent nerve terminals could internalize Cav2.2. After washout of N/OFQ for at least 20 min the effect of CVID (1 μm) on eEPSCs was determined (Fig. 5B). There was no significant difference in the effect of CVID on evoked EPSC amplitude in spinal cord slices incubated with N/OFQ compared with those incubated with vehicle (Fig. 5C) in either lamina I or lamina IIinner.
Discussion
This study has characterised a distinct subpopulation of rat sensory neurons that is highly responsive to N/OFQ and MOR agonists, and is IB4-negative and smaller than 20 μm in diameter. A large proportion of these neurons presumably represents small nociceptive peptidergic sensory neurons (e.g. Kitchener et al. 1993; Gerke & Plenderleith, 2001). Previous studies have reported varied distribution of ORL1R in sensory neurons. An in situ hybridisation study reported ORL1R mRNA expression in medium to large rat DRG neurons (Pettersson et al. 2002). Immunohistochemical studies in rat have reported enrichment of ORL1R-like immunoreactivity in small- to medium-sized DRG neurons (Chen & Sommer, 2006) or have found little selective localization at all (Monteillet-Agius et al. 1998; Altier et al. 2006). Previous physiological studies have also reported modest enrichment of responsiveness to N/OFQ in small- to medium-sized rat DRG neurons (Beedle et al. 2004) and similar enrichment was found in small mouse trigeminal ganglion neurons (Borgland et al. 2001). It is unlikely that the pre-incubation of isolated DRG neurons in IB4 affected the classification of subtypes because this procedure has been reported to have no effect on calcium currents or the actions of opioids on labelled DRG neurons (Wu & Pan, 2004; Wu et al. 2004).
The present identification of a subpopulation of small IB4-negative DRG neurons highly responsive to N/OFQ is of interest because the role of ORL1R in nociceptive and sensory processing remains unclear (Zeilhofer & Calò, 2003; Lambert, 2008). It has previously been reported that peptidergic and non-peptidergic neurons are involved in parallel pain pathways (Braz et al. 2005). IB4-positive neurons were found to be nociceptive and project preferentially to lamina IIinner of the dorsal horn, whilst IB4-negative neurons were found to synapse onto lamina I projection neurons (Kitchener et al. 1993; Gerke & Plenderleith, 2001). These two regions of the spinal cord then project to different supraspinal regions involved in processing pain (Braz et al. 2005). Furthermore, these two populations of neurons receive sensory information preferentially from different targets; IB4-positive neurons receive mostly cutaneous input and peptidergic, IB4-negative neurons predominantly innervate muscle and vice versa (Plenderleith & Snow, 1993; Wang et al. 1998). The present findings therefore suggest a preferential role of the ORL1R-N/OFQ system in peptidergic sensory pathways. This is supported by the findings of significantly greater inhibition of eEPSCs by N/OFQ in lamina I, which is preferentially innervated by peptidergic (IB4-negative) primary afferent fibres than lamina IIinner where IB4-positive fibres preferentially terminate (Kitchener et al. 1993; Braz et al. 2005).
By identifying a highly selective subpopulation of DRG neurons with >80% of cells highly responsive to N/OFQ, it was possible to test whether activation of this receptor down-regulates Cav2.2 in native neurons as has been reported in cultured cells (Altier et al. 2006; Evans et al. 2010). This was achieved by direct measurement of HVA ICa density in small, IB4-negative DRG neurons, as well sensitivity to the highly selective N-type VGCC blocker, CVID in these neurons and primary afferent terminals (Lewis et al. 2000, 2012; Rycroft et al. 2007). Although many previous studies have used ω-conotoxins GVIA or MVIIA as specific N-type VGCC inhibitors, we chose CVID for its higher selectivity for Cav2.2 than other ω-conotoxins, as well as irreversibility of inhibition (Lewis et al. 2000, 2012). Furthermore, our previous study demonstrated a robust and reproducible inhibitory effect of CVID on eEPSCs recorded in spinal cord that did not differ from MVIIA (Rycroft et al. 2007). In the present study, no functional evidence was found for internalisation of the N-type VGCC in DRG cell bodies or their nerve terminals in the dorsal horn. Previous studies utilised imaging and electrophysiological techniques in the tsA-201 cell line overexpressing both ORL1 and Cav2.2 (Altier et al. 2006; Evans et al. 2010). However, in native DRG neurons, only imaging studies were performed (Altier et al. 2006). Inability to test this electrophysiologically was presumably due to the lack of information regarding ORL1R (and MOR) expression in different populations of sensory neurons. The present study has overcome this limitation by developing criteria to select DRG neurons that express highly effective coupling of ORL1 to HVA ICa in nearly all neurons. Small IB4-negative DRG neurons also express a large proportion of Cav2.2 (Figs 3 and 4), as reported elsewhwere (Wu & Pan, 2004). Using this approach, no evidence could be found for either a reduced VGCC current density or proportion of N-type channels contributing to HVA ICa after prolonged exposure to high concentrations of N/OFQ. Although these findings are at odds with the findings of Altier et al. (2006) and Evans et al. (2010), whole-cell patch-clamp recording is arguably the most sensitive and reliable method for determing a functional change in surface expression of VGCC current density and, using selective channel toxins, determining changes in VGCC subtypes.
Exposure of small IB4-negative DRG neurons to a supramaximal concentration of N/OFQ while continuously monitoring HVA ICa at room temperature could be criticized because the experiment was performed for 10 min at room temperature, which might inhibit endocytosis. Alternatively or additionally, diffusion of a critical intracellular mediator into the patch pipette could have impaired endocytosis of Cav2.2.
However, this experiment did establish that exposure to a high concentration of N/OFQ reliably produced functional desensitization of ORL1R, which is closely related to endocytosis for many GPCRs (e.g. Dang & Christie, 2012). It might be expected that recovery of ORL1R function after desensitization would be very prolonged due to endocytosis and recycling or degradation. The time-course for resensitization of ORL1R is unknown but, if it resembles related opioid receptors, would require more than 60 min for complete recovery of function (Dang & Williams, 2004; Dang et al. 2011; Quillinan et al. 2011) which would not be feasible to study by examining modulation of ICa in DRG neurons.
These potential limitations would not apply to prolonged (30 min) pre-incubation of DRG neurons at 37°C in N/OFQ or N/OFQ plus met-enkephalin, which also produced no reduction in VGCC current density or proportion of N-type HVA ICa. It is most unlikely that the calcium channel expression was restored during the brief recovery period between termination of pre-incubation in N/OFQ and determination of HVA ICa current density because Altier et al. (2006) reported in tsA cells that endocytosis of Cav2.2 was followed by targeting for lysosomal degradation of the channel.
This study also found no evidence for down-regulation of Cav2.2 following prolonged N/OFQ incubation in nerve terminals of DRG neurons in spinal cord slices. This is especially relevant as the N-type VGCC, along with the P/Q-type, is responsible for triggering neurotransmitter release and is highly localised on DRG nerve terminals found in the dorsal horn of spinal cord (Heinke et al. 2004). The proportion of inhibition of primary afferent eEPSCs found here using CVID was also consistent with the inhibition reported using CVID, MVIIA (Rycroft et al. 2007) or GVIA at C-fibre afferents (Heinke et al. 2004).
It is difficult to reconcile the present findings with those of Altier et al. (2006), particularly in DRG neurons. It is possible that endocytosis and down-regulation of Cav2.2 is mediated by activation of ORL1 under our conditions but only in a neuronal subpopulation we did not record from, i.e. IB4- positive or larger neurons. If this is the case, then the internalisation mechanism is not expressed by small peptidergic neurons or any primary afferent nerve terminals. We also cannot rule out the possibility that the internalization mechanism, if present, cannot be detected in acutely isolated neurons and spinal cord slices. However Altier et al. (2006) also presented correlative immunohistochemical evidence for Cav2.2 internalization in acutely isolated DRG neurons, so this is an unlikely reason for the discrepancy. It is also possible, but seems unlikely, that Cav2.2 internalization and degradation did occur in our experiments but was balanced by an equally increased rate of de novo insertion of N-type channels into the surface membrane. A potential control for this in future studies in cell culture might be prolonged incubation (∼40 h) in gabapentin, which disrupts trafficking of the channel to the membrane (Hendrich et al. 2008).
In conclusion, the present study has identified a subpopulation of rat small IB4-negative DRG neurons that project to the superficial dorsal horn of the spinal cord and are very responsive to both N/OFQ and opioid agonists. However, no evidence was found for a previously proposed prolonged down-regulation of N-type VGCCs following exposure to high concentrations of N/OFQ in these neurons or their central nerve terminals in spinal cord slices. Down-regulation of N-type VGCCs following exposure to high concentrations of N/OFQ therefore appears to be of no physiological significance in native sensory neurons.
Acknowledgments
This study was supported by National Health and Medical Research Council of Australia (NH & MRC). M.J.C. is a NH & MRC Senior Principal Research Fellow (511914). Donation of ω-conotoxin CVID by Professor Richard J Lewis, University of Queensland is gratefully acknowledged. Advice from Professor Mark Connor on DRG dissociation and recording is gratefully acknowledged.
Glossary
Abbreviations
- BSA
bovine serum albumin
- CVID
ω-conotoxin CVID
- DAMGO
[Tyr-D-Ala-Gly-MePhe- Gly-ol]enkephalin
- DRG
dorsal root ganglion
- ORL1R
opioid receptor-like receptor 1
- N/OFQ
nociceptin/orphanin FQ
- HBS
Hepes-buffered saline
- HVA
high-voltage activated
- IB4
isolectin B4
- ME
[Met5]-enkephalin
- MOR
μ-opioid receptor
- TRPV1
transient receptor potential channel subfamily V member 1
- VGCC
voltage-gated calcium channel
Author contributions
S.S.M. designed and performed the DRG experiments, analysed and interpreted the data, drafted the manuscript and revised it critically for important intellectual content, I.A.N. and B.K.R. performed and analysed spinal cord experiments, M.J.C. contributed to conception, design and analysis of the experiments, drafting the article and revising it critically for important intellectual content. All authors approved the final version.
References
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, et al. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci. 2004;7:118–125. doi: 10.1038/nn1180. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Christie MJ. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J Physiol. 2001;536:35–47. doi: 10.1111/j.1469-7793.2001.t01-1-00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel 'pain' pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. J Peripher Nerv Syst. 2006;11:232–240. doi: 10.1111/j.1529-8027.2006.0093.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. Activation of the nociceptin opioid system in rat sensory neurons produces antinociceptive effects in inflammatory pain: involvement of inflammatory mediators. J Neurosci Res. 2007;85:1478–1488. doi: 10.1002/jnr.21272. [DOI] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol. 1996a;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol. 1996b;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 2004;145:2876–2885. doi: 10.1210/en.2004-0062. [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain. 2004;110:236–245. doi: 10.1016/j.pain.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Dang VC, Chieng B, Azriel Y, Christie MJ. Cellular morphine tolerance produced by βarrestin-2-dependent impairment of μ-opioid receptor resensitization. J Neurosci. 2011;31:7122–7130. doi: 10.1523/JNEUROSCI.5999-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci. 2004;24:7699–7706. doi: 10.1523/JNEUROSCI.2499-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, Zamponi GW. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem. 2010;285:1032–1040. doi: 10.1074/jbc.M109.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke MB, Plenderleith MB. Binding sites for the plant lectin Bandeiraea simplicifolia I-isolectin B4 are expressed by nociceptive primary sensory neurones. Brain Res. 2001;911:101–104. doi: 10.1016/s0006-8993(01)02750-0. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Nakata E, Morita A, Mizuno K, Yamamura K, Kato A, Ohashi K. Discovery of {1-[4-(2-{hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl}-1H-benzimidazol-1-yl)pip eridin-1-yl]cyclooctyl}methanol, systemically potent novel non-peptide agonist of nociceptin/orphanin FQ receptor as analgesic for the treatment of neuropathic pain: design, synthesis, and structure-activity relationships. Bioorg Med Chem. 2010;18:7675–7699. doi: 10.1016/j.bmc.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Heinke B, Balzer E, Sandkühler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci. 2004;19:103–111. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, et al. Pharmacological disruption of calcium channel trafficking by thea2d ligand gabapentin. Proc Nat Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Larsson KP, Olsen UB, Hansen AJ. Nociceptin is a potent inhibitor of N-type Ca2+ channels in rat sympathetic ganglion neurons. Neurosci Lett. 2000;296:121–124. doi: 10.1016/s0304-3940(00)01640-2. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Dutertre S, Vetter I, Christie M. Conus venom peptide pharmacology. Pharmacol Rev. 2012 doi: 10.1124/pr.111.005322. in press. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Nielsen KJ, Craik DJ, Loughnan ML, Adams DA, Sharpe IA, et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J Biol Chem. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- Liebel JT, Swandulla D, Zeilhofer HU. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br J Pharmacol. 1997;121:425–432. doi: 10.1038/sj.bjp.0701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Monteillet-Agius G, Fein J, Anton B, Evans CJ. ORL-1 and mu opioid receptor antisera label different fibers in areas involved in pain processing. J Comp Neurol. 1998;399:373–383. doi: 10.1002/(sici)1096-9861(19980928)399:3<373::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Minami T, Matsumura S, Takeshima H, Reinscheid RK, Civelli O, Ito S. The opioid peptide nociceptin/orphanin FQ mediates prostaglandin E2-induced allodynia, tactile pain associated with nerve injury. Eur J Neurosci. 2006;23:995–1004. doi: 10.1111/j.1460-9568.2006.04623.x. [DOI] [PubMed] [Google Scholar]
- Pettersson LM, Sundler F, Danielsen N. Expression of orphanin FQ/nociceptin and its receptor in rat peripheral ganglia and spinal cord. Brain Res. 2002;945:266–275. doi: 10.1016/s0006-8993(02)02817-2. [DOI] [PubMed] [Google Scholar]
- Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. Recovery from μ-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci. 2011;31:4434–4443. doi: 10.1523/JNEUROSCI.4874-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol. 2008;579:141–148. doi: 10.1016/j.ejphar.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Rycroft BK, Vikman KS, Christie MJ. Inflammation reduces the contribution of N-type calcium channels to primary afferent synaptic transmission onto NK1 receptor-positive lamina I neurons in the rat dorsal horn. J Physiol. 2007;580:883–894. doi: 10.1111/j.1469-7793.2000.t01-1-02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Alessandri M, Murari G. Agonist-induced internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Cell Mol Life Sci. 2002;59:2172–2183. doi: 10.1007/s000180200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Qasem AR. Nociceptin-induced internalization of the ORL1 receptor in human neuroblastoma cells. Neuroreport. 2001;12:3159–3163. doi: 10.1097/00001756-200110080-00035. [DOI] [PubMed] [Google Scholar]
- Suder P, Kotlinska J, Smoluch MT, Sallberg M, Silberring J. Metabolic fate of nociceptin/orphanin FQ in the rat spinal cord and biological activity of its released fragment. Peptides. 1999;20:239–247. doi: 10.1016/s0196-9781(98)00165-x. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586:515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rivero-Melián C, Robertson B, Grant G. Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience. 1994;62:539–551. doi: 10.1016/0306-4522(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Wang HF, Robertson B, Grant G. Anterograde transport of horseradish-peroxidase conjugated isolectin B4 from Griffonia simplicifolia I in spinal primary sensory neurons of the rat. Brain Res. 1998;811:34–39. doi: 10.1016/s0006-8993(98)00916-0. [DOI] [PubMed] [Google Scholar]
- Wu Z-Z, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- Wu Z-Z, Pan HL. High voltage-activated Ca2+ channel currents in isolectin B4-positive and -negative small dorsal root ganglion neurons of rats. Neurosci Lett. 2004;368:96–101. doi: 10.1016/j.neulet.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Calò G. Nociceptin/orphanin FQ and its receptor–potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–429. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]


