Abstract
Using specific inhibitors established that angiogenesis in the ovarian follicle and corpus luteum is driven by vascular endothelial growth factor. Recently, it has been demonstrated that the Notch ligand, delta-like ligand 4 (Dll4) negatively regulates vascular endothelial growth factor-mediated vessel sprouting and branching. To investigate the role of Dll4 in regulation of the ovarian vasculature, we administered a neutralizing antibody to Dll4 to marmosets at the periovulatory period. The vasculature was examined on luteal d 3 or d 10: angiogenesis was determined by incorporation of bromodeoxyuridine, staining for CD31 and cell death by staining for activated caspase-3. Ovulatory progesterone rises were monitored to determine effects of treatment on luteal function and time to recover normal cycles in a separate group of animals. Additionally, animals were treated in the follicular or midluteal phase to determine effects of Dll4 inhibition on follicular development and luteal function. Controls were treated with human IgG (Fc). Corpora lutea from marmosets treated during the periovulatory period exhibited increased angiogenesis and increased vascular density on luteal d 3, but plasma progesterone was significantly suppressed. By luteal d 10, corpora lutea in treated ovaries were significantly reduced in size, with involution of luteal cells, increased cell death, and suppressed plasma progesterone concentrations. In contrast, initiation of anti-Dll4 treatment during the midluteal phase produced only a slight suppression of progesterone for the remainder of the cycle. Moreover, Dll4 inhibition had no appreciable effect on follicular development. These results show that Dll4 has a specific and critical role in the development of the normal luteal vasculature.
Angiogenesis and vascular remodeling are rare in most healthy adult tissues but are critical for normal cyclical ovarian and uterine function (1–6). Dysregulated vascularization is associated with ovarian disorders such as polycystic ovary syndrome (7) and ovarian hyperstimulation syndrome (8, 9). Hence, it is important to elucidate how the microvasculature of the normal female reproductive system is controlled and identify targets for manipulation in conditions with abnormal vascularization.
In previous studies we have established the importance of vascular endothelial growth factor in ovarian angiogenesis by inhibiting its action with a neutralizing antibody (10) or vascular endothelial growth factor (VEGF) Trap (Aflibercept; Regeneron Pharmaceuticals, Tarrytown, NY), (11–16) at selected specific stages of the ovulatory cycle of the marmoset monkey. A critical role for VEGF and its receptors in ovarian angiogenesis has also been demonstrated in macaques and in rodents (17–22). These studies also revealed the importance of VEGF in maintaining the function of the ovary, i.e. sex steroid secretion into the blood.
Although VEGF is the principal stimulator of endothelial cell proliferation, the formation of a hierarchical network of vessels requires coordinated interplay of various angiogenic and angiostatic factors (23, 24). A distinct and critical role in regulating cell-fate determination and patterning of the vascular system has emerged for the delta-Notch signaling pathway. Of the many genes involved in vasculogenesis and angiogenesis, haploid insufficiency results in embryonic lethality only for VEGF and the specific Notch ligand, delta-like ligand 4 (Dll4), implicating Dll4 as a vascular-specific Notch ligand critically involved in development of the vascular system (25). Dll4 is primarily expressed in endothelial cells and acts specifically by regulating the differentiation and activity of tip cells present at the growing front of new vessels (26–28). Dll4 is also expressed in stalk cells of new vessels, where it plays a role in regulating endothelial cell proliferation. Dll4 is induced by VEGF in angiogenic vessels where it functions as part of a negative feedback loop to limit excessive angiogenic sprouting in response to VEGF in developing tissues, e.g. retina (26–28) and in pathological/tumor vessels (29–31). Inhibition of Dll4 in vivo in mouse tumor models leads to increased vascularity (29–31). However, tumor growth is decreased because these vessels are functionally defective (29–31). Consequently, potent inhibitors of Dll4 have been developed based on the premise that inhibition of Dll4 leads to development of nonfunctional blood vessels (31, 32).
The cyclical angiogenesis that takes place in the ovarian follicle and corpus luteum (33–36) has provided an excellent model in which to study the role of individual factors in the angiogenic process (1–6). Notch proteins and ligands have been localized by in situ hybridization (37) and immunohistochemistry in the rodent ovary (38, 39) and human endometrium (40) and because their sites of expression include the vasculature, a role for the Notch signaling pathway in ovarian neovascularization has been proposed (38).
The aim of this study was to determine the physiological role of Dll4 in the primate ovary by examining the effects of pharmacological inhibition of Dll4 on formation of the follicular and luteal vasculature using treatment schedules employed previously with VEGF inhibitors (10, 11). We used a potent neutralizing monoclonal antibody (REGN577), which neutralizes Dll4 by blocking its ability to bind and activate Notch receptors (predominantly Notch 1 and Notch 4 in the vasculature). Dll4 and Notch are thought to act primarily in trans (ligand and receptor on adjacent cells); Dll4 is normally anchored to the cell membrane, and binding to Notch in the membrane-anchored state is required to induce conformational changes in Notch that allow for enzymatic cleavage of the receptor, leading to release of the Notch intracellular domain from the plasma membrane into the cytoplasm, followed by translocation of the intracellular domain to the nucleus where it modulates gene expression (41). The antibody was administered to marmosets at three different stages of the ovulatory cycle. After treatment, ovaries were dual stained with bromodeoxyuridine (BrdU) and CD31 to assess the proliferation rate of endothelial cells and with CD31 alone to evaluate blood vessel morphology and distribution. In addition, the longer-term effects of treatment on luteal function and subsequent ovulation were assessed in nonterminal studies. It was predicted that stringent pharmacological neutralization of Dll4 would result in increased angiogenesis, but that potential associated abnormalities in microvascular structure and function might result in the uncoupling of the conventional link between vascular development and ovarian function.
Materials and Methods
Animals
The study was approved by the local Primate Ethical Committee and carried out under Project License PPL 60/2472 (UK Home Office). Adult female marmosets (Callithrix jacchus), weighing 380–500 g, were housed with vasectomized males. Ovulation was determined by measuring plasma progesterone profiles in blood collected three times per week and deemed to have occurred when progesterone increased to more than 32 nmol/liter followed by a progressive further increase (14). Marmosets exhibiting at least one ovulatory cycle immediately before being recruited were treated. Marmosets were injected with 1 μg prostaglandin (PG)F2α analog (cloprostenol; Planate, Coopers Animal Health Ltd, Crewe, UK), im on d 13–d 15 of the luteal phase to synchronize ovulation 9–11 d later (14). Treatment regimens were selected to cover specific stages of follicular or luteal development, encompassing terminal studies in which ovaries were collected at the end of the experimental period, and nonterminal studies in which the effects of treatment on ovarian function and time to restoration of normal cycles could be evaluated.
Effect of Dll4 antibody in the periovulatory period on early luteal angiogenesis
Because the early developing corpus luteum is the site of the most intense sprouting angiogenesis in the body (1–4, 33–36), studies were first performed targeting the luteal phase. To determine the acute effects of Dll4 inhibition on luteal function and angiogenesis, marmosets (n = 4) were treated on the day of expected ovulation (designated luteal d 0) with a Dll4 antibody (REGN 577) (5 mg/kg sc), and the treatment was repeated on luteal d 2. REGN577 is a recombinant, fully humanized anti-Dll4 monoclonal (IgG1 isotype) produced in Chinese hamster ovary cells. It binds human, mouse, and monkey Dll4 with similar affinities and has been shown to block Notch-1 mediated biological activity in human and mouse Dll4 dependent cell-based assays (Rudge, J.S., unpublished data). The antibody was dissolved in 50 mm Tris, 150 mm NaCl, pH 7.5, and injected at a volume of approximately 0.5 ml. Control marmosets were recruited randomly and injected with recombinant human Fc (Fc) (5 mg/kg sc) (n = 4) on an identical schedule.
Blood sample collection was increased to once daily starting on the day before start of the treatment. On d 3 after the start of treatment, animals were injected iv with 20 mg BrdU in saline 1 h before sedation with 200 μl ketamine hydrochloride (Parke-Davis Veterinary; Pontypool, Gwent, UK) and 200 μl Saffan (Alfaxan; Jurox, London, UK). After perfusion with 4% neutral buffered formalin (NBF), the ovaries were removed immediately, weighed, and placed in NBF for 24 h before transfer into 70% ethanol, dehydrated, and embedded in paraffin.
Effect of Dll4 antibody treatment in the periovulatory period on midluteal microvasculature
Marmosets were treated identically as for experiment 1 (Fc, n = 4; Dll4 antibody, n = 5), but collection of blood samples was continued until luteal d 10, at which time the animals were treated with BrdU and ovaries collected as described above.
Effect of Dll4 antibody in the periovulatory period on luteal function and recovery of normal cyclicity
The effect of Dll4 inhibition on luteal function and recovery of normal cyclicity was investigated by treating marmosets with Fc (n = 4) or Dll4 antibody (n = 4) as in experiment 1. Blood samples were collected until one posttreatment luteal phase rise was observed.
Effect of Dll4 antibody at the midluteal phase on luteal function
This experiment examined the effects of Dll4 antibody on the functioning corpus luteum to determine the impact on the established luteal vasculature. Marmosets were treated with Dll4 antibody or Fc on luteal d 8 and d 10 (n = 4 per group). Blood samples were collected daily for 5 d and subsequently three times per week until a normal posttreatment luteal phase rise in plasma progesterone was observed.
Effects of Dll4 antibody on follicular angiogenesis and development
Finally, the effects of immunoneutralization of Dll4 on follicular development was evaluated by treatment with anti-Dll4 for the duration of the follicular phase. To synchronize follicular recruitment, selection and ovulation during treatment cycles, marmosets were injected with PGF2α analog on d 13–d 15 of the luteal phase to induce luteolysis. This is followed by follicle selection on cycle d 5 and ovulation between d 9 and d 11 (15). The day of PG injection was also the time of initiation of treatment, designated follicular d 0. Marmosets (n = 5) were treated with Dll4 antibody on d 0, and on follicular d 3 and d 7. Control marmosets were injected with Fc (n = 5) on this schedule. On d 10 (the time of anticipated ovulation) animals were injected iv with BrdU, perfused, and ovaries were collected as above.
Hematoxylin and eosin (H&E) and immunohistochemical staining
Ovaries were serially sectioned (5 μm), and representative sections were stained with H&E to identify the major structures. Sections containing these structures were selected for immunohistochemical studies.
Methods for immunohistochemical detection of proliferating cells by BrdU (using mouse anti-BrdU, diluted 1:20; Roche Molecular Biochemicals, Indianapolis, IN) and endothelial cells by CD31 (using mouse antihuman CD31, diluted 1:30; DAKO, Cambridgeshire, UK) were as described previously (11, 14). Sections stained for BrdU or CD31 were visualized using nitroblue tetrazolium (NBT). BrdU sections were lightly counterstained with hematoxylin.
For detection of proliferating endothelial cells, dual staining was obtained by immunohistochemistry for BrdU and CD31. CD31 was detected as above and visualized with fast red (Sigma, Poole, UK). Sections were then washed with Tris-buffered saline before incubation with a sheep antibody against BrdU at 1:5000 (Fitzgerald, Concord, MA) overnight at 4 C. After postincubation washes with Tris-buffered saline, a biotinylated rabbit antisheep secondary antibody (Vector, Peterborough, UK) was added, followed by ABC-AP (DAKO). After incubation with the ABC-AP complex, slides were transferred to NBT buffer for 15 min before visualization with NBT. Reactions were stopped in water, and the slides cleared in xylene and mounted in pertex. No counterstain was used in BrdU/CD31 and CD31 sections.
To determine the localization and number of dying cells, an antibody to activated caspase-3 (Asp175; New England Biolaboratories, Hitchen, UK) was used as described previously (14). Visualization was achieved by 3–3′-diaminobenzidine, and sections were counterstained with hematoxylin.
Quantitative image analysis
Quantitative image analyses of luteal area, vascular density measurements, and cell proliferation were performed using an Olympus BH2 microscope, Spot Insight QE camera, and Image-Pro Plus version 4.5 for Windows (Media Cybernetics, Silver Spring, MD) (14). Captured images were thresholded, and the total area of each corpus luteum was outlined. Central cavities in the corpora lutea were not included in area measurements. All corpora lutea were analyzed under × 40 magnification for all stains and parameters. Where animals had ovulated two or more corpora lutea, single values were calculated by taking the mean of the measurements obtained on all the corpora lutea in that animal.
Relative endothelial cell area was determined by thresholding the CD31-stained area and calculating this area as a percentage of total luteal area.
Cellular proliferation was assessed by measuring the staining of BrdU-positive nuclei in the total luteal area and expressed as a percentage of the area of each corpus luteum. More than 84% of proliferating cells in the corpus luteum are endothelial (35). The number of caspase-3 positive cells in each corpus luteum was counted.
Assays
Plasma concentrations of progesterone were measured as described previously (11). Plasma levels of Dll4 antibody were determined in three marmosets from the nonterminal midluteal phase study and in two animals in the follicular phase group using a two-site ELISA. The capture protein was recombinant macaca fascicularis-mfDll4mychis (REGN545). The antibody used for the standard curve was the same as that present in the sample, antihuman Dll4 (REGN577). The reporter antibody was antihuman Fc linked to horseradish peroxidase. Concentrations of REGN577 were calculated in μg/ml.
Statistical analyses
The significance of differences in plasma progesterone between groups was determined by ANOVA followed by Bonferroni's posttest with P < 0.05 being considered significant. Other parameters were compared using a two-tailed unpaired nonparametric t test with P < 0.05 being taken as the level of significance.
Results
Effect of Dll4 antibody in the periovulatory period on early luteal angiogenesis
Ovaries were collected on luteal d 3. Paired ovarian weights were 309 ± 71 mg in controls and 230 ± 23 mg in Dll4 antibody-treated marmosets, a decrease that was not statistically significant. H&E staining showed that recently formed corpora lutea were present in the ovaries of both treated and control marmosets (e.g. Fig. 1, panels A and B, respectively); numbers of new corpora lutea per animal being two, three, three, and three for the four control animals and one, two, two, and three for the treated group. Luteal area on d 3 was not significantly different between groups (Fig. 1C). The expected quota of antral follicles was also present. Ovaries from three animals in both control and treated groups exhibited some hemorrhagic follicles or corpora lutea.
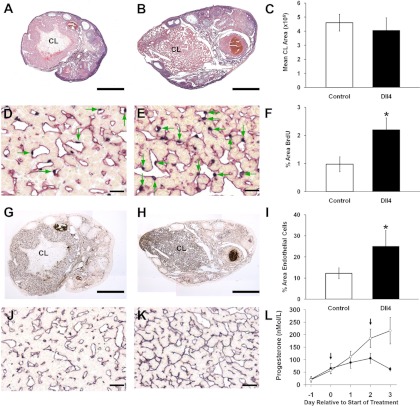
Fig. 1.
Marmoset ovaries stained with H&E after control Fc treatment (panel A) or inhibition of Dll4 during the early luteal phase (panel B). Each ovary exhibits a recently formed corpus luteum and measurement of luteal area (C) showed no significant effect of treatment. Dual staining for BrdU and CD31 (black nuclei, red cytoplasm, respectively) in corpora lutea from, control (panel D) and Dll4 antibody-treated animal (panel E) shows the high proportion of proliferating cells that are endothelial (green arrows) in both groups. Note the increased incidence of these cells in the corpora lutea from the treated marmoset. Histogram (F) shows a significant increase (P < 0.05) in area of BrdU staining after Dll4 treatment. Immunostaining for CD31 in control ovary (panel G), and Dll4 antibody-treated animal (panel H). Both show a well-developed microvasculature, but that in the corpora lutea of the treated marmoset is exceptionally dense, a feature that is depicted in a higher power magnification (J and K). I, The quantification of CD31 immunocytochemistry expressed as percentage area of corpus luteum stained in control (open bar) and treated (closed bar). Dll4 treatment caused a significant (P < 0.05) increase in area of CD31 staining. L, Plasma progesterone concentrations in Fc control (○) and Dll4 antibody-treated marmosets (●). Treatment started on the day of expected ovulation (d 0) and was repeated on d 2. Dll4 antibody treatment was associated with a significant suppression of progesterone by d 3 (n = 4 per group). Scale bar, 500 μm (A, B, G, and H) and 50 μm (D, E, J, and K). CL, Corpus luteum.
Dual staining of BrdU and CD31 localized proliferating endothelial cells in the corpora lutea of both control and treated marmosets (Fig. 1, panels D and E, respectively). However, dual staining was strikingly more prominent in the treated group (Fig. 1E). Quantitative analysis demonstrated a statistically significant increase (P < 0.05) in angiogenesis was associated with Dll4 inhibition (Fig. 1F).
CD31 staining alone revealed the extent of the microvascular tree in the corpora lutea (see low-power magnification in Fig. 1, G and H). Quantification of the area of CD31 staining within each corpus luteum confirmed a significant increase (P < 0.05) on d 3 after anti-Dll4 treatment (Fig. 1I). In the corpora lutea of the Dll4 antibody-treated marmosets, blood vessels appear to have invaded almost every possible space between the hormone-producing cells and appeared more elongate than in controls (Fig. 1, J and K).
Control marmosets exhibited a gradual rise in plasma progesterone concentration typical of the early luteal phase (Fig. 1L). In Dll4 antibody-treated marmosets, plasma progesterone concentrations rose normally on d 1, but began to diverge from controls on d 2 and were significantly lower (P < 0.001) than controls by 3 d after start of treatment (Fig. 1L).
Effect of Dll4 antibody treatment in the periovulatory period on midluteal microvasculature
Ovaries were collected on luteal d 10. Ovarian weight was significantly reduced (P < 0.05) by Dll4 antibody treatment from 306 ± 35 mg in controls to 182 ± 20 mg. Prominent corpora lutea were observed macroscopically on the ovaries of all Fc control marmosets, and this was confirmed by examination of H&E-stained sections (Fig. 2A). Numbers of corpora lutea per animal were three, four, three, and three for the four control animals. In the treated group, one of the marmosets had ovaries with three apparently healthy corpora lutea. However, in the remaining four treated animals no normal appearing, mature corpora lutea were present (Fig. 2B). Rather, two types of luteal tissues were identified, the first characterized by very small, regressed corpora lutea that contained involuted luteal cells with little apoptosis. These were considered to have originated from the previous cycle in which luteolysis was achieved and were not studied further. In addition, larger luteal areas (two, two, two, and three per animal) were observed which were deemed to be the corpora lutea of the treatment cycle that had been responsible for progesterone secretion at the start of the treatment phase. These were significantly smaller (P < 0.05) than the healthy corpora lutea seen in control ovaries (Fig. 2, B and C). H&E staining revealed a degree of involution of the hormone-producing cells and increased incidence of apoptotic fragments (data not shown). This involution could be demonstrated on sections stained with BrdU and counterstained with hematoxylin (Fig. 2, D and E). These sections also show that whereas BrdU staining was present in control corpora lutea predominantly in clearly defined blood vessels (Fig. 2D), in corpora lutea from treated animals BrdU staining was rarely associated with structures that could be defined as normal blood vessels (Fig. 2E). Numbers of proliferating cells were highly variable in corporal lutea of treated marmosets and not significantly different from controls (Fig. 2, E and F). Apoptosis, as confirmed by staining for activated caspase-3, was rare in control corpora lutea (Fig. 2G) and increased significantly (P < 0.0001) in corpora lutea from the treated group (Fig. 2, H and I). CD31 staining revealed that as expected corpora lutea of controls developed an extensive micovascular tree (Fig. 2, J and L) whereas in treated marmosets, CD31 staining was more dense, but diffuse (Fig. 2, K and M) and unsuitable for quantification.
Fig. 2.
Marmoset ovaries on d 10 of the luteal phase stained with H&E after control Fc treatment (panel A) or inhibition of Dll4 during the early luteal phase (panel B). A normal corpus luteum is central to the control ovary whereas in the ovary of the treated animal small corpora lutea are shown (*). C, Quantification of total corpus luteum area was significantly lower in the treated group. Immunohistochemistry for BrdU in corpora lutea from control (panel D) and Dll4 antibody-treated animal (panel E). Note the moderate incidence of proliferating cells (black nuclei) that are endothelial in the control and the healthy appearance of the hormone-producing cells whereas the corpus luteum from the treated marmoset shows increased cell density as a result of involution of hormone-producing cell nuclei and cytoplasm. BrdU stained cells are evident. F, Areas of BrdU staining in both groups were not significantly different. Immunostaining for activated caspase-3 (brown) is demonstrated in control corpus luteum (panel G) and Dll4 antibody-treated animal (panel H). The control shows an isolated positive cell surrounded by healthy cells. In contrast the corpus luteum of the treated marmoset shows numerous positive cells that when quantified (panel I) show a significant increase compared with controls. CD31 staining of whole ovaries is illustrated in ovaries from control (panel J) and after inhibition of Dll4 (panel K). Note the extensive mocrovascular tree in the two corpora lutea in the control ovary whereas staining for CD31 is dense in the treated ovary but the corpora lutea (*) are regressed. N, Plasma progesterone concentrations in Fc control (○) and Dll4 antibody-treated marmosets (●). Treatment started on the day of expected ovulation (d 0) and was repeated on d 2. Dll4 antibody treatment was associated with a significant suppression of progesterone by d 3 that continued until d 10 when ovaries were collected (n = 4 and 5) for Fc and Dll4 treated, respectively. Scale bar, 500 μm (A, B, J, and K) and 50 μm (D, E, G, H, L, and M). CL, Corpus luteum; bv, blood vessel.
Control marmosets exhibited a sustained rise in plasma progesterone concentrations typical of the early luteal to midluteal phase (Fig. 2N). In Dll4-antibody-treated marmosets, progesterone concentrations rose as normal on d 1, confirming the occurrence of ovulation, but began to diverge from controls on d 2 and were significantly lower (P < 0.001) than controls by 3 d after treatment (Fig. 2N). Progesterone remained suppressed for the remainder of the treatment period, being at follicular phase levels after d 7.
Interestingly, on luteal d 10, relatively large antral follicles were present in the ovaries of marmosets treated with anti-Dll4 (see Fig. 5A) but not in control ovaries (Fig. 2A).
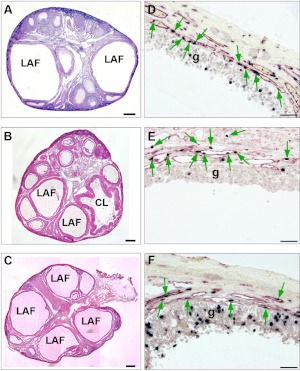
Fig. 5.
A–C, H&E-stained marmoset ovaries showing that large antral follicles (LAF) can develop despite inhibition of Dll4. A, Ovary from a marmoset on d 10 of the luteal phase after treatment with an antibody to Dll4 on luteal d 0 and d 2. Note the presence of two large antral follicles (LAF), in marked contrast to the restricted follicular growth expected at this stage of the cycle (compare with Fig. 2A). B, Ovary from a control marmoset treated with Fc for the duration of the follicular phase. Note the emergence of three large antral follicles and a recently ovulated follicle (CL). C, Ovary after treatment with Dll4 antibody throughout the follicular phase showing emergence of large antral follicles. D–-F, High-power magnifications of the wall of a large antral follicle in respective ovaries shown in panels A–C dual stained with BrdU (black nuclei) and CD31 (red cytoplasm). Note that the development of the vasculature within the thecal wall, shown by CD31 and the proliferating endothelial cells (shown by green arrows) appears to be similar to the control despite treatment with Dll4 antibody. Proliferating cells (black nuclei) in the granulosa cell layer (g) are also evident in each LAF. Scale bar, 500 μm (A–C) and 50 μm (D–F). CL, Corpus luteum.
Effect of Dll4 antibody in the periovulatory period on luteal function and recovery of normal cycles
Control marmosets treated with Fc at the periovulatory period exhibited the expected postovulatory profile in plasma progesterone secretion, with progesterone being elevated for some 21 d (Fig. 3A). In contrast, marmosets treated with Dll4 antibody at the time of ovulation exhibited a transient rise in plasma progesterone after ovulation with levels being suppressed for the remainder of the luteal phase (Fig. 3A), confirming the results obtained in the two previous experiments. Overall, progesterone concentrations were significantly (P < 0.01) suppressed by the treatment. However, on about d 15, toward the end of the luteal phase, plasma progesterone levels began to rise again in the Dll4 antibody-treated marmosets. In view of the presence of large antral follicles by luteal d 10 in animals treated in the periovulatory phase (Fig. 5A), it is likely that this progesterone rise was due to ovulation associated with the early resumption of follicular development. Interestingly, in three of the four treated marmosets, this second progesterone rise was found to be transitory (e.g. Fig. 3B), suggesting that luteal function after the first posttreatment ovulation remained impaired. In every case, these transitory progesterone increases were followed by a follicular phase of normal length and a postovulatory rise in progesterone of normal magnitude and duration (e.g. Fig. 3B).
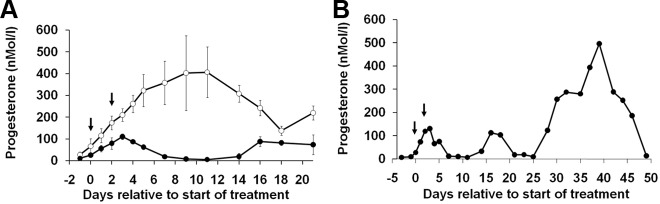
Fig. 3.
A, Effect of treatment with Fc control (○) or Dll4 antibody (●) on the day of expected ovulation (d 0) and d 2 on plasma progesterone concentrations. Dll4 antibody treatment was associated with a significant suppression of progesterone by d 3 after which time progesterone secretion declined to follicular phase concentrations before a rise around d 16. B, A representative progesterone profile from a marmoset treated with Dll4 antibody, illustrating failure of normal luteal progesterone rise followed by an apparent ovulation around d 15, which resulted in a short period of progesterone secretion. This was followed by a normal posttreatment luteal phase beginning on d 28. Fc controls (n = 4) and treated with Dll4 antibody (n = 3). Numbers are means ± sem.
Effect of Dll4 antibody in the midluteal phase Dll4 antibody on luteal function
Control marmosets treated with Fc at the midluteal period continued to have the expected luteal profile in plasma progesterone secretion, being elevated for the duration of the luteal phase (Fig. 4A). Dll4 antibody treatment was without significant effect on the progesterone profile overall. However, closer examination of the data revealed that after initiation of treatment, progesterone levels appeared to decline at a faster rate in marmosets treated with Dll4 antibody, such that values on d 4 and d 5 after treatment were significantly lower (P < 0.05) than in controls. In three of four treated marmosets, a progesterone rise indicative of ovulation occurred, some 10 d after progesterone had reached follicular phase levels (i.e. indicative of a follicular phase of normal duration) (Fig. 4B). In the remaining animals, the progesterone rise was short lived, but was followed by a normal progesterone profile after a further 10-d period.
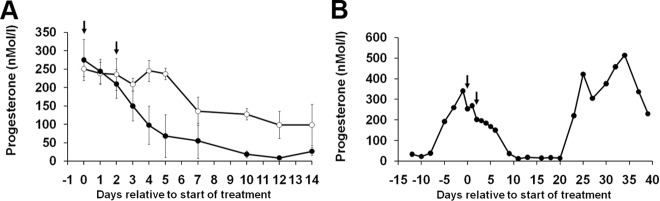
Fig. 4.
A. Effect of treatment with Fc control (○) or Dll4 antibody (●) on d 8 and d 10 of the luteal phase on plasma progesterone concentrations. Dll4 antibody treatment was associated with a significant suppression of progesterone on d 4 and d 5 after treatment. B, A representative progesterone profile from a marmoset treated with Dll4 antibody to show luteal regression was followed by a follicular phase of normal duration and progesterone secretion associated with normal ovulation (n = 4 per group). Numbers are means ± sem.
Plasma from this group confirmed high concentrations of antibody to Dll4. When antibody was administered by two injections 2 d apart, plasma concentrations were consistently more than 10 μg/ml on d 1, d 2, d 3, and d 7 and between 3–11 μg/ml on d 14 before falling to less than 1 μg/ml by d 20.
Effects of Dll4 antibody on follicular angiogenesis and development
In contrast to the clear evidence for induction of early luteal regression in animals treated with anti-Dll4 during the early periovulatory period, H&E-stained sections of ovaries collected during the midluteal phase revealed an additional striking difference to ovaries of control marmosets. Whereas control ovaries contained only small antral follicles (e.g. Fig. 2A), the ovaries of the four marmosets with regressed corpora lutea contained two or three healthy large antral follicles (e.g. Fig. 5A). Moreover, in the treated marmoset having corpora lutea that had yet to fully regress, one ovary contained two ovulating follicles, whereas the other had one ovulating follicle. The vasculature of these antral and ovulating follicles appeared normal. This observation suggested that, in contrast to the corpus luteum, follicular vascular development was not affected by pharmacological inhibition of Dll4. To test this hypothesis, a final experiment was conducted to investigate the effects of Dll4 antibody treatment over the duration of the follicular phase.
When evaluated on follicular d 10, weights of ovaries were not significantly different between control (290 ± 61 mg) and treated animals (226 ± 17 mg). In controls, the predominant structures on follicular d 10 were preovulatory follicles (n = 10) or recently ovulated follicles (n = 8) (e.g. Fig. 5B). Treatment with anti-Dll4 for the duration of the follicular phase had no major effect on follicular development. Three marmosets had ovaries containing large antral, preovulatory follicles (n = 3, 2, and 2) with proliferating cells in the granulosa cell layer (e.g. Fig. 5, C and D), whereas one had five recently ovulated follicles. In the remaining treated animal the ovaries were more typical of the midfollicular phase, containing numerous medium-sized antral follicles. Examination of sections dual stained with BrdU and CD31 revealed that in the thecal layer of large antral follicles from the group treated with Dll4 antibody the pattern of CD31 staining and BrdU incorporation appeared similar to that in controls (Fig. 5, D–F).
By d 10, progesterone levels had risen above follicular phase levels in four of the controls (61 ± 25 nmol/liter) and in one of the treated animals (14 ± 4 nmol/liter), a difference that was statistically significant (P < 0.02).
When plasma concentrations of antibody to Dll4 were examined on d 3, d 5, d 7, and d 10 after treatment on d 0, d 3, and d 7, they were found to be consistently more than 20 μg/ml.
Discussion
VEGF has been shown to play a critical role in normal ovarian angiogenesis and function (1–6), but the requirement for other factors with the potential to regulate the morphogenesis of the vasculature in this tissue remains to be fully elucidated (4, 23, 42). To date, pharmacological manipulation of these other factors has not produced the marked impact on ovarian angiogenesis seen for VEGF antagonists, at least not in primates. We now show that Dll4 also plays an indispensable physiological role in ovarian angiogenesis and function. However, in contrast to VEGF, Dll4 appears to play a crucial role in normal luteal, but not follicular, angiogenesis.
Treatment with the Dll4-neutralizing antibody in the periovulatory period resulted in the failure of the ovary to secrete its normal quota of progesterone. In the luteal microvasculature, the number of proliferating endothelial cells was increased by treatment and, as a result, the area occupied by the endothelium in the corpora lutea was significantly increased by d 3 after treatment. Given that the early corpus luteum is already the most active site of physiological angiogenesis in the adult (1), the resultant increase in endothelial proliferation after neutralization of Dll4 is remarkable. Increasing vasculature in the corpus luteum is normally associated with efficient secretion of progesterone (1–3), and these findings are the first to dissociate this relationship. From studies on the effects of Dll4 inhibition in tumor models, it may be assumed that the paradoxical decline in progesterone secretion is the result of the newly formed vessels being nonfunctional (29–31). Surprisingly, in the marmosets studied further, for 10 d into the luteal phase, the treatment was found to have a marked deleterious effect on the structure of both vascular and nonvascular elements in the corpus luteum. In keeping with the previous observation, the functional capacity of the tissue to secrete progesterone remained markedly impaired; by 7 d into the luteal phase, plasma progesterone had fallen to follicular phase levels in all treated marmosets, a potential indicator of premature luteolysis.
In confirmation, morphological analysis of the corpora lutea revealed premature structural luteolysis in four of the five treated marmosets. The corpora lutea were small in size with hormone-producing cells exhibiting shrinkage of the nucleus and cytoplasm. These changes were also associated with increased cell death. The incidence of caspase-3 staining was variable, but because apoptosis is a rapid phenomenon, in contrast to other features of luteal involution that take place over a longer period in primates (43–46), it is possible that the variation in caspase-3 reflects asynchronous structural luteolysis. However, in respect to the fall in progesterone, all treated marmosets exhibited a similar profile.
The observation of large antral follicles on luteal d 10 in marmosets treated with anti-Dll4 during the periovulatory period was a particularly unanticipated finding. In control marmosets, the presence of very small antral follicles is a feature during the midluteal phase. However, evidence of follicular development to the preovulatory stage was evident in four marmosets treated with Dll4 antibody, whereas the remaining animal had three recently ovulated follicles. This suggests that 1) the removal of the normal negative feedback effect of luteal progesterone is the principal trigger for follicular development in the Dll4 antibody treated animals, as it is in the normal cycle; and 2) in contrast to luteal angiogenesis, Dll4 inhibition did not appreciably impair angiogenesis requisite for the development of large antral follicles (5, 16, 19, 20).
Two further experiments supported the observation that follicular development could proceed to ovulation despite pharmacological neutralization of endogenous Dll4. First, treatment throughout the follicular phase did not prevent the development of preovulatory or recently ovulated follicles. Second, when marmosets were given Dll4 antibody in the periovulatory period in a nonterminal study, a progesterone rise occurred around 16 d after treatment in three of four marmosets. Given the fact that this treatment regimen causes rapid functional and structural luteolysis, it is likely that this rise was indicative of ovulation that occurred as a consequence of development of ovulatory follicles during the treatment period. The fact that this rise was only transitory in this first, aborted recovery cycle suggests that the low levels of Dll4 antibody present in the circulation at this time were sufficient to again induce abnormal sprouting angiogenesis and subsequent luteolysis. The resumption of normal ovulatory cycles, as judged by the restitution of luteal phases characterized by sustained elevations in plasma progesterone of normal magnitude and duration, was observed in treated animals shortly after circulating concentrations of anti-Dll4 fell to nondetectable levels.
By around d 8 after ovulation, the intense angiogenesis of the early luteal phase has subsided and the luteal microvascular tree is established (14, 35). It had been anticipated that administering Dll4 antibody during this postangiogenic phase would not affect luteal function. Indeed, plasma progesterone concentrations were unaffected for the first 3 d after treatment was initiated, but after d 4 there was a trend to more rapid decrease in progesterone levels. Taken together with the observations of structural luteolysis on d 10 after treatment in the early luteal phase this indicates that inhibition of Dll4 also exerts a deleterious effect on luteal function, and possibly structure, at later stages of luteal development, albeit to a much lesser extent.
Having previously described the effects of inhibition of VEGF on both follicular and luteal angiogenesis using the same treatment regimens (10, 11, 14–16) these results can be compared with the inhibition of Dll4. Whereas inhibition of either VEGF or Dll4 leads to disruption of the normal ovarian cycle, there are major differences in outcome with respect to effects on follicular and luteal angiogenesis, development, morphology, and cell viability. With respect to effects on follicular development, Aflibercept (VEGF Trap) treatment markedly inhibited endothelial cell proliferation in the thecal vessels and prevented development of follicles beyond the early antral stage (11, 16). In contrast, Dll4 antibody treatment appeared to have no major effect on follicular angiogenesis, and preovulatory follicles emerged despite the continuous presence of high levels of anti-Dll4 antibody in the blood for the duration of the follicular phase. VEGF is known to induce endothelial proliferation and migration, most typically in the context of angiogenic sprouting from existing vessels (29–31). However, VEGF also plays a key role in promoting other forms of angiogenesis, such as increase in the caliber of existing or undifferentiated vessels, followed by the formation of daughter vessels by intussusception (47, 48). In sprouting angiogenesis, Dll4 activity is known to be up-regulated by VEGF and to act downstream of the initial angiogenic drive to control vessel proliferation and branching (26), by negatively regulating tip cell formation as well as the proliferation of the more proximal stalk endothelial cells (27, 28). Timely development of the luteal vasculature is highly dependent upon initiation of a robust, well-organized sprouting angiogenic response to rapidly invest the avascular, newly formed luteal body with a blood supply. However, in addition to sprouting angiogenesis, follicular vascular expansion involves other forms of angiogenesis, such as vessel expansion and intussusception (49). The results of the present study suggest that, in contrast to VEGF, Dll4 does not play a significant role in these other forms of angiogenesis.
Although VEGF or Dll4 inhibition both disrupted normal luteal development and function, their specific effects on luteal angiogenesis were strikingly different. Inhibition of VEGF during the early luteal phase reduces endothelial cell proliferation by 90%, leading to a decrease in endothelial cell area and associated decline in plasma progesterone secretion (10, 11). In marked contrast, inhibition of Dll4 over the same period led to an increase in endothelial cell proliferation, sprouting angiogenesis, and endothelial cell area. However, this treatment too had a suppressive effect on plasma progesterone levels. In marmosets, administration of VEGF antibody during the luteal phase was associated with predominately healthy hormone-producing cells (10). This contrasts markedly with Dll4 inhibition, which resulted in hormone-producing cells in various states of regression. Thus, whereas inhibition of VEGF appeared to leave the corpus luteum in a resting state, inhibition of Dll4 led to cell death. The explanation for these events is likely to be similar to that proposed to explain the effects of Dll4 blockade in experimental models of solid tumor growth. Here too, the vascular sprouting, endothelial cell proliferation, and vessel density within the tumors are all increased, but the tumors paradoxically decrease in size, effects attributable to the overelaboration of poorly formed vessels that show reduced perfusion (29–31). The results of the present study suggest that a similar scenario obtains in the developing luteal vasculature.
Our conclusion, that inhibition of Dll4 has major effects on early luteal angiogenesis and subsequent luteal function, is supported by emerging studies of the Notch-signaling pathway in rodents. Intrabursal administration of a γ-secretase inhibitor, which nonselectively inhibits the Notch-signaling pathway, decreased progesterone secretion and increased caspase-3 in the corpora lutea of pregnant rats (39). Furthermore, the observation that follicular angiogenesis is spared after Dll4 inhibition, compared with effects on the corpus luteum, is supported by studies in mice recently reported in abstract form (50, 51).
Further studies will be required to more fully elucidate the role of Dll4 in the function of the hypothalamic-pituitary-ovarian axis in primates; these studies will likely require the use of an alternate primate species because accurate measurement of estrogens and pituitary gonadotropins is problematic in the marmoset. Observations on effects of treatment on plasma estradiol would be of particular importance in determining whether the appearance of normal follicular development and angiogenesis, as observed in the present study, are associated with normal follicular steroidogenic capacity. However, it should be noted that we found no appreciable effect of Dll4 inhibition on uterine weight or angiogenesis in the marmoset uterus, when evaluated in the late proliferative and early luteal phase groups (data not shown), suggesting that the effects of acute Dll4 inhibition on follicular steroidogenesis, if any, are likely to be subtle. Here it is also important to note that, at present, there is little evidence to indicate that acute inhibition of Dll4/Notch function, as employed in the present study, would produce an appreciable affect on the morphology of the mature, quiescent vasculature of most organs. In contrast, stringent, chronic inhibition of Dll4/Notch signaling with high-affinity antibodies (52) or gene deletions can lead to vascular abnormalities in some tissues (53, 54). Thus longer-term treatment may also be required to fully elucidate the role of the Dll4/Notch pathway in other components of the reproductive axis.
That in vivo inhibition of Dll4 in mouse tumor models leads to decreased tumor growth even though tumor vascularity is increased, has been a major discovery in oncology (30–32). The present findings on the effects of Dll4 blockade on the corpus luteum replicates this paradoxical phenomenon in a normal adult tissue, whereby increased angiogenesis is associated with structural regression. However, the effects of Dll4 inhibition on angiogenesis are clearly heterogeneous, at least in normal tissues, as evidenced by the lack of appreciable effect of Dll4 blockade on follicular development. Here, we propose that the marked difference in the susceptibility of luteal and follicular angiogenesis to Dll4 inhibition may reflect the predominance of sprouting angiogenesis in the developing corpus luteum and nonsprouting forms of VEGF-mediated angiogenesis in the maturing follicle. Such observations emphasize the need to rigorously study the effects of novel modulators of angiogenesis on normal tissues, to more fully elucidate the physiological roles of their targets.
Acknowledgments
We thank Mike Millar and Sheila McPherson, Medical Research Council Human Reproductive Sciences Unit, for valuable assistance with histology, staff of the R.V. Short Building, Medical Research Council Human Reproductive Science Unit, for animal care, and Donna Hylton, Regeneron Pharmaceuticals, for assistance in conducting the Dll4 antibody ELISA.
This work was supported by the core grant (U.1276.00.002.00003.01) to Medical Research Council Human Reproductive Sciences Unit (to H.M.F.).
Disclosure Summary: J.S.R. and S.J.W. are employed by and hold an equity interest in Regeneron Pharmaceuticals, Inc. H.M.F., J.M.H., D.A., and K.R.M. have nothing to declare.
Footnotes
- BrdU
- Bromodeoxyuridine
- Dll4
- delta-like ligand 4
- H&E
- hematoxylin and eosin
- NBT
- nitroblue tetrazolium
- PG
- prostaglandin
- VEGF
- vascular endothelial growth factor.
References
- 1. Reynolds LP, Killilea SD, Redmer DA. 1992. Angiogenesis in the female reproductive system. FASEB J 6:886–892 [PubMed] [Google Scholar]
- 2. Hazzard TM, Stouffer RL. 2000. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol 14:883–900 [DOI] [PubMed] [Google Scholar]
- 3. Stocco C, Telleria C, Gibori G. 2007. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- 4. Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. 2009. Angiogenesis and vascular function in the ovary. Reproduction 138:869–881 [DOI] [PubMed] [Google Scholar]
- 5. Fraser HM, Duncan WC. 2009. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod Fertil Dev 21:377–392 [DOI] [PubMed] [Google Scholar]
- 6. Kaczmarek MM, Schams D, Ziecik AJ. 2005. Role of vascular endothelial growth factor in ovarian physiology-an overview. Reprod Biol 5:111–136 [PubMed] [Google Scholar]
- 7. Delgado-Rosas F, Gaytán M, Morales C, Gómez R, Gaytán F. 2009. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum Reprod 24:1142–1151 [DOI] [PubMed] [Google Scholar]
- 8. Gómez R, Simón C, Remohi J, Pellicer A. 2002. Vascular endothelial growth factor receptor 2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology 143:4339–4348 [DOI] [PubMed] [Google Scholar]
- 9. Rodewald M, Herr D, Duncan WC, Fraser HM, Hack G, Konrad R, Gagsteiger F, Kreienberg R, Wulff C. 2009. Molecular mechanisms of ovarian hyperstimulation syndrome: paracrine reduction of endothelial claudin 5 by hCG in vitro is associated with increased endothelial cell permeability. Hum Reprod 24:1191–1199 [DOI] [PubMed] [Google Scholar]
- 10. Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. 2000. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology 141:995–1000 [DOI] [PubMed] [Google Scholar]
- 11. Wulff C, Wilson H, Rudge JS, Wiegand SJ, Lunn SF, Fraser HM. 2001. Luteal angiogenesis: prevention and intervention by treatment with vascular endothelial growth factor trap A40. J Clin Endocrinol Metab 86:3377–3386 [DOI] [PubMed] [Google Scholar]
- 12. Fraser HM, Wilson H, Rudge JS, Wiegand SJ. 2005. Single injections of vascular endothelial growth factor trap block ovulation in the macaque and produce a prolonged, dose-related suppression of ovarian function. J Clin Endocrinol Metab 90:1114–1122 [DOI] [PubMed] [Google Scholar]
- 13. Fraser HM, Wilson H, Morris KD, Swanston I, Wiegand SJ. 2005. Vascular endothelial growth factor Trap suppresses ovarian function at all stages of the luteal phase in the macaque. J Clin Endocrinol Metab 90:5811–5818 [DOI] [PubMed] [Google Scholar]
- 14. Fraser HM, Wilson H, Wulff C, Rudge JS, Wiegand SJ. 2006. Administration of vascular endothelial growth factor Trap during the 'post-angiogenic' period of the luteal phase causes rapid functional luteolysis and selective endothelial cell death in the marmoset. Reproduction 132:589–600 [DOI] [PubMed] [Google Scholar]
- 15. Taylor PD, Wilson H, Hillier SG, Wiegand SJ, Fraser HM. 2007. Effects of inhibition of vascular endothelial growth factor at time of selection on follicular angiogenesis, expansion, development and atresia in the marmoset. Mol Hum Reprod 13:729–736 [DOI] [PubMed] [Google Scholar]
- 16. Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. 2002. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology 143:2797–2807 [DOI] [PubMed] [Google Scholar]
- 17. Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. 1998. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 4:336–340 [DOI] [PubMed] [Google Scholar]
- 18. Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. 2001. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab 86:768–772 [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann RC, Xiao E, Bohlen P, Ferin M. 2002. Administration of antivascular endothelial growth factor receptor 2 antibody in the early follicular phase delays follicular selection and development in the rhesus monkey. Endocrinology 143:2496–2502 [DOI] [PubMed] [Google Scholar]
- 20. Zimmermann RC, Hartman T, Kavic S, Pauli SA, Bohlen P, Sauer MV, Kitajewski J. 2003. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. J Clin Invest 112:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hazzard TM, Xu F, Stouffer RL. 2002. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkey. Biol Reprod 67:1305–1312 [DOI] [PubMed] [Google Scholar]
- 22. Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, Sauer MV, Kitajewski J, Zimmermann RC. 2005. The vascular endothelial growth factor (VEGF)/VEGF receptor pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology 146:1301–1311 [DOI] [PubMed] [Google Scholar]
- 23. Clapp C, Thebault S, Jeziorski MC, Martínez De La Escalera G. 2009. Peptide hormone regulation of angiogenesis. Physiol Rev 89:1117–1215 [DOI] [PubMed] [Google Scholar]
- 24. Bicknell R, Harris AL. 2004. Novel angiogenic signalling pathways and vascular targets. Annu Rev Pharmacol Toxicol 44:219–238 [DOI] [PubMed] [Google Scholar]
- 25. Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. 2004. Haploinsufficiency δ-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101:15949–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. 2007. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104:3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445:776–780 [DOI] [PubMed] [Google Scholar]
- 28. Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. 2007. The Notch ligand δ-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104:3225–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. 2006. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444:1032–1037 [DOI] [PubMed] [Google Scholar]
- 30. Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. 2006. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–1087 [DOI] [PubMed] [Google Scholar]
- 31. Thurston G, Noguera-Troise I, Yancopoulos GD. 2007. The δ paradox: DLL4. blockade leads to more tumor vessels but less tumour growth. Nat Rev Cancer 7:327–331 [DOI] [PubMed] [Google Scholar]
- 32. Thurston G, Kitajewski J. 2008. VEGF and δ-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer 99:1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christenson LK, Stouffer RL. 1996. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology 137:367–374 [DOI] [PubMed] [Google Scholar]
- 34. Gaytán F, Morales C, García-Pardo L, Reymundo C, Bellido C, Sánchez-Criado JE. 1999. A quantitative study of changes in the human corpus luteum microvasulature during the menstrual cycle. Biol Reprod 60:914–919 [DOI] [PubMed] [Google Scholar]
- 35. Dickson SE, Fraser HM. 2000. Inhibition of early luteal angiogenesis by gonadotropin-releasing hormone antagonist treatment in the primate. J Clin Endocrinol Metab 85:2339–2344 [DOI] [PubMed] [Google Scholar]
- 36. Wulff C, Dickson SE, Duncan WC, Fraser HM. 2001. Angiogenesis in the human corpus luteum: simulated early pregnancy by HCG treatment is associated with both angiogenesis and vessel stabilization. Hum Reprod 16:2515–2524 [DOI] [PubMed] [Google Scholar]
- 37. Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J. 2001. Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev 109:355–361 [DOI] [PubMed] [Google Scholar]
- 38. Vorontchikhina MA, Zimmermann RC, Shawber CJ, Tang H, Kitajewski J. 2005. Unique patterns of Notch1, Notch4 and jagged 1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expr Patterns 5:701–709 [DOI] [PubMed] [Google Scholar]
- 39. Hernandez F, Peluffo MC, Stouffer RL, Irusta G, Tesone M. 2011. Role of the DLL4-Notch system in PGEF2 α induced luteolysis in the pregnant rat. Biol Reprod 84:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mazella J, Liang S, Tseng L. 2008. Expression of delta-like protein 4 in the human endometrium. Endocrinology 149:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bray SJ. 2006. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689 [DOI] [PubMed] [Google Scholar]
- 42. Xu F, Stouffer RL. 2005. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod 72:1352–1358 [DOI] [PubMed] [Google Scholar]
- 43. Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sánchez-Criado JE, Gaytán F. 2000. Different patterns of structural luteolysis in the human corpus luteum of menstruation. Hum Reprod 15:2119–2128 [DOI] [PubMed] [Google Scholar]
- 44. Young FM, Illingworth PJ, Lunn SF, Harrison DJ, Fraser HM. 1997. Cell death during luteal regression in the marmoset monkey (Callithrix jacchus). J Reprod Fertil 111:109–119 [DOI] [PubMed] [Google Scholar]
- 45. Fraser HM, Lunn SF, Harrison DJ, Kerr JB. 1999. Luteal regression in the primate: different forms of cell death during natural and gonadotropin-releasing hormone antagonist or prostaglandin analogue-induced luteolysis. Biol Reprod 61:1468–1479 [DOI] [PubMed] [Google Scholar]
- 46. Rowe AJ, Morris KD, Bicknell R, Fraser HM. 2002. Angiogenesis in the corpus luteum of early pregnancy in the marmoset and the effects of vascular endothelial growth factor immunoneutralization on establishment of pregnancy. Biol Reprod 67:1180–1188 [DOI] [PubMed] [Google Scholar]
- 47. Baum O, Suter F, Gerber B, Tschanz SA, Buergy R, Blank F, Hlushchuk R, Djonov V. 2010. VEGF-A promotes intussusceptive angiogenesis in the developing chicken chorioallantoic membrane. Microcirculation 17:447–457 [DOI] [PubMed] [Google Scholar]
- 48. Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. 2000. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability/vascular endothelial growth factor. Lab Invest 80:99–115 [DOI] [PubMed] [Google Scholar]
- 49. Macchiarelli G, Jiang JY, Nottola SA, Sato E. 2006. Morphological patterns of angiogenesis in ovarian follicle capillary networks. A scanning electron microscopy study of corrosion cast. Microsc Res Tech 69:459–468 [DOI] [PubMed] [Google Scholar]
- 50. Garcia C, Gomez R, Kitajewski J, Carrie S, Pellicer A, Zimmermann RC. 2011. Notch (N1) signalling regulates sprouting angiogenesis (SA) in corpora lutea (CL) through tip cells (TC) and stalk cells (SC). 58th Annual Meeting SGI, Miami Beach, FL, 2011, Suppl Rep Sci 18:O-060, p 173A [Google Scholar]
- 51. Levine BA, Jovanovic V, Sauer CM, Shawber CJ, Gomez R, Sauer MV, Kitajewski J, Zimmermann RC. 2011. Role of Notch in gonadotropin-dependent folliculogenesis in the rodent. 58th Annual Meeting SGI, Miami Beach, FL, 2011, Suppl Rep Sci 18:S-163 p 341A [Google Scholar]
- 52. Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. 2010. Chronic Dll4 blockade induces vascular neoplasms. Nature 463:E6–E7 [DOI] [PubMed] [Google Scholar]
- 53. Liu Z, Turkoz A, Jackson EN, Corbo JC, Engelbach JA, Garbow JR, Piwnica-Worms DR, Kopan R. 2011. Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J Clin Invest 121:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ryeom SW. 2011. A cautionary tale of side effects of Notch 1 inhibition. J Clin Invest 121:508–509 [DOI] [PMC free article] [PubMed] [Google Scholar]