Abstract
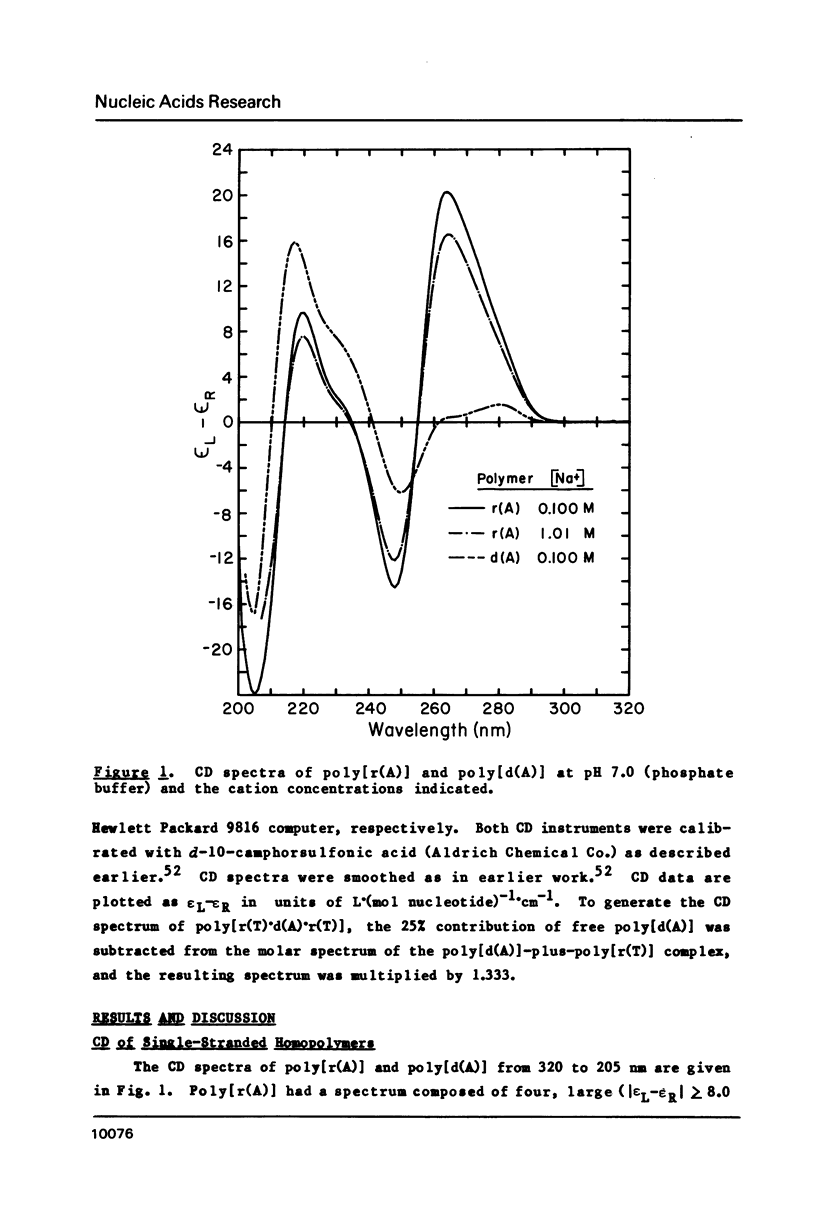
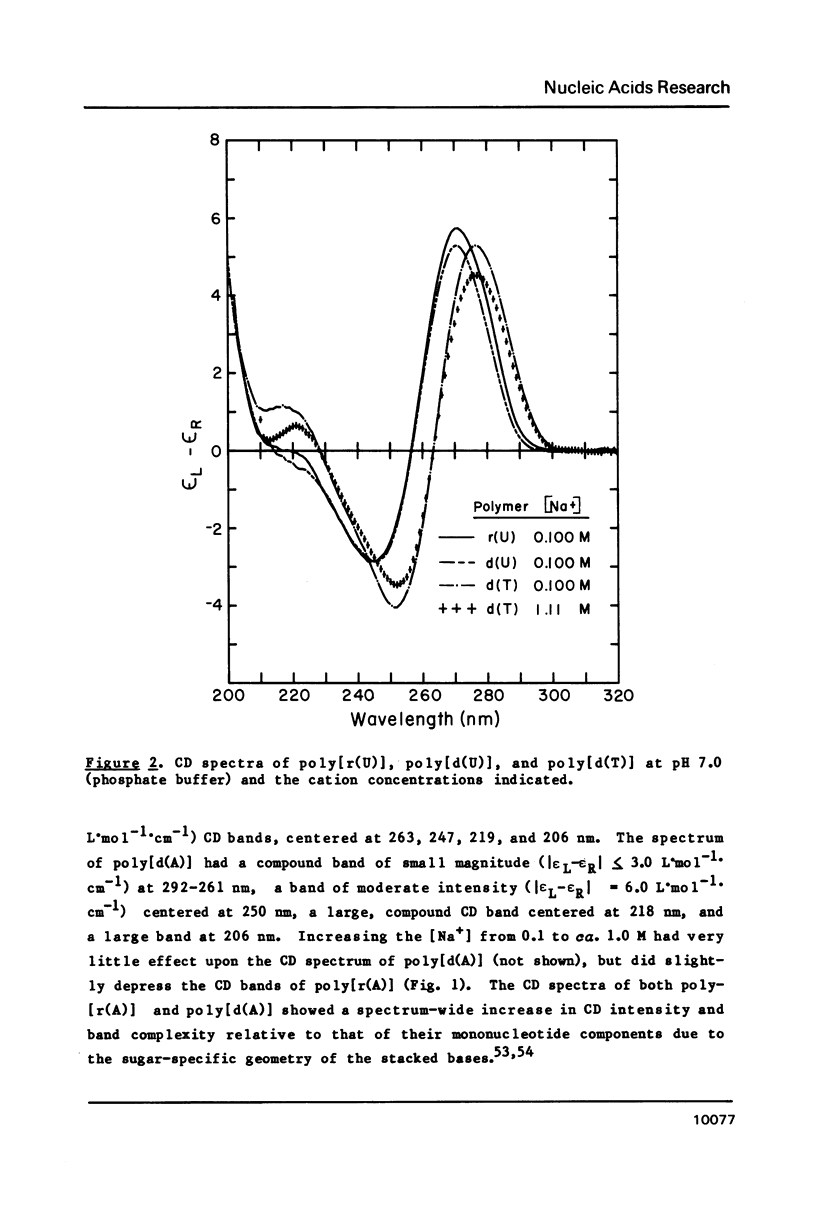
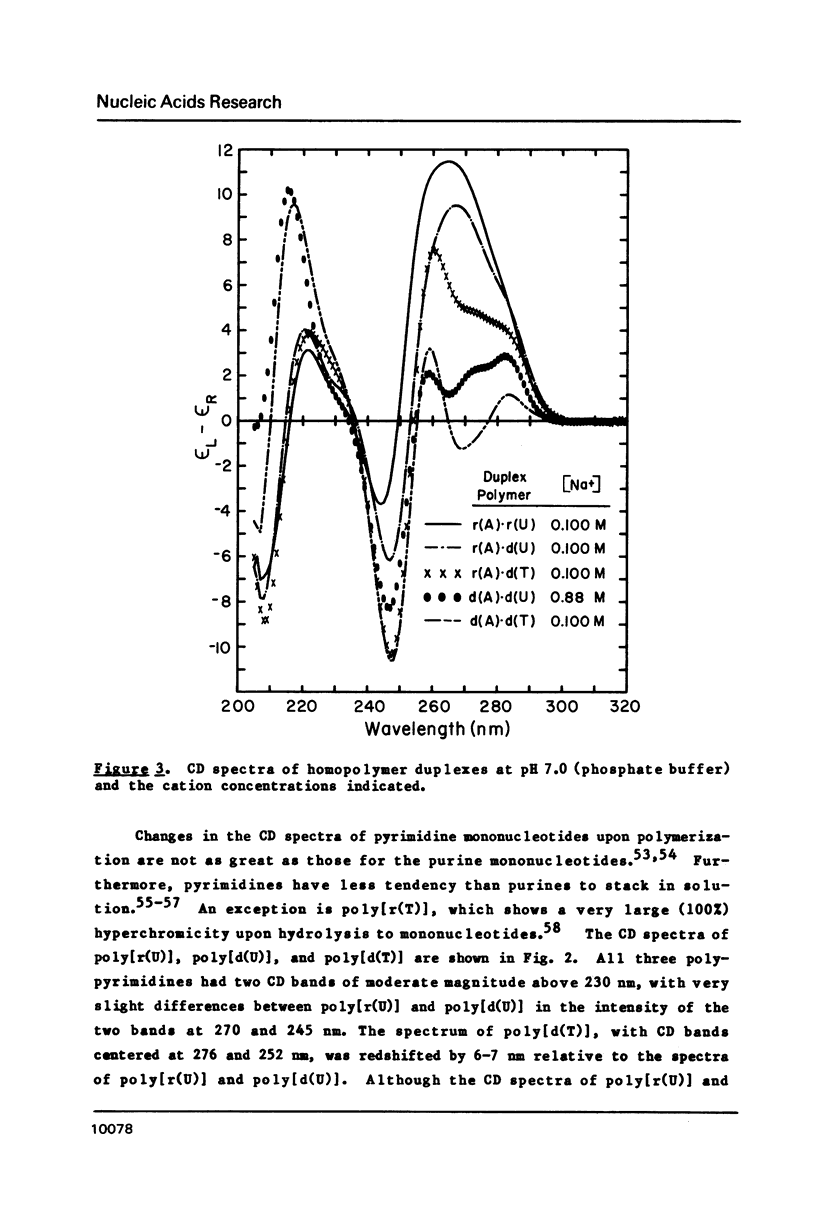
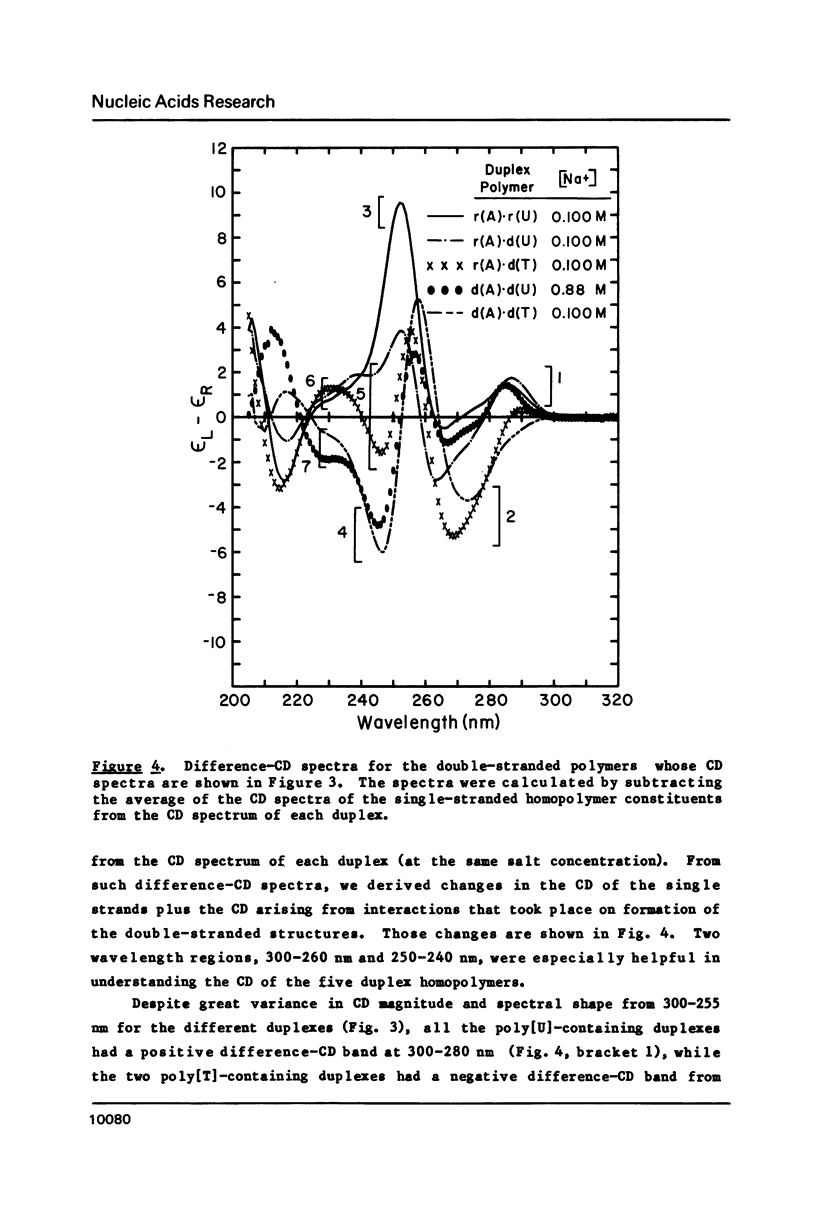
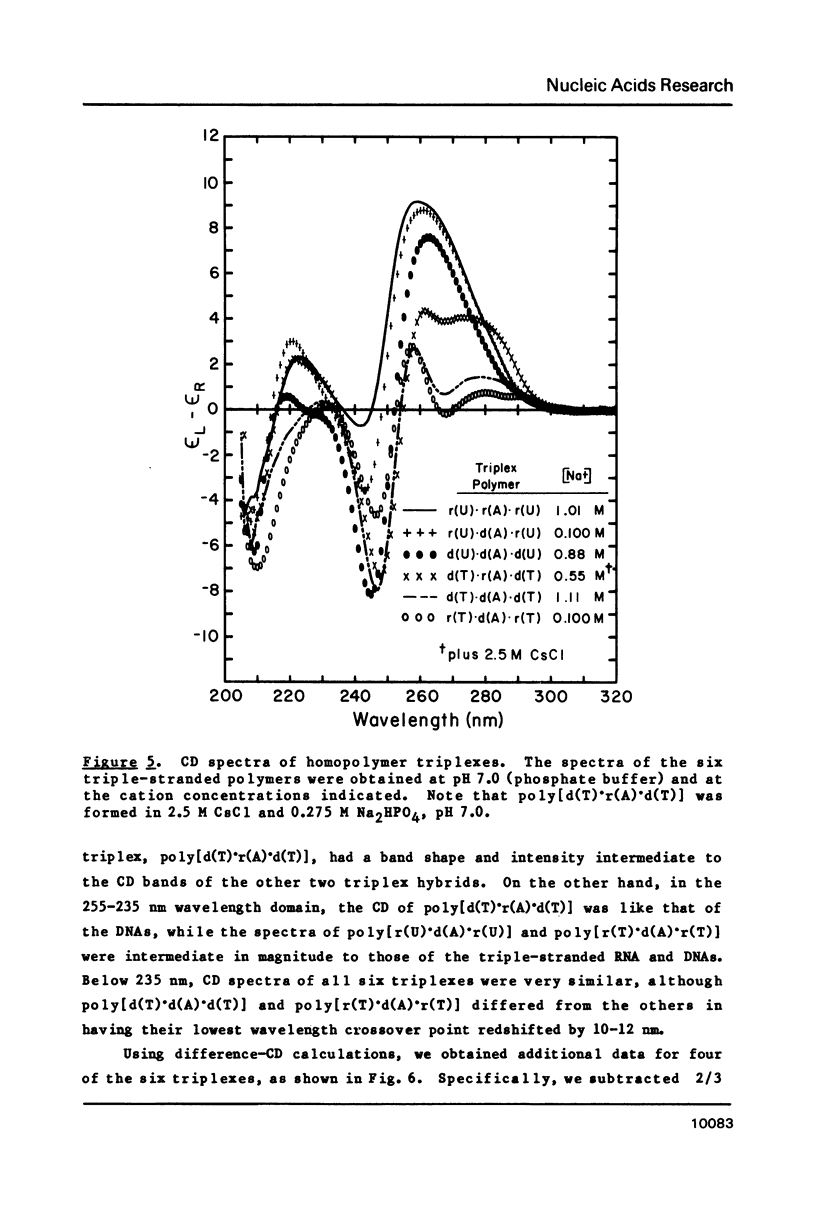
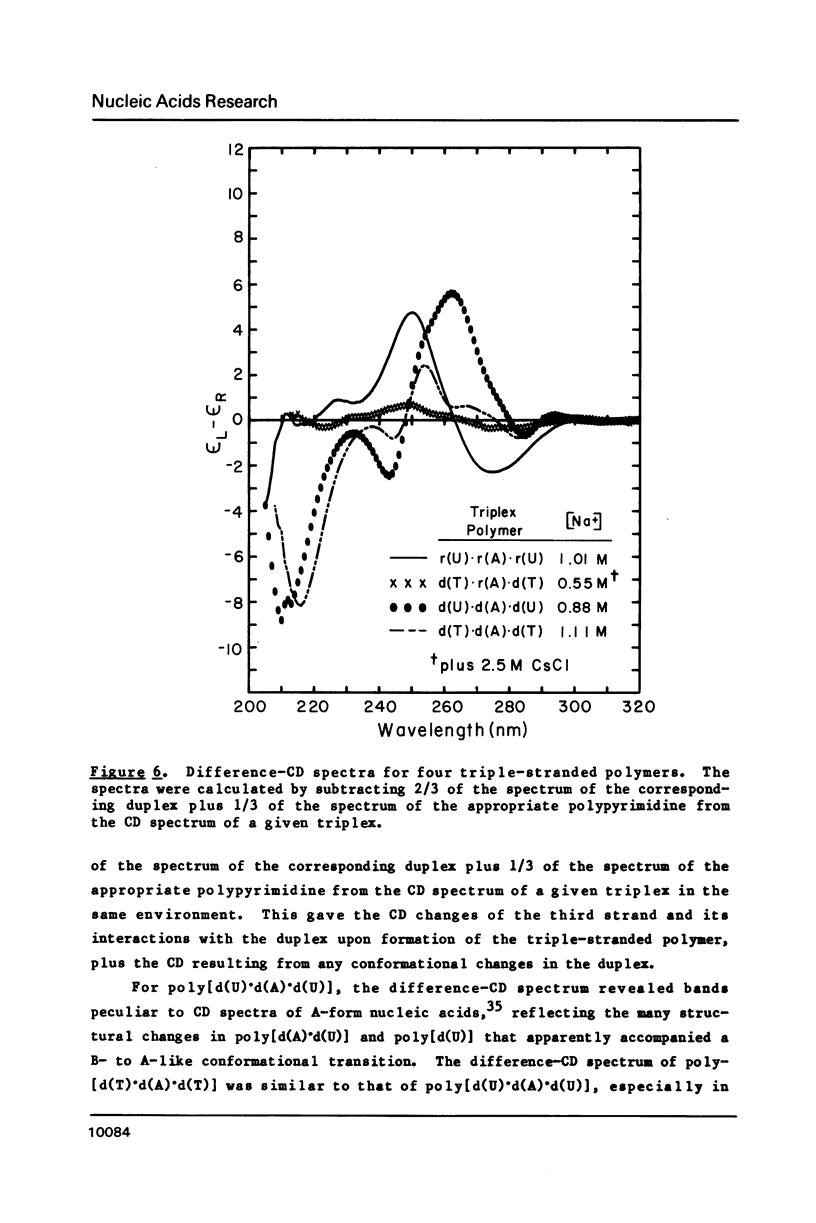
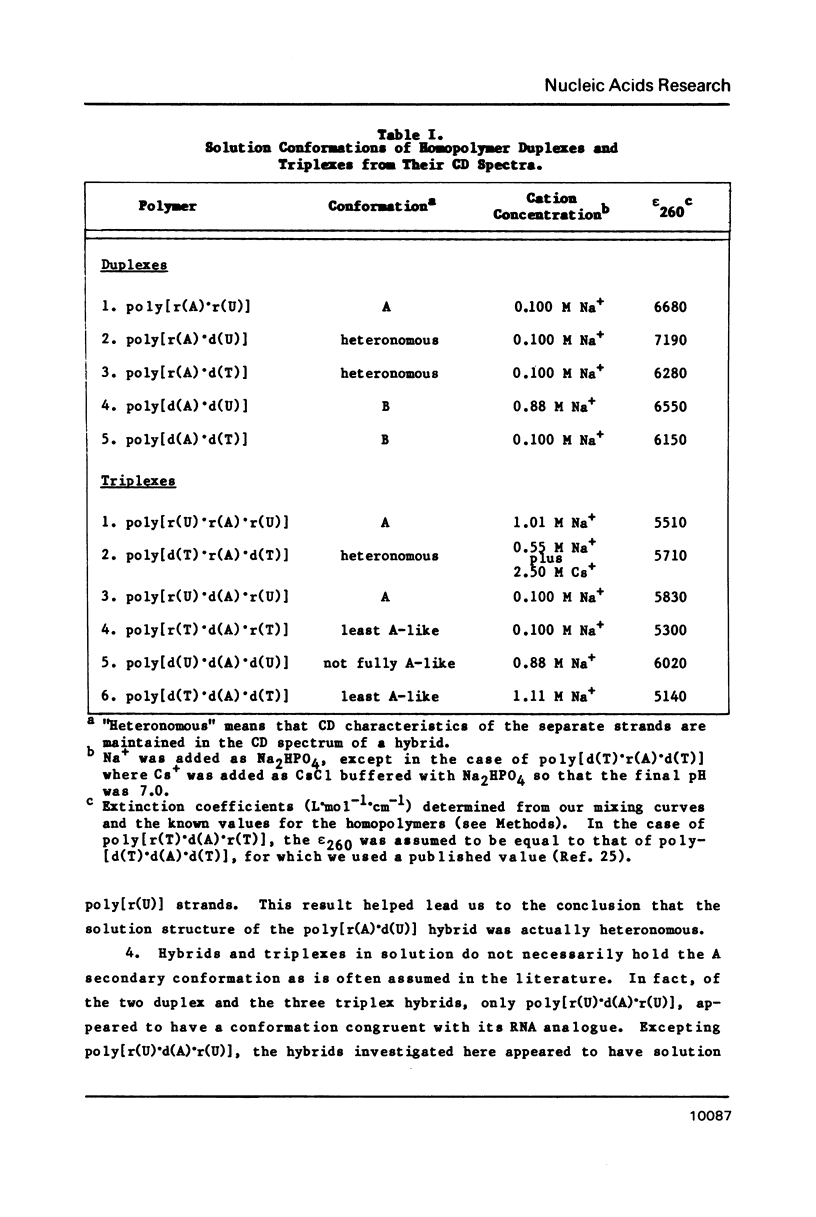
CD spectra and difference-CD spectra of (a) two DNA X RNA hybrid duplexes (poly[r(A) X d(U)] and poly[r(A) X d(T)]) and (b) three hybrid triplexes (poly-[d(T) X r(A) X d(T)], poly[r(U) X d(A) X r(U)], and poly[r(T) X d(A) X r(T)]) were obtained and compared with CD spectra of six A X U- and A X T-containing duplex and triplex RNAs and DNAs. We found that the CD spectra of the homopolymer duplexes above 260 nm were correlated with the type of base pair present (A-U or A-T) and could be interpreted as the sum of the CD contributions of the single strands plus a contribution due to base pairing. The spectra of the duplexes below 235 nm were related to the polypurine strands present (poly-[r(A)] or poly[d(A)]). We interpret the CD intensity in the intermediate 255-235 nm region of these spectra to be mainly due to stacking of the constituent polypurine strands. Three of the five hybrids (poly[r(A) X d(U)], poly[r(A) X d(T)], and poly[d(T) X r(A) X d(T)]) were found to have heteronomous conformations, while poly[r(U) X d(A) X r(U)] was found to be the most A-like and poly[r(T) X d(A) X r(T)], the least A-like.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Bond P. J., Selsing E., Smith P. J. Models of triple-stranded polynucleotides with optimised stereochemistry. Nucleic Acids Res. 1976 Oct;3(10):2459–2470. doi: 10.1093/nar/3.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Bond P. J. Structures for Poly(U)-poly(A)-poly(U)triple stranded polynucleotides. Nat New Biol. 1973 Jul 25;244(134):99–101. doi: 10.1038/newbio244099a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Millane R. P., Park H. S. DNA-RNA hybrid secondary structures. J Mol Biol. 1986 Apr 20;188(4):631–640. doi: 10.1016/s0022-2836(86)80011-0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W., Dover S. D., Fuller W., Hodgson A. R. Structures of synthetic polynucleotides in the A-RNA and A'-RNA conformations: x-ray diffraction analyses of the molecular conformations of polyadenylic acid--polyuridylic acid and polyinosinic acid--polycytidylic acid. J Mol Biol. 1973 Dec 5;81(2):107–122. doi: 10.1016/0022-2836(73)90183-6. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Barszcz D., Shugar D. Complexes of poly-ribothymidylic acid with poly-adenylic acids and some properties of poly-deoxyriboadenylic acid. Eur J Biochem. 1968 Jun;5(1):91–100. doi: 10.1111/j.1432-1033.1968.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Behling R. W., Kearns D. R. 1H two-dimensional nuclear Overhauser effect and relaxation studies of poly(dA).poly(dT) Biochemistry. 1986 Jun 3;25(11):3335–3346. doi: 10.1021/bi00359a037. [DOI] [PubMed] [Google Scholar]
- Behling R. W., Kearns D. R. Determination of NOE "silent" dipolar interactions between magnetically equivalent nuclei: application to poly(dA).poly(dT). Biopolymers. 1985 Jul;24(7):1157–1167. doi: 10.1002/bip.360240705. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Beychok S. Circular dichroism of biological macromolecules. Science. 1966 Dec 9;154(3754):1288–1299. doi: 10.1126/science.154.3754.1288. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Fresco J. R. Polynucleotides. VII. Spectrophotometric study of the kinetics of formation of the two-stranded helical complex resulting from the interaction of polyriboadenylate and polyribouridylate. J Mol Biol. 1966 Aug;19(1):145–160. doi: 10.1016/s0022-2836(66)80057-8. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Edmondson S. P., Gray D. M. A circular dichroism study of the structure of Penicillium chrysogenum mycovirus. Nucleic Acids Res. 1983 Jan 11;11(1):175–192. doi: 10.1093/nar/11.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Greve J., Maestre M. F., Moise H., Hosoda J. Circular dichroism study of the interaction between T4 gene 32 protein and polynucleotides. Biochemistry. 1978 Mar 7;17(5):887–893. doi: 10.1021/bi00598a022. [DOI] [PubMed] [Google Scholar]
- Gupta G., Sarma M. H., Sarma R. H. Secondary structure of the hybrid poly(rA).poly(dT) in solution. Studies involving NOE at 500 MHz and stereochemical modelling within the constraints of NOE data. J Mol Biol. 1985 Nov 20;186(2):463–469. doi: 10.1016/0022-2836(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., Altona C. A conformational study of nucleic acid phosphate ester bonds using phosphorus-31 nuclear magnetic resonance. Nucleic Acids Res. 1979 Mar;6(3):1135–1149. doi: 10.1093/nar/6.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. B., Maestre M. F. Temperature-dependent reversible transition of poly(dCdG).poly(dCdG) in ethanolic and methanolic solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2127–2139. doi: 10.1002/bip.360231103. [DOI] [PubMed] [Google Scholar]
- Higuchi S. Interactions of poly-N6-methyladenylic acid and N6-substituted-9-methyladenines with poly-5-bromouridylic acid: a novel base-pairing. J Biomol Struct Dyn. 1985 Feb;2(4):675–682. doi: 10.1080/07391102.1985.10506315. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Poly(2-aminoadenylic acid): interaction with poly(uridylic acid). Biochemistry. 1976 Aug 24;15(17):3783–3795. doi: 10.1021/bi00662a022. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Circular dichroism and its empirical application to biopolymers. Methods Biochem Anal. 1985;31:61–163. doi: 10.1002/9780470110522.ch2. [DOI] [PubMed] [Google Scholar]
- Jollès B., Laigle A., Chinsky L., Turpin P. Y. The poly dA strand of poly dA.poly dT adopts an A-form in solution: a UV resonance Raman study. Nucleic Acids Res. 1985 Mar 25;13(6):2075–2085. doi: 10.1093/nar/13.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi N., Marck C., Rousseau N., Leng M., De Clerq E., Guschlbauer W. Polynucleotide helix geometry and stability. Spectroscopic, antigenic and interferon-inducing properties of deoxyribose-, ribose-, or 2'-deoxy-2'-fluororibose-containing duplexes of poly(inosinic acid) . poly(cytidylic acid). J Biol Chem. 1982 Feb 25;257(4):1924–1928. [PubMed] [Google Scholar]
- Krueger W. C., Li L. H., Moscowitz A., Prairie M. D., Petzold G., Swenson D. H. Binding of CC-1065 to poly- and oligonucleotides. Biopolymers. 1985 Aug;24(8):1549–1572. doi: 10.1002/bip.360240811. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Johnson D. A., Morgan A. R. Complexes formed by (pyrimidine)n . (purine)n DNAs on lowering the pH are three-stranded. Nucleic Acids Res. 1979 Jul 11;6(9):3073–3091. doi: 10.1093/nar/6.9.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Reverse transcription of retroviral genomes: mutations in the terminal repeat sequences. J Virol. 1985 Feb;53(2):447–455. doi: 10.1128/jvi.53.2.447-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis R. E., Alderfer J. L. Halogenated nucleic acids: effects of 5-fluorouracil on the conformation and properties of a polyribonucleotide and its constituents. Biopolymers. 1986 Apr;25(4):571–600. doi: 10.1002/bip.360250405. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Lang B. F. Mitochondrial class II introns encode proteins related to the reverse transcriptases of retroviruses. Nature. 1985 Aug 15;316(6029):641–643. doi: 10.1038/316641a0. [DOI] [PubMed] [Google Scholar]
- Milman G., Langridge R., Chamberlin M. J. The structure of a DNA-RNA hybrid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1804–1810. doi: 10.1073/pnas.57.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Murray N. L., Morgan A. R. Enzymatic and physical studies on the triplex dTn.dAn.rUn. Can J Biochem. 1973 Apr;51(4):436–449. doi: 10.1139/o73-051. [DOI] [PubMed] [Google Scholar]
- O'Brien E. J., MacEwan A. W. Molecular and crystal structure of the polynucleotide complex: polyinosinic acid plus polydeoxycytidylic acid. J Mol Biol. 1970 Mar 14;48(2):243–261. doi: 10.1016/0022-2836(70)90159-2. [DOI] [PubMed] [Google Scholar]
- O'Connor T., Bina M. The structure of triple helical poly(U).poly(A).poly(U) studied by Raman spectroscopy. J Biomol Struct Dyn. 1984 Dec;2(3):615–625. doi: 10.1080/07391102.1984.10507595. [DOI] [PubMed] [Google Scholar]
- Ott D. G., Kerr V. N., Hansbury E., Hayes F. N. Chemical synthesis of nucleoside triphosphates. Anal Biochem. 1967 Dec;21(3):469–472. doi: 10.1016/0003-2697(67)90323-5. [DOI] [PubMed] [Google Scholar]
- Rainen L. C., Stollar B. D. Antisera to poly(A)-poly(U)-poly(I) contain antibody subpopulations specific for different aspects of the triple helix. Biochemistry. 1977 May 3;16(9):2003–2007. doi: 10.1021/bi00628a038. [DOI] [PubMed] [Google Scholar]
- Reid D. G., Salisbury S. A., Brown T., Williams D. H., Vasseur J. J., Rayner B., Imbach J. L. Use of inter-proton nuclear Overhauser effects to assign the nuclear magnetic resonance spectra of oligodeoxynucleotide and hybrid duplexes in aqueous solution. Eur J Biochem. 1983 Sep 15;135(2):307–314. doi: 10.1111/j.1432-1033.1983.tb07654.x. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- SHUGAR D., SZER W. Secondary structure in poly-ribothymidylic acid. J Mol Biol. 1962 Nov;5:580–582. doi: 10.1016/s0022-2836(62)80134-x. [DOI] [PubMed] [Google Scholar]
- Solie T. N., Schellman J. A. The interaction of nucleosides in aqueous solution. J Mol Biol. 1968 Apr 14;33(1):61–77. doi: 10.1016/0022-2836(68)90281-7. [DOI] [PubMed] [Google Scholar]
- Steely H. T., Jr, Gray D. M., Lang D., Maestre M. F. Circular dichroism of double-stranded RNA in the presence of salt and ethanol. Biopolymers. 1986 Jan;25(1):91–117. doi: 10.1002/bip.360250108. [DOI] [PubMed] [Google Scholar]
- Swierkowski M., Shugar D. Poly 5-ethyluridylic acid, a polyuridylic acid analogue. J Mol Biol. 1970 Jan 14;47(1):57–67. doi: 10.1016/0022-2836(70)90401-8. [DOI] [PubMed] [Google Scholar]
- Warshaw M. M., Cantor C. R. Oligonucleotide interactions. IV. Conformational differences between deoxy- and ribodinucleoside phosphates. Biopolymers. 1970;9(9):1079–1103. doi: 10.1002/bip.1970.360090910. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Harrell J. T. Characteristics and variations of B-type DNA conformations in solution: a quantitative analysis of Raman band intensities of eight DNAs. Biochemistry. 1986 May 6;25(9):2664–2671. doi: 10.1021/bi00357a056. [DOI] [PubMed] [Google Scholar]
- Wierzchowski K. L., Zielenkiewicz A., Miller P. S. Calorimetric study of 2U:1A three stranded complexes formed between poly(U) and adenine dinucleotides: ApA and diastereoisomers of nonionic adenine dideoxyribonucleoside methyl phosphonate. Biopolymers. 1984 Nov;23(11 Pt 1):2361–2382. doi: 10.1002/bip.360231117. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Behe M. J. Salt-induced Z-A-Z transition sequence in the mixed ribo-deoxyribo copolymer poly(rG-dC) X poly(rG-dC). Proc Natl Acad Sci U S A. 1984 Dec;81(23):7284–7287. doi: 10.1073/pnas.81.23.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Kakiuchi N., Guschlbauer W. Differential stabilization by netropsin of inducible B-like conformations in deoxyribo-, ribo- and 2'-deoxy-2'-fluororibo-adenosine containing duplexes of (dA)n . (dT)n and (dA)n . (dU)na. Nucleic Acids Res. 1982 Mar 11;10(5):1721–1732. doi: 10.1093/nar/10.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. A RNA.DNA hybrid that can adopt two conformations: an x-ray diffraction study of poly(rA).poly(dT) in concentrated solution or in fibers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):78–82. doi: 10.1073/pnas.78.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka B., Bollum F. J., Shugar D. Polydeoxyribouridylic acid and its complexes with polyribo- and deoxyriboadenylic acids. J Mol Biol. 1969 Nov 28;46(1):169–183. doi: 10.1016/0022-2836(69)90064-3. [DOI] [PubMed] [Google Scholar]


