Abstract
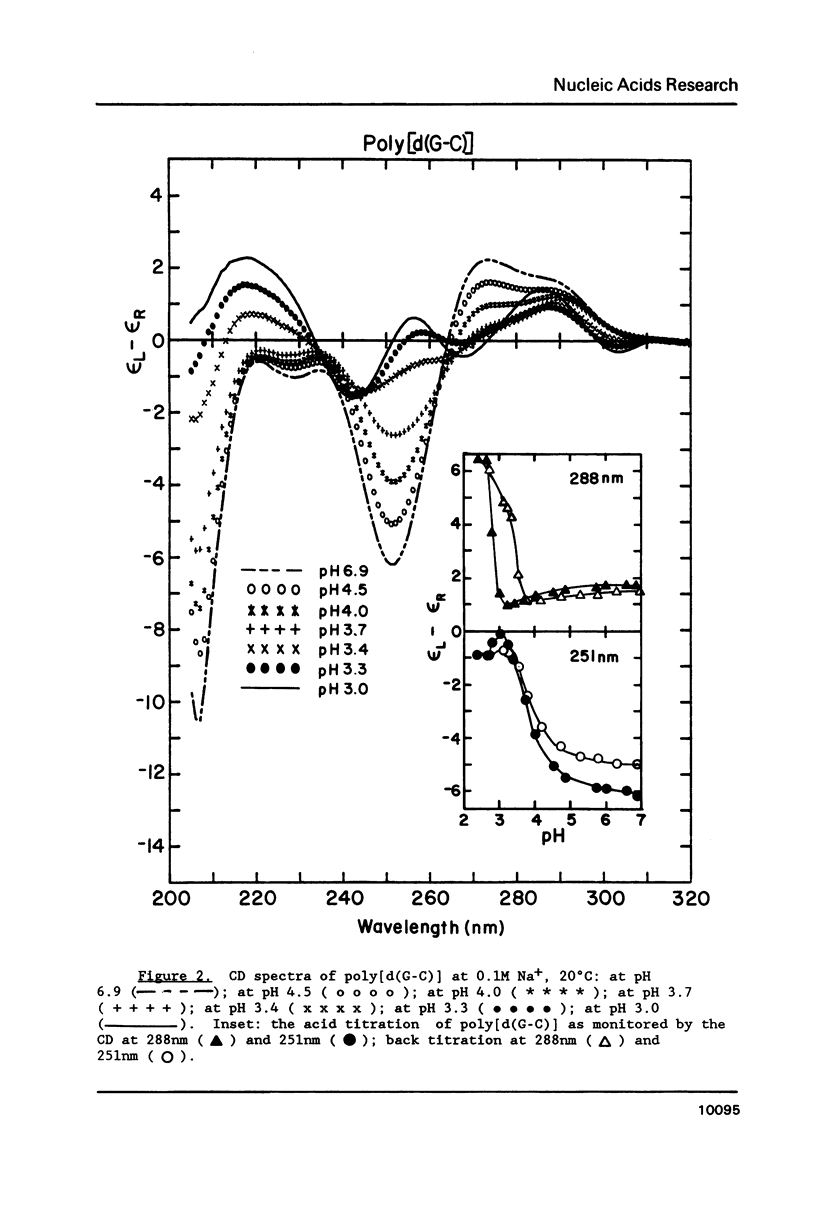
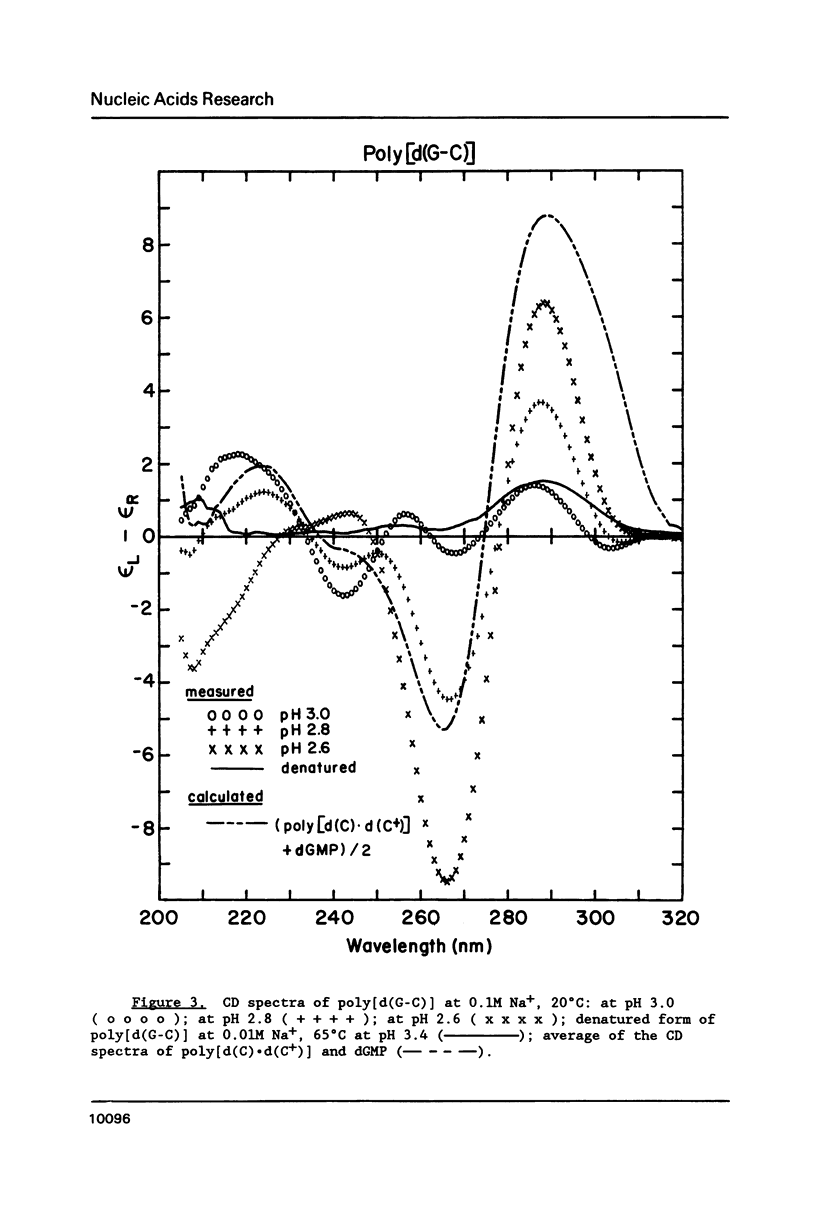
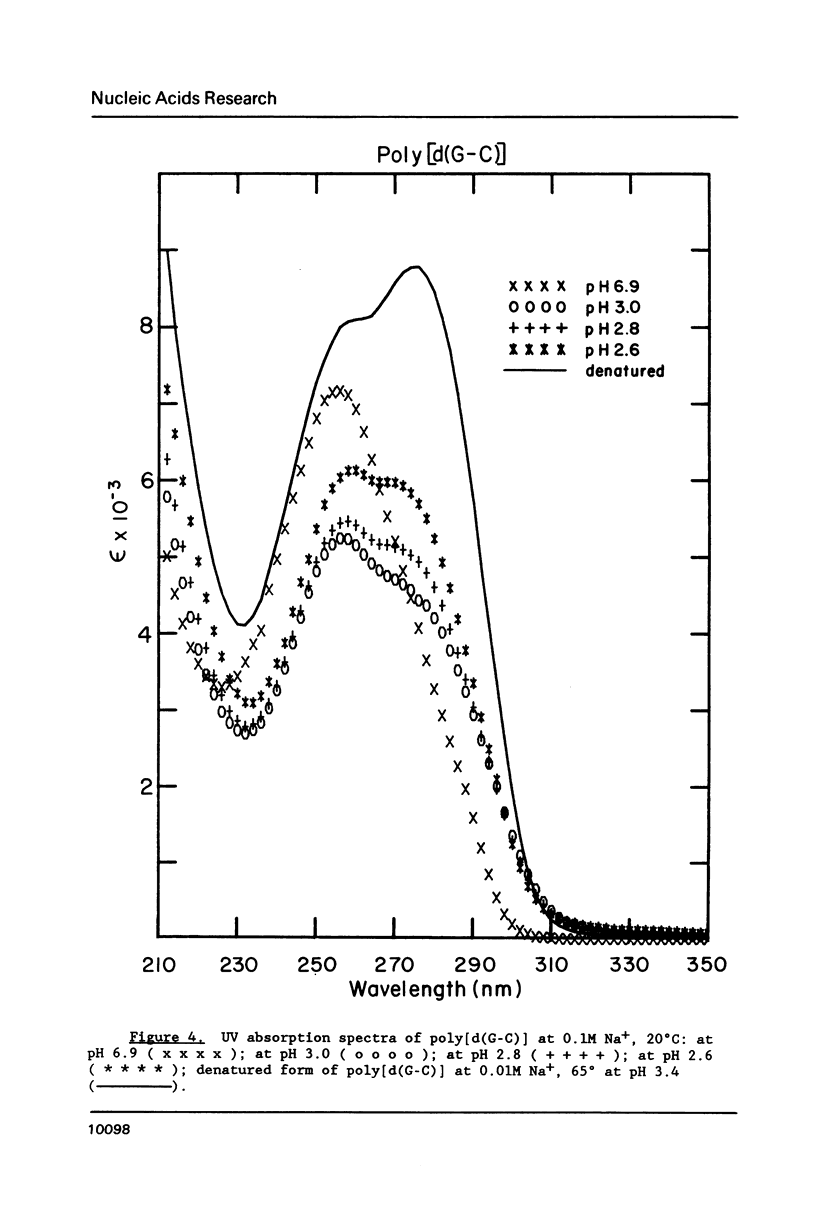
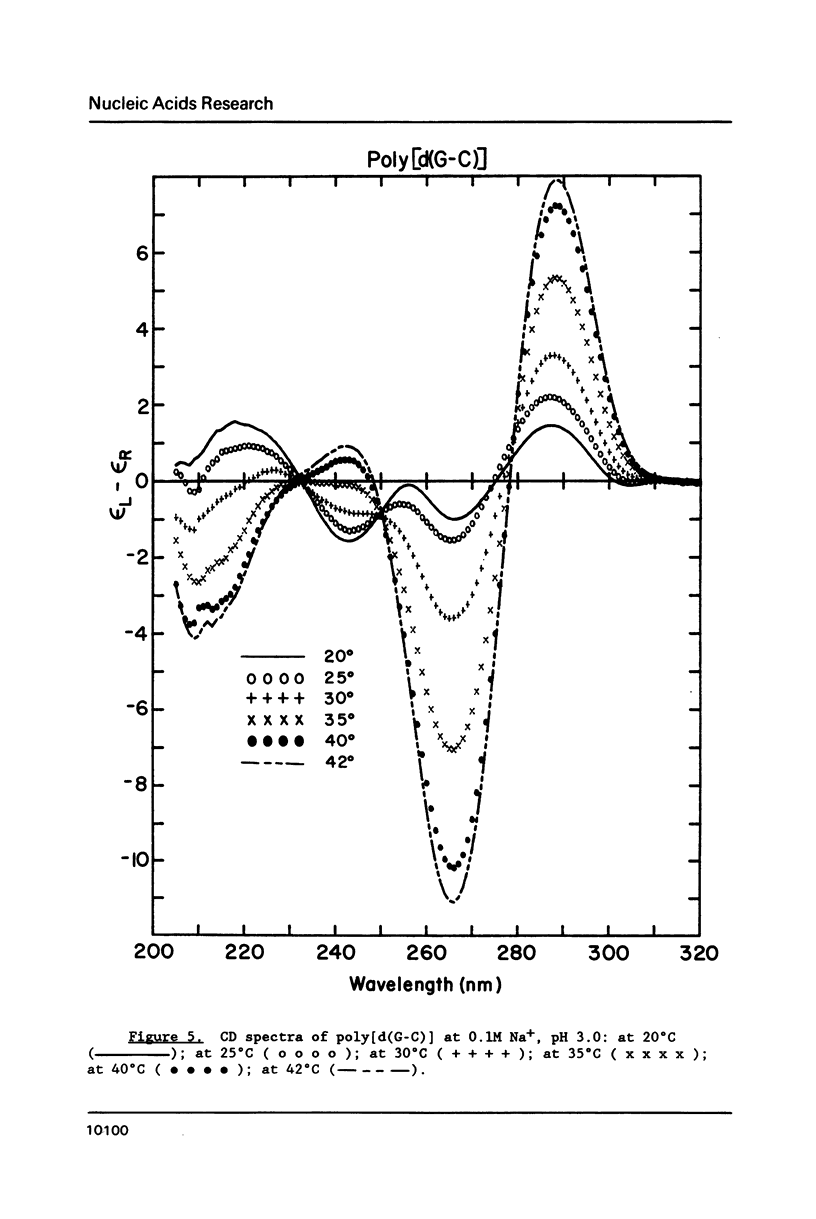
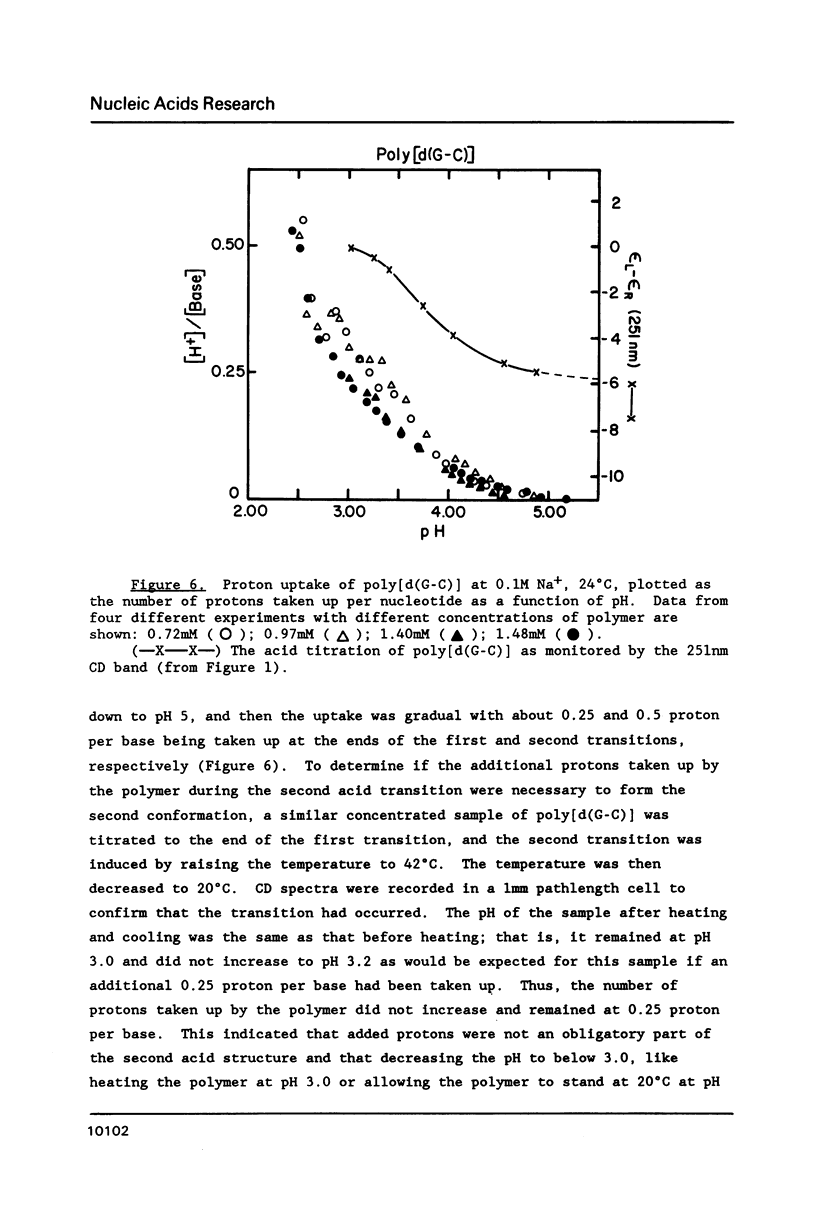
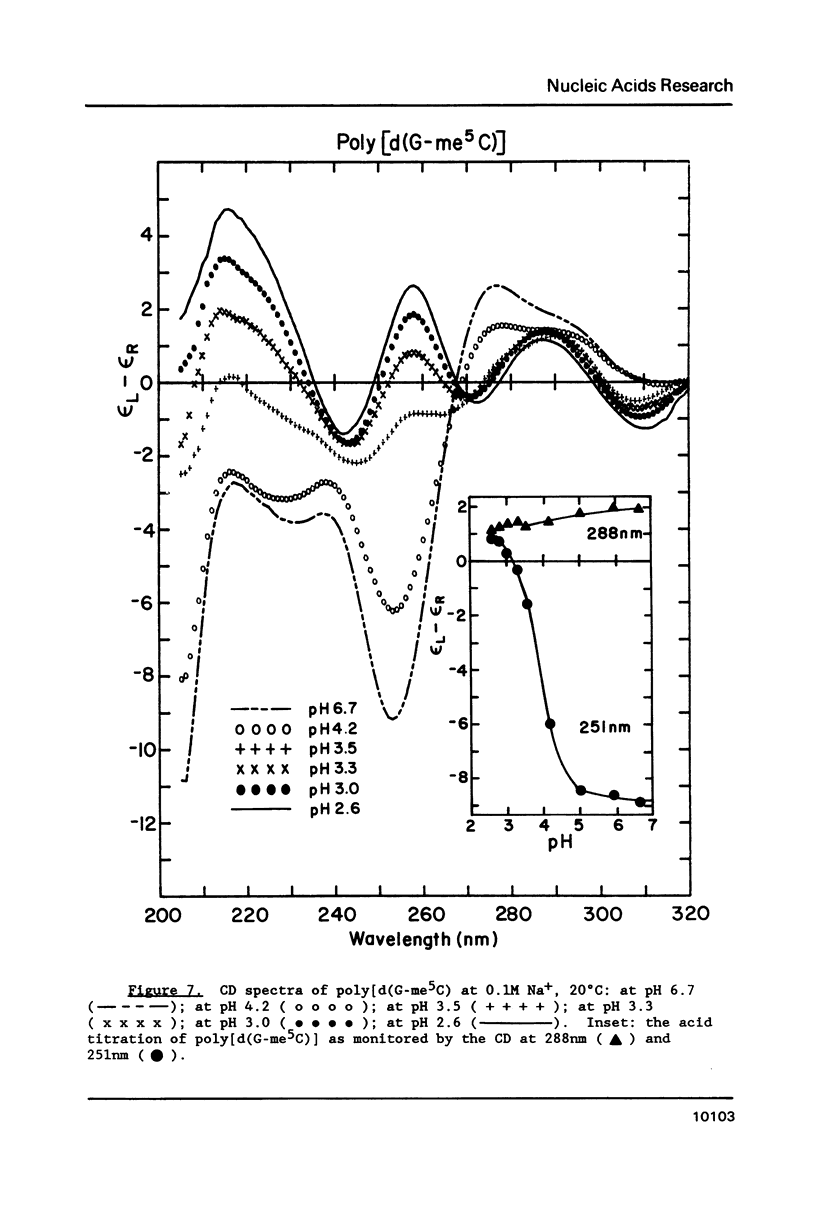
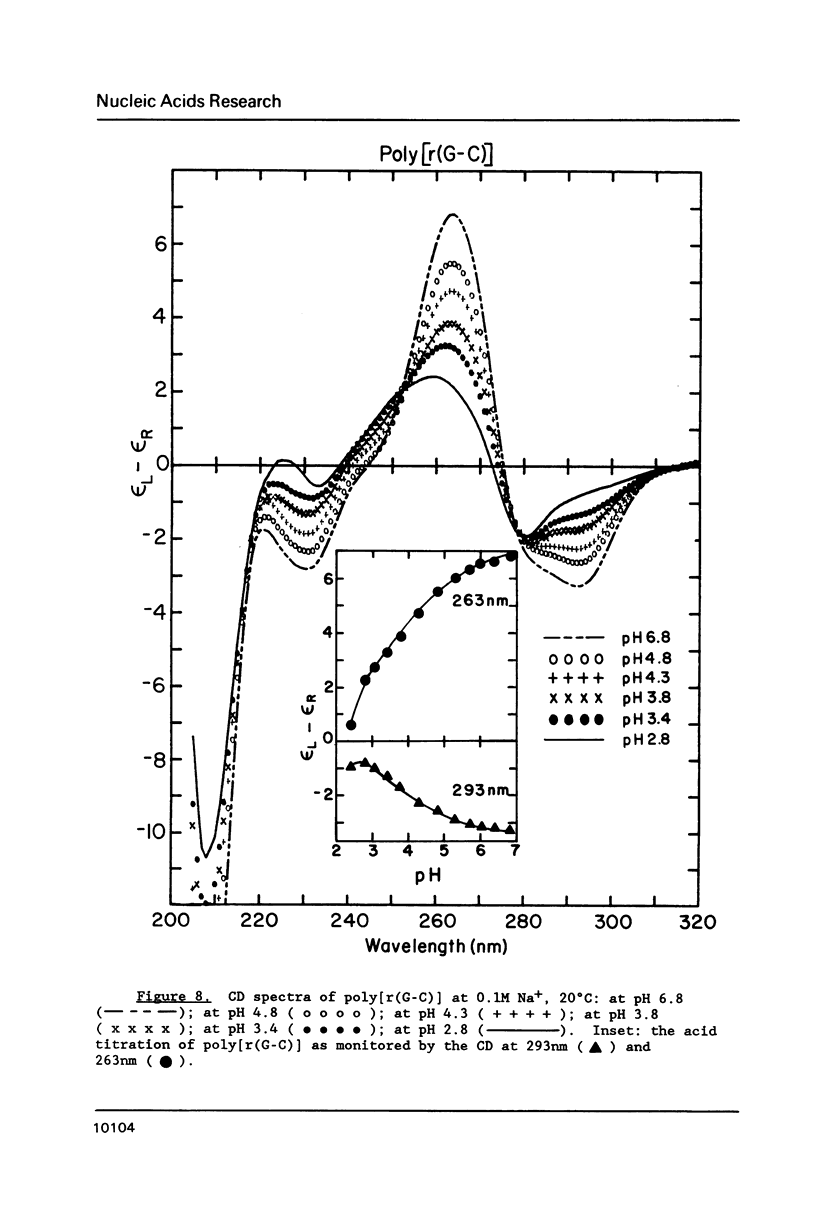
Circular dichroism (CD) and UV absorption data showed that poly[d(G-C)] (at 0.09M NaCl, 0.01M Na+ (phosphate), 20 degrees C) underwent two conformational transitions upon lowering of the pH by the addition of HCl. The first transition was complete at about pH 3.0. The second transition was complete upon lowering the pH to 2.6 or upon raising the temperature, at pH 3.0, to about 40 degrees C. There was no indication of denaturation during either transition. The CD spectrum for the second acid conformation had large CD bands including a positive one at 288nm, a characteristic associated with C X C+ base-pairs. Electron microscopy showed no significant formation of condensed supramolecular aggregates corresponding to the first or second acid forms of poly[d(G-C)]. On the basis of spectral data, electron microscopy, and proton-uptake measurements, we propose models for the secondary structures that poly[d(G-C)] adopts in its two acid conformations.
Full text
PDF

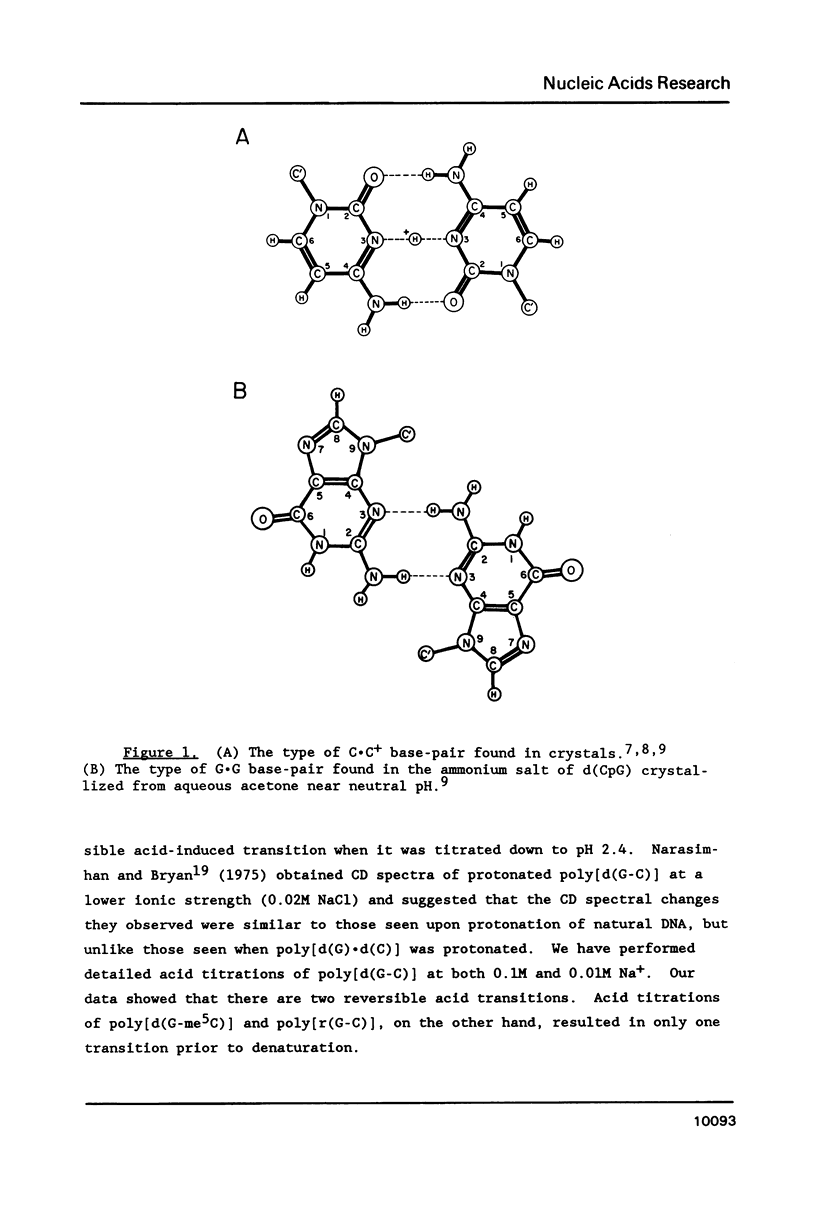












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKINRIMISI E. O., SANDER C., TS'O P. O. Properties of helical polycytidylic acid. Biochemistry. 1963 Mar-Apr;2:340–344. doi: 10.1021/bi00902a028. [DOI] [PubMed] [Google Scholar]
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M., Gray D. M., Patrick M. H., Ratliff R. L. Photochemical demonstration of stacked C.C+ base pairs in a novel DNA secondary structure. Biochemistry. 1985 Mar 26;24(7):1676–1683. doi: 10.1021/bi00328a016. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Castleman H., Specthrie L., Makowski L., Erlanger B. F. Electronmicroscopy and circular dichroism of the dynamics of the formation and dissolution of supramolecular forms of Z-DNA. J Biomol Struct Dyn. 1984 Oct;2(2):271–283. doi: 10.1080/07391102.1984.10507566. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Base protonation facilitates B-Z interconversions of poly(dG-dC) X poly(dG-dC). Biochemistry. 1984 Dec 4;23(25):6159–6165. doi: 10.1021/bi00320a041. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Conformational lability of poly(dG-m5dC):poly(dG-m5dC). Nucleic Acids Res. 1986 Jun 25;14(12):5081–5097. doi: 10.1093/nar/14.12.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. H., Thomas G. J., Jr Raman spectral studies of nucleic acids. XVI. Structures of polyribocytidylic acid in aqueous solution. Biopolymers. 1977 Apr;16(4):765–789. doi: 10.1002/bip.1977.360160406. [DOI] [PubMed] [Google Scholar]
- Courtois Y., Fromageot P., Guschlbauer W. Protonated polynucleotide structures. 3. An optical rotatory dispersion study of the protonation of DNA. Eur J Biochem. 1968 Dec 5;6(4):493–501. doi: 10.1111/j.1432-1033.1968.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Cruse W. B., Egert E., Kennard O., Sala G. B., Salisbury S. A., Viswamitra M. A. Self base pairing in a complementary deoxydinucleoside monophosphate duplex: crystal and molecular structure of deoxycytidylyl-(3'-5')-deoxyguanosine. Biochemistry. 1983 Apr 12;22(8):1833–1839. doi: 10.1021/bi00277a014. [DOI] [PubMed] [Google Scholar]
- Gray C. W., Brown R. S., Marvin D. A. Adsorption complex of filamentous fd virus. J Mol Biol. 1981 Mar 15;146(4):621–627. doi: 10.1016/0022-2836(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Cui T., Ratliff R. L. Circular dichroism measurements show that C.C+ base pairs can coexist with A.T base pairs between antiparallel strands of an oligodeoxynucleotide double-helix. Nucleic Acids Res. 1984 Oct 11;12(19):7565–7580. doi: 10.1093/nar/12.19.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Hamilton F. D., Vaughan M. R. The analysis of circular dichroism spectra of natural DNAs using spectral components from synthetic DNAs. Biopolymers. 1978 Jan;17(1):85–106. doi: 10.1002/bip.1978.360170107. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Vaughan M. Circular dichroism spectra show that repeating dinucleotide DNAs may form helices in which every other base is looped out. Nucleic Acids Res. 1980 Aug 25;8(16):3695–3707. doi: 10.1093/nar/8.16.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. B., Maestre M. F. Temperature-dependent reversible transition of poly(dCdG).poly(dCdG) in ethanolic and methanolic solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2127–2139. doi: 10.1002/bip.360231103. [DOI] [PubMed] [Google Scholar]
- INMAN R. B. TRANSITIONS OF DNA HOMOPOLYMERS. J Mol Biol. 1964 Sep;9:624–637. doi: 10.1016/s0022-2836(64)80171-6. [DOI] [PubMed] [Google Scholar]
- Karstadt M., Krakow J. S. Azotobacter vinelandii ribonucleic acid polymerase. X. Some physical properties of the alternating copolymers of inosinic and cytidylic residues and of guanylic and cytidylic acid residues. J Biol Chem. 1970 Feb 25;245(4):752–758. [PubMed] [Google Scholar]
- Lee J. S., Johnson D. A., Morgan A. R. Complexes formed by (pyrimidine)n . (purine)n DNAs on lowering the pH are three-stranded. Nucleic Acids Res. 1979 Jul 11;6(9):3073–3091. doi: 10.1093/nar/6.9.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J., Morgan A. R. Poly(pyrimidine) . poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984 Aug 24;12(16):6603–6614. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefler C. F., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. 3. Preparation of poly N-acetyldeoxyguanylate and polydeoxyguanylate. J Biol Chem. 1969 Feb 25;244(4):594–601. [PubMed] [Google Scholar]
- Narasimhan V., Bryan A. M. Temperature-induced perturbations in the circular dichroic spectrum of the synthetic polymer poly[d(G-C)]. Biochim Biophys Acta. 1976 Jul 16;435(4):433–437. doi: 10.1016/0005-2787(76)90209-4. [DOI] [PubMed] [Google Scholar]
- Narasimhan V., Bryan A. M. The unique protonated conformation of poly d (G-C) as detected by circular dichroism studies. FEBS Lett. 1975 Jun 1;54(1):49–52. doi: 10.1016/0014-5793(75)81065-9. [DOI] [PubMed] [Google Scholar]
- O'Connor T., Mansy S., Bina M., McMillin D. R., Bruck M. A., Tobias R. S. The pH-dependent structure of calf thymus DNA studied by Raman spectroscopy. Biophys Chem. 1982 Apr;15(1):53–64. doi: 10.1016/0301-4622(82)87016-6. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Haniford D. B., Morgan A. R. A structural basis for S1 nuclease sensitivity of double-stranded DNA. Cell. 1985 Aug;42(1):271–280. doi: 10.1016/s0092-8674(85)80122-7. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Ughetto G., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986 Jun 6;232(4755):1255–1258. doi: 10.1126/science.3704650. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud M., Arndt-Jovin D. J., Zarling D. A., Jovin T. M. Immunological detection of left-handed Z DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J. 1984 Apr;3(4):721–731. doi: 10.1002/j.1460-2075.1984.tb01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz M., Barton J. K., Magliozzo C. C., Tucker D., Lafer E. M., Stollar B. D. Lack of Z-DNA conformation in mitomycin-modified polynucleotides having inverted circular dichroism. Proc Natl Acad Sci U S A. 1983 May;80(10):2874–2878. doi: 10.1073/pnas.80.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Sundaralingam M. X-ray-structure of a cytidylyl-3',5'-adenosine-proflavine complex: a self-paired parallel-chain double helical dimer with an intercalated acridine dye. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1852–1856. doi: 10.1073/pnas.77.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Venner H. Protonation of cytosine in DNA. Biopolymers. 1966 Dec;4(10):1073–1079. doi: 10.1002/bip.1966.360041004. [DOI] [PubMed] [Google Scholar]


