Abstract
Background
Geriatric assessment is a multidisciplinary diagnostic process that evaluates the older adult’s medical, psychological, social, and functional capacity. No systematic review of the use of geriatric assessment in oncology has been conducted. The goals of this systematic review were: 1) to provide an overview of all geriatric assessment instruments used in the oncology setting; 2) to examine the feasibility and psychometric properties of those instruments; and 3) to systematically evaluate the effectiveness of geriatric assessment in predicting or modifying outcomes (including the impact on treatment decision making, toxicity of treatment, and mortality).
Methods
We searched Medline, Embase, Psychinfo, Cinahl, and the Cochrane Library for articles published in English, French, Dutch, or German between January 1, 1996, and November 16, 2010, reporting on cross-sectional, longitudinal, interventional, or observational studies that assessed the feasibility or effectiveness of geriatric assessment instruments. The quality of articles was evaluated using relevant quality assessment frameworks.
Results
We identified 83 articles that reported on 73 studies. The quality of most studies was poor to moderate. Eleven studies examined psychometric properties or diagnostic accuracy of the geriatric assessment instruments used. The assessment generally took 10–45min. Geriatric assessment was most often completed to describe a patient’s health and functional status. Specific domains of geriatric assessment were associated with treatment toxicity in 6 of 9 studies and with mortality in 8 of 16 studies. Of the four studies that examined the impact of geriatric assessment on the cancer treatment decision, two found that geriatric assessment impacted 40%–50% of treatment decisions.
Conclusion
Geriatric assessment in the oncology setting is feasible, and some domains are associated with adverse outcomes. However, there is limited evidence that geriatric assessment impacted treatment decision making. Further research examining the effectiveness of geriatric assessment on treatment decisions and outcomes is needed.
In North America and Europe, the majority of persons who receive a cancer diagnosis every year are aged 65 years or older (1–3). Cancer treatment decision making for older adults is often complicated by the presence of comorbidities and psychosocial factors. The US National Comprehensive Cancer Network (NCCN) and the International Society of Geriatric Oncology (SIOG) (4,5) have recommended that some form of geriatric assessment be conducted to help cancer specialists determine the best treatment for their older patients. Despite their recommendations, neither organization has indicated what constitutes the best form of assessment.
Geriatric assessment has been used in geriatric medicine since the 1980s (6). The aim of geriatric assessment in a traditional geriatric population is to identify current health problems and to guide interventions to reduce adverse outcomes and to optimize the functional status of older adults (7–9). A traditional geriatric assessment is not an intervention in itself but rather aims to identify opportunities for intervention. A geriatric assessment conducted in the oncology setting may not have the same goals as a traditional geriatric assessment, because the latter was never intended to help identify the best cancer treatment (10). The SIOG and NCCN recommend that a geriatric assessment be used to help select the best cancer treatment for an older patient with cancer (11–13). Oncology clinics see many more older adults each day compared with clinics that specialize in geriatric medicine, and the concerns of patients attending each type of clinic are often quite different (10). The feasibility and effectiveness of geriatric assessments in the oncology setting might also be very different compared with the geriatric medicine setting. Furthermore, the older cancer population is heterogeneous in terms of cancer type, cancer stage, and disease and treatment trajectories. These factors might affect the feasibility and efficacy of geriatric assessment in the oncology setting.
There has been only one review published to date on the use of geriatric assessment in older cancer patients. That review (4) was based on a literature search of MEDLINE up to February 2003 and was limited to English-language articles. It is not clear which data were abstracted and by whom, and how the quality assessment of the included studies was conducted. Similarly, descriptions of the included studies were not reported. Numerous geriatric assessment studies have been published since the publication of that review.
The objectives of this systematic review were: 1) to provide an overview of all geriatric assessment instruments that have been developed and/or are in use in the oncology setting for older adults with cancer; 2) to examine the feasibility of geriatric assessment instrument use in the oncology setting (ie, time needed to complete, proportion of patients with complete assessments), and the psychometric properties or diagnostic accuracy of the instruments (ie, reliability and validity, sensitivity and specificity); and 3) to systematically evaluate the impact of geriatric assessment instruments on the treatment decision-making process and their effectiveness in predicting cancer and treatment outcomes. The outcomes of interest were chosen a priori as part of the review protocol according to Cochrane review methodology as described in the Cochrane Handbook for Systematic Reviews of Interventions (14) and included mortality, complications and toxicity of treatment, health and functional status (ie, impact on activities of daily living), use of inpatient and outpatient care, use of geriatric assessment to avoid complications of treatment, and the impact on cancer treatment decisions and approaches. Geriatric assessment is typically used to predict functional status, use of care, and mortality (7–9). We included prediction of complications and toxicity of treatment and impact on planned cancer treatment as outcomes of interest in this review based on suggestions by experts and SIOG and NCCN that they may be impacted by the use of a geriatric assessment (11–13).
Methods
Data Sources
This review was based on a systematic comprehensive search of six databases: OVID MEDLINE (1950 to October week 4, 2010); PubMed (January 1, 2008, to November 16, 2010); OVID EMBASE (January 1, 1980, to 2010 week 44); OVID PsycINFO (January 1, 1987, to November week 1, 2010); CINAHL (January 1, 1982, to November 16, 2010); and the Cochrane Library (searched on November 6, 2011). We considered articles in English, Dutch, French, or German that were published or in press between January 1, 1996, and November 16, 2010, for inclusion in this review.
A study was eligible for inclusion if it: 1) reported on older patients (mean or median age of study participants 65 years or older) who were diagnosed with cancer (any type of cancer, including hematological malignancies) and being seen in oncology clinics (outpatient oncology or hematology clinics or inpatient oncology or hematology units); 2) reported on cross-sectional, longitudinal, observational, or interventional studies that either assessed the feasibility of the use of tools or instruments or the effectiveness of geriatric assessment tools on any of the aforementioned outcome measures; and 3) was written in English, French, Dutch, or German. We excluded editorials, case studies, reviews, and expert opinion papers and studies that were published as abstracts only.
The following sets of keywords or free text words were used in combination with subject headings where available: cancer (cancer* or neoplasm* OR oncolog*) AND geriatric assessment (geriatric or elderly or frailty or aged and assessment* or evaluation* or consultation*; or consultation service for senior adults or geriatric oncology module or frailty marker*). The literature search was performed by an experienced university librarian (ES).
Process of Study Selection
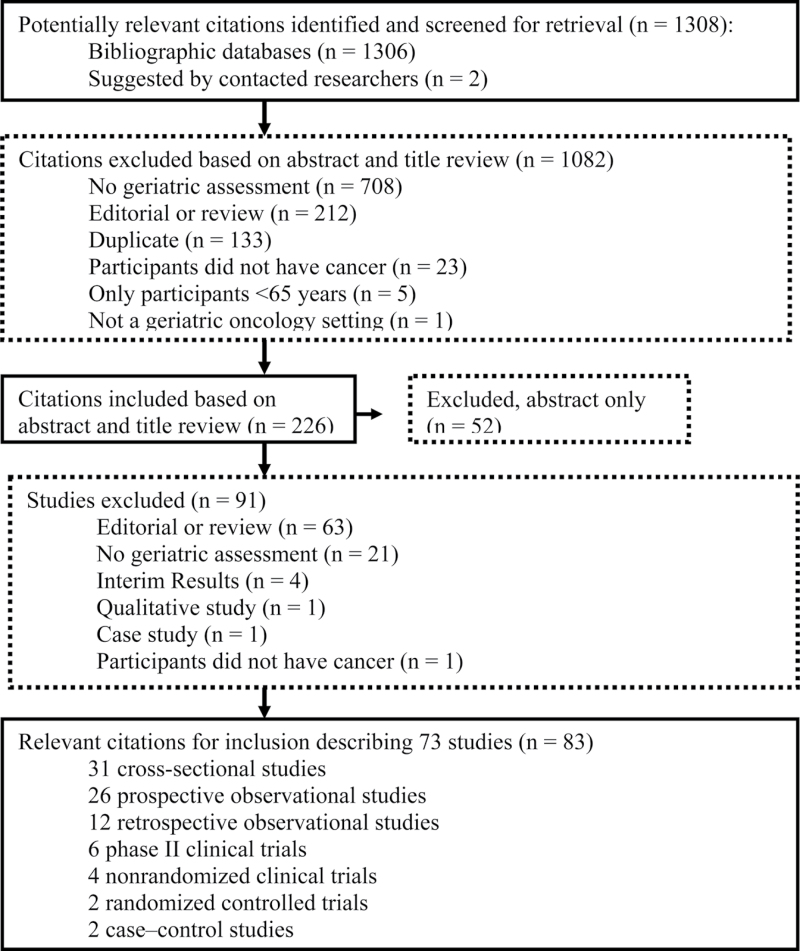
The studies were selected in two steps (Figure 1). In the first step, an initial selection based on titles and abstracts was done independently by two authors (MP and JH) using the inclusion and exclusion criteria. When at least one reviewer was uncertain about whether the article fulfilled the inclusion criteria, the abstract was included for full-text review. In the second step, the full text was reviewed independently by the same authors. Disagreements between reviewers were resolved by consensus (this process was used for eight studies). If multiple articles reported similar results, only the article with the most complete information was retained. For all studies identified as abstract only (n = 50), we attempted to contact the authors by e-mail to determine whether the full-text study had been published. For eight abstracts, no e-mail address was found. Of the 42 authors who were contacted, 19 did not respond, six e-mails were undeliverable, 15 authors responded that the studies and/or manuscripts were still in progress, and two authors informed us that their manuscripts were accepted for publication and were included in this systematic review. We also reviewed the reference lists of all selected articles to identify any additional relevant articles, but no additional studies were identified. When an article referred to additional publications for more details on study methods and design, those publications were also obtained.
Figure 1.
Flow chart of study selection.
Data Abstraction
Data were abstracted by the same reviewers using a data abstraction form that was created with Excel software (Microsoft Corporation, Redmond, WA). The abstracted information included the study design, aim of the study, location of the study, sampling method, source of data, recruitment, participant inclusion criteria, the characteristics of included study participants, the name used for the geriatric assessment, the instrument(s) used, instrument feasibility, results of the study, outcomes of the assessment, and details about the statistical analysis. If any aspect of the study design was unclear, we attempted to contact the authors of the study by e-mail. For two of 19 studies, no e-mail address could be found, and for one study, the email was undeliverable, leaving 16 authors that could be contacted. Of the 16 authors contacted, five did not respond whereas 11 responded and provided additional details.
Quality Assessment
The Reporting of Observational Studies in Epidemiology (STROBE), the Meta-analysis of Observational Studies in Epidemiology (MOOSE), and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were used by two reviewers (MP and JH) to assess the quality of the included studies (15–17). Any disagreement, which involved 421 (18%) of 2324 assessed quality items, was resolved though consensus. However, because this is the first systematic review on the use of geriatric assessment in oncology, no study was excluded based on the quality assessment.
Results
A total of 1308 abstracts were initially identified for possible inclusion (Figure 1). Based on the review of the abstracts, 226 citations were included for full-text review. Of those 226 articles, 83 reporting on 73 studies were included. Of the 83 articles included, three were written in French, and 80 were written in English.
Quality Assessment
The quality of most studies was poor to moderate based on MOOSE and PRISMA guidelines for reporting (Supplementary Table 1, available online). Of the 59 studies that were not chart reviews, 51 (86%) did not report a response rate (18–70), and all but one (38) also did not report the reasons for refusal to participate in study. Therefore, the extent of selection bias could not be assessed. Furthermore, of the 73 studies, 13 (18%) did not describe the study design (18,20,27,37,42,53,57,64,65,67,69,71,72), and 12 (16%) did not describe the setting in which the study was conducted (20,22–24,33,42,44,49,51,53,61,70). Among the 28 prospective studies, nine (32%) did not describe the method of follow-up (20,22,23,27,33,35,37,39–41) . The amount of missing data was not described in 41 (67%) of 61 studies (excluding studies that reported having complete data) (20–23,25,27,33–35,39–45,47,51,53,54,60–63,66,68–71,73–84), and 41 of 58 studies (excluding studies that reported no missing data or how missing data were handled) did not describe how the study authors dealt with missing data (20–23,25,27,33,37–39,41–47,49,51,53,54,57,58,60–64,68–72,74–85). For 12 (16%) of the 73 studies, the statistical methods were inadequately described (19,20,37,42,52,59,67,69,72,84,86,87). Three (4%) of the 73 studies did not describe all of the measurement instruments used in the study (ie, geriatric assessment instruments, outcomes, predictors) (20,42,52).
Characteristics of the Included Studies
The characteristics of the 73 studies reported by the included articles are presented in Supplementary Table 2 (available online). Twenty-five studies were conducted in North America: 23 in the United States (25,30–33,37,38,43,46,52,54,58,62,64,73,74,77,78,80,81,83,85,86, 88–92) and two in Canada (93–95). Forty-three studies were conducted in Europe: 19 in Italy (20,26,34–36,40,42, 44,51,53,57,59,61,63,67,69,70,76,79), 14 in France (19,27–29,39,45,48,72,82,84,87, 96–98), three in Spain (47,55,56), two in Germany (41,68,99), one in the United Kingdom (22,23), one in Norway (71,75), one in Greece (24), one in the Netherlands (18), and one in Austria (65). Two studies were conducted in Asia: one in Japan (100) and one in Korea (50). One study was conducted in Australia (66), and two studies were conducted in multiple countries (21,49,60).
Of the 73 studies that included geriatric assessment, 28 (38%) were prospective observational studies (18–41,71,73–76,94,95,100), 31 (42%) were cross-sectional observational studies (42–68,77,78,85,88–90,93,99), and 14 (19%) were retrospective studies or chart reviews (69,70,72,79–84,86,87,91,92,96–98). None of the reviewed studies was a randomized controlled trial specifically designed to examine the effectiveness of geriatric assessment.
In studies that investigated a new drug or treatment regimen (26,28,30,39,59,67,70), geriatric assessment was employed for the most part to describe study participants. Geriatric assessment was included in seven nonrandomized clinical drug trials (24,26,28,30,59,67,70) and no randomized controlled drug trial.
Most of the studies recruited participants either through convenience sampling (25 studies) (24–26,30–32,36,37,39,51,52,59,65, 67,69,73,74,76–78,84,88–90,93–95,97,99) or by consecutive sampling (32 studies) (18,19,21,27,33–35,40,41,45–48,55,57,58,60–64,66,68,71,75,79,80,82,85,86,91,92,96,98,100) techniques. Three studies used other sampling methods (29,38,54), and in 13 studies the method used for recruitment was not clear or not reported (20,22,23,42–44,49,50,53,56,70,72,81,83,87). However, 11 (15%) of 73 studies failed to report clear and explicit inclusion and recruitment procedures criteria (20,22–24,42,44,53,57,65,67,77,89,90).
Sample sizes ranged from 10 (36) to 12 480 (54) participants. Response rates ranged from 53% (73) to 100% (100). The age range of participants was 65–99 years.
Overview of All Geriatric Assessment Instruments Developed and/or Used in the Oncology Setting for Older Adults with Cancer
Setting of the geriatric assessments. In 61 of the 73 studies, the geriatric assessment was conducted in a hospital (18–21,24,25,27–41,43–8,50–53,55–69,71,72,74–79,81–90,92–100). In 11 studies, patients underwent geriatric assessment during admission or stay at inpatient ward (21,38,41,60,61,63,65,68,69,79,82,86,92), and participants in 11 studies were evaluated during initial or routine clinic visits (33,34,46–48,56,62,64,74,77,88). In four studies, the geriatric assessment took place either at inpatient admission or in the outpatient clinic (57,72,93–95).
Domains included in the geriatric assessment. Table 1 presents an overview of the domains included in geriatric assessments, and Supplementary Table 3 (available online) presents the detailed content and domains of the geriatric assessment used in each study. Of the 73 studies, 68 included measures of basic activities of daily living (18–41,41,42,44–51,53–63,65–67,69–72,74–83,85–100), and 65 included instrumental activities of daily living (18–28,30–39,41,44–63,65–72,74–95,97–99).
Table 1.
Domains of geriatric assessment included in the 73 studies that examined geriatric assessment in the oncology setting
| Domain | No. of studies that included the domain | The most frequently used questionnaire or instrument to assess the domain* | Frequency of use†, No. (%) |
| Activities of daily living | 68 | Katz index | 38 (56) |
| Instrumental activities of daily living | 65 | Lawton scale | 40 (62) |
| Comorbidity | 58 | Charlson comorbidity index; | 20 (34) |
| Cumulative Illness Rating Scale (including Cumulative Illness Rating Scale–Geriatrics) | 18 (31) | ||
| Cognitive functioning | 53 | Mini Mental State Examination | 41 (77) |
| Depression | 52 | Geriatric Depression Scale (any version) | 35 (67) |
| Nutritional assessment | 40 | Mini Nutritional Assessment (including short form); | 16 (40) |
| Body mass index | 15 (38) | ||
| Performance status | 37 | Eastern Oncology Collaborative Group scale; | 20 (54) |
| Karnofsky scale | 12 (32) | ||
| Fall risk assessment | 27 | Self-reported falls | 14 (52) |
*Both instruments were used in more than 20% of the studies.
†Among studies that included the domain.
A total of 58 studies included a comorbidity domain (18–25,27–32,34,35,38–48,50,51,54,55,57,59–61,63,65–68, 71–77,79,80,82,84–87,89–91,93–100). Cognitive functioning was evaluated in 53 studies (18–21,25,27–29,31,32,34–36,38,39,41,44, 45,47,49–58,60–62,64–67,69,71,72,74–77,79–84,86–88,91–95,97–100). Assessment of depression, anxiety, or general mood was a component of geriatric assessment in 52 studies (19–23, 26–33,36,39,42,44,47–50,52,53,57,58,60–67,69,71–77,79–85, 87–95,97,98).
A nutritional assessment was conducted in 40 studies (18–20, 25,27–29,32–36,38,39,43,45,47,48,50,51,53,55–57,63,65,66,71, 74–76,79,82,84,87–90,92–97,99,100), and 27 studies assessed the risk of falls (19,25,27,38,42,43,45,47,48,50,52,54,58,61,63,66, 72,76–78,80,82,84,88–96,98,99). Performance status was assessed in 37 studies (20,21,24,30–32,34,35,39,41,44, 46–48, 50,51,53,55,56,60–71,74,75,86–90,93–98,100).
Information about the use of prescription medications was collected from patients in 22 studies (19,25,28,29,39,47,48,50–52,55,56,63,66,71,72,75,78,82,84,85,92,98,99), and in 14 of these 22 studies, the information obtained included the total number of prescriptions (25,29,39,47,48,51,52,63,66, 71,75,78,82,84,85,92). In 24 studies (19,22,23,25,28,38,48,50–52,55,56,63,65,66,72,73,78,84–86,88–90,93,97,98), geriatric assessment included the availability of social support and living arrangements, such as the availability of a caregiver. The most commonly used objective measure of physical function was gait speed, which was included in 15 studies (25,29,43,45,50,52,61,64,65,72,78,84,87,88,93–95). Patient characteristics that were less often incorporated into geriatric assessments included symptoms [assessed using a symptom inventory, two studies (22,23,85); fatigue or energy levels, seven studies (21,25,37,43,60,85,93–95); pain, three studies (37,66,85); quality of life, seven studies (22,23,31,32,37,50,65,68,74); grip strength, five studies (43,64,87,93–95); distress, three studies (66,85,89,90); and self-rated health, two studies (54,73)].
In 30 of 73 studies, the results of geriatric assessment were summarized in a summary score (18,20,24,26,34,40,42,43,47,49–51,54,55,66,70,72–76,78,79,84,93–96,98,99). In 12 of those studies (20,24,40,47,50,54,75,79,84,96,98,99), the summary score used was the classification of fit, vulnerable, and frail developed by Balducci and Stanta (101). In this classification, frail refers to patients who are generally unfit for cancer treatment (defined as those with any of the following characteristics: older than 85 years, more than two disabilities, multiple comorbidities, or the presence of geriatric syndromes) and should receive best supportive care or palliative treatment; fit (defined as patients who are independent and have no clinically significant comorbid conditions) indicates patients who should receive standard therapy; and vulnerable (defined as patients with one or two clinically significant comorbid conditions and/or instrumental activities of daily living disability but no activities of daily living disability) refers to patients for which the standard treatment should be adjusted.
Feasibility and Psychometric Properties of Geriatric Assessment
Feasibility of geriatric assessment. Thirty studies reported some aspect of the feasibility of the geriatric assessment, such as time needed to complete the assessment and/or who (study author, patient themselves, or others) conducted the assessment (21,25,26,32,36,37,40,45,46,50,52,55–58,60,66,69,73–75,77,78,84,85,87–89,93,94,99). In most of these studies, the assessment was done through a face-to-face interview and generally took 10–45 minutes. Among studies that reported how many participants refused the assessment (26,74,78,94,95), only a small number of participants refused the assessments (Table 2). In six studies (33,66,69,85,88,89), geriatric assessment was done using self-administered surveys. However, only four of those studies (66,85,88,89) reported on feasibility, and each showed that it was acceptable (more than 75% of participants could complete the survey without assistance, and participants were satisfied with length of questionnaires and content).
Table 2.
Overview of the results of the feasibility of the assessments as reported in the article*
| First author, year (reference) | Sample size | Location and timing of geriatric assessment† | Time needed to complete the assessment | Assessment completed by | Results of the geriatric assessment | Other information about feasibility |
|---|---|---|---|---|---|---|
| Geriatric assessment studied in a prospective observational study design | ||||||
| Aaldriks, 2011 (18) | 202 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | 10% were frail by MMSE score, 32% by MNA score, 37% by GFI score, and 15% by IQCODE score | NR |
| Aparicio, 2011 (19) | 21 | During admission or stay at inpatient ward, before chemotherapy | NR | Gastroenterologist | MGA (CGA): 43% (38%) had mental status abnormality, 43% (43%) depression, 48% (33%) dependence, 67% (71%) nutrition problems, 62% (52%) comorbidities, 38% (48%) polypharmacy, 33% (33%) living situation (including caregiver support and fall hazards in the home), and 65% (50%) low hemoglobin levels or creatinin clearance | NR |
| Arnoldi, 2007 (20) | 153 | Outpatients, timing NR | NR | NR | 109 were not frail, 30 borderline, and 14 frail. The functional status in all three groups was not severely compromised | NR |
| Audisio, 2008 (21)‡ | 460 | During admission, before surgery | PACE was administered in a 20-min interview | Trial nurse or student physician | Of the 90% classified as having a PS score of 0 or 1, 11% had ADL disability, 11% MMSE score <24, 23% GDS score >4, 28% moderate or severe BFI score and 35% IADL dependence, and 61% had an abnormal outcome on at least one other PACE component | NR |
| Bailey, 2003 (22), 2004 (23)§ | 337 | Location NR, before treatment and after treatment | NR | NR | NR | NR |
| Bamias, 2007 (24) | 34 | In hospital, not specified if in- or outpatient setting, before chemotherapy, after treatment | NR | NR | 68% had PS score >2, 65% had comorbidities, median VES-13 score was 6. Two patients were classified as group 1 (PS score 0), 24 in group 2 (PS score 1), and 6 in group 3 (PS score 2 and 3) | NR |
| Bylow, 2008 (25)|| | 50 | In hospital, after at least 3 mo of ADT | NR | NR | 24% and 42% had impairments in ADL and IADL, respectively; 24% had abnormal SPMSQ score, 14% had fatigue, and 8% were nutritionally deficient. 56% had abnormal SPPB findings and 22% had fallen in the previous 3 mo | 50/58 completed assess- ment |
| Castagneto, 2004 (26) | 25 | In hospital, not specified if in- or outpatient setting, before chemotherapy and after three courses of chemotherapy and at the end of treatment | NR | NR | 2 patients had ADL disability, 6 patients IADL disability, 4 patients scored positive on the GDS. 11 patients were fully independent according to CGA parameters | Two patients refused CGA evaluation |
| Chaibi, 2011 (27) | 161 | In hospital, not specified if in- or outpatient setting, before chemotherapy, after tumor board recommendation | NR | NR | 47% had at least one comorbidity, 32% had ADL disability and 67% had IADL disability, 40% were at risk for malnutrition, and 25% were malnourished, 76% had geriatric interventions, 28% had higher dose intensity after CGA, and adherence to planned dose intensity was possible for 71% of patients | NR |
| Clough-Gorr, 2010 (73) | 660 | Location NR, after surgery | 45min (average) | Physicians | 42% had CCI score ≥1, 85% had good self-rated health, 21% were obese, 37% had ≥1 physical limitation, 69% had good mental health, 51% had good level of social support, 43% had deficits in ≥3 domains | NR |
| Extermann, 2004 (74) | 15 | Before chemotherapy, before radiation, after surgery at initial Senior Adult Oncology Program outpatient visit | NR | Multidisciplinary team | Median number of comorbidities was 5; 10 patients were at pharmacological risk, 5 were at psychosocial risk, and 8 were at nutritional risk. Patients had an average of six problems at baseline and three new problems during follow-up | 2/15 refused assessment |
| Freyer, 2004 (28) | 26 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | 26 patients were included, MGA done for 19 patients (reasons why the 7 other patients were not assessed, NR) | NR |
| Freyer, 2005 (29) | 83 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | Study author | 73.5% completely independent at home, 40% on ≥4 drugs per day, mean MMSE score 27 | NR |
| Fukuse, 2005 (100) | 120 | In hospital, not specified if in- or outpatient setting, before surgery | NR | Study authors | 65% had one or more comorbidities, 12.5% had a BMI <18.5 and 14.2% had a BMI >25kg/m2 (1.8% had PS score <2 and 89.7 had no ADL disability. 91.4% had a normal MMSE score | NR |
| Hurria, 2006 (30) | 20 (19 were evaluable) | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | Median ADL score = 18 (maximum 18), median IADL score = 20 (maximum 21), median KPS score = 80, median CCI score = 3, and median GDS score = 2 | NR |
| Hurria, 2006 (32)¶ | 50 (49 were evaluable) | In hospital, not specified if in- or outpatient setting, before chemotherapy, at start and 6 mo after completion of treatment | NR | Investigator, who was also physician, or other member of study team | Pretreatment median scores: ADL = 17; IADL = 21; GDS = 2; CCI = 3; FACT-B: physical wellbeing = 26, social wellbeing = 26, emotional wellbeing = 20, functional wellbeing = 22, breast scale = 27, and total = 117. Mean BMI = 28kg/m2 | NR |
| Hurria, 2006 (31)¶ | 31 (28 participated in neuro psycholo- gical tests) | Before chemotherapy, at start, and 6 mo after completion of treatment | NR | NR | Of 28 patients, 3 scored ≥2 SD below the published norms on two or more neuropsychological tests at baseline and 6 mo after chemotherapy; 8 patients scored ≥2 SD below published norms for two or more neuropsychological tests | NR |
| Kothari, 2011 (33) | 60 | Outpatient preoperative clinic visit, before surgery | NR | Patient completed questionnaire | One patient died within 30 d of surgery. Major complications were observed in 8 patients and 6 patients were discharged to a location other than home | NR |
| Kristjansson, 2010 (71,75)# | 182 | Location in hospital, not mentioned if in- or outpatient, before surgery | 20–80 min | Investigator, who was also a physician | 21 patients were classified as fit, 81 as intermediate, and 76 as frail according to a modified Balducci classification; 28 patients had ADL dependency, 41 had severe comorbidity, 11 took ≥8 medications/d, 16 had malnutrition, 12 had cognitive impairment, and 18 had depression. 3 died after surgery, 107 experienced complications, 83% of which were severe | Patients with some degree of cognitive impairment were interviewed in presence of their caregiver, data with regard to functional status was confirmed by nursing home staff or hospital staff |
| Marenco 2008 (34) | 571 | Initial outpatient visit, before treatment | NR | NR | 18% had BMI<21kg/m2, mean CIRS score = 17, mean KPS score = 68; 28% had ADL disability, mean IADL score = 9, mean SPMSQ score = 1 | NR |
| Marinello, 2008 (35) | 110 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | 50% had CIRS score >6; 55% had SPMSQ score of 0; 78% did not live alone; most had good ADL, IADL, and KPS scores (no results reported); 66% experienced some treatment failure, 13% died, 40% had grade 3 or 4 toxicity, and 17% had treatment interrupted | NR |
| Massa, 2006 (36) | 10 | In hospital, not specified if in- or outpatient setting, at baseline and after 4, 8, and 12wk of treatment | The authors indicated that assess-ment was “brief” | NR | At baseline, 4 patients had a MMSE score <23 | NR |
| Massa (76) | 75 | In hospital, not specified if in- or outpatient setting, before treatment | NR | NR | 26 patients were classified as fit, 23 as intermediate, and 26 as frail (unclear how defined) | NR |
| Presant, 2005 (37) | 26 | In hospital, not specified if in- or outpatient setting, before chemotherapy | 10–15min | Performed by medical assistant after only 15min of training; however, some scales completed incorrectly and not evaluable (rates of evaluable responses: pain 83%, energy 96%, QOL 91%, longer ADL and IADL forms both 52% | Mean scores: ADL 22, IADL 18, pain 1.4, energy 2.1, QOL 2.3 | Study authors reported that patients found the question- naire easy to complete and useful in communi- cating symptoms to physicians; easy to administer and short time for completion; completed by patient or patient plus family with no additional help |
| Puts, 2010 (94), 2011 (95)** | 112 | During visit to outpatient clinic or during admission, before treatment | Mean 45min (IQR = 40–55 min) | Investigators | 88% had ≥1 frailty marker, 54% had mobility impairment, 45% were physically inactive,40% had poor nutritional status, 28% had fatigue, 24% had cognitive impairment, 23% had mood disturbance, 21% had low grip strength, 35% had IADL disability, and 11% had ADL disability | 92% did not feel interview was too long, 78% had complete assess- ments |
| Rao, 2005 (38) | 99 | During admission or stay at inpatient ward | NR | NR | 27 patients received usual in- or outpatient care, 19 received geriatric inpatient and usual outpatient care, 28 received usual inpatient and geriatric outpatient care, and 25 received geriatric in- and outpatient care | NR |
| Tredan, 2007 (39) | 83 (Trial I), 75 (Trial II) | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | Presence of clinical symptoms of depression, abnormal MMSE scores, and number of medications taken daily were more frequent in CC group than in CP group; at least 1 IADL dependency was reported among 38 patients in CP group, none in CC group, median HADS score = 12 in CP group | NR |
| Tucci, 2009 (40) | 84 | In hospital, not specified if in- or outpatient setting, before surgery, before radiation | 15 min | Physician and registered nurse | 50% were classified as fit and 50% as unfit (Balducci classification) | NR |
| Wedding, 2007 (41)†† | 427 | During admission before chemotherapy | NR | NR | In 427 patients, 35% had an ADL score <100% (indicating disability), 28.4% had an IADL score <8 (indicating disability), and 30% had ≥1 comorbidities | NR |
| Geriatric assessment studied in a cross-sectional study design | ||||||
| Bearz, 2007 (42) | 22 | NR | NR | NR | 5 patients were scored as unfit, 8 patients were scored as frail, and 9 were scored as fit using the investigators’ own classification scheme (frail = patients aged ≥80 y, or patients aged ≥70 y with ≥3 grade 3 comorbidities, or patients with 1 grade 4 comorbidity and an ADL disability in ≥1 items or a geriatric syndrome | NR |
| Bylow, 2011 (43) | 134 | In hospital, not specified if in- or outpatient setting, case patients received at least 6 mo of ADT | NR | Data were from patients and medical chart | Using the modified Fried frailty criteria, 8.7% of patients were frail, 56.6% were prefrail vs 2.9% and 48.8%, respectively, in the control group (men with a history of prostate cancer after surgery or radiation, not on ADT and with no evidence of disease using PSA). 32% of patients vs 24% of control subjects had SPPB score <10. 14.3% of patients had reported a fall in the previous 6 mo vs 2.8% of control subjects | NR |
| Di Mauro, 2000 (44) | 108 | In hospital, not specified if in- or outpatient setting, timing NR | NR | NR | Average Satariano and Ragland comorbidity score was 2.5 in the cancer patients, 33% had depressive symptoms, 21% had an MMSE score <24 | NR |
| Dujon, 2006 (45) | 41 | In hospital, not specified if in- or outpatient setting, before treatment | 30min (average) | Two investigators, who were also physicians | 50% had ADL disability and 95% had IADL disability, 29% had a MMSE score <24, 17% had a PINI score >20, average CCI score was 2.7 | NR |
| Extermann, 1998 (46) | 203 | Initial visit to Senior Adult Oncology Program | NR | Multidisciplinary team | 79% had no ADL disability, 44% had no IADL disability, 31% had ECOG PS score of 0, 64% had a CCI score of 0, and 6% had a score of 0 on CIRS-G | NR |
| Girones, 2010 (47) | 91 | Follow-up visit in outpatient oncology clinic | 30–40 min | Investigator, who was also a physician | 4% had no ADL disability, 37% had no IADL disability, 10% had PS score of 2, median CCI score was 2, 28% had a geriatric syndrome, 37% were defined as frail according to the Balducci classification | NR |
| Girre, 2008 (48) | 105 | In geriatric oncology clinic, timing NR | 10 min | Investigator, who was also a physician | 58% were independent in ADL, 46% were independent in IADL, 20% had good nutritional status, 20% had impaired mobility, 53% had depressive symptoms, 33% had ≥2 comorbid conditions, 74% took ≥3 drugs | NR |
| Hurria, 2005 (88) | 43 agreed to participate (40 partici- pated) | The assessment was completed in physician’s office during outpatient visit | Mean time to complete = 27min (SD = 10min, range = 8–45min) | Patient and interviewer together | 63% had the maximum IADL score, 28% reported one or more falls, 8% reported clinically significant anxiety or depression, 45% had limitations in social activities, 5% had low BMI, and 48% reported weight loss | 78% did not need assistance to complete, 83% said the assessment was easy to understand, 90% were satisfied with the length of the question- naire, 100% stated no items were upsetting |
| Hurria, 2007 (89)‡‡ | 250 (245 completed survey) | The patients were mailed the questionnaire prior to appointment or received it at their appointment | Mean time to complete = 15min (SD = 10min, range = 2–60min). ESL patients took most time | Patient | Mean ADL score 12 (maximum 14), 49% had IADL disability, 74% had KPS score >70%, 21% had a fall, 94% had ≥1 comorbidity, 21% rated their distress score >5, 20% were underweight, and 26% had lost weight | 78% completed without assistance; of those who needed assistance, 19% got it from friends or family. 94% said that the question- naire was easy to under- stand and 91% were satisfied with its length. 89% had complete question- naires |
| Hurria, 2009 (90)‡‡ | 245 | Patients were mailed the questionnaire prior to appointment or received it at their appointment | Mean time to complete = 15min (SD = 10min, range = 2–60min). ESL patients took most time | Patient | 41% reported a distress score of ≥4 | |
| Ingram, 2002 (85) | 154 | Questionnaire was sent 2wk prior to scheduled appointments for initial consultations and follow-up appointments | NR | Patient | Mean number of medications was 6, mean number of comorbidities was 5, 69% had ADL disability, 58% had IADL disability, mean pain score was 4.2 (range = 0–10), 76% rated their health as fair or poor, 32% and 26% scored positive for depression and anxiety, respectively | Response rate to mail question- naire was 64% |
| Kellen, 2010 (49) | 113 | NR | It took 15min to complete the three screening instruments, and 30min for the CGA | Trained medical staff | GFI classified 31% as vulnerable, the VES-13 classified 49% as vulnerable (classification by aCGA NR) | NR |
| Kim, 2011 (50) | 65 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | Trained geriatric nurse | 25% had CCI score ≥2, 23% had ADL disability, 14% had IADL disability, 51% had mild cognitive impairment, 40% had depression. Frail patients had statistically significantly poorer PS and worse global health and QOL scores compared with nonfrail patients | NR |
| Luciani, 2010 (51) | 419 | In hospital, not specified if in- or outpatient setting, before treatment | NR | NR | 53% were vulnerable according to the VES-13, 30% had ADL disability, and 25% had IADL disability | NR |
| Lynch, 2007 (52) | 85 | In hospital, not specified if in- or outpatient setting, timing NR | NR | Social work intern | Most frequently reported need was emotional support, followed by caregiver support and transportation issues | NR |
| Mantovani, 2004 (53) | 84 older cancer patients, 59 adult cancer patients | In hospital, not specified if in- or outpatient setting, timing NR | NR | NR | 15% of elderly patients had severe functional impairment, 46% had IADL disability, 16% had depression according to BDI scores, 41% had MMSE score <24, 29% had MNA score <12 | NR |
| Mohile, 2007 (78)|| | 58 agreed to participate and 50 had data | In hospital, not specified if in- or outpatient setting, timing NR | NR | NR | 50% were impaired according to the VES-13 score (60% according to CGA) | 50/58 had complete assessment |
| Mohile, 2009 (54) | 12 480 | NR | NR | Investigator used data from databases | Persons with a history of cancer had a higher prevalence of ADL and IADL disabilities and geriatric syndromes, low self-rated health, a VES-13 score >3, and frailty according to the Balducci classification compared with persons without cancer | NR |
| Molina- Garrido, 2011 (55) | 41 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | Investigator who was also physician | 37% had ADL disability, 46% had IADL disability, 2% were at social risk, 46% had no comorbidity, 42% had 1 comorbidity, 10% had 2 comorbidities, and 2% had 3 comorbidities, 20% had a cognitive deficit using the Pfeiffer scale, 34% were at risk of malnutrition, and 39% took >4 drugs | NR |
| Molina- Garrido, 2011 (56) | 99 | After oncology service referral, during outpatient visit, timing NR | Mean time needed to complete CGA = 12.87min (range = 9.5–20min) | Investigator who was also a physician | 87.5% were at risk of frailty, 65.3% were ADL dependent, 75% were IADL dependent, 29.3% had some degree of cognitive impairment, 46.7% were at risk of malnutrition | Patients’ opinions regarding length of survey: very long (36.4%), short (0%), suitable (63.6%); difficulty: difficult (30.3%), acceptable (69.7%), easy (0%) |
| Monfardini, 1996 (57) | 30 | During admission or stay at inpatient ward, during routine visits | Mean = 27.4 min (range = 20–45min) | Two physicians | Patients were moderately disabled, had several depressive symptoms and good cognitive functioning. No actual numbers reported | NR |
| Overcash, 2007 (58) | 165 | Patients seen at Senior Adult Oncology Program, not specified if in- or outpatient setting, timing NR | 30 min | Interview with trained data collectors | 37 patients had experienced a fall | NR |
| Overcash 2008 (77) | 352 | Patients seen at Senior Adult Oncology Program outpatient clinic | 30 min | Interview with trained data collectors | The population was divided into three groups: no treatment, treatment, and geriatric. Mean ADL scores were 17.5, 17.6, and 16.7, respectively; mean GDS scores were 2.1, 2.9, and 2.4, respectively; mean MMSE scores were 28.4, 27.9, and 25.0, respectively; and percentages with a fall were 25%, 33%, and 42%, respectively. | NR |
| Pignata, 2008 (59) | 26 | In hospital, not specified if in- or outpatient setting, before chemotherapy | NR | NR | 65.4% had no ADL disability, 69.2% had at least 1 IADL disability, and most patients had at least 1 comorbidity, 50% had 2 or more comorbidities | NR |
| Pope, 2006 (60)‡ | 460 | During admission, before surgery | PACE was administered in a 20-min interview | Trial nurse or student physician | 33.3% had 1 or more comorbidities. 85.0% and 59.8% were independent in ADL and IADL, respectively; 87.8% had normal MMSE score, 73.3% were not depressed, 69% had no or mild fatigue, and 91% had PS score <2 | |
| Repetto, 2002 (61) | 363 | During admission or stay at inpatient ward | 20min (average) | Data used in assessment was obtained from medical chart and patient questionnaire | 74% had PS score <2, 86% were independent in ADL and 52% were independent in IADL. 41% had 1 or more comorbidities, 27% had abnormal MMSE scores, and 40% had 1 or more depressive symptoms | NR |
| Retornaz, 2008 (93) | 50 | Patients were assessed for the study when they were admitted or during initial or routine outpatient follow-up visit | NR | Investigator who was also a physician | 12% were completely independent, 42% had frailty markers but no disability, 30% had an IADL disability but no ADL disability, and 16% had an ADL disability. The most prevalent frailty markers were nutrition (62%), mobility (58%), physical inactivity (42%), cognition (42%), grip strength (26%), mood (22%), and fatigue (12%) | NR |
| Roche, 1997 (62) | 50 | After initial visit to geriatric oncology outpatient clinic | NR | Patients were seen in the geriatric oncology clinic, NR who conducted the assessment | 74% had no ADL disability and 56% had disability in IADL functioning. 27% showed cognitive deficits, 24% were considered to be depressed. The study participants who were not receiving active cancer treatment were more functionally impaired in ADL (P = .006) and IADL (P = .004) compared with those who were receiving active cancer treatment | NR |
| Serraino, 2001 (63) | 303 | During admission or stay at inpatient ward | NR | Interview with geriatrician | 17% had ADL disability, 59% had IADL disability, and 13% had limitations in taking medications. 54% of patients aged <80 y had PS score of 2–4 compared with 22% of patients aged 65–69 y (P < .001); presence of comorbidity was the same for these two age groups; frequency of IADL limitations more pronounced in oldest group aged ≥80 y of elderly patients compared with those aged 65–69 y (P = .03) | NR |
| Siegel, 2006 (64) | 25 | At outpatient clinic visit, timing NR | Assessment (three performance tests) took <5min | NR | Most had ECOG PS score of 1, the variance was highest for grip strength, less for TUG, and least for the Tinetti test. Among patients with ECOG PS score of 1, these measures were able to further identify subgroups with different functional status | NR |
| Stauder, 2010 (65) | 78 | During admission or stay at inpatient ward | NR | NR | Median values: KPS score = 90, ADL score = 100, WHO PS score = 1, VES-13 score = 2, IADL score = 7, GSD score = 7.5, CCI score =1, CIRS-G score = 5.5, MMSE score = 27, BMI = 24.7kg/m2 | NR |
| To, 2010 (66) | 200 | Location NA, before initial medical oncology visit | The first 100 patients needed 17min (average) to complete | Patients completed a questionnaire that was mailed prior to the first appointment | 45% had ADL disability and 41% had IADL disability, 35% had KPS score <70, 22% had a fall, 34% had weight loss >5% in the last 6 mo, 26% had limited social support, 39% received some support service, 22% had memory problems; 60% were classified as vulnerable, 28% as fit, and 13% as frail using own classification scheme (4–5 factors of assessment of concern = frail, 1–3 factors of concern = vulnerable, and 0 factors = fit). Those who were frail had worse functional status | 84% reported complete satisfaction with length, style, and clarity. Patients or proxies were expected to complete questionnaire before appointment, but in some cases, a geriatric oncology nurse assisted |
| Venturino, 2000 (67) | 45 | In hospital, not specified if in- or outpatient setting, timing NR | NR | NR | Descriptive (% of patients): 11.2% had PS score ≥2, 20% was ADL dependent (impaired in at least 1 item), and 51.2 % was IADL dependent (impaired in at least 1 item). Of all patients, 46.7% screened GDS positive and 24.5% scored impaired on the MMS. Of all patients, 64.4% had arthrosis or arthritis, 44.4% had hypertension, 35.5% had vascular diseases, 31.1% had digestive disease, and 28.8% had CNS diseases (excluding stroke) | NR |
| Wedding, 2007 (68)†† | 477 | Admitted to hospital, before chemotherapy | NR | NR | In group A (elderly cancer patients), 36.8% needed help with IADL, 27.5% had a KPS score of 10%–70%, and 37% had 2 or more comorbidities. In group B (younger cancer patients), 18.7% needed help with IADL, 18.5% had a KPS score of 10%–70%, and 16% had 2 or more comorbidities. In group C (elderly noncancer patients), 24.2% needed help with IADL, 14% had a KPS score of 10%–70%, and 42 % had two or more comorbidities | NR |
| Wedding, 2007 (99) | 200 | During routine oncology visit in outpatient setting | Median duration of assessment 20min (range = 9–47min) | Two physicians | 50% had maximum ADL score, 54% had maximum IADL score, 43% had poor nutritional status or were at risk, 8% had cognitive impairment using MMSE score, 23% had increased risk of falls, 16% had ≥2 comorbidities. According to the Balducci classification, 25% were fit, 25.5% were vulnerable, and 49.5% were frail. Physicians identified 64% as fit, 32.4% as vulnerable, and 3.2% as frail. The CGA identified a mean of 1.3 problems in those identified as fit, 2.3 problems in those identified as vulnerable, and 4.2 problems in those identified as frail | NR |
| Geriatric assessment studied in retrospective studies or chart reviews | ||||||
| Barthelemy, 2011 (98) | 192 (93 underwent geriatric assess- ment) | After hospital referral, not clear when and where the assessment took place | NR | NR | 36 patients were fit, 47 were vulnerable, and 10 were frail using the Balducci classification. Median age of fit patients was 75.4 y, vulnerable patients 80.3 y, and frail patients 87.4 y | NR |
| Basso, 2008 (79) | 117 | Admitted to medical oncology ward, before chemotherapy | NR | Multidisciplinary team | 33.3% were fit, 32.5% were vulnerable, and 34.2% were frail using the Balducci classification. 39.3% received an “elderly friendly” regimen, the others received a standard regimen | NR |
| Cudennec, 2007 (72) | 124 | During admission or stay at inpatient ward, outpatient (not specified) | Within 1 h | NR | Assessment was done in 82% of inpatients and 18% of outpatients presenting with gastrointestinal cancer. Average MMSE score was 23, 43% had probable depression, 40% had abnormal TUG score, 26% required a more thorough geriatric evaluation | NR |
| Cudennec, 2010 (84) | 57 | In hospital, not specified if in- or outpatient setting, before treatment decision | The Simplified Geriatric Evaluation took 1h (average) | NR | All patients lived at home and took on average 6.8 drugs per day, 51% had MMSE score <26, 47% were suspected of having depression, 68% were at risk for falls, and 44% had loss of autonomy. 5% were classified as fit, 68% were intermediate, and 42% were vulnerable. All patients in the fit group were considered able to receive optimal treatment, compared with none in vulnerable group and some in the intermediate group | NR |
| Flood, 2006 (92) | 119 | During admission to hospital | NR | Data from medical chart | Of the 11 patients who had a positive GDS score, 7 had depression documented by physician team. 42 patients had an abnormal Clock Construction Test score and 25 patients had an abnormal Short Blessed Test score, but 36% of all patients had cognitive impairment according to treating team. Of all patients, 45% had ADL disability and 74% had IADL disability, 87% were able to return home, 35% had a history of weight loss | NR |
| Fratino, 1999 (69) | 363 | During admission or stay at inpatient ward | NR | Patient filled out questionnaire and data from chart | 26% had a poor PS score, 41% had comorbid conditions, 14% had ADL limitations, 48% had IADL limitations, 27% had poor MMSE scores, and 40% had depressive symptoms | NR |
| Garman, 2004 (86) | 102 admitted, 36 with cancer | During admission or stay at inpatient ward | NR | Data from medical chart | The mean number of comorbid conditions was 4.6, the mean number of symptoms was 2.5, and the mean KPS score was 55%. 53% had cognitive impairment | NR |
| Koroukian, 2006 (91)§§ | 2552 | Location NR, during admission to Medicare Home Health Care | NR | Investigator used databases | The proportions of patients with no comorbidity, disability, or geriatric syndromes were 26.4% (breast cancer), 12% (prostate cancer), and 14% (colorectal cancer). The proportions with comorbidity, disability, and geriatric syndromes were 11.7%, 24.7%, and 15.7%, respectively. With increasing age, the proportion of persons with no comorbidity, disability, or geriatric syndromes declined | NR |
| Koroukian, 2010 (80)§§ | 1009 | Location NR, during admission to Medicare Home Health Care | NR | Investigator used databases | 15% had 1 functional limitation, 22% had ≥2 functional limitations, 31% had 1 geriatric syndrome, 17% had ≥2 geriatric syndromes, 29% had 1 comorbidity, 22% had ≥2 comorbidities | NR |
| Overcash, 2005 (81), 2006 (83) || || | 352 | In hospital, not specified if in- or outpatient setting, at initial visit to Senior Adult Oncology Program | NR | Chart review | 500 charts were reviewed, no other information presented | NR |
| Retornaz, 2008 (82) | 183 | Admitted to hospital, before chemotherapy | NR | Chart review | 67% admitted for acute medical problems and 33% admitted for diagnosis. More than 10% had geriatric syndromes, 60% took ≥3 medications, 53% had ADL disability and 64% had IADL disability, 67% had mobility impairments and malnutrition, 50% had depressive symptoms | NR |
| Rollot-Trad, 2008 (97) | 54 | In hospital, not specified if in- or outpatient setting, timing NR | NR | Chart review | 74% had a CCI score of 0–3, 22% had a CCI score of 4–5, and 2% had a CCI score >5; 39% took 4 or more medications, 69% had social support, 98% lived at home, 24% were depressed; 61% were independent in ADL, 63% were independent in IADL, 27% had an MMSE score <24 | NR |
| Sorio, 2006 (70) | 17 | In hospital, not specified if in- or outpatient setting, timing NR | NR | NR | 11 patients were considered not to have an increased risk for adverse outcomes (also called geriatric risk in this study) and 6 patients had a geriatric risk score of 1, which was defined as: PS 2, taking more than two medical treatments, and/or ADL or IADL disability | NR |
| Terret, 2004 (87) | 60 | Patients seen in geriatric oncology program (not specified if in- or outpatient setting), before treatment | Mini-CGA lasted 90–120 min | NR | 66% had an ADL disability and 87% had an IADL disability; all patients had clinically significant comorbidity; 50% were at risk for falls; 67 had a GDS score <15, 45% had cognitive disorders, and 65% were malnourished or at risk of malnutrition | NR |
| Yonnet, 2008 (96) | 363 | In hospital, not specified if in- or outpatient setting, timing NR | NR | Chart review | According to the Standardized Geriatric Evaluation (Evaluation Gériatrique Standardisée) score, patients aged ≥70 y had statistically significantly more disability, higher CCI score, underwent radiotherapy and chemotherapy less often, and had symptomatic treatment more compared with the patients aged <70 y. Those who were frail (Balducci classification) received more treatment consisting of only radiation compared with those classified as fit or vulnerable, whereas those classified as fit most often received chemotherapy alone or in combination with surgery and radiation | NR |
*NA = not applicable; NR = not reported; aCGA = abbreviated geriatric assessment; ADL = activities of daily living; ADT = androgen deprivation therapy; AGS = American Geriatric Society; BDI = Beck Depression Inventory; BFI = Brief Fatigue Inventory; BMI = body mass index; BUN = blood urea nitrogen; CC = carboplatin and cyclophosphamide; CP = carboplatin and paclitaxel; CGA = comprehensive geriatric assessment; CCI = Charlson comorbidity index; CIRS-G = Cumulative Illness Rating Scale–Geriatric; DLCL = diffuse large cell lymphoma; ECOG = Eastern Collaborative Group Oncology; PS = performance status; ESL = English as a second language; FACT-B = Functional Assessment Cancer Treatment–Breast; GDS = Geriatric Depression Scale; GFI = Groningen frailty indicator; HADS = Hospital Anxiety and Depression Scale; IADL = instrumental activities of daily living; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; IQR = interquartile range; KPS= Karnofsky Performance Status; MGA = multidimensional geriatric assessment; MMSE = Mini Mental State Examination; MNA = Mini Nutritional Assessment; NSI = nutritional risk screening; OARS = Older Americans Resources and Services; PACE = Preoperative Assessment of Cancer in the Elderly; PINI = Prognostic Inflammatory and Nutrition Index; PS = Performance Status; PPT = physical performance test; QOL = quality of life; SPMSQ = Short Portable Mental Screening Questionnaire; SPPB = Short Physical Performance Battery; TUG = Timed Up and Go test; VES-13 = Vulnerable Elder Survey-13 items; SIC = Satariano comorbidity index.
†Location = inpatient or outpatient setting; timing of geriatric assessment = before, during, or after treatment.
‡Articles reporting on the same study.
§Articles reporting on the same study.
||Articles reporting on the same study.
¶Articles reporting on the same study.
#Articles reporting on the same study.
**Articles reporting on the same study.
††Articles reporting on the same study.
‡‡Articles reporting on the same study.
§§Articles reporting on the same study.
|| ||Articles reporting on the same study.
Psychometric properties and diagnostic accuracy of geriatric assessment instruments. Eleven studies (19,37,46,49,51,55,57,65,78,81,83,99) reported psychometric properties or diagnostic accuracy of the geriatric assessment (ie, validity, reliability, and/or sensitivity and specificity) (Table 3). Most of these studies examined diagnostic accuracy of one or more short geriatric assessment tools with those of a full geriatric assessment. However, because these studies compared different screening instruments with different forms of full geriatric assessment or used the same instruments but with different cutoffs, it was not possible to summarize the results in a quantitative manner. Nevertheless, two main findings emerged from our review of these studies. First, shorter forms of geriatric assessment generally had good diagnostic accuracy compared with a full geriatric assessment. For example, Aparicio et al. (19) found that concordance between individual domain scores from mini-geriatric assessment and from comprehensive geriatric assessment ranged from 66% to 83%. Second, four studies that compared the Vulnerable Elder Survey-13 items (VES-13) with a full geriatric assessment found that the former had excellent diagnostic accuracy, with an area under the curve that ranged from 0.83 to 0.90, sensitivity that ranged from 54% to 87%, and specificity that ranged from 70% to 89% (49,51,55,78).
Table 3.
Psychometric properties and/or diagnostic accuracy reported*
| First author, year (reference) | Test being assessed | Reference (gold) standard | Reliability | Validity | Test performance | Other comments | ||
|---|---|---|---|---|---|---|---|---|
| Geriatric assessment studied in a prospective observational study design | ||||||||
| Aparicio, 2011 (19) | Compared MGA with a more traditional CGA.MGA consisted of a 1-item dementia screening, 1-item depression screening, 5 ADL and IADL items, 1-item malnutrition screening, 12 comorbidity items, screening of medications, 3 environment items (mobility and social support), and hemoglobin level and creatinine clearance | CGA consisting of cognition (MMSE), depression (modified GDS), ADL (Katz index), IADL (Lawton-Brody scale), nutrition (MNA), comorbidity (CIRS-G), polypharmacy, social intervention (no tool specified), hemoglobin level and creatinine clearance. Cutoffs used in CGA: MMSE ≤24, modified GDS ≥1, ADL<6, IADL <100%, MNA <23.5, CIRS ≥3, polypharmacy (1 anticoagulant or 2 cardiovascular drugs or 2 psychotropic drugs or ≥10 total medications), no cutoff listed for social intervention, hemoglobin level <10g/dL, creatinine clearance <50mL/min | Concordance between MGA and CGA was 86% for mental status; 73% for depression, dependence, and environment; and 66% for nutrition, comorbidity, and polypharmacy | NA | NA | NA | ||
| Presant, 2005 (37) | GOM was validated over a 12-mo period in 300 patients. GOM included a 5-point pain scale, a 5-point global quality-of-life scale, a 5-point energy scale, and 7 IADL and 8 ADL items | Each patient was interviewed by the physician after they completed the GOM; content of the interview not specified | NA | Compared physician interview results with the results of the pain, global quality of life, and energy scales and found >90% consistency (consistency not defined) | NA | NA | ||
| Geriatric assessment studied in a cross-sectional study design | ||||||||
| Extermann, 1998 (46) | CIRS-G was compared with the CCI to test the performance of both instruments and their relationship with functional status | NA | Interrater correlation (two raters) was good or very good for all subscales; the test–retest reliability was excellent for the following scales: CCI, CIRS-G categories, total CIRS-G score, and comorbidity severity grade 3 or 4. The interrater correlations for the following scales were: CCI rho = 0.74; CIRS-G total score rho = 0.76. Test–retest reliability excellent: CCI rho = 0.86, CIRS-G total score rho = 0.95. Correlation between the two comorbidity indices was fair (range = 0.25–0.39). The correlation between CIRS-G comorbidity severity grade 3 or 4 and ADL was 0.27. There was low or no correlation between comorbidity and functional status variables | NA | NA | NA | ||
| Kellen, 2010 (49) | Compared GFI, VES-13, and a newly developed aCGA to a CGA. The aCGA consisted of a 4-item GDS, 3-item ADL, 4-item IADL, and cognition.Cutoffs to define vulnerability used in the aCGA: GFI ≥4, VES-13 ≥3, aCGA GDS 2, ADL 1, IADL 1, MMS 6 | CGA consisted of ADL (Barthel index), IADL (Lawton scale), cognition (MMSE), and mood (GDS). Cutoffs to define vulnerability for the tests included in the CGA were: Barthel index of 2, MMSE ≤24, IADL 2, GDS ≥8. Vulnerability was defined as impairments in ≥2 domains (ADL and IADL) or cognitively impaired | NA | NA | GFI: sensitivity = 39%, specificity =86%, PPV = 86%, NPV = 40%; VES-13: sensitivity = 61%, specificity = 78%, PPV = 85%, NPV =48%; aCGA (aggregate): sensitivity = 51%, specificity = 97%, PPV = 97%, NPV = 48%; ADL, IADL questions in aCGA had high sensitivity; GDS, ADL had highest NPV | All three screening instruments missed cases of vulnerability that were identified using the CGA | ||
| Luciani, 2010 (51) | Compared VES-13 with CGA as a whole and CGA items. Cutoff used to classify vulnerable: VES-13 ≥3 | The CGA consisted of comorbidity (CIRS-G), cognition (MMSE), nutrition (Mini MNA), ADL (Katz index), IADL (Lawton scale), and mood (GDS). Cutoffs used to define vulnerability using the different scales included in the CGA: CIRS-G ≥3, MMSE <24, Mini MNA <12, ADL ≤5, IADL ≤5. For the GDS, no cutoff score listed. No cutoff for vulnerable provided based on the CGA as a whole | Spearman correlation between VES-13 and CGA was 0.4, and Spearman correlation between VES-13 ADL and IADL was 0.5. Weak correlations with individual items of the CGA and the VES-13; Cronbach’s alpha ranged from 0.1 (CIRS-G) to 0.3 (MMSE) | NA | The AUC comparing CGA as a whole to VES-13 was 0.83, sensitivity was 0.87, and specificity was 0.62. The AUCs comparing the whole CGA to VES-13 and CGA items were: VES-13 0.83, CIRS-G 0.58, Mini MNA 0.67, MMSE 0.81, GDS 0.56. The AUC of the VES-13 compared to ADL and IADL scale was 0.9, sensitivity was 0.9, and specificity was 0.7 | NA | ||
| Mohile, 2007 (78) | The VES-13 was compared with CGA. Cutoff: VES-13 ≥3 | CGA consisted of ADL (Katz index), IADL (Lawton scale), SPPB comorbidity (CCI), total number of medications, social support (RAND MOS Social Support Scale), and cognition (SPMSQ). Cutoffs: ADL ≤14, IADL ≤12, SPPB <9, CCI >10, number of medications ≥5, social support <4, and SPMSQ >3 | The reliability of the VES-13 using the Pearson correlation coefficient was 0.92 | NA | Using ≥2 of 7 deficits as the cutoff for CGA, the AUC was 0.90. Reliability: Pearson correlation coefficient = 0.92; with CGA: sensitivity = 0.73, specificity = 0.86, PPV = 88.9, NPV = 66.7; AUC = 0.9 | The patients who were impaired on the VES-13 performed statistically significantly worse on all tests of the CGA with the exception of social functioning (P < .05) | ||
| Molina- Garrido, 2011 (55) | Compared the BQ and the VES-13 with a CGA. Cutoffs: BQ >0 and VES-13 ≥3 | Geriatric assessment consisted of ADL (Barthel index), IADL (Lawton-Brody scale), comorbidity (CCI), social support (Guijon Social Scale), cognition (Pfeiffer test), nutrition (NSI), total number of medications, and PS (ECOG). Cutoffs: ADL ≤60, IADL ≥12, CCI >10, social support ≥8, Pfeiffer test >3, NSI ≥21, total number of medications >4, and ECOG PS ≥2. Frailty on the basis of the CGA was defined as deficits in ≥2 domains | NA | BQ, detecting risk of frailty: sensitivity 59.1%, specificity 78.9%, PPV 76.5%, NPV 62.5%, ICC 0.67 (95% CI = 0.46 to 0.81, P < .001).Detecting risk of frailty VES-13: sensitivity 54.6%, specificity 100%, PPV 100%, NPV 65.5%, ICC 0.81 (95% CI = 0.68 to 0.9, P < .001) | NA | The predictive ability for frailty: VES-13 AUC = 0.88; BQ AUC = 0.72 | ||
| Monfardini, 1996 (57) | Compared MACE with SIP. MACE consisted of the following domains: demographic, socioeconomic status, characteristics of neoplasia, comorbidity, symptoms, use of services, cognition (MMSE), depressive symptoms (GDS), balance (FICSIT), physical function (PPT), disability (IADL, ADL, and WHO PS. | NA | Using multivariable analysis, disability (using WHO PS) was associated with the SIP global score. Using multivariable analysis, disability (using WHO PS) and PPT were associated with physical SIP score. Using multivariable analysis, disability (using WHO PS) number of symptoms, GDS and balance were associated with psychosocial SIP | The ICCs for interrater reliability ranged from 0.4 (GDS) to 1 (household composition). The ICCs for test–retest reliability ranged from 0.25 (FICSIT balance score) to 1 (household composition). Cronbach’s alpha ranged from 0.4 (MMSE) to 1 (IADL and ADL) | NA | They also examined the variance by measures included in the MACE and found that WHO PS explained 70% of variance in SIP global score and 83% of the variance in SIP psychosocial score | ||
| Stauder, 2010 (65) | Used exploratory factor analysis to determine the number of individual domains assessed in the geriatric assessment (construct validity). The geriatric assessment consisted of the WHO PS, the KPS, ADL (Barthel index), IADL (Lawton-Brody scale), TUG, 7-item PPT, VES-13, GDS, FACT-G, MMSE, CIRS-G, CCI, and Social Support (F-SozU) | NA | NA | Factor analysis showed that six domains—functional status (KPS, ADL, PS, VES-13); health-related QOL (FACT-G, GDS); the variables comorbidities (as measured with both the CCI and CIRS), social support (as measured with the F-SozU and social well-being subscale FACT-G), cognition (as measured with the MMSE), and nutrition (as measured using BMI)—together explained 77% of total variance. Almost all correlations among (sub-) scales belonging to the same factor (domain) were at least moderately high; Spearman correlation coefficient >0.4 | NA | NA | ||
| Wedding, 2007 (99) | Compared physician’s judgment to the geriatric assessment. The physicians were hematologists and oncologists with >10 y of experience who classified patients as fit, vulnerable, or frail | Geriatric assessment consisted of ADL (Barthel index), IADL (Lawton-Brody Index), nutrition (MNA), cognition (MMSE), comorbidity (CCI), and mobility (Tinetti test).Cutoffs: Barthel index <100%, IADL <8, MNA continuous score used (no cutoff), MMSE continuous score used (no cutoff), CCI continuous score used (no cutoff), Tinetti test <20 | NA | Sensitivity was 0.88 and specificity was 0.31. They also compared the physician’s judgment to the Balducci classification: sensitivity was 0.43 and specificity was 0.80 | NA | NA | ||
| Geriatric assessment studied in chart reviews | ||||||||
| Overcash, 2005 (81)† | An aCGA consisting of 15 items was developed and compared with CGA. The aCGA and CGA consisted of the same scales, but the aCGA used only a few items of each scale whereas the CGA used the entire scales. The aCGA consisted of 3 ADL items (Katz index), 4 IADL items (Lawton scale), cognition (4 MMSE items), and mood (4 GDS items) | The CGA consisted of ADL 6 items (Katz index), IADL 10 items (Lawton scale), cognition (MMSE 10 items), and mood (GDS 15 items) | Items with the highest item–total correlation were selected for the aCGA. For ADL that included items 1, 3, and 4 (item–total correlations >0.70), for IADL, that included items 3–5 and 7 (item–total correlation >0.79), for GDS that included items 3, 7, 8, and 12 (item–total correlations >0.49), and for MMSE that included items 3 and 8–10 (item–total correlation >0.41). The Cronbach’s alpha coefficients for abbreviated aCGA scales were: ADL 0.84, IADL 0.930, MMSE 0.70 and GDS 0.70. The Cronbach’s alpha coefficients for CGA were: ADL 0.81, IADL 0.90, MMSE 0.65, and GDS 0.77. The Pearson correlation to assess construct validity between aCGA and CGA was 0.93 for ADL, 0.96 for IADL, 0.84 for MMSE, and 0.86 for GDS | NA | NA | NA | ||
| Overcash, 2006 (83)† | Developed cutpoints for the aCGA to indicate when a CGA is needed. The aCGA and the CGA consisted of the same scales, but the aCGA used only a few items of each scale whereas the CGA used the entire scales. The aCGA consisted of 3 ADL items (Katz index), 4 IADL items (no tool specified), cognition (4 MMSE items), and mood (4 GDS items) | The CGA consisted of ADL 6 items (Katz index), IADL 10 items (no tool specified), cognition (MMSE 10 items), and mood (GDS 15 items) | Cronbach’s alpha (internal consistency): abbreviated IADL = 0.93, both abbreviated MMSE and GDS = 0.7; abbreviated ADL = 0.84. Scores on aCGA and CGA highly correlated (ADL abbreviated–full ADL scale correlation = 0.93, abbreviated–full IADL scale correlation = 0.96, abbreviated–full MMSE scale correlation = 0.84, and abbreviated–full GDS scale correlation = 0.86) | The sensitivity for GDS using cutoff 2 was 0.81, specificity was 0.90. The sensitivity for MMSE using cutoff 6 was 0.82 and specificity 0.91 | NA | If a patient scores ≥2 on the abbreviated GDS then the full GDS needs to be administered. If a patient scores ≤6 on the abbreviated MMSE, the entire MMSE needs to be administered. If a patient scores a deficit on either of the ADL or IADL scales, the full scale needs to be administered | ||
*aCGA = abbreviated comprehensive geriatric assessment; AUC = area under the curve; BQ = Barber questionnaire; ADL= activities of daily living; BMI = body mass index; CGA = comprehensive geriatric assessment; CCI = Charlson comorbidity index; CI = confidence interval; CIRS-G = Cumulative Illness Rating Scale–Geriatric; ECOG = Eastern Collaborative Group Oncology; FACT-G=Functional Assessment of Cancer–General; FICSIT = Frailty and Injuries: Cooperative Studies of Intervention Techniques; F-SozU = Questionnaire for the Assessment of Social Support; PS = performance status; GDS = Geriatric Depression Scale; GFI = Groningen frailty indicator; GOM = Geriatric Oncology Module; HADS = Hospital Anxiety and Depression Scale; IADL = instrumental activities of daily living; ICC = intraclass correlation coefficient; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; KPS = Karnofsky Performance Status; MACE = Multidimensional Assessment Protocol for Cancer in the Elderly; MGA = Mini Geriatric Assessment; MMSE = Mini Mental State Examination; MNA = Mini Nutritional Assessment; NA = not applicable; NPV = negative predictive value; NSI = nutritional risk screening; PPT = physical performance test; PPV = positive predictive value; QOL= quality of life; RAND MOS = Rand Corporation Medical Outcomes Survey; SIP = Sickness Impact Profile; SPMSQ = Short Portable Mental Screening Questionnaire; SPPB = Short Physical Performance Battery; TUG = Timed Up and Go test; VES-13 = Vulnerable Elder Survey-13 items; SIC = Satariano comorbidity index; WHO PS= World Health Organization performance status.
†Articles reporting on the same study.
In addition, one study (49) compared the Groningen frailty indicator to a full geriatric assessment; one study (55) compared the Barber questionnaire to a full geriatric assessment; and one study (99) compared expert physician judgment to the Balducci classification.
Effectiveness of Geriatric Assessments in Predicting Cancer and Treatment Outcomes
Thirty-seven studies (51%) examined at least one of the four a priori specified outcomes presented below. The outcomes use of geriatric assessment (followed by interventions) to avoid complications of treatment and health and functional status were not studied in the included studies. Below, the results for each of the studied outcomes are described.
Geriatric assessment and treatment decision. An important goal of geriatric assessment is to distinguish between older patients who are fit to undergo standard cancer treatments and frail older patients who would benefit from modified treatment or best supportive care. Only four studies (19,27,48,98), all conducted in France, examined the impact of geriatric assessment before the start of treatment on the cancer treatment plan (Table 4). In two studies (19,98), geriatric assessment did not influence the treatment decision, whereas in the other two studies (27,48), geriatric assessment led to changes in the treatment plan for 40%–50% of patients, mostly consisting of changes in the chemotherapy regimen. Of note, in the study by Girre et al. (48), the final treatment decision (which took into account the results of the geriatric assessment) was made by a doctor or team that was not the original doctor or team that conducted the geriatric assessment. In the study of Chaibi et al. (27), patients were rediscussed at tumor board, where the multidisciplinary team decided to change their treatment recommendation based on the results of the geriatric assessment.
Table 4.
Impact of geriatric assessment on cancer treatment decision-making process or treatment delivery*
| First author, year (reference) | Sample size for geriatric assessment | Impact of geriatric assessment on cancer treatment decision making | Impact of geriatric assessment on predicting cancer treatment delivery |
|---|---|---|---|
| Geriatric assessment studied in a prospective observational study design | |||
| Aaldriks, 2011 (18) | 202 | NA | Patients receiving <4 cycles more often had low MNA scores and low MMSE scores compared with those who received ≥4 cycles of chemotherapy |
| Aparicio, 2011 (19) | 21 | The MGA never modified the oncological treatment plan | Those with a higher number of MGA abnormalities completed treatment less often, those with <6 ADL (of 7 maximum) completed treatment less often |
| Chaibi, 2011 (27) | 134 | Geriatric assessment led to changes in the proposed treatment plan in 79 patients (49%), including delay of therapy (5 patients), less intensive therapy (29 patients), and more intensive therapy (45 patients) | NA |
| Freyer, 2005 (29) | 83 | NA | Predictors of receiving <6 cycles: ECOG PS ≥2, dependence, and symptoms of depression at baseline |
| Geriatric assessment studied in a cross-sectional study design | |||
| Girre, 2008 (48) | 105 | Geriatric oncology consultation led to modifications of treatment plan for 38.7% of patients. More modifications in treatment were made for those with low BMI (≤23kg/m2) (P = .029) and those who were depressed (P = .018); in 6 cases, the chemotherapy protocol was modified with use of different drugs because of comorbidity, functional status, or malnutrition; and in 7 cases, no chemotherapy was delivered | NA |
| To, 2010 (66) | 200 | No statistically significant difference in treatment intent between fit, vulnerable, or frail groups defined according to the geriatric assessment | NA |
| Geriatric assessment studied in retrospective studies and chart reviews | |||
| Barthelemy, 2011 (98) | 93 | The Balducci classification (fit, vulnerable, frail) had no impact on the chemotherapy proposed | NA |
| Cudennec, 2007 (72) | 124 | 26% required a more thorough geriatric evaluation after the short geriatric assessment was done; for 38 of 77 patients, chemotherapy was undertaken after the geriatric assessment but the authors did not mention if the geriatric assessment changed the treatment decision | NA |
| Cudennec, 2010 (84) | 57 | The decision based on the SGE matched the multidisciplinary group initial treatment decision for SGE group 1 (general good state) and group 3 (frail patients) (n = 18). The vulnerable group (group 2) was divided into 2 subgroups, 2+ (patients with no more than 2 stabilized comorbidities) and 2− (patients with more than 2 stabilized comorbidities or at least 2 poorly or nonstabilized comorbidities). The decision based on SGE matched with the initial treatment decision for 20 of 24 patients in group 2+ (and for 13 out of 15 in group 2−) | NA |
*ADL = activities of daily living; BMI = body mass index; CGA = comprehensive geriatric assessment; ECOG PS = Eastern Collaborative Oncology Group performance status; NA = not applicable; MGA = Mini Geriatric Assessment; MNA = Mini Nutritional Assessment; MMSE = Mini Mental State Examination; SGE = Simplified Geriatric Evaluation.
In a small pilot study of 15 breast cancer patients, Extermann et al. (74) reported that assessment and interventions influenced the oncological treatment, but it was not clear how or how often they influenced the outcome. The impact of geriatric assessment on the treatment decision was examined by Marenco et al. (34) in a prospective study with a variety of cancers and stages (n = 571), and by To et al. (66) in a cross-sectional study with diverse cancers and stages (n = 200). However, it is not clear how treatment decisions were specifically impacted (eg, increase in treatment dose or dose reduction was not reported) in these two studies. Three studies (27,74,84) have shown that geriatric assessment led to geriatric interventions, such as nutritional interventions and treatment of depression before the start of treatment.
Geriatric assessment and complications or toxicity of treatment. Table 5 lists all studies that examined complications or toxicity of treatment as an outcome of geriatric assessment. Nine studies (21,30–33,35,71,73,75,95,100) that examined the impact of geriatric assessment on complications of any type of cancer treatment did not use multivariable analysis techniques. Complications were generally defined as grade 3 or 4 treatment-related toxicity, treatment interruptions, and postoperative complications, such as wound infections. In five studies with mixed cancer diagnoses and stages and sample sizes that ranged from 60 to 660 participants (21,33,35,71,75,100), impairments in basic and instrument activities of daily living, comorbidity, poor mental health, poor social support, and cognitive functioning were associated with treatment complications. In a prospective observational study that included mixed cancer diagnoses and stages (n = 112), Puts et al. (95) reported that low grip strength was the only frailty marker (of seven measured) to predict treatment toxicity. Two other studies (30–32) with sample sizes of 20, 28, and 49 participants (most with breast cancer) showed no difference in treatment toxicities with regard to geriatric assessment variables. These studies may have lacked statistical power to detect statistically significant associations.
Table 5.
Predictive validity of geriatric assessment for treatment complications*
| First author, year (reference) | Type of statistical analysis used | Was multivariable analysis conducted and were adjustments appropriate? | Sample size, number of events (treatment studied) | Complications of treatment |
|---|---|---|---|---|
| Geriatric assessment studied in a prospective observational study design† | ||||
| Audisio, 2008 (21) | Cox regression (time was held constant for all) | Multivariable analysis was conducted. The variables that were statistically significant in univariate analyses were kept in the multivariable models, including age, sex, type and stage of cancer, and severity of surgery | 460, 16% had at least 1 major complication (surgery) | Statistically significant predictors of major complications: abnormal ASA risk score (RR = 1.96, 95% CI = 1.09 to 3.53). Predictors of hospital stay longer than that for the cancer-specific median stay: ADL dependence (RR = 2.01, 95% CI = 1.37 to 2.93), IADL dependence (RR = 1.58, 95% CI = 1.11 to 2.24), abnormal PS (RR = 1.64, 95% CI = 1.06 to 2.56).Statistically significant predictors of any complication:IADL dependence (RR = 1.43, 95% CI = 1.03 to 1.98), abnormal ECOG PS (RR = 1.64, 95% CI = 1.07 to 2.52), BFI moderate or severe fatigue (RR: 1.52, 95%CI = 1.09 to 2.12) |
| Clough-Gorr, 2010 (73) | Spearman correlation, t test, χ2 test, Cochran–Armitage test, logistic regression | Multivariable analysis was conducted. The variables that were statistically significant in univariate analyses were kept in the multivariable models, which included age, stage, comorbidity, and physical and social functioning | 660, 38 had poor treatment tolerance (all treatment) | Predictors of poor treatment tolerance: CCI ≥1 (OR = 2.49, 95% CI = 1.18 to5.25), MHI5 score <80 (OR = 2.36, 95% CI = 1.15 to 4.86), Social Support Scale score <80 (OR = 3.32, 95% CI = 1.44 to 7.66) |
| Fukuse, 2005 (100) | χ2 test, logistic regression | Multivariable analysis was conducted. The adjustments were not sufficient. The variables that were statistically significant in univariate analysis were selected for inclusion in multivariable analysis, which did not include age, sex, comorbidity, or cancer stage | 120, 17% had postoperative complications (surgery) | Best logistic regression models predicting postoperative complications: Model 1: Barthel index (P = .04), MMSE (P = .031); Model 2: Barthel index (P = .019), MMSE (P = .039), operation time (P = .016) |
| Hurria, 2006 (32)‡ | Repeated measures ANOVA | NA | 49, 53% had grade 3 or 4 toxicity (chemotherapy) | Development of toxicity did not affect any geriatric assessment domains during 6-month follow-up |
| Hurria, 2006 (30) | Regression analysis | NA | 20, 11 of 19 patients experienced ≥grade 3 toxicity (chemotherapy) | Lower IADL correlated with longer terminal half-life of bound docetaxel (P = .02); higher depression score correlated with higher volume distribution (P = .01) and longer terminal half-life of bound docetaxel (P = .01). There was no univariate association between geriatric assessment variables and toxicity |
| Hurria, 2006 (31)‡ | Paired sample t test | NA | 28, 11 patients declined in cognitive functioning (chemotherapy) | 91% received CMF chemotherapy. No statistically significant difference in geriatric assessment variables for those who experienced decline vs those with no decline |
| Kothari, 2011 (33) | Correlation analysis, Fisher exact test, and Wilcoxon rank sum test | NA | 60, in-hospital mortality was 4.8%, 13% had severe complications (surgery) | The following preoperative geriatric screens predicted surgical complications: IADL shopping disability (r = 0.332, P = .009), GDS Question 2 (have you dropped many of your activities/interests) (r = 0.270, P = .037) |
| Kristjansson, 2010 (75)§ | Logistic regression | Multivariable analysis was conducted. The adjustments were not sufficient, backward selection was used to select variables, and the multivariable models therefore did not include age, sex, comorbidity, or cancer stage | 178, 107 patients experienced complications, 83 had severe complications (surgery) | Frail patients were at higher risk of severe complications compared with nonfrail patients: OR 3.13 (95% CI = 1.65 to 5.92). In univariate analysis, frail patients had higher risks of any complication, severe complications, pulmonary complications, cardiac complications, anastomotic leak, delirium, reoperation, and readmission compared with nonfrail patients |
| Kirstjansson, 2010 (71)§ | Logistic regression | Multivariable analysis was conducted. The adjustments were appropriate. Variables that were statistically significant in univariate analysis at P = .10 were selected. The final multivariable model only included variables statistically significantly associated with the outcome at P < .05, which included age and comorbidity | 182, unclear how many patients had treatment complications (surgery) | Predictors of severe complications: ECOG PS 0 (referent), ECOG PS 1 (OR = 1.64, 95% CI = 0.29 to 1.12), ECOG PS 2 (OR = 4.41, 95% CI = 1.79 to 10.86), ECOG PS 3 (OR 8.58, 95% CI = 2.19 to 33.56); PPV = 64%, NPV = 65%. Predictors of any complications: ECOG PS 0 (referent), ECOG PS 1 (OR 2.62, 95% CI = 1.23 to 5.60), ECOG PS 2 (OR 6.77, 95% CI = 2.58 to 17.77), ECOG PS 3 (OR =7.95, 95% CI = 1.88 to 33.67);PPV = 74%, NPV = 66% |
| Marinello, 2009 (35) | Logistic and multinomial logistic regression | Multivariable analysis was conducted. The adjustments were appropriate. The authors used backward selection methods to select variables that were statistically significant at an alpha of .15. The final multivariable model included metastatic disease, comorbidity, and functional status | 110, 14 died, 40 had severe toxicity, and 19 had treatment interruption for other reasons (chemotherapy) | Factors included in final models of predictors of death, treatment toxicity, or treatment interruption (combined outcome): metastatic disease (OR = 2.44, 95% CI = 0.99 to 5.99), toxicity of treatment (OR = 1.82, 95% CI = 1.06 to 3.14), CIRS >6 (OR = 3.68, 95% CI = 1.47 to 9.20), and KPS ≥80 vs <80 (OR = 0.47, 95% CI = 0.24 to 0.94) |
| Puts, 2011 (95) | Logistic and Cox regression | Multivariable analysis was conducted. The adjustments were appropriate. Multivariable models included age, sex, comorbidity, extensive treatment received, stage of disease, and diagnosis | 112, 31 had severe treatment-related toxicity (all treatments) | Poor grip strength predicted treatment toxicity (OR = 4.93, 95% CI = 1.26 to 19.22) |
*NA = not applicable; NR = not reported; HR = hazard ratio; RR = relative risk; OR = odds ratio; CI = confidence interval; ANOVA= analysis of variance; ASA = American Society of Anesthesiologists; BFI = Brief Fatigue Inventory; CCI = Charlson comorbidity index; CIRS = Cumulative Illness Rating Scale; CMF= cyclophosphamide, methotrexate, and fluorouracil; gECOG PS = Eastern Collaborative Group Oncology performance status; GDS = Geriatric Depression Scale; IADL = instrumental activities of daily living; MHI 5 = Mental Health Index 5 items; MMSE = Mini Mental State Examination; NPV= negative predictive value; PPV= positive predictive value; PS = performance status.
†No cross-sectional or retrospective studies examined the predictive validity of geriatric assessment for treatment complications.
‡Articles reporting on the same study.
§Articles reporting on the same study.
Geriatric assessment and prediction of mortality. Table 6 lists all studies that examined mortality as an outcome of domains of geriatric assessment. Sixteen studies examined the ability of geriatric assessment domains to predict mortality: 13 studies were prospective (18,20,23,24,29,34,35,39–41,71,73,95), two were cross-sectional (79,80), one was retrospective (97), and all studies included a variety of cancer diagnoses and stages. The following geriatric assessment variables were associated with increased mortality across multiple studies (18,23,29,34,35,41,71,80): older age, inadequate finances, mental health, comorbidity, high medication use, high Groningen frailty indictor score, low Mini Nutritional Assessment score, and impairments in activities of daily living. The majority of these studies adjusted for important confounders, such as sex, age, type of malignancy, stage of cancer, and comorbidity. However, three studies with sample sizes of 54 to 182 reported that none of the geriatric assessment variables were independent predictors of mortality (71,95,97).
Table 6.
Predictive validity of geriatric assessment for mortality*
| First author, year (reference) | Type of statistical analysis used | Was multivariable analysis conducted and were adjustments appropriate? | Sample size, number of events | Mortality |
|---|---|---|---|---|
| Geriatric assessment studied in a prospective observational study design | ||||
| Aaldriks, 2011 (18) | Cox regression and paired sample t test to compare changes in geriatric assessment over time | Multivariable analysis was conducted. The adjustments were appropriate. The multivariable models included sex, age, purpose of chemotherapy, and type of malignancy | 202, only mean survival was reported for each group of geriatric assessment variables, no overall mean for survival for the whole sample | Those with a GFI score ≥4 (OR = 2.19, 95% CI = 1.42 to 3.39) and MNA <23 (OR = 1.80, 95% CI =1.17 to 2.18) had higher risk of dying after start of chemotherapy |
| Arnoldi, 2007 (20) | NR | Unclear | 153, 43 patients died | 43 patients died: 50% were frail, 37% borderline, and 23% nonfrail (frail vs nonfrail, P < .05) |
| Bailey, 2004 (23) | Logistic regression | Multivariable analysis was conducted. The adjustments were appropriate; variables that were statistically significant in univariate analyses were selected for inclusion in multivariable analysis. The multivariable models included age and comorbidity | 337, 18% died | Factors associated with death within 6 mo after the baseline interview: receiving treatment with palliative intent or no treatment vs surgery only (OR = 7.42, 95% CI = 3.36 to 15.16), ADL impairment vs independent (OR = 2.47, 95% CI = 1.30 to 4.68) |
| Bamias, 2007 (24) | Cox regression | Not stated if multivariable analysis was conducted, and if it was conducted, which adjustments were done | 34, 18 patients died | Longer median PFS in geriatric assessment groups 1 (no ADL or IADL disability, no comorbidities) and 2 (IADL disability or 1–2 comorbidities) compared with group 3 (ADL disability or ≥2 comorbidities (6.9 mo vs 1.9 mo, P = .005) |
| Clough- Gorr, 2010 (73) | Spearman correlation, t test, χ2 test, Cochran–Armitage test, logistic and Cox regression | Yes, multivariable analysis was conducted. Adjustments were appropriate; variables that were statistically significant in univariate analyses were selected for inclusion in multivariable analysis. The multivariable analysis included age, stage, comorbidity, and physical and social functioning | 660, 187 died | Predictors of mortality: age 70–79 y (HR = 1.83, 95% CI = 1.19 to 2.82) and age ≥80 y (HR = 4.20, 95% CI = 2.60 to 6.81) vs age 65–69 y, inadequate finances (HR = 1.89, 95% CI = 1.24 to 2.88), CCI ≥1 (HR = 1.38, 95% CI = 1.01 to 1.88), functional limitations (HR = 1.40, 95% CI = 1.01 to 1.93), MHI 5 score <80 (HR = 1.34, 95% CI = 1.01 to 1.85) |
| Freyer, 2005 (29) | Logistic and Cox regression; however only P values are reported (no risk estimates) | Multivariable analysis was conducted. It is unclear what adjustments were done in multivariable analysis | 83, not clear how many died | Predictors of poor PFS: depression (P < .003), FIGO stage IV (P < .04), initial nonoptimal surgery (P < .008). Predictors of poor OS: depression (P = .003), FIGO stage IV (P = .007), taking >6 drugs/day (P = .04) |
| Kirstjansson, 2010 (71) | Logistic and Cox regression | Multivariable analysis was conducted. Adjustments were appropriate; variables statistically significant in univariate analysis at P < .10 were selected for inclusion in multivariable analysis. The final multivariable model included age and comorbidity (OS also adjusted for stage) | 182, 26% died | Predictors of shorter OS: ECOG PS 0 (referent), ECOG PS 1 (HR = 2.42, 95% CI = 1.04 to 5.65), ECOG PS 2 (HR = 2.95, 95% CI = 1.12 to 7.73), ECOG PS 3 (HR = 9.69, 95% CI = 3.01 to 31.22) |
| Marenco, 2008 (34) | Logistic and Cox regression | Multivariable analysis was conducted. The adjustments were sufficient. The multivariable model included age, type of cancer, stage, sex, and comorbidity | 571, 412 patients died | Prognostic impact on survival of patient characteristic obtained at first visit: male sex (HR = 1.68, 95% CI = 1.31 to 2.15), KPS, per 10-point decline (HR = 0.8, 95% CI = 0.7 to 0.91) |
| Marinello, 2009 (35) | Logistic and multinomial logistic regression | Multivariable analysis was conducted. The adjustments were appropriate. Variables were selected by backward selection, variables that were statistically significant at P = .15 were included. The final model included sex, metastatic disease, comorbidity, functional status, and toxicity of treatment | 110, 14 died, 40 had severe toxicity, and 19 had treatment interruption for other reasons | Predictors of death: metastatic disease (OR = 20.96, 95% CI = 3.17 to 138.7); toxicity of treatment (OR = 2.8, 95% CI = 1.02 to 7.68); CIRS score >6 (OR = 6.46, 95% CI = 1.31 to 31.93) |
| Puts, 2011 (95) | Cox regression | Multivariable analysis was conducted. The adjustments were appropriate. The multivariable model included age, sex, comorbidity, extensive treatment received, stage of disease, and diagnosis | 112, 15 died during follow-up | None of the frailty markers was associated with OS |
| Tredan, 2007 (39) | Cox regression | Multivariable analysis was conducted. The adjustments were appropriate. Variables that were statistically significant in univariate analysis were included in the multivariable analysis. The multivariable analysis model included age, stage, and PS | 83 (trial 1) and 75 (trial 2), 43% died | Predictors of mortality: increasing age (HR = 1.07, 95% CI = 1.01 to 1.13), stage IV vs stage III (HR = 3.05, 95% CI = 1.58 to 5.89), depression (HR = 5.2, 95% CI = 2.46 to 10.99), carboplatin + paclitaxel vs carboplatin + cyclophosphamide (HR = 2.14, 95% CI = 1.1 to 4.15) |
| Tucci, 2009 (40) | Fisher exact test, Student t test, log rank tests | NA | 84, unclear how many patients died | Patients classified as fit using the Balducci classification had better median survival compared with those classified as vulnerable and frail (not reached vs 8 mo, P < .001); OS: 77.6% vs 23.8% (log-rank P < .001), 2-y PFS: 73.4% vs 21.7% (P < .001) |
| Wedding, 2007 (41) | Cox regression | Multivariable analysis was conducted. The adjustments were appropriate; variables were selected based on P value in the univariate analysis and included age, comorbidity, and type of tumor | 427, 61.4% died | Predictors of shorter survival: increasing age (HR = 1.02, 95% CI = 1.01 to 1.03), type of tumor (HR = 1.83, 95% CI = 1.31 to 2.55), WHO PS (HR = 1.45, 95% CI = 1.06 to 2.00), comorbidity level 3–4 vs none (HR = 1.42, 95% CI = 1.01 to 2.00) |
| Geriatric assessment studied in a cross-sectional study design | ||||
| Basso, 2008 (79) | Kaplan–Meier analysis, log-rank test | NA | 117, 74 patients died | Median survival: frail patients (6.4 mo), nonfrail patients (16.9 mo); statistically significantly different survival rates at 1 and 2 y. |
| Geriatric assessment studied in retrospective studies and chart reviews | ||||
| Koroukian, 2010 (80) | Logistic and Cox regression | Multivariable analysis was conducted. The adjustments were appropriate. The multivariable model included age, sex, race, cancer stage, comorbidity, functional limitations, and geriatric syndromes | 1009, not clear how many died | Predictors of overall mortality: ≥2 functional limitations (HR = 1.33, 95% CI = 1.10 to 1.62), ≥2 geriatric syndromes (HR = 2.34, 95% CI = 1.74 to 3.15) |
| Rollot-Trad, 2008 (97) | Cox regression | Multivariable analysis was conducted. It is unclear for what variables the multivariable analysis was adjusted | 54, the mortality rate was 41% | None of the variables was statistically significantly associated with survival in multivariable analysis |
*NA = not applicable; NR = not reported; HR = hazard ratio; RR = relative risk; OR = odds ratio; CI = confidence interval; CCI = Charlson comorbidity index; CIRS = Cumulative Illness Rating Scale; ECOG PS = Eastern Collaborative Group Oncology performance status; FIGO = International Federation of Gynecology and Obstetrics; GFI = Groningen frailty indicator; IADL = instrumental activities of daily living; MHI 5 = Mental Health Index 5 items; KPS = Karnofsky performance status; MNA = Mini Nutritional Assessment; OS = overall survival; PFS = progression-free survival; VES-13 = Vulnerable Elder Survey-13 items; WHO PS = World Health Organization performance status.
Six studies (20,24,40,42,76,79) examined the survival of patients categorized as frail, vulnerable, or fit rather than according to individual components of the geriatric assessment. These studies used tests such as χ2 or log rank tests but did not examine predictive validity using multivariable analytic techniques.
Three studies examined overall survival, progression-free survival, and/or response to treatment in relation to geriatric assessment in univariate analyses only. Bamias et al. (24) found no associations between the VES-13 score and overall survival, progression-free survival, or response to treatment. Basso et al. (79) found that the incidence of treatment interruption was higher and had less benefit in terms of response in patients classified as frail according to the Balducci classification. Massa et al. (76) reported better response in fit patients compared with frail patients (how the patients were classified as fit, intermediate, and frail was not described), but it is not clear what analysis was conducted.
Geriatric assessment and the use of care and other outcomes. Two studies (23,94) examined the association between domains of geriatric assessment and the use of care (Supplementary Table 4, available online). In a prospective study with 337 colorectal cancer patients that adjusted for age and sex but not for illness severity and comorbidity, Bailey et al. (23) found that patients who were older and had poorer mental health had greater use of social resources. In a prospective study that used seven markers of frailty markers and included a wide variety of cancers and stages, Puts et al. (94) reported that only one frailty marker, cognitive impairment, predicted visits to the emergency department after adjustment for confounders such as cancer type, cancer stage comorbidity, age, and sex. Five studies (22,23,34,66,91,98) reported that components of the geriatric assessment, such as age and functional status, were associated with the receipt of certain treatment modalities or regimens, such as surgery only.
Other outcomes studied included changes in functional status, distress, clinical response, and discharge to usual place of residence after hospital admission (Supplementary Table 4, available online).
Discussion
This is the first review, to our knowledge, to systematically summarize all available evidence with regard to the use and effectiveness of geriatric assessment in the oncology setting. The evidence summarized in this review suggests that it is feasible to conduct a geriatric assessment in a hospital setting in older patients with cancer. The use of a geriatric assessment in the hospital setting can identify many health and functional status issues that might not otherwise be known by the treating oncologist. In addition, several domains of geriatric assessment are associated with oncological outcomes, such as toxicity of treatment and mortality, even in heterogeneous study populations. The factors consistently associated with these outcomes include impairments in activities of daily living, comorbidity, and poor mental health. Because most of the studies included heterogeneous study populations and featured small sample sizes, they had limited ability to conduct subgroup analyses. Thus, it was not possible to compare the results for solid tumors vs hematological malignancies or for cancers with different prognoses or treatment trajectories (eg, adjuvant vs metastatic settings). Future studies in more homogeneous populations are needed to identify populations where geriatric assessment might be particularly useful in helping a physician select the cancer treatment, preventing adverse outcomes of cancer and its treatment.
We found that although many studies have incorporated some form of geriatric assessment to describe the patient population, fewer studies have examined the usefulness of geriatric assessment in terms of its ability to identify older adults at risk for adverse outcomes of cancer and its treatment. To date, no randomized controlled trial has been conducted to evaluate the effectiveness of geriatric assessment for distinguishing between fit and frail older adults to improve outcomes of cancer treatment compared with usual care in oncology. Nevertheless, experts in the field and SIOG (13,102) expect that by distinguishing between fit and more vulnerable and frail patients, treatment regimens can be adjusted to maximize the treatment effectiveness and avoid complications; however, this expectation still needs to be proven in a randomized controlled trial setting.
Even though no randomized controlled trial has examined the effectiveness of geriatric assessment in the oncology setting, the general principles of geriatric medicine and geriatric assessment are thought to apply to all older adults, including those with cancer. Published guidelines and the recommendations of groups such as the NCCN and the SIOG suggest that most clinicians accept the applicability of geriatric assessment in the oncology setting. However, we found no high-quality evidence (ie, from randomized controlled trials) that conducting a geriatric assessment and tailoring interventions based on its findings altered important patient outcomes in older cancer patients. Thus, based on the results of this systematic review, firm recommendations for implementing geriatric assessment and the type of geriatric assessment in routine clinical practice await additional study because the effectiveness of geriatric assessment in improving patient outcomes remains unclear. Geriatric assessment is not an intervention in and of itself. Rather, interventions that can improve patient outcomes are identified based on the geriatric assessment. The aim of the traditional geriatric assessment is to predict functional decline and falls in an older population with cognitive and functional impairments. Therefore, it is not surprising that in many of the reviewed studies, geriatric assessment was not useful in predicting oncology outcomes, such as treatment toxicity. Ceiling effects (ie, when most participants score the maximum score possible on a test because the test is unable to distinguish between individuals at the higher score range of the test), as reported by Hurria et al. (32), could explain the null effect of geriatric assessment in predicting outcomes in many of the studies that were included in this review. However, experts have recommended using geriatric assessment in clinical oncology practice because it is expected to improve care for older oncology patients by helping improve treatment selection, avoiding toxicity, and identifying undetected medical problems that can interfere with treatment (11,12,13). Future studies should carefully consider which outcomes are most relevant in this population and how the geriatric assessment can be used to identify opportunities for effective interventions. The necessary next step in geriatric oncology requires intervention studies based on geriatric assessment. A recent meta-analysis of 22 randomized controlled trials that evaluated the effect of geriatric assessment vs usual care on independence and discharge to usual residence after hospital admission for older adults admitted to the hospital showed those who received geriatric assessment prior to interventions were more likely to be alive and in their own homes at the 6-month follow-up and less likely to suffer death or deterioration (103). However, few of these patients had cancer.
This review has several strengths. We used systemic methods to identify all relevant studies, and two reviewers independently assessed the titles and abstracts by following the PRISMA statement. We also used various published quality assessment criteria to take into account different study designs included in this review. We attempted to synthesize the results in an unbiased and reproducible way. Our search strategy was inclusive: We did not exclude any study based on the methodological quality because this is the first systematic review providing a comprehensive overview of the use of geriatric assessment in the oncology setting.
This review also has several limitations. A meta-analysis was not possible because the studies were heterogeneous with respect to geriatric assessment instruments, methods, study populations, and outcomes. Furthermore, the findings are limited by the heterogeneous scientific quality of the studies included. Although we tried to contact all study authors if there were questions regarding the study , we were not successful in contacting all study authors, especially because some studies were published 15 years ago. It is thus possible that we rated some quality criteria of each of the individual studies during the quality assessment as unsatisfactory simply because they were not reported or because reporting guidelines such as STROBE and MOOSE for different study types were published more recently than some of the studies. In addition, cancer treatment options for older adults have changed because more elder-friendly treatments are being developed with less toxicity. These changes may have impacted the predictive validity results of the geriatric assessments reviewed in this systematic review. In addition, we did not examine the feasibility or effectiveness of geriatric assessment by cancer type or stage. Moreover, study participants ranged in age from 65 to 99 years. The results of this systematic review might be different for different patient populations. As more studies are conducted, future systematic reviews should take cancer type and stage into account to examine the effectiveness of geriatric assessment in improving patient outcomes for different tumor types and stages.
There are four fundamental barriers to advancing the field of geriatric oncology as identified through this systematic review. First and most important is the conceptual issue of the clinical value of a gold standard for geriatric assessment in the oncology setting. There is also no consensus regarding which domains should be included in geriatric assessment and how the instruments should best be designed and used in the oncology setting. The ability to compare newly developed, abbreviated, or otherwise-modified instruments with an idealized geriatric assessment is limited because the value of geriatric assessment in terms of predictive validity and impact on cancer treatment or patient outcomes is unclear. For example, the value of geriatric assessment has not been rigorously compared with usual care in the oncology setting, particularly with respect to the impact on treatment decision making or patient outcomes.
Second, there is no uniform approach to classifying patients in different risk groups. The most frequently used classification scheme is the fit–vulnerable–frail classification developed by Balducci and Stanta (101). This classification approach recommends standard therapy for fit patients, adjusted therapy for those classified as vulnerable, and best supportive care or palliative treatment for those classified as frail. Other studies have developed their own standards for classifying patients into different risk groups. Most authors have defined impairments in two or more domains of the geriatric assessment as criteria for classifying a patient as frail. These approaches are not necessarily in agreement with the concept of frailty as it is used in the geriatric medicine setting (104). In the latter context, frailty is not considered to be the endpoint of the continuum of fit to completely dependent; rather, it represents a state where an individual is independent but at high risk for developing disability. This inconsistent use of the concept of frailty by oncology and geriatric medicine may lead to confusion and hinder the translation of knowledge from research into clinical practice across different settings. In addition, this varied usage hampers research because study results cannot be compared across studies, both within geriatric oncology and across disciplines.
The third barrier is the lack of information about the psychometric properties of the tools used in the geriatric assessment. Most studies have used instruments that have been validated in the traditional geriatric medicine setting. The properties of these instruments may be different in the oncology setting because the psychometric properties are determined by the clinical population studied. The clinical population in the oncology setting might be different from the one in the geriatric medicine setting where the psychometric properties of these tools were studied. Older persons with moderate to severe disability or cognitive impairment are less likely to be referred to oncology clinics due to referral bias (105). Thus, most likely, the population in the oncology setting has less cognitive impairment and better functional status than the population in which these tools were developed and tested. Therefore, the psychometric properties of geriatric assessment instruments should be examined within the geriatric oncology setting. This would better allow clinicians and researchers to select or develop the most appropriate and effective tools to include in their geriatric assessment in the oncology setting.
Finally, the quality of reporting for studies in the field of geriatric oncology should be improved. Our quality assessment of the published studies suggests that researchers conducting future studies need to report more details on the study design, setting, response rates, and follow-up so that other researchers and clinicians can better evaluate the generalizability of the findings to their own settings.
Randomized controlled trials comparing the effectiveness of conducting geriatric assessment with standard oncological care on relevant oncology outcomes are urgently needed to move the field of geriatric oncology forward. Two studies (27,48) showed an impact of geriatric assessment on the cancer treatment decision, whereas two others did not (19,98); however, none of these studies was a randomized controlled trial. Several studies were published after the search for this systematic review was conducted (106–112). Two studies examined the impact of geriatric assessment on the treatment decision and showed that for the majority of patients geriatric assessment had no impact on the treatment decision (108,112). In addition, four studies that evaluated the predictive validity of geriatric assessment showed that geriatric assessment domains were predictive of cancer treatment outcomes, such as chemotherapy (106,109–112).
Although geriatric assessment is recommended to be used in clinical settings for older adults with cancer by both NCCN and SIOG (11,12,13), in a public health care system with finite resources to allocate to competing health care interventions, showing the (cost-) effectiveness of a geriatric assessment in improving oncology outcomes for older adults is necessary for it to become standard of care. Given that geriatric assessment has been recommended as the standard of care, the broad implementation of geriatric assessment in clinical settings is likely to improve oncology outcomes for older adults affected by cancer.
There is a dearth of studies examining the impact of geriatric assessment on the use of care, and this outcome should be included in future studies. In addition, no study has examined the impact of geriatric assessment on quality of life, which, for older adults with cancer, is an important consideration (113,114).
Thus, there is a need for studies with improved methodological quality, larger sample sizes, and longitudinal design to obtain evidence for the use of geriatric assessment in older patients who are seen in diverse oncology settings. Furthermore, given the costs of conducting multidisciplinary geriatric assessments and the large number of older adults being seen in oncology, there is a need for a short screening tool with good psychometric properties to identify older adults that can benefit from a more in-depth geriatric assessment. Several such tools have been developed for the geriatric oncology setting, including the G-8 (115) and the instrument developed by Hurria et al. (107), all of which are currently being investigated for this purpose. The effectiveness of such an approach—a screening tool for all older patients followed by an in-depth assessment of those deemed to be at risk—is not established and needs to be validated in randomized controlled trials. Of course, such screening tools will only be of value once randomized controlled trials have clearly demonstrated that resource-intensive comprehensive geriatric assessment is effective in improving outcomes compared with usual care in the oncology setting. Furthermore, organizations such as SIOG and NCCN that advocate for some form of geriatric assessment should articulate more clearly the current state of knowledge with regard to the benefits and impact of geriatric assessment on specific outcomes along with highlighting the current large gap in evidence.
Supplementary Material
Funding
This study was funded by the Canadian Institutes for Health Research, grant number KRS-103278 (to MTEP).
Notes
The funder had no role in the study. The authors report that they have no conflicts of interest.
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010 CA Cancer J Clin. 2010;60 (5):277– 300 [DOI] [PubMed] [Google Scholar]
- 2. Canadian Cancer Society’s Steering CommitteeCanadian Cancer Statistics 2011Toronto: Canadian Cancer Society; 2011; [Google Scholar]
- 3. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation J Clin Oncol. 2009;27 (17):2758– 2765 [DOI] [PubMed] [Google Scholar]
- 4. Extermann M, Aapro M, Bernabei R,, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55 (3):241– 252 [DOI] [PubMed] [Google Scholar]
- 5. Balducci L, Yates J. . General guidelines for the management of older patients with cancer. Oncology. 2000;14 (11A):221– 227 [PubMed] [Google Scholar]
- 6. Rubenstein LZ, Josephson KR, Wieland GD, et al. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med. 1984;311 (26):1664– 1670 [DOI] [PubMed] [Google Scholar]
- 7. Rubenstein LZ, Siu AL, Wieland D. Comprehensive geriatric assessment: toward understanding its efficacy Aging. 1989;1 (2):87– 98 [DOI] [PubMed] [Google Scholar]
- 8. Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials Lancet. 1993;342 (8878):1032– 1036 [DOI] [PubMed] [Google Scholar]
- 9. Wieland D, Ferrucci L. Multidimensional geriatric assessment: back to the future J Gerontol A Biol Sci Med Sci. 2008;63 (3):272– 274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurria A. We need a geriatric assessment for oncologists Nat Clin Pract Oncol. 2006;3 (12):642– 643 [DOI] [PubMed] [Google Scholar]
- 11. International Society of Geriatric Oncology Geriatric Assessment Tools.. http:// www.siog.org 2012. Accessed November 8, 2011.
- 12. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Senior Adult Oncology. Version 2. 2011http://www.nccn.org/professionals/physician_gls/pdf/senior.pdfAccessed November 8, 2011.
- 13. Droz JP, Balducci L, Bolla M,, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology BJU Int. 2010;106 (4):462– 469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S. The Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 15. von EE, Altman DG, Egger M,, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies J Clin Epidemiol. 2008;61 (4):344– 349 [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC,, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group JAMA. 2000;283 (15):2008– 2012 [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62 (10):1006– 1012 [DOI] [PubMed] [Google Scholar]
- 18. Aaldriks AA, Maartense E, le Cessie S,, et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy Crit Rev Oncol Hematol. 2011;79 (2):205– 212 [DOI] [PubMed] [Google Scholar]
- 19. Aparicio T, Girard L, Bouarioua N,, et al. A mini geriatric assessment helps treatment decision in elderly patients with digestive cancer. A pilot study Crit Rev Oncol Hematol. 2011;77 (1):63– 69 [DOI] [PubMed] [Google Scholar]
- 20. Arnoldi E, Dieli M, Mangia M, Minetti B, Labianca R. Comprehensive geriatric assessment in elderly cancer patients: an experience in an outpatient population Tumori. 2007;93 (1):23– 25 [DOI] [PubMed] [Google Scholar]
- 21. Audisio RA, Pope D, Ramesh HS,, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study Crit Rev Oncol Hematol. 2008;65 (2):156– 163 [DOI] [PubMed] [Google Scholar]
- 22. Bailey C, Corner J, Addington-Hall J,, et al. Treatment decisions in older patients with colorectal cancer: the role of age and multidimensional function Eur J Cancer. 2003;12 (3):257– 262 [DOI] [PubMed] [Google Scholar]
- 23. Bailey C, Corner J, Addington-Hall J, Kumar D, Haviland J. Older patients’ experiences of treatment for colorectal cancer: an analysis of functional status and service use Eur J Cancer. 2004;13 (5):483– 493 [DOI] [PubMed] [Google Scholar]
- 24. Bamias A, Lainakis G, Kastritis E,, et al. Biweekly carboplatin/gemcitabine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: report of efficacy, quality of life and geriatric assessment Oncology. 2007;73 (5–6):290– 297 [DOI] [PubMed] [Google Scholar]
- 25. Bylow K, Dale W, Mustian K,, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy Urology. 2008;72 (2):422– 427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castagneto B, Zai S, Marenco D,, et al. Single-agent gemcitabine in previously untreated elderly patients with advanced bladder carcinoma: response to treatment and correlation with the comprehensive geriatric assessment Oncology. 2004;67 (1):27– 32 [DOI] [PubMed] [Google Scholar]
- 27. Chaibi P, Magne N, Breton S,, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients Crit Rev Oncol Hematol. 2011;79 (3):302– 307 [DOI] [PubMed] [Google Scholar]
- 28. Freyer G, Lortholary A, Delcambre C,, et al. Unexpected toxicities in elderly patients treated with oral idarubicin in metastatic breast cancer: the GINECO experience Clin Oncol. 2004;16 (1):17– 23 [DOI] [PubMed] [Google Scholar]
- 29. Freyer G, Geay JF, Touzet S,, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study Ann Oncol. 2005;16 (11):1795– 1800 [DOI] [PubMed] [Google Scholar]
- 30. Hurria A, Fleming MT, Baker SD,, et al. Pharmacokinetics and toxicity of weekly docetaxel in older patients Clin Cancer Res. 2006;12 (20, pt 1):6100– 6105 [DOI] [PubMed] [Google Scholar]
- 31. Hurria A, Rosen C, Hudis C,, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study J Am Geriatr Soc. 2006;54 (6):925– 931 [DOI] [PubMed] [Google Scholar]
- 32. Hurria A, Hurria A, Zuckerman E,, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy J Am Geriatr Soc. 2006;54:1119– 1124 [DOI] [PubMed] [Google Scholar]
- 33. Kothari A, Phillips S, Bretl T, Block K, Weigel T. Components of geriatric assessments predict thoracic surgery outcomes J Surg Res. 2011;166 (1):5– 13 [DOI] [PubMed] [Google Scholar]
- 34. Marenco D, Marinello R, Berruti A,, et al. Multidimensional geriatric assessment in treatment decision in elderly cancer patients: 6-year experience in an outpatient geriatric oncology service Crit Rev Oncol Hematol. 2008;68 (2):157– 164 [DOI] [PubMed] [Google Scholar]
- 35. Marinello R, Marenco D, Roglia D,, et al. Predictors of treatment failures during chemotherapy: a prospective study on 110 older cancer patients Arch Gerontol Geriat. 2009;48 (2):222– 226 [DOI] [PubMed] [Google Scholar]
- 36. Massa E, Madeddu C, Lusso MR, Gramignano G, Mantovani G. Evaluation of the effectiveness of treatment with erythropoietin on anemia, cognitive functioning and functions studied by comprehensive geriatric assessment in elderly cancer patients with anemia related to cancer chemotherapy Crit Rev Oncol Hematol. 2006;57 (2):175– 182 [DOI] [PubMed] [Google Scholar]
- 37. Presant CA, Thompson E, Leberthon B, Vergara L, McGowen P. Effects of weekly paclitaxel or paclitaxel plus carboplatin on functionality and symptoms of geriatric patients with cancer as measured by a brief geriatric oncology module: a pilot experience Cancer. 2005;103 (12):2623– 2628 [DOI] [PubMed] [Google Scholar]
- 38. Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient J Gerontol A Biol Sci Med Sci. 2005;60 (6):798– 803 [DOI] [PubMed] [Google Scholar]
- 39. Tredan O, Geay JF, Touzet S,, et al. Carboplatin/cyclophosphamide or carboplatin/paclitaxel in elderly patients with advanced ovarian cancer? Analysis of two consecutive trials from the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens Ann Oncol. 2007;18 (2):256– 262 [DOI] [PubMed] [Google Scholar]
- 40. Tucci A, Ferrari S, Bottelli C,, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy Cancer. 2009;115 (19):4547– 4553 [DOI] [PubMed] [Google Scholar]
- 41. Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients J Cancer Res Clin Oncol. 2007;133 (12):945– 950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bearz A, Fratino L, Spazzapan S,, et al. Gefitinib in the treatment of elderly patients with advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2007;55 (1):125– 127 [DOI] [PubMed] [Google Scholar]
- 43. Bylow K, Hemmerich J, Mohile SG,, et al. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study Urology. 2011;77 (4):934– 940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di Mauro S, Distefano A, Di Fazio I, Leotta C, Malaguarnera M. On the importance of multidimensional evaluation of elderly oncologic patients Arch Gerontol Geriat. 2000;30 (1):63– 71 [DOI] [PubMed] [Google Scholar]
- 45. Dujon C, Azarian R, Azarian V, Petitpretz P. Lung cancer in the elderly: performance status and/or geriatric indices? Rev Mal Respir. 2006;23 (4, pt 1):307– 318 [DOI] [PubMed] [Google Scholar]
- 46. Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients J Clin Oncol. 1998;16 (4):1582– 1587 [DOI] [PubMed] [Google Scholar]
- 47. Girones R, Torregrosa D, az-Beveridge R. Comorbidity, disability and geriatric syndromes in elderly breast cancer survivors. Results of a single-center experience Crit Rev Oncol Hematol. 2010;73 (3):236– 245 [DOI] [PubMed] [Google Scholar]
- 48. Girre V, Falcou MC, Gisselbrecht M,, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci. 2008;63 (7):724–730 [DOI] [PubMed] [Google Scholar]
- 49. Kellen E, Bulens P, Deckx L,, et al. Identifying an accurate pre-screening tool in geriatric oncology Crit Rev Oncol Hematol. 2010;75 (3):243– 248 [DOI] [PubMed] [Google Scholar]
- 50. Kim YJ, Kim JH, Park MS,, et al. Comprehensive geriatric assessment in Korean elderly cancer patients receiving chemotherapy J Cancer Res Clin Oncol. 2011;137 (5):839– 847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luciani A, Ascione G, Bertuzzi C,, et al. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13 J Clin Oncol. 2010;28 (12):2046– 2050 [DOI] [PubMed] [Google Scholar]
- 52. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center Clin J Oncol Nurs. 2007;11 (6):929– 933 [DOI] [PubMed] [Google Scholar]
- 53. Mantovani G, Madeddu C, Gramignano G,, et al. Association of serum IL-6 levels with comprehensive geriatric assessment variables in a population of elderly cancer patients Oncol Rep. 2004;11 (1):197– 206 [PubMed] [Google Scholar]
- 54. Mohile SG, Xian Y, Dale W,, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries J Natl Cancer Inst. 2009;101 (17):1206– 1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Molina-Garrido MJ, Guillen-Ponce C. Comparison of two frailty screening tools in older women with early breast cancer Crit Rev Oncol Hematol. 2011;79 (1):51– 64 [DOI] [PubMed] [Google Scholar]
- 56. Molina-Garrido MJ, Guillen-Ponce C. Development of a cancer-specific comprehensive geriatric assessment in a University Hospital in Spain Crit Rev Oncol Hematol. 2011;77 (2):148– 161 [DOI] [PubMed] [Google Scholar]
- 57. Monfardini S, Ferrucci L, Fratino L,, et al. Validation of a multidimensional evaluation scale for use in elderly cancer patients Cancer. 1996;77 (2):395– 401 [DOI] [PubMed] [Google Scholar]
- 58. Overcash J. Prediction of falls in older adults with cancer: a preliminary study Oncol Nurs Forum. 2007;34 (2):341– 346 [DOI] [PubMed] [Google Scholar]
- 59. Pignata S, Breda E, Scambia G,, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study Crit Rev Oncol Hematol. 2008;66 (3):229– 236 [DOI] [PubMed] [Google Scholar]
- 60. Pope D, Ramesh H, Gennari R,, et al. Pre-operative assessment of cancer in the elderly (PACE): a comprehensive assessment of underlying characteristics of elderly cancer patients prior to elective surgery Surg Oncol. 2006;15 (4):189– 197 [DOI] [PubMed] [Google Scholar]
- 61. Repetto L, Fratino L, Audisio RA,, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study J Clin Oncol. 2002;20 (2):494– 502 [DOI] [PubMed] [Google Scholar]
- 62. Roche RJ, Forman WB, Rhyne RL. . Formal geriatric assessment, an imperative for the older person with cancer Cancer Pract. 1997;5 (2):81– 86 [PubMed] [Google Scholar]
- 63. Serraino D, Fratino L, Zagonel V. Prevalence of functional disability among elderly patients with cancer Crit Rev Oncol Hematol. 2001;39 (3):269– 273 [DOI] [PubMed] [Google Scholar]
- 64. Siegel AB, Lachs M, Coleman M, Leonard JP. Lymphoma in elderly patients: novel functional assessment techniques provide better discrimination among patients than traditional performance status measures Clin Lymphoma Myeloma. 2006;7 (1):65– 69 [DOI] [PubMed] [Google Scholar]
- 65. Stauder R, Moser K, Holzner B, Sperner-Unterweger B, Kemmler G. Six independent domains are defined by geriatric assessment in elderly cancer patients Crit Rev Oncol Hematol. 2010;74 (2):97– 105 [DOI] [PubMed] [Google Scholar]
- 66. To THM, Okera M, Prouse J, Prowse RJ, Singhal N. Infancy of an Australian geriatric oncology program-characteristics of the first 200 patients J Geriatr Oncol. 2010;1 (2):81– 86 [Google Scholar]
- 67. Venturino A, Comandini D, Granetto C,, et al. Formestane is feasible and effective in elderly breast cancer patients with comorbidity and disability Breast Cancer Res Treat. 2000;62 (3):217– 222 [DOI] [PubMed] [Google Scholar]
- 68. Wedding U, Rohrig B, Klippstein A,, et al. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients Support Care Cancer. 2007;15 (9):1097– 1104 [DOI] [PubMed] [Google Scholar]
- 69. Fratino L, Serraino D, Repetto L,, et al. Comprehensive geriatric assessment in elderly cancer patients. Preliminary results from the Italian Group of Geriatric Oncology-GIOG-er Rays. 1999;24 (2):53– 57 [Google Scholar]
- 70. Sorio R, Toffoli G, Crivellari D,, et al. Oral etoposide in elderly patients with advanced non small cell lung cancer: a clinical and pharmacological study J Chemother. 2006;18 (2):188– 191 [DOI] [PubMed] [Google Scholar]
- 71. Kristjansson SR, Jordhoy MS, Nesbakken A,, et al. Which elements of a comprehensive geriatric assessment (CGA) predict post-operative complications and early mortality after colorectal surgery? J Geriatr Oncol. 2010;1 (2):57– 69 [Google Scholar]
- 72. Cudennec T, Mitry E, Moulias S,, et al. Which patients should receive geriatric assessment before chemotherapy? Adv Gastrointest Cancer. 2007;5(4):3– 6 [Google Scholar]
- 73. Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up J Clin Oncol. 2010;28 (3):380– 386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Extermann M, Meyer J, McGinnis M,, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients Crit Rev Oncol Hematol. 2004;49 (1):69– 75 [DOI] [PubMed] [Google Scholar]
- 75. Kristjansson SR, Nesbakken A, Jordhoy MS,, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study Crit Rev Oncol Hematol. 2010;76 (3):208– 217 [DOI] [PubMed] [Google Scholar]
- 76. Massa E, Madeddu C, Astara G,, et al. An attempt to correlate a “Multidimensional Geriatric Assessment” (MGA), treatment assignment and clinical outcome in elderly cancer patients: results of a phase II open study. Crit Rev Oncol Hematol. 2008;66 (1):75– 83 [DOI] [PubMed] [Google Scholar]
- 77. Overcash JA, Beckstead J. Predicting falls in older patients using components of a comprehensive geriatric assessment Clin J Oncol Nurs. 2008;12 (6):941– 949 [DOI] [PubMed] [Google Scholar]
- 78. Mohile SG, Bylow K, Dale W,, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation Cancer. 2007;109 (4):802– 810 [DOI] [PubMed] [Google Scholar]
- 79. Basso U, Tonti S, Bassi C,, et al. Management of frail and not-frail elderly cancer patients in a hospital-based geriatric oncology program Crit Rev Oncol Hematol. 2008;66 (2):163– 170 [DOI] [PubMed] [Google Scholar]
- 80. Koroukian SM, Xu F, Bakaki PM,, et al. Comorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancer J Gerontol A Biol Sci Med Sci. 2010;65 (3):322– 329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis Crit Rev Oncol Hematol. 2005;54 (2):129– 136 [DOI] [PubMed] [Google Scholar]
- 82. Retornaz F, Seux V, Pauly V, Soubeyrand J. Geriatric assessment and care for older cancer inpatients admitted in acute care for elders unit Crit Rev Oncol Hematol. 2008;68 (2):165– 171 [DOI] [PubMed] [Google Scholar]
- 83. Overcash JA, Beckstead J, Moody L, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA) for use in the older cancer patient as a prescreen: scoring and interpretation Crit Rev Oncol Hematol. 2006;59 (3):205– 210 [DOI] [PubMed] [Google Scholar]
- 84. Cudennec T, Gendry T, Labrune S,, et al. Use of a simplified geriatric evaluation in thoracic oncology Lung Cancer. 2010;67 (2):232– 236 [DOI] [PubMed] [Google Scholar]
- 85. Ingram SS, Seo PH, Martell RE,, et al. Comprehensive assessment of the elderly cancer patient: the feasibility of self-report methodology J Clin Oncol. 2002;20 (3):770– 775 [DOI] [PubMed] [Google Scholar]
- 86. Garman KS, McConnell ES, Cohen HJ. Inpatient care for elderly cancer patients: the role for geriatric evaluation and management units in fulfilling goals for care Crit Rev Oncol Hematol. 2004;51 (3):241– 247 [DOI] [PubMed] [Google Scholar]
- 87. Terret C, Albrand G, Droz JP. Geriatric assessment in elderly patients with prostate cancer Clin Prostate Cancer. 2004;2 (4):236–240 [DOI] [PubMed] [Google Scholar]
- 88. Hurria A, Gupta S, Zauderer M,, et al. Developing a cancer-specific geriatric assessment: a feasibility study Cancer. 2005;104 (9):1998– 2005 [DOI] [PubMed] [Google Scholar]
- 89. Hurria A, Lichtman SM, Gardes J,, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice J Am Geriatr Soc. 2007;55 (10):1604– 1608 [DOI] [PubMed] [Google Scholar]
- 90. Hurria A, Li D, Hansen K,, et al. Distress in older patients with cancer J Clin Oncol. 2009;27 (26):4346– 4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care J Clin Oncol. 2006;24 (15):2304– 2310 [DOI] [PubMed] [Google Scholar]
- 92. Flood KL, Carroll MB, Le CV,, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit J Clin Oncol. 2006;24 (15):2298– 2303 [DOI] [PubMed] [Google Scholar]
- 93. Retornaz F, Monette J, Batist G,, et al. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: a pilot study J Gerontol A Biol Sci Med Sci. 2008;63 (5):518– 522 [DOI] [PubMed] [Google Scholar]
- 94. Puts MT, Monette J, Girre V,, et al. Does frailty predict hospitalization, emergency department visits, and visits to the general practitioner in older newly-diagnosed cancer patients? Results of a prospective pilot study Crit Rev Oncol Hematol. 2010;76 (2):142– 151 [DOI] [PubMed] [Google Scholar]
- 95. Puts MT, Monette J, Girre V,, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study Crit Rev Oncol Hematol. 2011;78 (2):138– 149 [DOI] [PubMed] [Google Scholar]
- 96. Yonnet S, Gazaille V, Grasset-Dupuy M,, et al. Age and management decisions in patients with primary lung cancer Rev Mal Respir. 2008;25 (3):295– 302 [DOI] [PubMed] [Google Scholar]
- 97. Rollot-Trad F, Lahjibi H, Lazarovici C,, et al. Haematological malignancies in older adults: experience in a geriatric acute care department Rev Med Interne. 2008;29 (7):541– 549 [DOI] [PubMed] [Google Scholar]
- 98. Barthelemy P, Heitz D, Mathelin C,, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals Crit Rev Oncol Hematol. 2011;79 (2):196– 204 [DOI] [PubMed] [Google Scholar]
- 99. Wedding U, Kodding D, Pientka L, Steinmetz HT, Schmitz S. Physicians’ judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy Crit Rev Oncol Hematol. 2007;64 (1):1– 9 [DOI] [PubMed] [Google Scholar]
- 100. Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients Chest. 2005;127 (3):886– 891 [DOI] [PubMed] [Google Scholar]
- 101. Balducci L, Stanta G. Cancer in the frail patient. A coming epidemic Hematol Oncol Clin North Am. 2000;14 (1):235– 250 xi [DOI] [PubMed] [Google Scholar]
- 102. Balducci L, Colloca G, Cesari M, Gambassi G. Assessment and treatment of elderly patients with cancer Surg Oncol. 2010;19 (3):117– 123 [DOI] [PubMed] [Google Scholar]
- 103. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;7:CD006211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bergman H, Ferrucci L, Guralnik J,, et al. Frailty: an emerging research and clinical paradigm issues and controversies J Gerontol A Biol Sci Med Sci. 2007;62 (7):731– 737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Townsley C, Pond GR, Peloza B,, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada J Clin Oncol. 2005;23 (16):3802– 3810 [DOI] [PubMed] [Google Scholar]
- 106. Hurria A, Togawa K, Mohile SG,, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study J Clin Oncol. 2011; 29 (25):3457– 3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hurria A, Cirrincione CT, Muss HB,, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401 J Clin Oncol. 2011;29 (10):1290– 1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Horgan AM, Leighl NB, Coate L,, et al. Impact and feasibility of a comprehensive geriatric assessment in the oncology setting: a pilot study [published online ahead of print March 17, 2011]. Am J Clin Oncol 2011. doi:10.1097/COC.0b013e318210f9ce [DOI] [PubMed] [Google Scholar]
- 109. Biesma B, Wymenga AN, Vincent A,, et al. Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin-gemcitabine or carboplatin-paclitaxel: NVALT-3 a phase III study Ann Oncol. 2011;22 (7):1520– 1527 [DOI] [PubMed] [Google Scholar]
- 110. Winkelmann N, Petersen I, Kiehntopf M,, et al. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma J Cancer Res Clin Oncol. 2011;137 (4):733– 738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clough-Gorr KM, Thwin SS, Stuck AE, Silliman RA. Examining five- and ten-year survival in older women with breast cancer using cancer-specific geriatric assessment. Eur J Cancer. 2012;48(6):805–812. doi: 10.1016/j.ejca.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Caillet P, Canoui-Poitrine F, Vouriot J,, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study J Clin Oncol. 2011; 20;29 (27):3636– 3642 [DOI] [PubMed] [Google Scholar]
- 113. Extermann M, Albrand G, Chen H,, et al. Are older French patients as willing as older American patients to undertake chemotherapy? J Clin Oncol. 2003;21 (17):3214– 3219 [DOI] [PubMed] [Google Scholar]
- 114. Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews BMJ. 1998;317 (7161):771– 775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bellera CA, Rainfray M, Mathoulin-Pelissier S,, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool [published onlineahead of print] Ann Oncol. 2012. doi: 10.1093/annonc/mdr587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.