Abstract
Osteosarcoma belongs tà the orphan diseases but is the most common primary malignant tumor of bone. Currently, the standard treatment for osteosarcoma requires both macroscopic surgical resection and postoperative multi-drug chemotherapy in neo-adjuvant and adjuvant settings. However, the 5-year event-free survival remains at a plateau of 60–70% of patients with non-metastatic osteosarcoma for over 30 years. Mifamurtide [liposomal muramyl tripeptide phosphatidylethanolamine (L-MTP-PE)] is a new agent. L-MTP-PE is a nonspecific immunomodulator, which is a synthetic analog of a component of bacterial cell walls. L-MTP-PE activates macrophages and monocytes as a potent activator of immune response. The addition of L-MTP-PE to standard chemotherapy improves the overall survival from 70 % to 78% and results in a one-third reduction in the risk of death from osteosarcoma. Recently, L-MTP-PE has been approved in Europe for the treatment of non-metastatic osteosarcoma with chemotherapy. According to preliminary clinical report, L-MTP-PE is well-tolerated and has little severe side effects. L-MTP-PE in combination with traditional treatment is expected to go mainstream and to be beneficial for patients with osteosarcoma.
Keywords: Acetylmuramyl-Alanyl-Isoglutamine; administration & dosage; adverse effects; analogs & derivatives; therapeutic use; Adjuvants, Immunologic; administration & dosage; adverse effects; therapeutic use; Antineoplastic Agents; adverse effects; therapeutic use; Bone Neoplasms; drug therapy; surgery; Disease Progression; Humans; Neoadjuvant Therapy; Osteosarcoma; drug therapy; surgery; Phosphatidylethanolamines; administration & dosage; adverse effects; therapeutic use
Keywords: osteosarcoma, liposomal muramyl tripeptide phosphatidylethanolamine, mifamurtide, macrophage, chemotherapy, innate immunity
1. Introduction
Osteosarcoma is the most common primary malignant bone tumor. It usually arises in the metaphyses of long bone in children and adolescents [1, 2]. Approximately 1000 new patients are seen per year in North America and a similar number in Europe [3]. The standard treatment of the primary osteosarcoma consists of macroscopic surgical resection and multi-agent chemotherapy in neo-adjuvant and adjuvant settings. Surgical techniques for osteosarcoma have improved and built up from amputations to limb-salvage surgery during the last decades [4]. However, it did not contribute to the improvement of both EFS and overall survival.
On the other hand, the value of chemotherapy for treatment of osteosarcoma is well established [5, 6]. There are currently four chemotherapeutic agents, which consist of doxorubicin, cisplatin, high-dose methotrexate with leucovorin rescue, and ifosfamide [7–9]. Vigorous chemotherapy treatment with these agents has significantly improved survival of the osteosarcoma patients over the past several decades [10]. It has been reported that EFS of osteosarcoma patients at 3–5 years has reached 60–70% in the non-metastatic condition. However, that in the metastatic relapse or recurrent conditions remained at 10–30% since early 1980s [4, 10–12]. None of the various chemotherapy agents and regimens has demonstrated any significant superiority yet [13]. No new anti-osteosarcoma agents have been developed in the intervening years [14]. Recently, Children’s Oncology Group carried out long-term follow-up of the key trial of chemotherapy with or without mifamurtide (liposomal muramyl tripeptide phosphatidyl ethanolamine [L-MTP-PE]). The proprietary product name of mifamurtide is Mepact® (Takeda). This group demonstrated that addition of L-MTP-PE to chemotherapy significantly improved overall survival at 6 years from 70% with chemotherapy alone to 78 % with chemotherapy and L-MTP-PE (p = 0.03) [15]. These data demonstrate that L-MTP-PE may have a potential role in the improvement of survival of the osteosarcoma patients remained on a plateau for over two decades [14, 16–18]. L-MTP-PE, a nonspecific immunomodulator, is a synthetic analog of a component of bacterial cell walls [19]. L-MTP-PE activates macrophages and monocytes as a potent activator of immune response [20, 21].
In this review, we will summarize the most recent findings about L-MTP-PE and its therapeutic application for non-metastatic osteosarcoma.
2. Pharmacology
MTP-PE is a stimulator of innate immunity and a synthetic molecule derived from muramyl dipeptide (MDP). MTP-PE results from the covalent addition of alanin and dipalmitoyl phosphatidyl ethanolamine to MDP (Figure 1), which is a peptidoglycan found in Gram-positive and Gram-negative bacterial cell walls [19].
Figure 1.
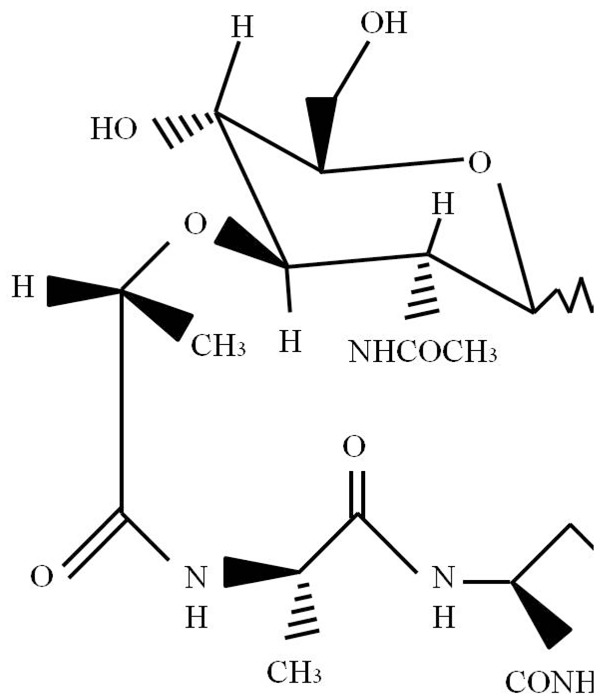
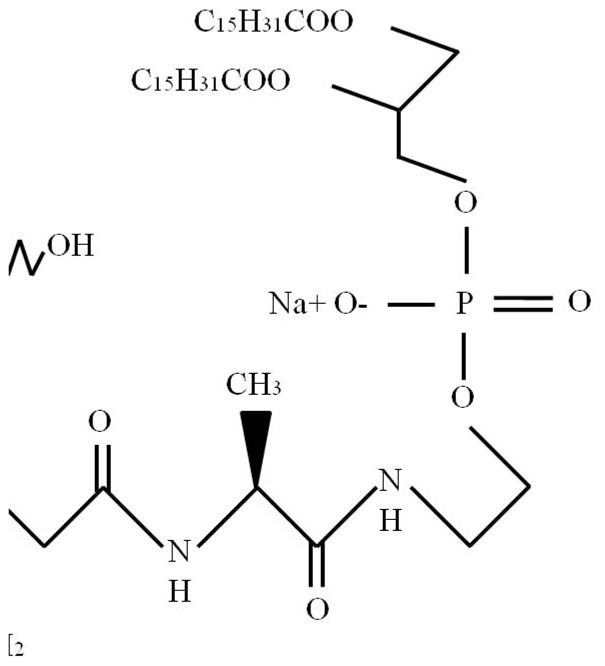
The molecular structure of liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE).
In vitro monocyte/macrophage activation by MTP-PE is related to the upregulation of tumoricidal activity and secretion of pro-inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, nitric oxide (NO), prostaglandin E2 (PGE2), and PGD2 [22–27]. NO, PGE2, and PGD2 are synthesized and released by murine Kupffer cells (liver macrophages) after in vitro MTP-PE exposure [28]. Moreover, L-MTP-PE induces the expression of adhesion molecules including lymphocyte function-associated antigen (LFA)-1, intracellular adhesion molecule (ICAM)-1, and human leukocyte antigen (HLA)-DR. These molecules could be closely related to interaction with tumor cells [19, 29].
MTP-PE is superior to MDP in the activation of human monocytes [20]. That is because the lipophilic properties of MTP-PE cause higher cell uptake via passive transfer through the cytoplasmic membrane. Indeed, the lipophilic MTP-PE could be efficiently incorporated in the lipid bilayer of liposomal structures and distributed primarily in the liver, spleen, and lungs after intravenous MTP-PE administration [20, 21, 30]. Thus, the intravenous MTP-PE encapsulated in liposomes has been developed to target delivery of the drug selectively to monocytes and macrophages, such as those in liver, spleen, and lungs [11, 14, 19]. These liposomes are composed of small lipid particles, which act as excellent transporters of lipophilic peptides [31]. The particle nature of liposomes converts the parent drug into “pro-drug”. Liposomes are concentric multi-lamellar vesicles with the lipid bilayers resembling an onion of particle size approximately 2–3 μm. Formulation of MTP-PE into these phospholipid vesicles enhances the activation of macrophages/monocytes tumoricidal properties and extends its existence in the lungs [20, 30]. In fact, an advantage of MTP-PE over MDP in the activation of human monocytes was demonstrated [20]. This advantage was ascribed to the benefit of the lipophilic properties of MTP-PE described earlier. In addition, the liposomal formulation (L-MTP-PE) has improved the safety profile of several drugs by modifying parent drug or solubilization agent toxicity [32]. Due to rapid mononuclear phagocytosis of the liposome transporter, L-MTP-PE has very rapid clearance from the blood only approximately 0.5% of L-MTP-PE remains in the plasma at the 5-min time point compared with 93% when administrated as the free form [33]. In humans, there is no evidence of accumulation of either liposomes or free MTP-PE after L-MTP-PE 4-mg treatment twice a week for 9 week [34]. The half-life of free MTP-PE can be estimated as 3–6 h from dog and rat studies. Additionally, no accumulation of phospho1ipids after repeated administration has been confirmed [30]. Due to such the rapid clearance, L-MTP-PE shows ten times lesser adverse event level than free MTP-PE in rabbits and dogs [19].
MTP-PE can bind to Toll-like receptor (TLR) 4 and activate extracellular-signal regulated kinase 1/2 (ERK 1/2), nuclear factor-kappa B (NF-κB) and adaptor protein (AP)-1 [27, 35]. However, the other receptor for MTP-PE may be intracellular [20]. Recently, MTP-PE has been reported as a specific ligand of nucleotide-binding oligomerization domain (Nod) 2 receptor. Nod2, an intracellular MDP sensor, is strongly expressed in monocytes, granulocytes, myeloid dendritic cells, and macrophages [36]. It induces NF-κB and influences the innate immune response [37]. L-MTP-PE is selectively phagocytosed by monocytes and macrophages after intravenous administration [38]. The phagocytic cells gradually degrade the liposomal vesicles. Then, MTP-PE is released into the cytosol where it interacts with Nod2 and activates the cells [20]. Thus, monocytes/macrophages activation by L-MTP-PE is considered to be mediated via Nod2 [14, 19] (Figure 2). In sum, preclinical studies showed that L-MTP-PE, in comparison with free MTP-PE, was more effective in terms of activating monocytes [20], was longer held in target organs [30], and was 10-fold less toxic [39].
Figure 2.
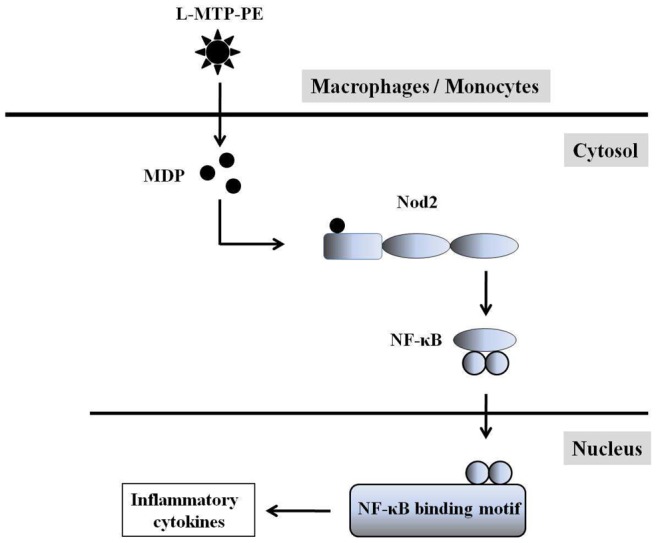
Nod2 is an intracellular MDP sensor. Monocytes/macrophages activation by L-MTP-PE is mediated via Nod2. L-MTP-PE is selectively phagocyted by monocytes and macrophages after intravenous administration. L-MTP-PE is released into the cytosol and degraded to MDP. Nod2 binding to MDP activates NF-κB and influences the innate immune response. Abbreviations: Nod2; nucleotide-binding oligomerization domain 2, MDP; muramyl dipeptide, L-MTP-PE; liposomal muramyl tripeptide phosphatidyl ethanolamine, NF-κB; nuclear factor-kappa B.
Monocytes activated by L-MTP-PE had no effect on non-tumorigenic cells [22, 24, 40] and even under conditions of co-cultivation with tumorigenic cells [22, 40]. Furthermore, peripheral blood monocytes from patients with cancer could be activated to similar levels of tumoricidal activity as monocytes from healthy volunteers [24, 37, 40, 41]. L-MTP-PE interacts with interferon (IFN)-γ to up-regulate tumoricidal activity and to induce secretion of cytokines such as TNF-α and IL-1β [19, 42–47]. The mechanism of this potentiation remains to be elucidated, but a 2-fold increase in liposome phagocytosis was observed after treatment of human monocytes with IFN-γ [47].
Activation of monocytes-mediated tumoricidal activity was investigated following in vivo treatment with L-MTP-PE in phase I and II clinical trials [34, 48, 49]. In the phase I study, 28 patients with metastatic cancer received increasing doses (1h intravenous administration) of L-MTP-PE (0.05–12.0 mg/m2) twice a week [34, 48]. Peripheral blood monocytes were harvested and examined ex vivo for cytotoxic activity against human A375 melanoma cells before L-MTP-PE therapy and at various time points during the 9-week treatment period. Activation of monocytes-mediated cytotoxic activity was seen in 24 (86%) of the 28 patients at some time points during the treatment period [48]. Monocytes-mediated tumoricidal activity remained for up to 96 hours after initial mifamurtide infusion [34]. In the phase II study, 16 patients with relapsed osteosarcoma received a 1h intravenous infusion of L-MTP-PE (2 mg/m2) twice a week for 12 weeks [49]. These patients had histologically confirmed osteosarcoma and pulmonary metastases that had developed during adjuvant chemotherapy or that were present at diagnosis and had persisted despite chemotherapy. The patients were pre-treated with surgical resection of visible and palpable lung metastases before participating in the study. Peripheral blood monocytes were harvested and examined ex vivo for cytotoxic activity against human A375 melanoma cells before L-MTP-PE therapy and during the 4-week treatment period. Monocytes-mediated tumoricidal activity was significantly increased in 80% of the patients evaluated. The peak cytotoxic activity was observed at 24 (n = 3), 72 (n = 4), and 96 (n = 1) hours post-infusion [49]. Similarly, monocytes and macrophages from non-human species demonstrated increased tumoricidal activity after in vitro [23, 26] and in vivo [26, 43, 50–52] treatment with L-MTP-PE or MTP-PE.
Although various chemotherapeutic agents and regimens have been examined for osteosarcoma, none of them demonstrates any clear superiority [13, 53]. Since L-MTP-PE was intended to be used in combination with adjuvant chemotherapy to surgery in the treatment of osteosarcoma, some researchers reported the effects of cytotoxic agents on the monocytes-mediated tumoricidal activity of L-MTP-PE [37, 54, 55]. These results showed that most chemotherapeutic drugs including the treatment of osteosarcoma (i.e., doxorubicin, cisplatin, high-dose methotrexate with leucovorin rescue, and ifosfamide) did not influence on macrophage activation by L-MTP-PE [37, 54, 55]. For example, doxorubicin [37, 54], cisplatin [37], methotrexate [37], or cyclophosphamide [37] administration to patients with osteosarcoma did not interfere with the ability of L-MTP-PE to enhance the monocytes-mediated tumoricidal activity from these patients in vitro. Furthermore, combination chemotherapy of doxorubicin and cyclophosphamide strongly suppressed monocytes-mediated tumoricidal activity by L-MTP-PE [37]. Similarly, simultaneous administration of ifosfamide and L-MTP-PE did not interfere with the ability of L-MTP-PE to activate an in vivo immune response according to the results of a phase IIb clinical study in patients with relapsed osteosarcoma (n = 9) [55]. The trial participants had histologically confirmed osteosarcoma and pulmonary metastases that had developed during adjuvant chemotherapy or that were present at diagnosis and had persisted despite chemotherapy. They were treated neoadjuvantly and/or adjuvantly with a combination of ifosfamide ( up to 8 cycles in total; each cycle consisted of 1.8 g/m2 for 5 days every 21 days) and L-MTP-PE (2 mg/m2 twice a week for 12 weeks and then once a week for 12 weeks). Up-regulations of serum TNF-α, IL-6, and IL-8 in patients treated with the combination therapy was not different from those in patients treated with L-MTP-PE alone [49]. In addition, monocytes-mediated tumoricidal activity was elevated at 24 and 72 hours after the initial of combination therapy, similar to the following L-MTP-PE alone [48]. In particular, combination treatment of ifosfamide and L-MTP-PE did not increase the toxicity of ifosfamide [55]. L-MTP-PE did not decrease the tumoricidal activity of ifosfamide or doxorubicin in three synergistic murine tumor models [56]. Furthermore, the suggested course of L-MTP-PE therapy did not worsen the identified renal (i.e., cisplatin, ifosfamide) or hepatic (i.e., methotrexate, ifosfamide) toxicities of the concurrently administered chemotherapies in phase III study in patients with osteosarcoma [57].
3. Clinical therapeutic efficacy
A phase III randomized prospective trial was conducted by the Children’s Cancer and Pediatric Oncology Groups (now collectively known as the Children’s Oncology Group) from 1993 to 1997 [10]. This central randomized trial in osteosarcoma, known as the Intergroup Study 0133 (INT study 0133), was recruited a total of 662 eligible patients aged ≤ 30 years with non-metastatic osteosarcoma whose primary tumors were considered to be resectable [15]. This study was designed to assess whether the addition of L-MTP-PE and/or ifosfamide to a standard chemotherapeutic regimen composed of three agents (i.e., doxorubicin, cisplatin and high-dose methotrexate with leucovorin rescue) would increase both EFS and overall survival in newly diagnosed patients with high-grade osteosarcoma. No significant improvement in EFS and overall survival was observed by the addition of ifosfamide in the dose and schedule used in this study (p = 0.934 and 0.992 respectively). However, significant improvement in EFS and overall survival were observed in patients randomized to receive L-MTP-PE (p = 0.030 and 0.039 respectively). These findings correspond to a 25% reduction in the risk of recurrence and a 30% reduction in the risk of death [19, 58]. Most notably, the 6-year overall survival improved from 70% without L-MTP-PE to 78% with it [15].
In the INT study 0133, all patients were intended to receive a similar backbone treatment called MAP (i.e., high-dose methotrexate with leucovorin rescue, doxorubicin/adriamycin, and cisplatin) with identical cumulative doses of high-dose methotrexate (12 times at doses of 12 g/m2), doxorubicin (6 times at doses of 75 mg/m2), and cisplatin (4 times at doses of 120 mg/m2). The randomized prospective study was conducted with a 2 × 2 factorial design with 4 treatment groups: (1) MAP, (2) MAP + L-MTP-PE, (3) MAP + ifosfamide, and (4) MAP + ifosfamide + L-MTP-PE. Ifosfamide was administered 5 times at a dose of 9 g/m2 per course. L-MTP-PE was administered at a dose of 2 mg/m2 basically. L-MTP-PE was administered intravenously twice a week for 12 weeks starting at week 12, and then weekly for additional 24 weeks starting at week 24. The duration of treatment was 20 weeks for patients randomly assigned to ‘MAP’ group, 27 weeks for patients randomly assigned to ‘MAP + ifosfamide’ group, and 36 weeks for patients randomly assigned to ‘MAP + L-MTP-PE’ group and ‘MAP + ifosfamide + L-MTP-PE’ [10, 15]. The results of INT study 0133 were analyzed and published in 2005 and 2008 [10, 15]. In 2005, Meyers et al. [10] reported their initial analysis of EFS without overall survival. According to their findings, there was no significant evidence of L-MTP-PE on EFS [10]. Later, this initial report was cited as inappropriate analyses. In 2008, as the subsequent analysis with longer follow-up, both overall survival and EFS were reported as the end points of the study [15]. This re-analysis reported improved overall survival with the addition of L-MTP-PE from 70% to 78% 6-year overall survival (p = 0.03) [15]. By contrast, in the analysis of EFS, there was no sufficient evidence of the interaction (p = 0.102) [15]. This makes the interpretation of INT 0133 study very complicated [59, 60] because the effect of treatment on overall survival is expected to be mediated through EFS. There were no differences in the frequency of favorable tumor necrosis, which is strongly associated with EFS and determined by modified Huvos grading [61], among those treatment groups. Therefore, the authors concluded that ifosfamide and the other chemotherapy agents are equivalent in their ability to contribute to favorable tumor necrosis. Furthermore, another study demonstrated that patients with metastatic osteosarcoma were given a good benefit by administration of a higher dose of ifosfamide (14 mg/m2) [62, 63] . Thus, the most effective combination of ifosfamide with L-MTP-PE may be a dose question. At any rate, many additional questions including the best way to combine chemotherapy and L-MTP-PE still remain, but the INT study 0133 clearly demonstrated the clinical benefit associated with L-MTP-PE in the treatment of osteosarcoma.
4. Dosage and Side effects
In European Union, L-MTP-PE is indicated in patients with high-grade, resectable, non-metastatic osteosarcoma after macroscopically complete surgical resection aged between 2 and 30 years [57]. It is currently recommended that L-MTP-PE is intravenously-administered over 1 hour twice weekly for an initial 12 weeks, followed by once weekly for an additional 24 weeks (total 48 infusions in 36 weeks) [15]. Since all patients recently receiving L-MTP-PE on a compassionate basis have had evidence of biologic activity at 2 mg/m2, premedication and use of the fixed 2 mg/m2 dose is suggested. The preparation and infusion of L-MTP-PE has recently been validated in numerous processes, and these are reproducible and easily performed in the outpatient setting.
L-MTP-PE therapy was generally well tolerated [34, 64]. The most commonly reported side effects are listed in Figure 3. Most of those side effects were mild or moderate severity [57]. In the phase I and II studies, there was no evidence of dose-dependent [65], cumulative [34], and organ-related [65] toxicity in association with L-MTP-PE. Furthermore, the majority of patients experienced side effects with the initial administration of the agent [34, 66]. The major side effects of L-MTP-PE administration are fever and chills. They are typically transient and generally respond to palliative treatment [57]. The possibility of anti-inflammatory drugs relieving these adverse effects has been investigated. Premedication such as ibuprofen [67] can help prevent the severity of fever and chills. However, high-dose ibuprofen (> 40 μg/ml) with L-MTP-PE down-regulates the antitumor effect and the production of IL-1 and TNF-α in monocytes [67]. The antitumor effect of L-MTP-PE was lost in a murine fibrosarcoma model using diclofenac [68]. These findings suggest that anti-inflammatory agents such as cyclooxygenase inhibitors can down-regulate the antitumor effect of L-MTP-PE. For ibuprofen-resistant cases, acetaminophen and/or meperidine are recommended as alternative agents [11]. Specifically, ibuprofen 200 or 400 mg is given as a premedication, If fever and/or chills occur, acetaminophen (15 mg/kg; up to 1000mg) may be given. If needed more, both acetaminophen and ibuprofen may be additionally utilized to improve the symptoms [15, 69].
Figure 3.
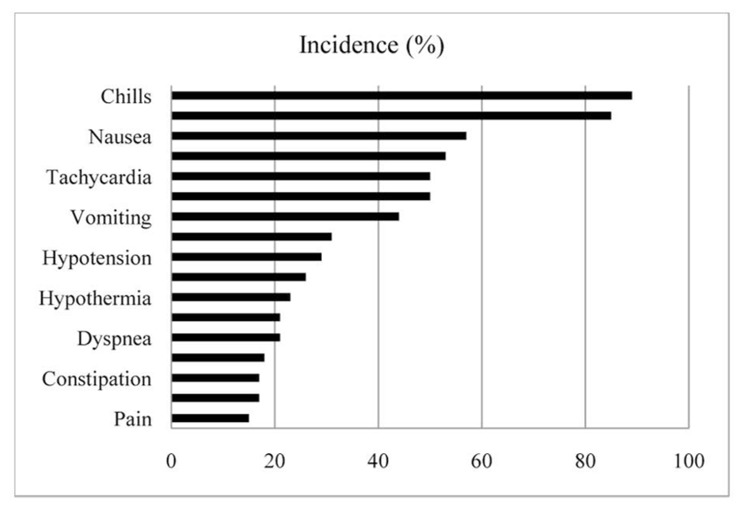
Tolerability of mifamurtide. Major side effects in 248 patients with advanced cancer including 51 patients with osteosarcoma [34, 57, 64–66].
5. Expert Opinion
The orphan disease limits the number of patients available to study. Osteosarcoma is an orphan disease with fewer than 1500–2000 new cases per year diagnosed in the USA similar to Europe. Indeed, except L-MTP-PE, only IFN-α study are undergoing as international cooperative trial for osteosarcoma adjuvant chemotherapy. The efficacy of IFN-α, which is initiated after postoperative chemotherapy, is investigated by EURAMOS 1 [38]. Unfortunately, this trial is still under the recruitment phase [17]. Therefore, L-MTP-PE, the first new agent approval for the treatment of osteosarcoma in over 20 years, is strongly expected to become ‘routine’ for both oncologists and patients with osteosarcoma. From now on, this will require future international prospective trials of L-MTP-PE in osteosarcoma treatment.
References
- 1.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–89. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 2.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiller CA, Bielack SS, Jundt G, et al. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–35. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Harris MB, Cantor AB, Goorin AM, et al. Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study. Med Pediatr Oncol. 1995;24:87–92. doi: 10.1002/mpo.2950240205. [DOI] [PubMed] [Google Scholar]
- 8.Kung FH, Pratt CB, Vega RA, et al. Ifosfamide/etoposide combination in the treatment of recurrent malignant solid tumors of childhood. A Pediatric Oncology Group Phase II study. Cancer. 1993;71:1898–903. doi: 10.1002/1097-0142(19930301)71:5<1898::aid-cncr2820710529>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Miser JS, Kinsella TJ, Triche TJ, et al. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5:1191–8. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 10.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Anderson P. Liposomal muramyl tripeptide phosphatidyl ethanolamine: ifosfamide-containing chemotherapy in osteosarcoma. Future Oncol. 2006;2:333–43. doi: 10.2217/14796694.2.3.333. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 13.Bacci G, Lari S. Adjuvant and neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov. 2001;86:253–68. [PubMed] [Google Scholar]
- 14.Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–49. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Ando K, Heymann D. Liposomal muramyl tripeptide phosphatidyl ethanolamine: a safe and effective agent against osteosarcoma pulmonary metastases. Expert Rev Anticancer Ther. 2008;8:151–9. doi: 10.1586/14737140.8.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Kopp L, Anderson N, et al. Novel bone cancer drugs: investigational agents and control paradigms for primary bone sarcomas (Ewing’s sarcoma and osteosarcoma) Expert Opin Investig Drugs. 2008;17:1703–15. doi: 10.1517/13543784.17.11.1703. [DOI] [PubMed] [Google Scholar]
- 18.Hughes DP. Novel agents in development for pediatric sarcomas. Curr Opin Oncol. 2009;21:332–7. doi: 10.1097/CCO.0b013e32832c94e2. [DOI] [PubMed] [Google Scholar]
- 19.Nardin A, Lefebvre ML, Labroquere K, et al. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–33. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- 20.Fogler WE, Fidler IJ. Comparative interaction of free and liposome-encapsulated nor-muramyl dipeptide or muramyl tripeptide phosphatidylethanolamine (3H-labelled) with human blood monocytes. Int J Immunopharmacol. 1987;9:141–50. doi: 10.1016/0192-0561(87)90088-9. [DOI] [PubMed] [Google Scholar]
- 21.Schroit AJ, Fidler IJ. Delivery of macrophage-augmenting factors encapsulated in liposomes for destruction of tumor metastases. Prog Clin Biol Res. 1982;102(pt A):347–55. [PubMed] [Google Scholar]
- 22.Kleinerman ES, Erickson KL, Schroit AJ, et al. Activation of tumoricidal properties in human blood monocytes by liposomes containing lipophilic muramyl tripeptide. Cancer Res. 1983;43:2010–4. [PubMed] [Google Scholar]
- 23.Fogler WE, Fidler IJ. Nonselective destruction of murine neoplastic cells by syngeneic tumoricidal macrophages. Cancer Res. 1985;45:14–8. [PubMed] [Google Scholar]
- 24.Galligioni E, Quaia M, Spada A, et al. Activation of cytolytic activity in peripheral blood monocytes of renal cancer patients against non-cultured autologous tumor cells. Int J Cancer. 1993;55:380–5. doi: 10.1002/ijc.2910550307. [DOI] [PubMed] [Google Scholar]
- 25.Asano T, McWatters A, An T, et al. Liposomal muramyl tripeptide up-regulates interleukin-1 alpha, interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-8 gene expression in human monocytes. J Pharmacol Exp Ther. 1994;268:1032–9. [PubMed] [Google Scholar]
- 26.Kurzman ID, Shi F, Vail DM, et al. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm. 1999;14:121–8. doi: 10.1089/cbr.1999.14.121. [DOI] [PubMed] [Google Scholar]
- 27.Dieter P, Ambs P, Fitzke E, et al. Comparative studies of cytotoxicity and the release of TNF-alpha, nitric oxide, and eicosanoids of liver macrophages treated with lipopolysaccharide and liposome-encapsulated MTP-PE. J Immunol. 1995;155:2595–604. [PubMed] [Google Scholar]
- 28.MacEwen EG, Kurzman ID, Vail DM, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:4249–58. [PubMed] [Google Scholar]
- 29.Asano T, McIntyre BW, Bednarczyk JL, et al. Liposomal muramyl tripeptide upregulates adhesion molecules on the surface of human monocytes. Oncol Res. 1995;7:253–7. [PubMed] [Google Scholar]
- 30.Fogler WE, Wade R, Brundish DE, et al. Distribution and fate of free and liposome-encapsulated [3H]nor-muramyl dipeptide and [3H]muramyl tripeptide phosphatidylethanolamine in mice. J Immunol. 1985;135:1372–7. [PubMed] [Google Scholar]
- 31.Cryan SA. Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 2005;7:E20–41. doi: 10.1208/aapsj070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11:8230–4. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 33.Gay B, Cardot JM, Schnell C, et al. Comparative pharmacokinetics of free muramyl tripeptide phosphatidyl ethanolamine (MTP-PE) and liposomal MTP-PE. J Pharm Sci. 1993;82:997–1001. doi: 10.1002/jps.2600821005. [DOI] [PubMed] [Google Scholar]
- 34.Murray JL, Kleinerman ES, Cunningham JE, et al. Phase I trial of liposomal muramyl tripeptide phosphatidylethanolamine in cancer patients. J Clin Oncol. 1989;7:1915–25. doi: 10.1200/JCO.1989.7.12.1915. [DOI] [PubMed] [Google Scholar]
- 35.Dieter P, Hempel U, Kamionka S, et al. Prostaglandin E2 affects differently the release of inflammatory mediators from resident macrophages by LPS and muramyl tripeptides. Mediators Inflamm. 1999;8:295–303. doi: 10.1080/09629359990306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81:935–8. doi: 10.1093/jnci/81.12.935. [DOI] [PubMed] [Google Scholar]
- 37.Kleinerman ES, Snyder JS, Jaffe N. Influence of chemotherapy administration on monocyte activation by liposomal muramyl tripeptide phosphatidylethanolamine in children with osteosarcoma. J Clin Oncol. 1991;9:259–67. doi: 10.1200/JCO.1991.9.2.259. [DOI] [PubMed] [Google Scholar]
- 38.Europeans Medicines Agency. Assessment report for Mepact. [Accessed 9.25, 2010]. at http://www.ema.europa.eu/humandocs/PDFs/EPAR/mepact/H-802-en6.pdf.
- 39.Fidler IJ, Brown NO, Hart IR. Species variability for toxicity of free and liposome-encapsulated muramyl peptides administered intravenously. J Biol Response Mod. 1985;4:298–309. [PubMed] [Google Scholar]
- 40.Fidler IJ, Jessup JM, Fogler WE, et al. Activation of tumoricidal properties in peripheral blood monocytes of patients with colorectal carcinoma. Cancer Res. 1986;46:994–8. [PubMed] [Google Scholar]
- 41.Sone S, Utsugi T, Tandon P, et al. Tumor cytotoxicity and interleukin 1 production of blood monocytes of lung cancer patients. Cancer Immunol Immunother. 1990;30:357–62. doi: 10.1007/BF01786885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinney CP, Tanguay S, Bucana CD, et al. Intravesical liposomal muramyl tripeptide phosphatidylethanolamine treatment of human bladder carcinoma growing in nude mice. J Interferon Cytokine Res. 1995;15:585–92. [PubMed] [Google Scholar]
- 43.Fidler IJ, Fan D, Ichinose Y. Potent in situ activation of murine lung macrophages and therapy of melanoma metastases by systemic administration of liposomes containing muramyltripeptide phosphatidylethanolamine and interferon gamma. Invasion Metastasis. 1989;9:75–88. [PubMed] [Google Scholar]
- 44.Goldbach P, Dumont S, Kessler R, et al. In situ activation of mouse alveolar macrophages by aerosolized liposomal IFN-gamma and muramyl tripeptide. Am J Physiol. 1996;270:L429–34. doi: 10.1152/ajplung.1996.270.3.L429. [DOI] [PubMed] [Google Scholar]
- 45.Utsugi T, Nii A, Fan D, et al. Comparative efficacy of liposomes containing synthetic bacterial cell wall analogues for tumoricidal activation of monocytes and macrophages. Cancer Immunol Immunother. 1991;33:285–92. doi: 10.1007/BF01756592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galligioni E, Santarosa M, Favaro D, et al. In vitro synergic effect of interferon gamma combined with liposomes containing muramyl tripeptide on human monocyte cytotoxicity against fresh allogeneic and autologous tumor cells. Tumori. 1994;80:385–91. doi: 10.1177/030089169408000514. [DOI] [PubMed] [Google Scholar]
- 47.Sone S, Tandon P, Utsugi T, et al. Synergism of recombinant human interferon gamma with liposome-encapsulated muramyl tripeptide in activation of the tumoricidal properties of human monocytes. Int J Cancer. 1986;38:495–500. doi: 10.1002/ijc.2910380407. [DOI] [PubMed] [Google Scholar]
- 48.Kleinerman ES, Murray JL, Snyder JS, et al. Activation of tumoricidal properties in monocytes from cancer patients following intravenous administration of liposomes containing muramyl tripeptide phosphatidylethanolamine. Cancer Res. 1989;49:4665–70. [PubMed] [Google Scholar]
- 49.Kleinerman ES, Jia SF, Griffin J, et al. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol. 1992;10:1310–6. doi: 10.1200/JCO.1992.10.8.1310. [DOI] [PubMed] [Google Scholar]
- 50.Talmadge JE, Schneider M, Collins M, et al. Augmentation of NK cell activity in tissue specific sites by liposomes incorporating MTP-PE. J Immunol. 1985;135:1477–83. [PubMed] [Google Scholar]
- 51.Xu Z, Fidler IJ. The in situ activation of cytotoxic properties in murine Kupffer cells by the systemic administration of whole Mycobacterium bovis organisms or muramyl tripeptide. Cancer Immunol Immunother. 1984;18:118–22. doi: 10.1007/BF00205745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith BW, Kurzman ID, Schultz KT, et al. Muramyl peptides augment the in vitro and in vivo cytostatic activity of canine plastic-adherent mononuclear cells against canine osteosarcoma cells. Cancer Biother. 1993;8:137–44. doi: 10.1089/cbr.1993.8.137. [DOI] [PubMed] [Google Scholar]
- 53.Bielack S, Kempf-Bielack B, Schwenzer D, et al. Neoadjuvant therapy for localized osteosarcoma of extremities. Results from the Cooperative osteosarcoma study group COSS of 925 patients. Klin Padiatr. 1999;211:260–70. doi: 10.1055/s-2008-1043798. [DOI] [PubMed] [Google Scholar]
- 54.Hudson MM, Snyder JS, Jaffe N, et al. In vitro and in vivo effect of adriamycin therapy on monocyte activation by liposome-encapsulated immunomodulators. Cancer Res. 1988;48:5256–63. [PubMed] [Google Scholar]
- 55.Kleinerman ES, Meyers PA, Raymond AK, et al. Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. J Immunother Emphasis Tumor Immunol. 1995;17:181–93. doi: 10.1097/00002371-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Killion JJ, Kleinerman ES, Wilson MR, et al. Sequential therapy with chemotherapeutic drugs and liposome-encapsulated muramyl tripeptide: determination of potential interactions between these agents. Oncol Res. 1992;4:413–8. [PubMed] [Google Scholar]
- 57.MEPACT 4 mg powder dor suspension for infusion: summary of product characteristics. Paris: IDM; 2010. [Google Scholar]
- 58.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 59.Hunsberger S, Freidlin B, Smith MA. Complexities in interpretation of osteosarcoma clinical trial results. J Clin Oncol. 2008;26:3103–4. doi: 10.1200/JCO.2008.17.3484. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 60.Bielack SS, Marina N, Ferrari S, et al. Osteosarcoma: the same old drugs or more? J Clin Oncol. 2008;26:3102–3. doi: 10.1200/JCO.2008.17.1108. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 61.Goorin AM, Harris MB, Bernstein M, et al. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol. 2002;20:426–33. doi: 10.1200/JCO.2002.20.2.426. [DOI] [PubMed] [Google Scholar]
- 62.Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104:2214–21. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 63.Lafleur EA, Koshkina NV, Stewart J, et al. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. 2004;10:8114–9. doi: 10.1158/1078-0432.CCR-04-0353. [DOI] [PubMed] [Google Scholar]
- 64.Urba WJ, Hartmann LC, Longo DL, et al. Phase I and immunomodulatory study of a muramyl peptide, muramyl tripeptide phosphatidylethanolamine. Cancer Res. 1990;50:2979–86. [PubMed] [Google Scholar]
- 65.Creaven PJ, Cowens JW, Brenner DE, et al. Initial clinical trial of the macrophage activator muramyl tripeptide-phosphatidylethanolamine encapsulated in liposomes in patients with advanced cancer. J Biol Response Mod. 1990;9:492–8. [PubMed] [Google Scholar]
- 66.Kleinerman ES, Gano JB, Johnston DA, et al. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol. 1995;18:93–9. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Fujimaki W, Griffin JR, Kleinerman ES. Effect of ibuprofen on monocyte activation by liposome-encapsulated muramyl tripeptide phosphatidylethanolamine (CGP 19835A): can ibuprofen reduce fever and chills without compromising immune stimulation? Cancer Immunol Immunother. 1993;36:45–51. doi: 10.1007/BF01789130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fedorocko P, Hoferova Z, Hofer M, et al. Administration of liposomal muramyl tripeptide phosphatidylethanolamine (MTP-PE) and diclofenac in the combination attenuates their anti-tumor activities. Neoplasma. 2003;50:176–84. [PubMed] [Google Scholar]
- 69.Kleinerman ES, Raymond AK, Bucana CD, et al. Unique histological changes in lung metastases of osteosarcoma patients following therapy with liposomal muramyl tripeptide (CGP 19835A lipid) Cancer Immunol Immunother. 1992;34:211–20. doi: 10.1007/BF01741788. [DOI] [PMC free article] [PubMed] [Google Scholar]


