Abstract
Hsp12p is considered to be a small heat shock protein and conserved among fungal species. To investigate the expression of this heat shock protein in the fungal pathogen Candida albicans we developed an anti-CaHsp12p antibody. We show that this protein is induced during stationary phase growth and under stress conditions including heat shock, osmotic, oxidative and heavy metal stress. Furthermore, we find that CaHsp12p expression is influenced by the quorum sensing molecule farnesol, the change of CO2 concentration and pH. Notably we show that the key transcription factor Efg1p acts as a positive regulator of CaHsp12p in response to heat shock and oxidative stress and demonstrate that CaHsp12p expression is additionally modulated by Hog1p and the cAMP-PKA signaling pathway. To study the function of Hsp12p in C. albicans we generated a null mutant, in which all four CaHSP12 genes have been deleted. Phenotypic analysis of the strain shows that CaHSP12 is not essential for stress resistance, morphogenesis or virulence when tested in a Drosophila model of infection. However, when overexpressed, CaHSP12 significantly enhanced cell-cell adhesion, germ tube formation and susceptibility to azole antifungal agents whilst desensitizing C. albicans to the quorum sensing molecule farnesol.
Introduction
Candida albicans is an ascomycete yeast which can be found in the gastrointestinal tract and the oral or vaginal mucosa of many otherwise healthy individuals [1]. However, it is also a major opportunistic fungal pathogen, causing superficial infections of mucosa and skin, or life-threatening invasive infections when either the innate or acquired immune system is compromised [1], [2].
Prompt sensing and adaptation to a wide range of environmental conditions are key for fungal survival in the various niches of the host and C. albicans has developed a fine-tuned response to stresses required for efficient host colonization [3], [4], [5], [6], [7]. The ability to undergo a reversible morphological transition in response to environmental changes is an additional advantage of C. albicans host adaptation. In fact, both stress response and polymorphism are considered major virulence factors of C. albicans [8], [9]. Previous studies have shown that the Hog1p MAPK signaling pathway plays an important role in regulation of stress response [8] whereas C. albicans polymorphism is controlled by multiple signaling pathways including the Efg1p-mediated cAMP pathway, Cph1p-mediated MAPK, Rim101p and Tup1p dependant pathways [6], [10], [11], [12].
Heat shock proteins, a group of molecular chaperones found in all organisms, accumulate upon a shift from physiological to higher temperatures. They are also induced by other stresses and thus allow the cells to survive under challenging conditions [13]. Heat shock proteins are classified based on their molecular weight [14]. Small heat shock proteins have a molecular mass ranging between 10 and 30 kDa and share a conserved sequence in their C-terminus called the α-cystallin domain [15], [16]. Yeast small heat shock proteins, including Hsp26p and Hsp30p, are induced under heat shock and during stationary growth phase [17], [18]. Over-expression of Hsp26p increases thermo-tolerance of yeast cells [19] and C. albicans Hsp30p has been shown to be induced upon exposure to the antifungal agent amphotericin B [20].
C. albicans HSP12 (CaHSP12) gene expresssion is regulated by changes in the concentrations of environmental CO2 and pH via the cAMP-dependent and Rim101p-dependent signaling cascades [21]. Other reports have shown that CaHSP12 is induced when cells are exposed to osmotic stress, oxidative stress, heavy metal stress and heat shock [8], [22], [23]. Additionally, CaHSP12 expression is regulated by quorum sensing molecules [24], upon hypoxic conditions [25], drug-resistance [26], [27], tissue invasion [28], the yeast-to-hyphal transition [29] and iron limitation [30]. HSP12 in Candida glabrata, which is the second most common cause of systemic candidiasis, is up-regulated in fluconazole-resistant mutants [31]. Finally, HSP12 orthologs in Cryptococcus neoformans which is another pathogenic fungus have role in polyene antifungal drug susceptibility and are regulated by the cAMP signaling pathway [32].
Despite this large amount of information gathered on HSP12 gene expression very little is known about its function in fungal species in general and nothing on the C. albicans Hsp12p protein in particular. In this study, we characterize CaHSP12 from the fungal pathogen C. albicans with respect to its gene structure, regulation of protein expression, function and virulence. We show that CaHsp12p is induced by stress and the quorum sensing molecule farnesol, and regulated by the change of CO2 concentration and pH. Notably, we identify the transcription factor Efg1p to be required for expression in response to heat shock and oxidative stress and demonstrate that expression of CaHsp12p is additionally regulated by the Hog1p and cAMP signaling pathways. We also present a comparative study on HSP12 expression in C. albicans, S. cerevisiae and C. glabrata in response to different stresses in general, and report differences among these yeast species when exposed to oxidative stress in particular. We find that Hsp12p is not essential for stress resistance, filamentation or virulence. However, when overexpressed, it enhances cell-cell aggregation, susceptibility to azole antifungal agents, and promotes farnesol tolerance.
Materials and Methods
Strains and growth conditions
The yeast strains used in this study are listed in Table 1. All strains were grown either in rich YEPD medium or in YNB minimal medium or YNB minimal medium buffered with 150 mM HEPES as described [5], [21]. All C. albicans and C. glabrata strains were grown at 37°C unless indicated otherwise. All S. cerevisiae strains were grown at 30°C.
Table 1. Yeast strains used in this study.
| Strain | Description | Genotype | Source |
| SC5314 | C. albicans laboratory wild-type strain | ||
| CAI4 | URA3 auxotrophic strain | ura3:: λimm434/ura3:: λimm434 | [33] |
| BWP17 | URA3, HIS1, ARG4 auxotrophic strain | ura3:: λimm434/ura3:: λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | [34] |
| CAI4-pFM2 | Wild-type strain transformed with pFM2 as the control in HSP12 overexpression experiment | ura3:: λimm434/ura3:: λimm434-(pFM2 URA3) | This study |
| BWT | With-type strain transformed with CIp30 as the control in CaHSP12 deletion experiment | ura3:: λimm434/ura3:: λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rps1-(CIp30 URA3, HIS1, ARG4) | This study |
| HSP12OE | CaHSP12 overexpressing strain | ura3:: λimm434/ura3:: λimm434-(CaHSP12-pFM2 CaHSP12, URA3) | This study |
| HSP12KO2 | Strain with two CaHSP12 alleles deleted | ura3:: λimm434/ura3:: λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG hsp12a::HIS1/hsp12a::hisG-URA3-hisG, HSP12b/HSP12b | This study |
| HSP12KO5 | CaHSP12 deletion strain transformed with CIp30 | ura3:: λimm434/ura3:: λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG hsp12a::HIS1/hsp12a::hisG, hsp12b::hisG/hsp12b::hisG rps1-(CIp30 URA3, HIS1, ARG4) | This study |
| HSP12C | CaHSP12 reconstitution strain | ura3:: λimm434/ura3:: λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG hsp12a::HIS1/hsp12a::hisG, hsp12b::hisG/hsp12b::hisG rps1-(CaHSP12-CIp30 CaHSP12, URA3, HIS1, ARG4) | This study |
| Cg2001 | C. glabrata wild-type | ||
| Cg2001TU | C. glabrata TRP1 URA3 auxotrophic strain | Δura3 Δtrp1 | [38] |
| Cg12KO | CgHSP12 deletion strain | Δura3 Δtrp1 ΔCghsp12::TRP1 (pEM13D URA3) | This study |
| Cg12C | CgHSP12 reconstitution strain | Δura3 Δtrp1 ΔCghsp12::TRP1 –(CgHSP12-pEM13D CgHSP12, URA3) | This study |
| BY4741 | S. cerevisiae HIS3 LEU2 MET15 URA3 auxotrophic strain | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | [63] |
Strain construction
For a comprehensive description of all methods see Text S1. Briefly, both HSP12 loci (designated CaHSP12a and CaHSP12b) present in the C. albicans genome were deleted by using Ura-blaster and HIS1 cassettes in BWP17 strain according to standard protocols [33], [34], [35]. Reconstitution strains (HSP12C) were constructed by integrating CIp30 containing a wild-type copy of CaHSP12 to the RP10 locus [36]. To construct the CaHSP12 overexpressing strain, HSP12OE, CaHSP12 was cloned downstream of the TEF2 promoter in pFM2 [3]. C. glabrata HSP12 was cloned, disrupted and reconstituted according to standard protocols [37], [38].
Anti-CaHsp12p antibody generation
CaHsp12p was expressed in E. coli and purified using GST-tag affinity chromatography. Purified CaHsp12p was then boiled at 95°C for 10 min before sending to Charles River Laboratories (Romans-sur-Isère, France). Antibody generation, protein expression in yeast and Western blotting are detailed in Text S1 and as previously described [5].
Phenotypic assays
Growth rate determination, cell-cell aggregation and adhesion studies using the XTT reduction assays, farnesol susceptibility studies, antifungal drug and stress sensitivity tests and virulence test using our previously published Drosophila model [7] are all describe in Text S1.
Results
C. albicans contains two CaHSP12 genes
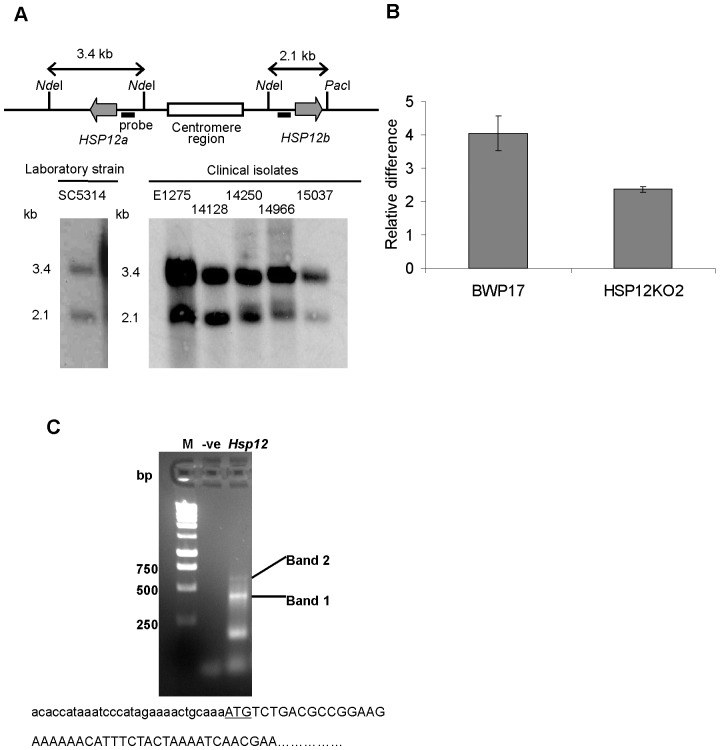
We identified two loci of CaHSP12, arranged in an inverted manner (CaHSP12a and CaHSP12b with GenBank Accession Nos. XM715434 and XM709485), in the C. albicans genome database. Both genes are located within 6.5 kb of each other in proximity to the centromere on chromosome 5. Their predicted open reading frames encode proteins that differ in only two amino acids. The homology of the upstream regions (1640 bp) of the two different loci is 99%. However, the 1000 bp downstream regions are only <50% identical. Due to this dissimilarity, different sizes of NdeI-PacI digested fragments of the two CaHSP12 copies (3.4 kb and 2.1 kb) are obtained and visualized in Southern blots (Figure 1A). We confirmed that not only the C. albicans type strain, SC5314, but equally five additional clinical isolates carry two loci of different CaHSP12 (Figure 1A). Database sequences show that there are two HSP12 genes present in the genome of Candida dublinensis, which is closely related to C. albicans, but only one in S. cerevisiae, C. glabrata, Candida tropicalis, Candida guiliermondii, Candida lusitaniae and Cryptococcus neoformans. To determine if both copies of CaHSP12 are expressed, qRT-PCR was carried out in a strain in which one copy of CaHSP12 had been deleted (HSP12KO2). This showed a reduction of CaHSP12 expression by 50% compared to the parent strain (Figure 1B) suggesting that both copies are expressed in C. albicans.
Figure 1. HSP12 differs among yeast species.
(A) Two CaHSP12 genes have been identified in C. albicans SC5314 and five clinical isolates by Southern blot. (B) Both alleles of CaHSP12 are transcriptionally expressed. The transcription level of CaHSP12 is assessed by qRT-PCR of total RNA obtained from the strain with absence of one CaHSP12 gene (HSP12KO2) and its parental strain (BWP17). The error bars represent the S.D. of triplicate independent reactions. P value<0.01, two-sided unpaired student t-test. (C) 5′ RACE analysis of CaHSP12. Two DNA bands (band 1 and band 2) with the expected size above 250 bp were sequenced. The sequencing shows that the 5′ untranslated region (in lowercase) contains 29 bp nucleotides and only the second start codon (underlined) of CaHSP12 can be identified. M: 1 kb DNA ladder; -ve: negative control of PCR without template; HSP12: 5′RLM-RACE PCR product of CaHSP12.
C. albicans HSP12 contains two putative start codons
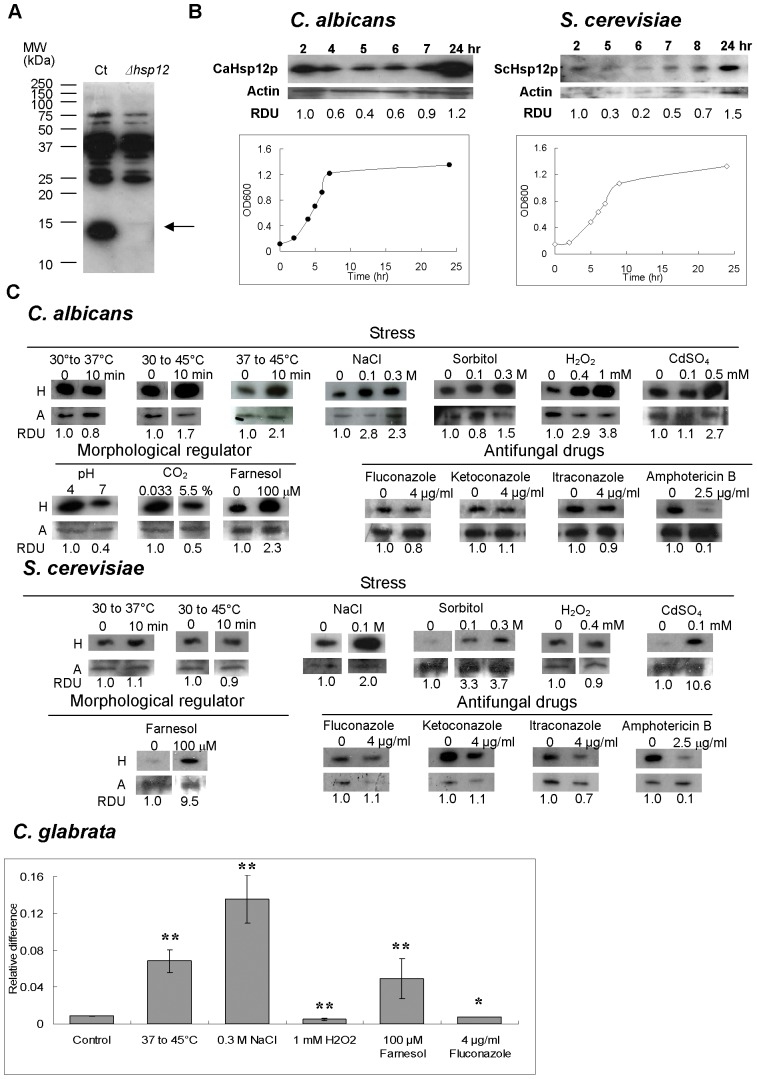
Bioinformatics analysis identified two putative start codons (ATG) for both CaHSP12 loci while only one is found in HSP12 from other fungal species. Translation from the first start codon would produce a 168 amino acid protein corresponding to a 18.0 kDa protein whereas translation from the second would lead to a 127 amino acid, 13 kDa, protein. The origin of transcription was determined by analysis of the 5′ end of CaHSP12 mRNA via sequence analysis of 5′ RACE reaction products (Figure 1C). This revealed that the 5′ start point of the CaHSP12 transcript is present at position −29 from the second start codon (Figure 1C). Western blot analysis, using an anti-Hsp12p antibody, identified a signal with a size of 13 kDa (Figure 2A).
Figure 2. Hsp12p is regulated in response to a wide range of stresses.
(A) CaHsp12p is recognized by a polyclonal antibody. The anti-CaHsp12p antibody was tested by using Western blot analysis against protein samples from the C. albicans CAI4 control strain (Ct) and the Cahsp12 null mutant (Δhsp12). The arrow indicates the 13 kDa band of CaHsp12p which is present in CAI4, but absent in the Cahsp12 null mutant. (B) Induction of Hsp12p in C. albicans and in S. cerevisiae during stationary growth. Total protein was extracted at the indicated time points from C. albicans CAI4 at 37°C or S. cerevisiae BY4741 at 30°C. Western blots were probed with anti-Hsp12p antibody and showed a band corresponding to the expected size of 13 kDa. Blots were probed with anti-actin antibody as loading control. Growth curves with sampling time points (open or solid dots) are shown. RDU: relative densitometry units. (C) Hsp12p is regulated in response to diverse conditions. Hsp12 protein level in C. albicans CAI4 or S. cerevisiae BY4741 was assayed using Western blot and a band of the expected size (13 kDa) was detected. H: anti-Hsp12p antibody. A: anti-actin antibody (equal protein loading control). RDU: relative densitometry units. CgHSP12 transcript level was determined by qRT-PCR with total RNA extracted from the Cg2001TU strain. The transcript level was normalized to the Act1 control. The error bars represent the S.D. of triplicate independent reactions. **P value<0.01, * P value>0.3, two-sided unpaired student t-test.
C. albicans Hsp12p is regulated in response to a wide range of stresses
To study C. albicans cells that were exposed to different stresses we raised an anti-CaHsp12p antibody (Figure 2A). Furthermore, we compared the expression of Hsp12p between C. albicans and S. cerevisiae using an S. cerevisiae anti-Hsp12 antibody. Finally, we studied the expression of CgHSP12 in C. glabrata, which is phylogenetically closely related to S. cerevisiae.
Heat shock proteins in fungi are synthesized at high levels during stationary phase growth [14], [17], [39] and we show that protein expression of CaHsp12p is highly induced in stationary phase but not in exponentially growth of C. albicans (Figure 2B). We also show that ScHsp12p is increased in stationary phase (Figure 2B), confirming previous northern blot analysis of ScHSP12 transcript by Praekelt and Meacock [14].
Transcription of HSP12 has been shown to be induced under stress in both S. cerevisiae and C. albicans [8], [14], [22], [23], [40], [41]. Hence, we examined the response of Hsp12p to stress at the protein level. Western blot analysis showed that CaHsp12p is induced by heat shock (from 30°C to 45°C or from 37°C to 45°C), however no induction of CaHsp12p was observed when shifting the temperature from 30°C to 37°C (Figure 2C). Apart from heat shock, CaHsp12p is also induced in osmotic stress such as sodium chloride (NaCl) and sorbitol, oxidative stress such as hydrogen peroxide (H2O2) , and the heavy metal cadmium (Cd2+) (Figure 2C). We noted that even exposure to low doses of NaCl (0.1 M) or H2O2 (0.4 mM) resulted in significant induction of CaHsp12p expression (Figure 2C). However, CaHsp12p is slightly increased when grown in high concentrations of sorbitol (0.3 M) (Figure 2C). Expression is only enhanced in cells treated with higher levels of heavy metal Cd2+ (0.5 mM), but not in the lower doses (0.1 mM Cd2+) (Figure 2C). Similar to CaHsp12p, ScHsp12p is induced by osmotic stress such as low doses of NaCl (0.1 M) and sorbitol (0.1 M), and heavy metal Cd2+ (0.1 mM) (Figure 2C). However, we found that ScHsp12p is not induced when shifting cells from 30°C to either 37°C or 45°C (Figure 2C). Interestingly, unlike CaHsp12p, ScHsp12p was not regulated by H2O2 (Figure 2C). Analysis of C. glabrata HSP12 transcript levels revealed an 8-fold induction following heat shock from 37°C to 45°C (Figure 2C). CgHSP12 was 15-fold up-regulated after exposure to 0.3 M NaCl (Figure 2C) but the transcript level was slightly decreased (2-fold) following exposure to 1 mM H2O2 (Figure 2C). Finally we show that C. albicans Hsp12 protein is down-regulated by physiological CO2 and pH (Figure 2C).
C. albicans, S. cerevisiae and C. glabrata Hsp12p is induced by the quorum sensing molecule farnesol
Using qRT-PCR, Davis-Hanna et al. have previously shown that transcription of CaHSP12 was influenced when C. albicans was grown in the presence of the quorum sensing molecule farnesol [24]. Consistent with this work we show that CaHsp12p protein levels sharply increase in response to 100 µM farnesol (Figure 2C). Intriguingly, ScHsp12p is also highly induced (Figure 2C), and CgHSP12 is 6-fold increased upon exposure to farnesol (Figure 2C).
Polyene but not azole antifungal agents impact on CaHsp12p expression
Previous work by Coste et al has shown that the promoter of CaHSP12 contains a cis-acting drug-responsive element (DRE)-like region with four mismatches [26]. Additionally, CaHSP12 was found to be up-regulated in azole-resistant strains [27], [42]. Moreover, CaHSP12 is induced when the cells are exposed to fluphenazine, which can also induce multidrug transporter genes [27]. However, there is no direct evidence showing if CaHSP12 is regulated by antifungal drugs. Therefore, we investigated whether CaHsp12p is regulated when the cells were treated with 4 µg ml−1 of the azole drugs fluconazole, ketoconazole, itraconazole, or 2.5 µg ml−1 of the polyene antifungal agent amphotericin B. No significant change of CaHsp12p level was been found when C. albicans was treated with azole antifungal drugs (Figure 2C). Interestingly, CaHsp12p is down-regulated upon exposure to amphotericin B (Figure 2C). C. glabrata HSP12 is also not regulated when the cells were exposed to 4 µg ml−1 fluconazole whereas S. cerevisiae Hsp12p is slightly down-regulated in the presence of itraconazole and significantly decreased upon exposure to amphotericin B (Figure 2C).
Hsp12p expression is regulated by the Hog1p stress response and cAMP-PKA signaling pathway
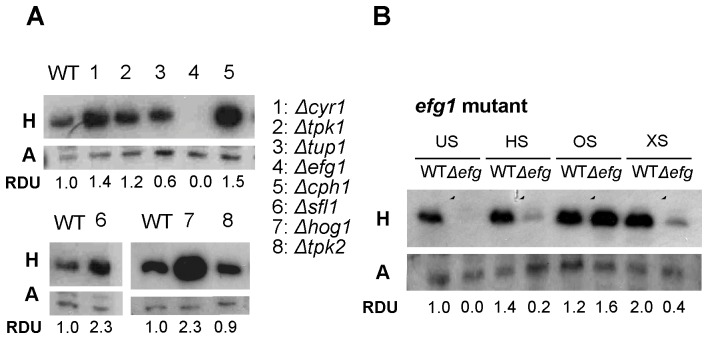
The mechanisms of CaHsp12p regulation during stress response and yeast-to-hyphae transition in C. albicans are unclear. Therefore, we determined if CaHsp12p expression is influenced by protein kinases or key transcription factors which are involved in stress response and regulating filamentation. To this end we monitored expression in the hog1 [8], cyr1 [7], tpk1 [43], tpk2 [43], efg1 [44], cph1 [44], tup1 [45], and sfl1 [46] mutants. Notably, expression of CaHsp12p was repressed in the efg1 mutant (Figure 3A), suggesting that Efg1p functions as an activator of CaHsp12p. CaHsp12p was also slightly repressed in the tup1 mutant. In contrast, elevated levels of CaHsp12p were observed in the hog1, cyr1, cph1 and, sfl1. The level of CaHsp12p was slightly increased in tpk1 but not tpk2 mutants in unstressed conditions, suggesting that Hog1p, Cyr1p, Cph1p, Sfl1p and Tpk1p but not Tpk2p, repress the production of CaHsp12p (Figure 3A).
Figure 3. Expression of CaHsp12p in C. albicans mutant strains.
(A) CaHsp12p expression is regulated by the Hog1p stress response and cAMP-PKA signalling pathway. CaHsp12p was isolated from mutant strains after growing until mid-log phase and its level analyzed by Western blot. Equal protein loading was assessed by probing the blot with anti-actin antibody. H: anti-CaHsp12p; A: anti-actin; RDU: relative densitometry units. (B) EFG1 is required for the induction of CaHsp12p in response to heat shock and oxidative stress. Western blot show the level of CaHsp12p in the efg1 mutant in unstressed condition (US) or following exposure to heat shock from 37°C to 45°C (HS), osmotic stress, 0.3 M NaCl, (OS) or oxidative stress, 1 mM H2O2, (XS). Equal protein loading was assessed by probing the blot with anti-actin antibody. H: anti-CaHsp12p; A: anti-actin; RDU: relative densitometry units.
Efg1p is required for the expression of CaHsp12p during heat shock and oxidative but not osmotic stress
In order to determine the role of Hog1p and cAMP signaling pathway on the regulation of CaHsp12p in response to stress, expression of CaHsp12p was examined in the hog1, cyr1, tpk1, tpk2 and efg1 deletion mutants after exposure to heat shock from 37°C to 45°C, 0.3 M NaCl and 1 mM H2O2. Western blots showed that CaHsp12p levels are reduced in the efg1 mutant following heat shock and exposure to NaCl, but not H2O2. This suggested that Efg1p is required for the expression of CaHsp12p under heat shock and oxidative stress, but not to osmotic stress (Figure 3B). These result indicated that there are distinct mechanisms for osmotic stress response and for heat and oxidative stress response in C. albicans. The level of CaHsp12p expression remained high in the hog1, cyr1 and tpk1 deletion mutants exposed to stress (Figure S1). Also, the level of CaHsp12p was not changed between the control strain and the tpk2 deletion mutant under stress (Figure S1).
HSP12 is not essential for growth, stress resistance or virulence
To gain insight into the function of Hsp12p in both C. albicans and C. glabrata we constructed hsp12 null mutants in both species. This required deletion of all four HSP12 alleles in C. albicans, and the single gene in C. glabrata. Determination of the growth rates or cell adhesion of the Cahsp12 and the Cghsp12 deletion mutants did not reveal any differences when compared with their control strains (Figure S2). Furthermore, similar growth on medium supplemented with either osmotic stressors such as sodium chloride, sorbitol; oxidative stressors such as H2O2, menadione; cell wall and cell membrane stressors such as Congo red, calcofluor white, caffeine and SDS or antifungal drugs such as itraconazole, ketoconazole, fluconazole and amphotericin B did not reveal differences in survival or growth (Figure S3 and S4). The Cahsp12 deletion mutant did not show any difference in germ tube formation when compared with its control strain (Figure S5). The results indicate that Hsp12p is not essential for the growth, cell adhesion, filamentation and stress resistance in C. albicans or C. glabrata under standard laboratory conditions.
In order to study whether CaHsp12p is essential for virulence of C. albicans, a virulence test of the Cahsp12 null mutant was carried out in a Toll deficient Drosophila line as previously described by us [7]. There was no significant difference in the survival of flies infected with either the Cahsp12 null mutant or its control strain (Figure S6), indicating that deletion of CaHSP12 did not affect the virulence of C. albicans.
Overexpression of CaHSP12 enhances cell aggregation
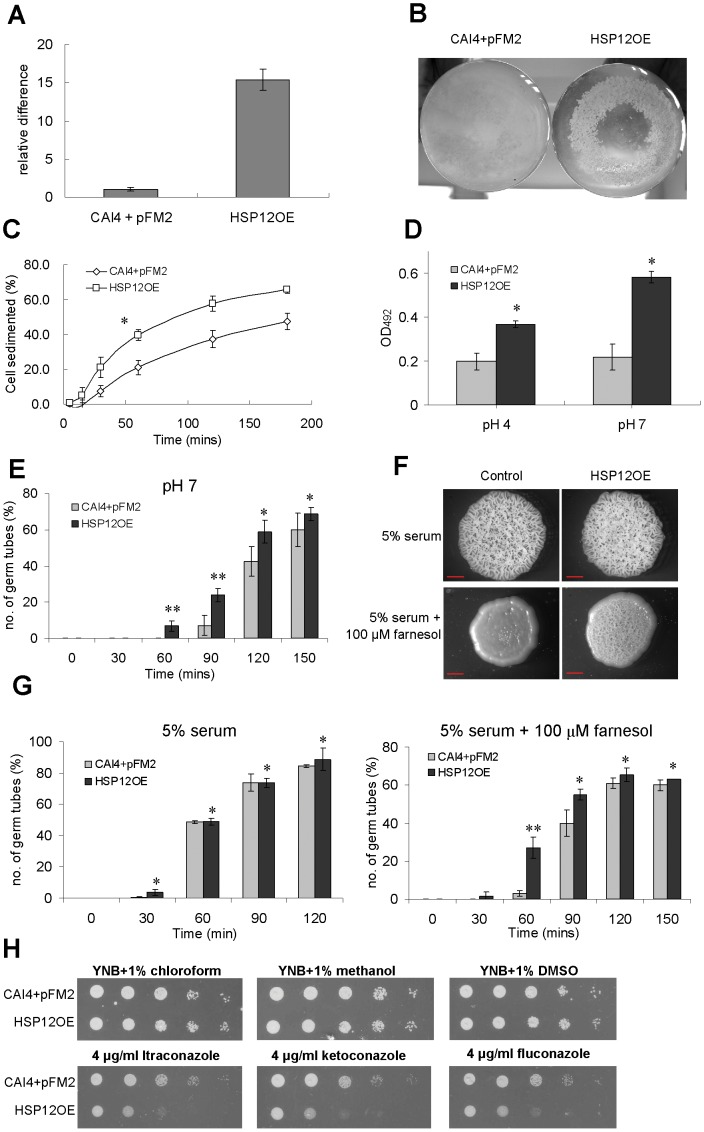
To explore the function of CaHsp12 protein further, the gene was expressed under the control of the native TEF2 promoter generating C. albicans HSP12OE. Overexpression was confirmed by using qRT-PCR, showing that the expression of CaHSP12 is increased by 15-fold (Figure 4A). The elevated level of Hsp12p in HSP12OE was also seen in Western blot analysis (Figure S7). Although we observed no alterations in stress resistance, including heat shock, osmotic and oxidative stress (Figure S8), HSP12OE was found to form clumps of cells when grown at pH 7 in liquid medium (Figure 4B). Susequently, cell-aggregation was quantified [47] and show that HSP12OE rapidly sedimented to the bottom of the cuvettes if grown at pH 7 and/or 5.5% CO2 (Figure S9). Since high pH and CO2 are conditions which promote C. albicans filamentation [3], [4], [6], [7], [40] we investigated if the cells continue to aggregate at pH 4, a condition where C. albicans did not filament. Under this condition HSP12OE settled quicker than the control (Figure 4C), demonstrating that cell aggregation was not secondary to filamentation. Cell adhesion of HSP12OE was also tested using the microtitre plate cell adhesion XTT reduction assay [48], [49]. This showed that HSP12OE adhesion to plastic is much stronger when compared to the control (Figure 4D). Interestingly, cell adhesion of HSP12OE, but not of the control, was further enhanced at pH 7, suggesting that the overexpressing phenotypes were influenced by the environmental pH.
Figure 4. Overexpression of CaHSP12 in C. albicans.
(A) qRT-PCR analysis of the CaHSP12 transcripts in HSP12OE. The level of transcripts was normalized to ACT1. The error bars represent the S.D. of triplicate independent reactions (B) Overexpression of CaHSP12 induced cell clumping. The control CAI4+pFM2 and HSP12OE were grown at pH 7. (C) Overexpression of CaHSP12 promoted cell aggregation which was independent from filamentation. CAI4+pFM2 and HSP12OE were grown at pH 4 for 4 h. Aggregation was then measured. The graphs were plotted by the percentage of cells sedimented against time. Results represent the means of three biological replicates with S.D. *P value<0.05, versus control strain, two-sided unpaired student t-test. (D) Overexpression of CaHSP12 enhanced cell adhesion at pH 4 or pH 7. HSP12OE and CAI4+pFM2 were grown on the flat-bottomed 96-well polystyrene plates and incubated at 37°C for 24 h. The adherent cells were quantified using the XTT reduction assay. The error bars were calculated from the S.D. of the triplicates. *P value<0.01, versus control strain, two-sided unpaired student t-test. (E) Overexpression of CaHSP12 promoted filamentation at pH 7. The percentage of the germ tube formation was counted every 30 min. The results presented are the means of three biological replicates with the S.D. **P value<0.01, * P value>0.05 versus control strains, two-sided unpaired student t-test. (F) Overexpression of CaHSP12 impacts on farnesol susceptibility. Cells were spotted onto 5% serum YEPD plates supplemented with or without 100 µm farnesol. Scale bar, 200 µm. (G) CAI4+pFM2 and HSP12OE were incubated in YNB supplemented with 5% serum with or without 100 µM farnesol. Germ tube formation was quantified every 30 min. The error bars were calculated from the S.D. of the triplicates. **P value<0.01, *P value>0.05 versus control strains, two-sided unpaired student t-test. (G) Overexpression of CaHSP12 increases susceptibility to azole antifungal drugs. 10-fold dilutions were spotted onto YNB plates containing 4 µg ml−1 itraconazole, ketoconazole and fluconzaole. YNB plates supplemented with 1% chloroform, methanol and DMSO act as control.
Overexpression of HSP12 desensitizes C. albicans to the quorum sensing molecule farnesol and enhances susceptibility to azole antifungal agents
When induced at pH 7 HSP12OE filamented earlier, compared to the control strain (Figure 4E). In fact germ tube formation in HSP12OE started after 60 min as opposed to 90 min in the control strain. Since the quorum-sensing molecule farnesol specifically interferes with the yeast-to-hyphal transition of C. albicans [50] we investigated its effect on HSP12OE. In the presence of 100 µM farnesol the formation of rough colonies in the control was completely inhibited but filamentation was only marginally affected in HSP12OE colonies (Figure 4F). When quantified in liquid filamentation assays, 100 µM farnesol reduced early-stage germ tube formation to a lesser degree in HSP12OE when compared to the control strain (Figure 4G). Since serum-induced germ tube formation in HSP12OE was not enhanced (Figure 4G), we directly attribute the observed phenotype to a reduced response to farnesol. Overexpression of CaHsp12p specifically enhanced susceptibility to the azole antifungal agents itraconazole, ketoconazole and fluconazole (Figure 4H) but not amphotericin B and rapamycin (Figure S8).
Discussion
Although small heat shock proteins are found in most organisms [51] their functions are still poorly understood. Previously, we have shown that expression of the C. albicans small heat shock gene, HSP12, is regulated by physiologically levels of CO2 and pH [21]. Interestingly these studies revealed that the S. cerevisiae orthologue of HSP12 is unaffected by the change of pH, suggesting different mechanisms of adaptation between the two yeasts [21].
Here we characterize CaHSP12 from the fungal pathogen C. albicans and show that it has both similar and different features when compared with other fungal species. CaHSP12 differs from other fungal species for carrying two different loci of the gene, both of which are transcriptionally expressed. We found that CaHsp12p and ScHsp12p are diversely regulated in response to oxidative stress. C. albicans has a greater level of resistance to oxidative stress than S. cerevisiae [40] which may be due to the fact that it has to cope with oxidative stress when phagocytosed by macrophages. Similar to other heat shock proteins, CaHsp12p is induced in stationary phase [17]. Previously it has been suggested that an increased degree of environmental stress resistance is correlated to the entry into stationary phase favoring long term viability [52], [53]. CaHsp12p was strongly induced subsequent to heat shock and a bioinformatics approach revealed several heat shock elements (HSE) in the upstream non-coding region. Nicholls et al have shown that the heat shock factor-1 Hsflp is activated under heat shock and required for the expression of heat shock genes, by specifically binding to HSE [54]. In fact microarray analysis showed that CaHSP12 is highly up-regulated, in a Hsf1p-dependent manner, in response to heat shock [54]. Additionally, chromatin immunoprecipitation (ChIP) revealed that ScHsp12p is targeted by Hsf1p in S. cerevisiae [55].
We identified E-boxes in the CaHSP12 promoter and demonstrated that the transcriptional regulator Efg1p is required not only for C. albicans Hsp12p baseline expression in unstressed condition but also under heat and oxidative stress. Previous studies have shown that Efg1p is required for heat stress adaptation, so it is possible that regulation of Hsp12p in response to heat shock is dependent on Efg1p. However, induction of Hsp12p is still found in the efg1 mutant and additional pathways may be involved in the regulation. Interestingly, Efg1p is not required for the expression of Hsp12p under osmotic stress. This suggests that there are different mechanisms for C. albicans in response to different stresses. Hog1p for example is known to play a role in response to osmotic and oxidative stress in C. albicans [8]. Unexpectedly, we found CaHsp12p to be repressed by Hog1p. One could speculate that dephosphorylated Hog1p represses the CaHsp12p expression whereas stress-mediated phosphorylation of Hog1p can abolish the repression [8].
We found that CaHsp12p protein is also regulated by changes of environmental CO2 and pH confirming our previous results investigating CaHSP12 mRNA levels [21]. Furthermore we found CaHsp12p protein levels to be significantly affected by the quorum-sensing molecule farnesol, which blocks the yeast-to-hyphal transition via the cAMP-dependent signaling cascade [46], [56], [57], [58], [59], confirming results reported by Davis-Hanna et al. [24]. Hall et al. recently showed that farnesol directly inhibits the adenylyl cyclase, Cyr1p [46], [59] suggesting a link between CaHsp12p expression and farnesol inhibition of Cyr1p. This is consistent with the elevated levels of CaHsp12p found in the cyr1 mutant. Overexpression of Hsp12p desensitized cells to the effect of farnesol. Although the mechanism of farnesol tolerance is still unclear, Hsp12 protein may have a role in protecting the targets of farnesol or the components of farnesol response pathways [46], [59].
Since ambient pH, CO2 and farnesol are all signals which impact on C. albicans filamentation, it was hypothesized that the expression of CaHsp12p is required during morphogenesis. However, deletion of HSP12 showed that the gene is not essential for C. albicans stress resistance, filamentation and virulence. However, HSP12 overexpression did enhance the early stage of hyphal formation and reduced the effect of farnesol on the inhibition of filamentation. This suggests that CaHsp12p may have a facilitating role in hyphal formation.
Overexpression of CaHSP12 increased the sensitivity of the cells to several azole antifungal drugs. The action of azoles on fungi is mediated by depletion of ergosterol, which results in the alteration of membrane fluidity [60]. In S. cerevisiae, ScHsp12p is known to influence plasma membrane fluidity enhancing the stability of the cell membrane [61]. Overexpression also enhanced cell adhesion. Interestingly the actions of adhesion are mediated by cell wall proteins [1] and ScHsp12p has been shown to be localized in cell wall [62]. CaHsp12p has 43% homology to the amino-terminal region of ScHsp12p, thus it is feasible to speculate that CaHsp12p is present in the cell wall and as a heat shock protein it may have a role in protection of cell wall proteins. Localization studies of CaHsp12p in vivo in response to stress and during the yeast-to-hyphal transition are required to address this further.
We show the CaHsp12p is significantly regulated under a wide range of stimuli, but is not essential for C. albicans to survive in those conditions. This raises the possibility that other proteins with similar functions may compensate for the inactivation of CaHsp12p in the Cahsp12 null mutant. Our overexpression studies point to the potential role of CaHsp12p in protecting the targets of farnesol, the cell membrane and cell wall protection. Therefore, identifying protein partners of CaHsp12p should be of interest and reveal additional information on its biological function.
Supporting Information
Expression of Ca Hsp12p in C. albicans mutant strains. Western blot analysis showing that levels of CaHsp12p remained high in hog1, cyr1, tpk1 mutants and unchanged in tpk2 mutant when heat shocked from 37°C to 45°C, 0.3 M NaCl or 1 mM H2O2. H: anti-Hsp12p antibody. A: anti-actin antibody (equal protein loading control). RDU: relative densitometry units.
(TIF)
Deletion of HSP12 does not affect growth rate and cell adhesion. (A) No significant change in the growth rates of the Cahsp12 (HSP12KO5) and Cghsp12 (Cg12KO) null mutants was observed. The overnight cultures were diluted into the OD600 of 0.1 and incubated at 37°C. The OD600 of the cells was measured at the indicated time points. The growth curves of strains were plotted in the OD600 against time. Triplicate biological experiments have been performed. The error bars represent the S.D. of the triplicate independent experiments. (B) The Cahsp12 and the Cghsp12 null mutant displayed the same ability of cell adhesion as controls in the XTT reduction assay. The strains were grown on the flat-bottomed 96-well polystyrene plates and incubated at 37°C for 24 h. The adherent cells were quantified using the XTT reduction assay. The results presented are the means of three biological replicates with standard derivation. *P value>0.05 versus controls, two-sided unpaired student t-test.
(TIF)
Ca HSP12 is not essential for C. albicans in resistance to stresses and antifungal drugs. Overnight cultures were diluted in YEPD liquid to an OD600 of 2. For heat shock test, the cells were heated at 55°C for 2 min and 10-fold dilutions of the cells were spotted onto YEPD. For other stress tests, the cells at 10-fold dilutions were spotted onto YNB plates containing stress or antifungal agents as indicated. The cultural plates were incubated at 37°C for 24 h. The YNB plates supplemented with 1% chloroform, methanol and DMSO act as control of itraconazole, ketoconazole and fluconazole which were dissolved in chloroform, methanol and DMSO.
(TIF)
Deletion of CgHSP12 did not affect resistance to stress and antifungal drugs. The overnight cultures were diluted in YEPD liquid to an OD600 of 2, and heat shock at 55°C for 2 min. The cells at 10-fold dilutions were spotted onto YEPD plates and incubated at 37°C for 24 h. For other stress tests, the cells at 10-fold dilutions were spotted onto YNB plates containing stress or antifungal agents as indicated. The cultural plates were incubated at 37°C for 24 h. The YNB plates supplemented with 1% chloroform, methanol and DMSO act as control of itraconazole, ketoconazole and fluconazole.
(TIF)
Deletion of Ca HSP12 does not interfere with filamentation at pH 7 in 5.5% CO2. The Cahsp12 deletion strain and its controls were incubated in YNB minimal medium at pH 7 in 5.5% CO2 at 37°C. The cell morphology of the strains was observed by a light microscopy. The percentage of the germ tube formation was counted under the microscopy every 30 min. The germ tube formation of the Cahsp12 null mutant had no significant difference to the controls. Results presented are the means of three biological replicates with standard derivation. P value>0.1 versus controls, two-sided unpaired student t-test.
(TIF)
Deletion of Ca HSP12 does not influence the virulence of C. albicans in the Toll mutant fruit fly. 15 flies per experimental group were injected with the C. albicans strains. The flies were then incubated at 30°C for 40 h. The numbers of the living flies were counted at the indicated time. The results are calculated from the means of three biological replicates with the standard derivations. *P value>0.1, versus control strains (BWT or HSP12C), two-sided unpaired student t-test.
(TIF)
Western blot analysis of the CaHsp12p expression in HSP12OE. CaHsp12p was expressed higher in the HSP12OE when compared to wild-type. The blot was hybridised with the anti-Hsp12p antibody and the anti-actin antibody, served as the control for equal protein loading as described in text S1.
(TIF)
Overexpression of CaHSP12 does not affect growth under stresses and exposure to antifungal agents. For the heat shock assay, the overnight cultures were diluted to OD600 of 2 and shifted to 55°C for 2 min. The 10-fold serial dilutions of the heat shock cells were spotted onto YEPD plates and incubated at 37°C for 24 h. For other stress studies, the overnight cultures at the OD600 of 2.0 were diluted 10-fold serially. The dilutions (5 µl) were spotted onto YNB plates supplemented with stress and antifungal agents as indicated. The plates were incubated at 37°C for 24 h.
(TIF)
Overexpression of Ca HSP12 promotes cell aggregation at pH 7 in air or 5.5% CO2. The strains were grown at (A) pH 7 in air; (B) pH 7 in 5.5% CO2. Total 1 ml of the culture was settled to the bottom of the cuvettes. The OD600 corresponding to the cells at the upper part of the cuvettes was measured at the time points indicated. The graphs were plotted by the percentage of cell sedimented against time. Results represent the means of three biological replicates with standard derivation. *P value<0.05, versus control strain, two-sided unpaired student t-test.
(TIF)
Acknowledgments
We thank Peter Meacock, University of Leicester, and Mick Tuite, School of Biosciences University of Kent for providing/sharing the anti-ScHsp12p antibody. We thank all the researchers who were so generous to send us their yeast mutant strains, listed in Table 1, used in this study. Fabien Cottier is thanked for his help during all phases of the project.
Funding Statement
Medical Research Council (MRC)(http://www.mrc.ac.uk/index.htm); Biotechnology and Biological Sciences Research Council (BBSRC)(http://www.bbsrc.ac.uk/home/home.aspx); the University of Kent (http://www.kent.ac.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mavor AL, Thewes S, Hube B (2005) Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets 6: 863–874. [DOI] [PubMed] [Google Scholar]
- 2.Hosking S (1999) Fungi as animal pathogens. In: Oliver RP, Schweizer M, editors. Molecular fungal biology. Cambridge: Cambridge University Press. pp. 322–340.
- 3. Mühlschlegel FA, Fonzi WA (1997) PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol 17: 5960–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Barkani A, Kurzai O, Fonzi WA, Ramon A, Porta A, et al. (2000) Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans . Mol Cell Biol 20: 4635–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cottier F, Raymond M, Kurzai O, Bolstad M, Leewattanapasuk W, et al. (2012) The bZIP transcription factor Rca1p is a central regulator of a novel CO2 sensing pathway in yeast. PLoS Pathog 8: e1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, et al. (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall RA, De Sordi L, Maccallum DM, Topal H, Eaton R, et al. (2010) CO2 acts as a signalling molecule in populations of the fungal pathogen Candida albicans . PLoS Pathog 6: e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J (2004) A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans . Mol Biol Cell 15: 4179–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiteway M, Bachewich C (2007) Morphogenesis in Candida albicans . Annu Rev Microbiol 61: 529–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, et al. (1998) Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun 66: 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis D, Wilson RB, Mitchell AP (2000) RIM101-dependent and-independent pathways govern pH responses in Candida albicans . Mol Cell Biol 20: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kadosh D, Johnson AD (2005) Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell 16: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnie JP, Carter TL, Hodgetts SJ, Matthews RC (2006) Fungal heat-shock proteins in human disease. FEMS Microbiol Rev 30: 53–88. [DOI] [PubMed] [Google Scholar]
- 14. Praekelt UM, Meacock PA (1990) HSP12, a new small heat shock gene of Saccharomyces cerevisiae: analysis of structure, regulation and function. Mol Gen Genet 223: 97–106. [DOI] [PubMed] [Google Scholar]
- 15. de Jong WW, Leunissen JA, Voorter CE (1993) Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol 10: 103–126. [DOI] [PubMed] [Google Scholar]
- 16.Garstel M, Vajdy E, Buchner J (1997) The small Hsps - an overview. In: Gething M, editor. Guidebook to the molecular chaperones and protein-folding catalysts. Oxford: Oxford University Press. pp. 269–272.
- 17. Panaretou B, Piper PW (1992) The plasma membrane of yeast acquires a novel heat-shock protein (hsp30) and displays a decline in proton-pumping ATPase levels in response to both heat shock and the entry to stationary phase. Eur J Biochem 206: 635–640. [DOI] [PubMed] [Google Scholar]
- 18. Carmelo V, Sa-Correia I (1997) HySP26 gene transcription is strongly induced during Saccharomyces cerevisiae growth at low pH. FEMS Microbiol Lett 149: 85–88. [DOI] [PubMed] [Google Scholar]
- 19. Bentley NJ, Fitch IT, Tuite MF (1992) The small heat-shock protein Hsp26 of Saccharomyces cerevisiae assembles into a high molecular weight aggregate. Yeast 8: 95–106. [DOI] [PubMed] [Google Scholar]
- 20. Liu TT, Lee RE, Barker KS, Wei L, Homayouni R, et al. (2005) Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans . Antimicrob Agents Chemother 49: 2226–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheth CC, Mogensen EG, Fu MS, Blomfield IC, Mühlschlegel FA (2008) Candida albicans HSP12 is co-regulated by physiological CO2 and pH. Fungal Genet Biol 45: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 22. Enjalbert B, Nantel A, Whiteway M (2003) Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol Biol Cell 14: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, et al. (2006) Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans . Mol Biol Cell 17: 1018–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA (2008) Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF (2006) Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol 361: 399–411. [DOI] [PubMed] [Google Scholar]
- 26. Coste AT, Karababa M, Ischer F, Bille J, Sanglard D (2004) TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2 . Eukaryot Cell 3: 1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karababa M, Coste AT, Rognon B, Bille J, Sanglard D (2004) Comparison of gene expression profiles of Candida albicans azole-resistant clinical isolates and laboratory strains exposed to drugs inducing multidrug transporters. Antimicrob Agents Chemother 48: 3064–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, et al. (2007) In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol 63: 1606–1628. [DOI] [PubMed] [Google Scholar]
- 29. Nantel A, Dignard D, Bachewich C, Harcus D, Marcil A, et al. (2002) Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol Biol Cell 13: 3452–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lan CY, Rodarte G, Murillo LA, Jones T, Davis RW, et al. (2004) Regulatory networks affected by iron availability in Candida albicans . Mol Microbiol 53: 1451–1469. [DOI] [PubMed] [Google Scholar]
- 31. Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, et al. (2006) Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol 61: 704–722. [DOI] [PubMed] [Google Scholar]
- 32. Maeng S, Ko YJ, Kim GB, Jung KW, Floyd A, et al. (2010) Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot Cell 9: 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fonzi WA, Irwin MY (1993) Isogenic strain construction and gene mapping in Candida albicans . Genetics 134: 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson RB, Davis D, Mitchell AP (1999) Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181: 1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wendland J (2003) PCR-based methods facilitate targeted gene manipulations and cloning procedures. Curr Genet 44: 115–123. [DOI] [PubMed] [Google Scholar]
- 36. Dennison PM, Ramsdale M, Manson CL, Brown AJ (2005) Gene disruption in Candida albicans using a synthetic, codon-optimised Cre-loxP system. Fungal Genet Biol 42: 737–748. [DOI] [PubMed] [Google Scholar]
- 37. Weig M, Haynes K, Rogers TR, Kurzai O, Frosch M, et al. (2001) A GAS-like gene family in the pathogenic fungus Candida glabrata . Microbiology 147: 2007–2019. [DOI] [PubMed] [Google Scholar]
- 38. Kitada K, Yamaguchi E, Arisawa M (1995) Cloning of the Candida glabrata TRP1 and HIS3 genes, and construction of their disruptant strains by sequential integrative transformation. Gene 165: 203–206. [DOI] [PubMed] [Google Scholar]
- 39. Petko L, Lindquist S (1986) Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell 45: 885–894. [DOI] [PubMed] [Google Scholar]
- 40. Jamieson DJ, Rivers SL, Stephen DW (1994) Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology 140 (Pt 12) 3277–3283. [DOI] [PubMed] [Google Scholar]
- 41. Varela JC, Praekelt UM, Meacock PA, Planta RJ, Mager WH (1995) The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol Cell Biol 15: 6232–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cowen LE, Nantel A, Whiteway MS, Thomas DY, Tessier DC, et al. (2002) Population genomics of drug resistance in Candida albicans . Proc Natl Acad Sci U S A 99: 9284–9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bockmühl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF (2001) Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans . Mol Microbiol 42: 1243–1257. [DOI] [PubMed] [Google Scholar]
- 44. Eckert SE, Heinz WJ, Zakikhany K, Thewes S, Haynes K, et al. (2007) PGA4, a GAS homologue from Candida albicans, is up-regulated early in infection processes. Fungal Genet Biol 44: 368–377. [DOI] [PubMed] [Google Scholar]
- 45. Braun BR, Johnson AD (1997) Control of filament formation in Candida albicans by the transcriptional repressor TUP1 . Science 277: 105–109. [DOI] [PubMed] [Google Scholar]
- 46. Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, et al. (2011) The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans . Eukaryot Cell 10: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eboigbodin KE, Biggs CA (2008) Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromolecules 9: 686–695. [DOI] [PubMed] [Google Scholar]
- 48. Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP (2003) Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol 41: 2961–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hiller E, Heine S, Brunner H, Rupp S (2007) Candida albicans Sun41p, a putative glycosidase, is involved in morphogenesis, cell wall biogenesis, and biofilm formation. Eukaryot Cell 6: 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, et al. (2001) Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67: 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12: 842–846. [DOI] [PubMed] [Google Scholar]
- 52. Sanchez Y, Taulien J, Borkovich KA, Lindquist S (1992) Hsp104 is required for tolerance to many forms of stress. EMBO J 11: 2357–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elliott B, Futcher B (1993) Stress resistance of yeast cells is largely independent of cell cycle phase. Yeast 9: 33–42. [DOI] [PubMed] [Google Scholar]
- 54. Nicholls S, Leach MD, Priest CL, Brown AJ (2009) Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Mol Microbiol 74: 844–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hahn JS, Hu Z, Thiele DJ, Iyer VR (2004) Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24: 5249–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL (2002) Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68: 5459–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Westwater C, Balish E, Schofield DA (2005) Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot Cell 4: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nickerson KW, Atkin AL, Hornby JM (2006) Quorum sensing in dimorphic fungi: farnesol and beyond. Appl Environ Microbiol 72: 3805–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hogan DA, Mühlschlegel FA (2011) Candida albicans developmental regulation: adenylyl cyclase as a coincidence detector of parallel signals. Curr Opin Microbiol 14: 682–686. [DOI] [PubMed] [Google Scholar]
- 60. Smriti, Krishnamurthy SS, Prasad R (1999) Membrane fluidity affects functions of Cdr1p, a multidrug ABC transporter of Candida albicans . FEMS Microbiol Lett 173: 475–481. [DOI] [PubMed] [Google Scholar]
- 61. Welker S, Rudolph B, Frenzel E, Hagn F, Liebisch G, et al. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol Cell 39: 507–520. [DOI] [PubMed] [Google Scholar]
- 62. Motshwene P, Karreman R, Kgari G, Brandt W, Lindsey G (2004) LEA (late embryonic abundant)-like protein Hsp 12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae . Biochem J 377: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, et al. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of Ca Hsp12p in C. albicans mutant strains. Western blot analysis showing that levels of CaHsp12p remained high in hog1, cyr1, tpk1 mutants and unchanged in tpk2 mutant when heat shocked from 37°C to 45°C, 0.3 M NaCl or 1 mM H2O2. H: anti-Hsp12p antibody. A: anti-actin antibody (equal protein loading control). RDU: relative densitometry units.
(TIF)
Deletion of HSP12 does not affect growth rate and cell adhesion. (A) No significant change in the growth rates of the Cahsp12 (HSP12KO5) and Cghsp12 (Cg12KO) null mutants was observed. The overnight cultures were diluted into the OD600 of 0.1 and incubated at 37°C. The OD600 of the cells was measured at the indicated time points. The growth curves of strains were plotted in the OD600 against time. Triplicate biological experiments have been performed. The error bars represent the S.D. of the triplicate independent experiments. (B) The Cahsp12 and the Cghsp12 null mutant displayed the same ability of cell adhesion as controls in the XTT reduction assay. The strains were grown on the flat-bottomed 96-well polystyrene plates and incubated at 37°C for 24 h. The adherent cells were quantified using the XTT reduction assay. The results presented are the means of three biological replicates with standard derivation. *P value>0.05 versus controls, two-sided unpaired student t-test.
(TIF)
Ca HSP12 is not essential for C. albicans in resistance to stresses and antifungal drugs. Overnight cultures were diluted in YEPD liquid to an OD600 of 2. For heat shock test, the cells were heated at 55°C for 2 min and 10-fold dilutions of the cells were spotted onto YEPD. For other stress tests, the cells at 10-fold dilutions were spotted onto YNB plates containing stress or antifungal agents as indicated. The cultural plates were incubated at 37°C for 24 h. The YNB plates supplemented with 1% chloroform, methanol and DMSO act as control of itraconazole, ketoconazole and fluconazole which were dissolved in chloroform, methanol and DMSO.
(TIF)
Deletion of CgHSP12 did not affect resistance to stress and antifungal drugs. The overnight cultures were diluted in YEPD liquid to an OD600 of 2, and heat shock at 55°C for 2 min. The cells at 10-fold dilutions were spotted onto YEPD plates and incubated at 37°C for 24 h. For other stress tests, the cells at 10-fold dilutions were spotted onto YNB plates containing stress or antifungal agents as indicated. The cultural plates were incubated at 37°C for 24 h. The YNB plates supplemented with 1% chloroform, methanol and DMSO act as control of itraconazole, ketoconazole and fluconazole.
(TIF)
Deletion of Ca HSP12 does not interfere with filamentation at pH 7 in 5.5% CO2. The Cahsp12 deletion strain and its controls were incubated in YNB minimal medium at pH 7 in 5.5% CO2 at 37°C. The cell morphology of the strains was observed by a light microscopy. The percentage of the germ tube formation was counted under the microscopy every 30 min. The germ tube formation of the Cahsp12 null mutant had no significant difference to the controls. Results presented are the means of three biological replicates with standard derivation. P value>0.1 versus controls, two-sided unpaired student t-test.
(TIF)
Deletion of Ca HSP12 does not influence the virulence of C. albicans in the Toll mutant fruit fly. 15 flies per experimental group were injected with the C. albicans strains. The flies were then incubated at 30°C for 40 h. The numbers of the living flies were counted at the indicated time. The results are calculated from the means of three biological replicates with the standard derivations. *P value>0.1, versus control strains (BWT or HSP12C), two-sided unpaired student t-test.
(TIF)
Western blot analysis of the CaHsp12p expression in HSP12OE. CaHsp12p was expressed higher in the HSP12OE when compared to wild-type. The blot was hybridised with the anti-Hsp12p antibody and the anti-actin antibody, served as the control for equal protein loading as described in text S1.
(TIF)
Overexpression of CaHSP12 does not affect growth under stresses and exposure to antifungal agents. For the heat shock assay, the overnight cultures were diluted to OD600 of 2 and shifted to 55°C for 2 min. The 10-fold serial dilutions of the heat shock cells were spotted onto YEPD plates and incubated at 37°C for 24 h. For other stress studies, the overnight cultures at the OD600 of 2.0 were diluted 10-fold serially. The dilutions (5 µl) were spotted onto YNB plates supplemented with stress and antifungal agents as indicated. The plates were incubated at 37°C for 24 h.
(TIF)
Overexpression of Ca HSP12 promotes cell aggregation at pH 7 in air or 5.5% CO2. The strains were grown at (A) pH 7 in air; (B) pH 7 in 5.5% CO2. Total 1 ml of the culture was settled to the bottom of the cuvettes. The OD600 corresponding to the cells at the upper part of the cuvettes was measured at the time points indicated. The graphs were plotted by the percentage of cell sedimented against time. Results represent the means of three biological replicates with standard derivation. *P value<0.05, versus control strain, two-sided unpaired student t-test.
(TIF)