Abstract
The use of electrical muscle stimulation to treat denervated muscle prior to delayed reinnervation has been widely debated. There is evidence showing both positive and negative results following different protocols of electrical stimulation. In this study we investigated the role electrical stimulation has on muscle reinnervation following immediate and delayed nerve repair using motor unit estimation techniques. Rat gastrocnemius muscle was denervated and repaired using the peroneal nerve either immediately or following three-months with and without electrical stimulation. Motor unit counts, average motor unit sizes, and maximum compound action potentials were measured three-months following peroneal nerve repair. Motor unit counts in animals that were denervated and stimulated were significantly higher than those that were denervated and not stimulated. Both average motor unit sizes and maximum compound action potentials showed no significant differences between denervated and denervated-stimulated animals. These results provide evidence that electrical stimulation prior to delayed nerve repair increases muscle receptivity to regenerating axons and may be a worthwhile treatment for peripheral nerve injuries.
I. INTRODUCTION
Despite the ability for peripheral nerves to regenerate following injury, complete functional recovery is rare [1]–[3]. The best case scenario for treating nerve injuries is immediate surgical repair; however, this is not always possible [1]–[4]. As soon as a nerve is injured or transected, the muscle has lost its functional connection to the nervous system and is termed denervated. As the length of time of muscle denervation increases, the chance for complete functional recovery significantly diminishes. Progressive muscle atrophy usually follows long-term denervation with a loss of muscle mass, muscle spindles, force, motor function and an increase in collagenization and fibrosis of the tissue [2], [3], [5]. Other important factors contributing to poor recovery are incomplete or inappropriate muscle reinnervation and decreased receptivity of muscle to reinnervation [5], [6]. One of the potential maintenance treatments for denervated muscle is electrical stimulation (ES) following injury. The issue with this treatment is that it is not widely accepted due to a lack of standards and questions of efficacy [7]. Many studies have shown both positive and negative results. Some beneficial effects include an increase in muscle fiber area, maintenance of fatiguability properties, and increases in muscle force [8]–[10]. Some negative effects include reduced intramuscular axonal sprouting [11], [12] and compromised reinnervation [7]. Others have shown no benefits for the use of ES prior to delayed nerve repair [13]. Because of the wide range of positive and negative effects there is still no consensus regarding the optimal electrical muscle stimulation protocol. Some studies have utilized a 24-hour paradigm employing implanted stimulators [14], [15], while others have shown that short term (20 minutes to 5 hours daily) stimulation provides just as much benefit [8]. Recently, our group has shown that a 1 hour per day stimulation protocol was effective at significantly increasing muscle weight, force, and fiber area in one month denervated rat gastrocnemius muscle [16]. In a second more comprehensive study we have looked at the longer term effects of electrical stimulation in both three month denervated and immediately repaired muscle. This paper specifically addresses the issue of ES and reinnervation using motor nerve stimulation and motor unit number estimation.
II. METHODS
A. Animals and Surgical Procedures
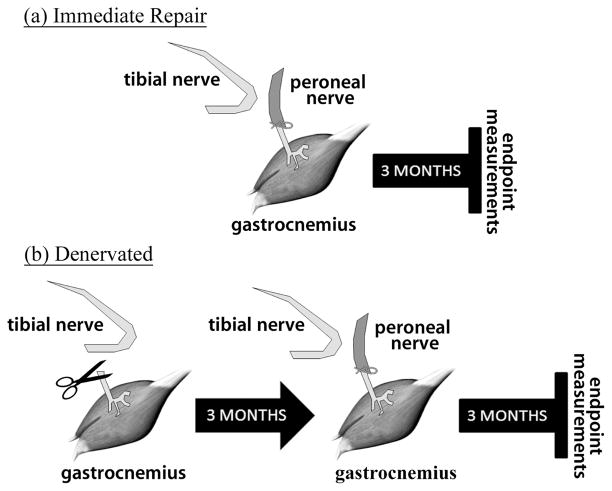
The experiments were performed on male Lewis rats (Charles River, Quebec, Canada) weighing between 200–250g. This strain was chosen as it shows the least self-mutilation following surgery [17]. All housing, surgical procedures, analgesia and assessments were performed according to the Canadian Council on Animal Care Guidelines, using protocols approved by the Animal Care Committee at McMaster University. Thirty-two animals were randomly assigned to one of four groups: immediate repair (IR), immediate repair with electrical stimulation (IR+ES), denervated (DEN), or denervated with electrical stimulation (DEN+ES). Each group had the right gastrocnemius muscle denervated, as described previously [2], by cutting the tibial nerve approximately 13 mm from its entry point into the gastrocnemius muscle (Fig. 1). The free distal stump was ligated to minimize extraneous innervation from other axons, and the proximal stump of the nerve was inserted and sutured into the biceps femoris muscle to avoid reinnervation from proximal tibial nerve axons [2]. Teflon coated, stainless steel (Cooner Wire, AS 631) stimulating electrodes with ends bared of insulation were implanted into the belly of the right gastrocnemius muscle in all groups using an electrode suture complex to minimize electrode migration [18]. For both the IR and IR+ES groups the peroneal nerve was transected and connected directly to the distal tibial stump. For DEN and DEN+ES groups, the peroneal to tibial union was delayed by three months after the initial denervation. Following the peroneal to tibial repair, the muscles were allowed to recover for three months before final motor unit assessments were made.
Fig. 1.
Surgical procedure and time points for experimental muscles. (a) immediate peroneal to tibial repair followed by 3 months of recovery. (b) complete denervation followed by a delayed (3 month) peroneal to tibial repair and a 3 month recovery period. At the end of the recovery period functional measurements are taken. Both of these groups were duplicated and included 1 month of electrical stimulation in the immediate repair case and 3 months of electrical stimulation in the denervated case. Animals in all groups had electrodes implanted into the belly of the muscle.
B. Electrical Muscle Stimulation
The stimulation paradigm featured a 1 hour session per day using 400 ms of 100 Hz frequency, 200 μs per phase biphasic pulses followed by 6 seconds of rest. Stimulus amplitudes were adjusted to maintain a strong visual contraction. The paradigm was delivered using a custom designed stimulator capable of stimulating five rats simultaneously using the protocol mentioned above. Details of the stimulator design and stimulation paradigm are discussed elsewhere [16]. Animals in the IR+ES group received one month of electrical stimulation following the initial surgery. The rationale for this is that following peroneal to tibial union the muscle is without any functional connections for approximately one month. This is the estimated time it takes for regenerating motor axons from the peroneal nerve to reach the muscle and form functional connections. Animals in the DEN+ES group received three months of stimulation following the initial denervation surgery and one month following the peroneal to tibial repair surgery.
C. Motor Unit Number Estimation (MUNE)
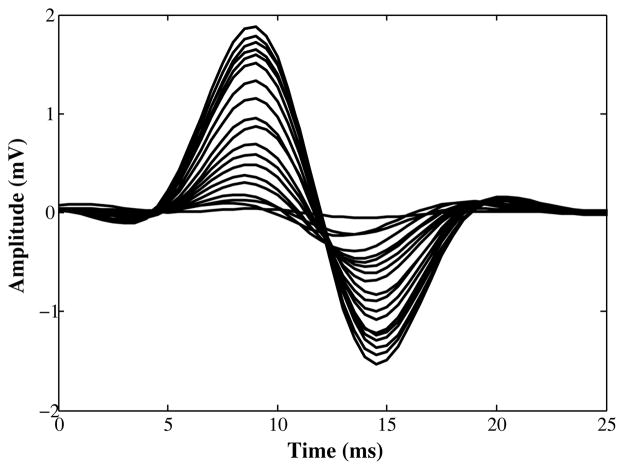
Motor unit estimation was performed on all animals following the three-month recovery period after peroneal to tibial repair. Two needle recording electrodes were placed in the gastrocnemius muscle and two stimulating hook electrodes on the proximal peroneal nerve. Stimulation pulses of 50 μs duration were delivered using an isolated stimulator and a custom designed EMG amplifier was used to record M-wave responses at a sampling rate of 8 Khz. A custom written LabVIEW program was used to trigger, record, and analyze the results. M-wave pattern recognition for template formation in this technique was accomplished using a 3-level wavelet decomposition classification scheme [20]. Maximum compound muscle action potentials (CMAP) were first recorded and then twenty templates were recorded for each leg in the animal by gradually increasing the stimulus amplitude from threshold. Motor unit numbers were then estimated using the incremental method [19]. This involved dividing the area of the maximum CMAP by the average motor unit action potential contribution. The stimulating electrode was then moved a few millimeters proximally and the procedure repeated. Motor unit counts were averaged over these two trials. Fig. 2 shows a typical result of the properly characterized templates using wavelet pattern recognition.
Fig. 2.
A typical template set for a fully innervated rat gastrocnemius muscle
Average motor unit action potential (MUAP) size was found by taking the largest template peak to peak value and dividing it by the number of steps required to reach that value. To ensure that there were no contributions to the motor unit count from the previously cut tibial nerve, stimulating electrodes were placed proximal to the level of the initial denervation and 50 μs stimulus pulses were delivered. The acquisition of an M-wave by the recording electrodes would suggest ectopic innervation and hence that animal’s results would be discarded.
D. Statistical Methods
To determine any significant differences between the groups a one-way ANOVA was performed followed by a Tukey post hoc test. Significance was defined as p<0.05.
III. RESULTS
Two animals died during the initial surgery and three animals had noticeable ectopic innervation upon proximal tibial nerve stimulation. The latter three animals were excluded from any analysis.
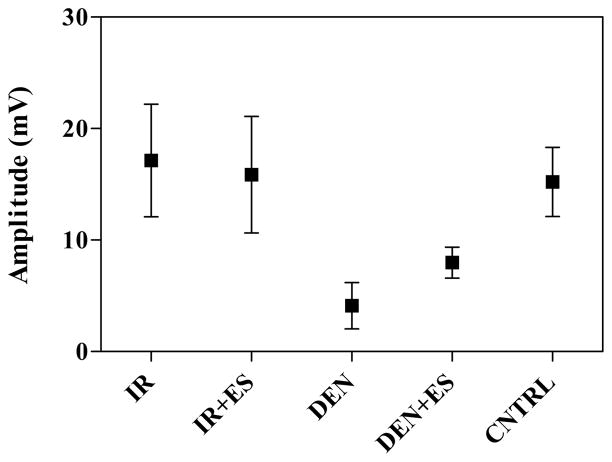
Fig. 3 shows the motor unit estimates from all the groups. One month of stimulation in the IR+ES group provided no additional increases in motor unit numbers when compared to the unstimulated group (IR). Interestingly, three months of electrical stimulation in the DEN+ES group resulted in motor unit numbers not significantly different from either IR group. Most importantly, all three groups had values significantly greater than the DEN group, p<0.05. The fully innervated control group (CNTRL), which is the untreated contralateral limb in each animal, is shown for comparison.
Fig. 3.
An estimation of the number of motor units shown for the immediately repaired (IR), immediately repaired with electrical stimulation (IR+ES), denervated (DEN), denervated with electrical stimulation (DEN+ES), and unoperated gastrocnemius muscle from the contralateral limb (CNTRL). Error bars represent standard deviation.
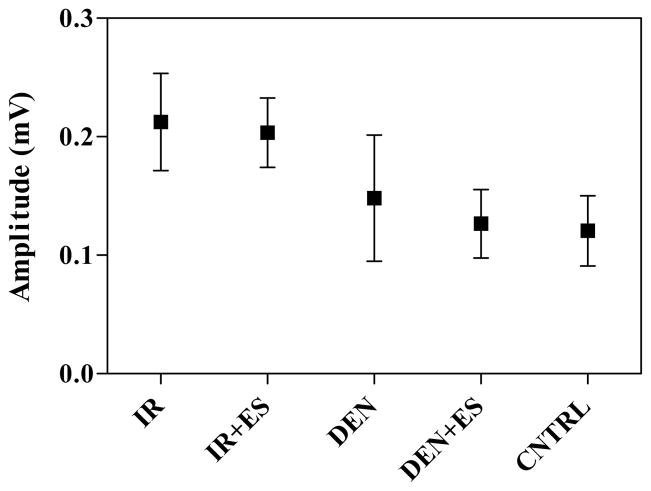
Fig. 4 shows the average MUAP size. The post hoc test found no significant differences between the groups because of the large variability in each group’s results. However, there is clearly a trend in that both the DEN and DEN+ES groups have smaller average values than both IR groups.
Fig. 4.
Average peak-to-peak MUAP amplitude values. Error bars represent standard deviation.
The last measure to be evaluated was the maximum peak-to-peak amplitude of the compound muscle action potential (Fig 5). There was no significant difference between both IR groups; however, both of these were significantly greater than both the DEN and DEN+ES groups (p<0.05). There was also no statistical difference between the DEN and DEN+ES groups although the mean values for the stimulated group were approximately 30% greater than for the unstimulated.
Fig. 5.
Maximum peak-to-peak amplitude values for the compound muscle action potentials. Error bars represent standard deviation.
IV. DISCUSSION
This study provides clear evidence that electrical stimulation of denervated muscle plays a positive role in functional recovery. Motor unit numbers were not significantly different than the best-case scenario: immediate repair. Interestingly, one month of electrical muscle stimulation provided no added benefit in the immediate repair case. This may be due to the fact that the majority of motor axons found in the peroneal nerve create functional connections with the muscle and there is little room for improvement. However, these values were much less than the typical motor unit count in the gastrocnemius. Fully innervated and untreated gastrocnemius muscles in our animals have motor unit counts approximately 2 times that of our IR and IR+ES groups (155±32 vs. 82±20 and 80±20, respectively). This is consistent with a study that showed the tibial nerve has about twice as many motor axons as the peroneal nerve [21]. Our motor unit numbers in the control leg are also similar to those published by other authors [22].
We expected that the average MUAP size for the DEN group would be smaller than that of the DEN+ES group. Our rationale was that because of the severe fiber atrophy and fibrosis taking place over three months of denervation, the muscle would not be as receptive to reinnervation. Consequently, motor axons that do make their way towards the muscle are forming connections with a small number of extremely atrophied fibers. Our results from a previous study [16] showed that after only one month the untreated denervated muscle had significantly smaller average muscle fiber sizes compared to a denervated-stimulated and fully innervated muscle. MUAPs from these motor units should then be smaller than MUAPs from a motor unit containing the same number of muscle fibers but with a much larger fiber area. Our results are not consistent with this hypothesis as both the DEN and DEN+ES MUAPs were the same size.
Our results do, however, show that significantly fewer axons were able to form functional connections with the denervated muscle fibers. One explanation for the larger than expected average MUAP size for this group may be that, during the three month recovery period following peroneal repair, those fewer motor axons that have made early functional connections with the denervated muscle will have had additional time to sprout collateral branches to the remaining denervated fibers and to further increase the size of the motor unit. Indeed, preliminary histological observations of muscle fiber area from this study also indicate that the DEN+ES group has a much larger fiber area than the DEN group. That is, the denervated untreated MUAPs are larger than expected because these motor units have a greater number of small muscle fibers. CMAP values provide an indication of the size of muscle fibers and consequently the force output. The results were as expected with the DEN+ES group being higher (although not significantly) than the DEN group. Control values for CMAP were not statistically different than either of the immediate repair groups. However, histological examination shows larger fiber areas in control muscles than in both IR and IR+ES groups indicating larger force output. Indeed, preliminary measurements of tetanic force confirm this result.
V. CONCLUSION
Contrary to what other studies have shown [13], electrical stimulation of denervated muscle prior to nerve repair provides beneficial effects over a longer period of time with both increased motor unit numbers and higher CMAP values. The data from this study may provide clinicians with the information necessary to start investigating electrical stimulation as supplementary treatment for peripheral nerve injuries.
Acknowledgments
The authors wish to thank Matthew Macdonald and Mary Susan Thompson for their contributions to the histological aspect of this study. We would also like to thank Larissa Schudlo and Christine Gabardo for their help with the animal muscle stimulation.
This work was supported in part by a grants #IMH-87057 and #CPG-99371 from the Canadian Institute of Health Research.
Contributor Information
Michael P. Willand, School of Biomedical Engineering, McMaster University, Hamilton, ON, L8S 4K1, Canada.
Michael Holmes, Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, L8S 4K1, Canada.
James R. Bain, Department of Surgery (Division of Plastic Surgery), McMaster University, Hamilton, ON, L8S 4K1, Canada
Margaret Fahnestock, Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, L8S 4K1, Canada.
Hubert de Bruin, School of Biomedical Engineering, McMaster University, Hamilton, ON, L8S 4K1, Canada.
References
- 1.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15(5 Pt 2):3886–95. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–10. doi: 10.1016/s0306-4522(00)00577-7. 2. [DOI] [PubMed] [Google Scholar]
- 3.Veltri K, Kwiecien JM, Minet W, Fahnestock M, Bain JR. Contribution of the distal nerve sheath to nerve and muscle preservation following denervation and sensory protection. J Reconstr Microsurg. 2005;21:57–70. doi: 10.1055/s-2005-862783. discussion 71–4. 1. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, JKWM The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997 Jul;20(7):858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Elsohemy A, Butler R, Bain JR, Fahnestock M. Sensory protection of rat muscle spindles following peripheral nerve injury and reinnervation. Plast Reconstr Surg. 2009 Dec;124:1860–1868. doi: 10.1097/PRS.0b013e3181bcee47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bain JR, Hason Y, Veltri K, Fahnestock M, Quartly C. Clinical application of sensory protection of denervated muscle. J Neurosurg. 2008;109:955–61. doi: 10.3171/JNS/2008/109/11/0955. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberstein A, Eberstein S. Electrical stimulation of denervated muscle: is it worthwhile? Med Sci Sports Exerc. 1996;28:1463–9. doi: 10.1097/00005768-199612000-00004. 12. [DOI] [PubMed] [Google Scholar]
- 8.Ashley Z, Sutherland H, Russold MF, Lanmuller H, Mayr W, Jarvis JC, Salmons S. Therapeutic stimulation of denervated muscles: the influence of pattern. Muscle Nerve. 2008;38:875–86. doi: 10.1002/mus.21020. 1. [DOI] [PubMed] [Google Scholar]
- 9.Dow DE, Cederna PS, Hassett CA, Kostrominova TY, Faulkner JA, Dennis RG. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve. 2004;30:77–86. doi: 10.1002/mus.20054. 1. [DOI] [PubMed] [Google Scholar]
- 10.Kern H, Salmons S, Mayr W, Rossini K, Carraro U. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve. 2005;31:98–101. doi: 10.1002/mus.20149. 1. [DOI] [PubMed] [Google Scholar]
- 11.Brown MC, Holland RL, Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam SL, Archibald V, Jassar B, Tyreman N, Gordon T. Increased neuromuscular activity reduces sprouting in partially den-ervated muscles. J Neurosci. 2001 Jan;21(2):654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dow DE, Cederna PS, Hassett CA, Dennis RG, Faulkner JA. Electrical stimulation prior to delayed reinnervation does not enhance recovery in muscles of rats. Restor Neurol Neurosci. 2007;25:601–10. 5–6. [PubMed] [Google Scholar]
- 14.Dennis RG, Dow DE, Faulkner JA. An implantable device for stimulation of denervated muscles in rats. Med Eng Phys. 2003;25:239–53. doi: 10.1016/s1350-4533(02)00193-5. 3. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaidis SC, Williams HB. Muscle preservation using an implantable electrical system after nerve injury and repair. Microsurg. 2001;21:241–7. doi: 10.1002/micr.1047. 6. [DOI] [PubMed] [Google Scholar]
- 16.Willand MP, Lopez JP, de Bruin H, Fahnestock M, Holmes M, Bain JR. A new system and paradigm for chronic stimulation of denervated rat muscle. J Med Biol Eng. 2011 Apr;31:2–92. doi: 10.5405/jmbe.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr MM, Best TJ, Mackinnon SE, Evans PJ. Strain differences in autotomy in rats undergoing sciatic nerve transection or repair. Ann Plas Surg. 1992 Jun;28(6):538–544. [PubMed] [Google Scholar]
- 18.Ichihara K, Venkatasubramanian G, Abbas JJ, Jung R. Neuro-muscular electrical stimulation of the hindlimb muscles for movement therapy in a rodent model. J Neurosci Methods. 2009;176:213–24. doi: 10.1016/j.jneumeth.2008.09.015. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvador J, de Bruin H. The use of the wavelet transform in EMG m-wave pattern classification. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2304–2307. doi: 10.1109/IEMBS.2006.259534. [DOI] [PubMed] [Google Scholar]
- 20.Galea V, de Bruin H, Cavasin R, McComas AJ. The numbers and relative sizes of motor units estimated by computer. Muscle Nerve. 1991 Nov;14(11):1123–1130. doi: 10.1002/mus.880141114. [DOI] [PubMed] [Google Scholar]
- 21.Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- 22.Xiong G, Guan Y, Hong Y, Zhang J, Guan H. Motor unit number estimation may be a useful method to evaluate motor function recovery after spinal cord transection in rats. Spinal Cord. 2010 May;48(5):363–366. doi: 10.1038/sc.2009.141. [DOI] [PubMed] [Google Scholar]