Abstract
Many surgical procedures require the placement of an inert or tissue-derived implant deep within the body cavity. While the majority of these implants do not become colonized by bacteria, a small percentage develops a biofilm layer that harbors invasive microorganisms. In orthopaedic surgery, unresolved periprosthetic infections can lead to implant loosening, arthrodeses, amputations and sometimes death. The focus of this review is to describe development of an implant in which an antibiotic tethered to the metal surface is used to prevent bacterial colonization and biofilm formation. Building on well-established chemical syntheses, studies show that antibiotics can be linked to titanium through a self-assembled monolayer of siloxy amines. The stable metal-antibiotic construct resists bacterial colonization and biofilm formation while remaining amenable to osteoblastic cell adhesion and maturation. In an animal model, the antibiotic modified implant resists challenges by bacteria that are commonly present in periprosthetic infections. While the long-term efficacy and stability is still to be established, ongoing studies support the view that this novel type of bioactive surface has a real potential to mitigate or prevent the devastating consequences of orthopaedic infection.
Keywords: Antibiotics, bacteria, Staphylococcal aureus, vancomycin, titanium, implants, orthopedic
I. Introduction
Infection continues to plague all medical disciplines that rely on implantation of a foreign object. In orthopaedics, while a diversity of organisms are present in deep infection, the dominant bacteria are Gram-positive pathogens [1], with Staphylococcus aureus (S. aureus) and the coagulase-negative staphylococci especially prevalent [2–5]. Established treatments rely on controlled release of antibiotics [6–10], or, more recently, the release of silver ions from the implant surface [11–19]. While both treatments have considerable strengths, limitations such as tissue toxicity have prompted a search for alternatives that are antibacterial over the lifetime of the implant. The objective of this review is to examine factors leading to orthopaedic infection and consider the efficacy of immobilized antibiotics for long-term prevention of implant-associated infections.
II. Orthopaedic Infections
The incidence of infection depends on the surgical site and the procedure. Transcutaneous fracture fixation pins have a 2–30% chance of infection [15, 20–22], bone supplementation can be as high as 13%[23–24], spinal infections are in the 2–5% range [25–26], and depending on the center, infection after arthroplasty can be significantly less than 1–2%[27–31]. In cases of devastating trauma despite aggressive antibiotic prophylaxis and delay of hardware placement, infection occurs frequently [32–34].
Pathogen colonization of hardware is enhanced by the host response to implantation. Specifically, the host rapidly coats implanted materials with serum proteins that promote cell recruitment and tissue repair. Unfortunately, these same serum proteins are used by pathogens for adhesion and virulence [35–37]. The problem is further compounded by activation of an inflammatory response as well as the complement system upon presentation of these adsorbed proteins. These events, including attenuated activation of phagocytic cells, blunt the local immune response creating an opportunity for pathogen colonization [38–40]. In addition, the proteins adsorbed onto the implant can promote bacterial adhesion to the implant [5, 41–42]. Another complication is added as the bacteria use the adsorbed serum fibronectin and vitronectin to interact with adjacent cells. In some cases, these interactions lead to cellular internalization which can initiate a chronic infection [43–46] as antimicrobial penetration into infected mammalian cells may be insufficient to eradicate the pathogen [47–50]. Internalization is not limited to professional phagocytic cells—HeLa cervical carcinoma cells [51], aortic endothelial cells, fibroblasts and osteoblasts, among others harbor bacteria, in vitro [52–57]. Finally, the presence of a bacterial biofilm (a community of surface adherent bacteria encased in a polymeric complex) protects microorganisms from antibiotic activity and from immune cell surveillance. The avidity with which these pathogens colonize implants, their recalcitrance to antibiotic treatment in an adherent state, and the possibility that they can persist in the tissue despite implant removal, make prevention rather than treatment of infection of paramount importance.
Since surfaces that tend to be biocompatible often promote bacterial colonization, it raises the question, how can infection be avoided? Clearly, the first defense is rigid adherence to sterile techniques. Clean room environments have been shown to lower orthopaedic infection rates to below 1% [58]. A robust host response is also required. Approximately 20 years ago, Gristina [59], suggested that there was a “race to the surface” between the cells from the host tissue and the invading pathogens. Conditions are optimum for the host when mammalian cells rapidly populate the implant prior to contamination by pathogens. In the process, the adhering mammalian cells remodel the serum proteins adherent to the implant surface and initiate formation of either a fibrous or osseous interface with the implant, to ultimately minimize the inflammation associated with the foreign body reaction to the implant [38, 40]. In concert with tissue formation, immune cell surveillance, although attenuated in the peri-implant space, serves to protect the site from bacterial colonization. Such events would be expected to promote host cell survival and prevent bacterial biofilm formation, thereby providing a level of protection that would be optimum for orthopaedic implants.
The tissue infection paradigm has been elegantly modeled in vitro by Subbiahdoss, et al. [60–62]. These researchers showed that when challenged with bacterial toxins, flow conditions promoted cell survival and allowed examination of the competition between bacteria and cell for surfaces. Using this flow system, pre-incubation of the surface with bacteria supports osteoblast-like cell adhesion, albeit with a more rounded morphology and very limited proliferative span. On the other hand, when cells first populate the surface, the numbers of adherent bacteria decrease as coverage from adherent osteoblast-like cells increase. Overall, this in vitro model suggests that bacterial presence limits osteoblast-like cell population of the implant whereas adhesion of osteoprogenitor cells to the implants limits bacterial adhesion. These studies provide an in vitro competition model for major non-immune events modulating implantation. In this “race for the surface,” the importance of fostering the initial bone ingrowth to counteract bacterial colonization was deemed to be of critical importance. Addition of macrophages to a bacterial-cell system allows an in vitro measure of a simplified integration of the activated macrophage and its control of bacterial colonization, allowing the mammalian cells to have the advantage in the race to the surface.
Clinically, early contamination, especially during the initial implantation period, is assumed to occur peri-operatively through introduction of the pathogen either on the implant or into the surgical site. Under these conditions, microorganisms would be present at the surface during the initial post-operative stages which include clot formation and dissolution prior to stem cell commitment and osteoblast colonization of the implant. This situation may be particularly devastating as the host cell response to implantation includes complement activation, recruitment of phagocytic cells whose activity quickly becomes attenuated in the presence of the large foreign object, and release of inflammatory cytokines [63]. Together, these events increase the probability for successful bacterial propagation. To mimic this situation, Schwarz and his colleagues set up a peri-prosthetic infection model in which the implant was already populated with bacteria at the time of surgery [64]. Infection ensues as the colonizing bacteria further activate (in addition to the foreign body reaction) the immune system to result in tissue degradation, sinus formation, and dissemination of bacteria into the surrounding tissues.
Later infections may arise due to persistence of an indolent infection (seeded in the peri-operative period) or hematogenous seeding of the implant by a low-level bacterial contamination such as transient bacteremias experienced with urinary tract infections.
With revision arthroplasty (replacement of the implant due to aseptic loosening or infection), infection and re-infection are more prevalent. Because even low levels of bacterial colonization can induce implant loosening, some cases of aseptic loosening are now considered to be due to subclinical contamination of the site. Infection rates can be greater than 10% for revision of septic joints [65], even with aggressive antimicrobial treatment. One explanation is that adjacent tissues can harbor bacteria [66–68] so that removal of the implant, debridement of the bone, and aggressive antimicrobial treatment may be inadequate to remove all of the bacterial contamination. In these cases, placement of an implant may become impossible.
III. Implant Colonization
Except in cases of trauma where probable sources of infection are obvious, there still is debate concerning the origin of the contaminating organisms. Implant colonization can occur peri-operatively as detailed above or hematogenously later in the lifetime of the implant. The hematogenous spread may occur when low numbers of bacteria are transiently dispersed throughout the body, such as occurs during urinary tract infections or dental procedures. Under ideal circumstances, these blood-borne bacteria are cleared by the host immune system before they lodge in vulnerable sites. Unfortunately, transient bacteremias of 100 or fewer microorganisms are capable of successfully colonizing an implant surface [40, 68–69]. From this perspective, the limiting factor is time not organism dose.
Importantly, bacterial adherence to the implant initiates metabolic and phenotypic changes that render the adherent pathogen resistant to antibiotics as well as protecting it from immune surveillance [70]. As immune surveillance may already be compromised due to the presence of the implant, protection of the bacteria may be even more effective. With respect to antibiotics, even with antibiotic concentrations that are 20–100X greater than the minimum inhibitory concentration (MIC), some adherent bacteria can still persist on implants, in vitro [71–72]. Fortunately, some antibiotics, such as vancomycin and daptomycin, appear to be somewhat effective against biofilms [73–74].
This apparent antibiotic resistance is initiated upon adhesion to an implant when bacteria change from a rapidly growing, non-adherent planktonic state to a sessile organism. Generally, these adherent bacteria secrete and then become encased in a thick biofilm matrix that serves to further protect them against host assault. Within this biofilm, channels exist for nutrient exchange, and, as in any renewing structure, there is both proliferation and death [41, 75–77]. This latter event results in the biofilm being a rich repository of DNA. Exchange of biofilm-trapped DNA between bacterial species can cause transfer of traits, in particular those responsible for true antibiotic resistance [41, 77].
An important corollary to the presence of a mature biofilm on an implant is the sloughing off of bacteria into the environment. These bacteria can then colonize the surrounding tissue [66–68], adhering to ECM proteins present in the tissue as well as initiating cellular processes that cause pathogen internalization [36, 55, 78–81]. Thus, once implant colonization has occurred, continuing infection is propagated not only through the bacteria disseminated from the biofilm on the implant, but through adherence and colonization of contiguous tissues.
In summary, adhesion of bacteria to a surface and subsequent biofilm formation promotes metabolic, phenotypic, and genotypic changes that make their eradication extremely difficult. Moreover, continuous seeding of surrounding tissues increases the probability that infection will continue. For these reasons, the single most important target for prevention of implant-associated infections is eradication of bacteria before they can populate the implant/tissue environment.
IV. Implant Designs to Minimize Bacterial Colonization
Depending on surgical requirements, orthopaedic implants can be comprised of a single material, as in fracture fixation hardware and spinal hardware (metals or polymers such as polyethylarylketone), multiple materials, such as the combinations of plastics or ceramics and metals used for hip and knee implants (which are often accompanied by cements or morselized bone to enhance implant fit) or complex proteins and minerals as found in allograft bone. It is important to note that all implants are not designed to be osseointegrated. Unfortunately, all implants are prone, to some degree, to bacterial colonization. The propensity for bacterial colonization does, to some extent, depend on the material. However the general rule of thumb is that in accord with the “race for the surface” scenario, i.e., enhanced protein adsorption will ultimately result in increased [osseo]integration and resistance to infection; however during the early stages of this process, these self-same properties predispose the implant to bacterial colonization.
At the material level, the composition of the implant has some impact on bacterial colonization. For instance, in vivo, stainless steel is colonized more readily than titanium, perhaps due to differences in osseointegration [82–83] which may reflect differences in protein adsorption to the surface. Hydrophobicity/hydrophilicity, surface composition, and texturing all impact bone ingrowth and bacterial adhesion [84–86]; of these, the most important is surface hydrophobicity [87–89]. Texturing of surfaces at the micro level through processes such as sand-blasting and at the macro level through introduction of beads, sintering, etc., have improved osseointegration of the neck of joint replacement stems [90–93]. Additionally, application of calcium phosphate, especially sintered hydroxyapatite is common [94–95], with conflicting reports as to its efficacy in stabilizing bone growth around the implant [94, 96–97]. There is some debate whether any of these textured surfaces or hydroxyapatite layers alter the frequency or extent of bacterial colonization [98] [85, 99–101]. However, while hydroxyapatite coatings themselves may or may not be permissive for bacterial colonization, they do serve as ready depots for adsorption of antibiotics for in situ release [102–104]. In one application, adsorption of gentamicin to hydroxyapatite resulted in sufficient antibiotic release to allow short-term prophylaxis [105–106].
At the other end of the size spectrum, nano-texturing is being introduced to foster drug delivery and bone ingrowth. The effect of nanotexture on bacterial colonization appears to depend on size, texture, and spacing [107–109], which will also affect serum protein adsorption and mammalian cell adhesion. An interesting topography has been based on the natural diamond-like texturing of shark skin, which shows a 50–80% decrease in colonization [110]. In vivo experiments will be required to determine if nanotexturing promotes tissue formation and remodeling while decreasing bacterial adhesion and biofilm formation.
V. Treatment Of Surgical Infection
While low, the orthopaedic infection rate reflects the surgical technique, the health and age of the patient, the physical dimensions and state of the surgical site and the implant material [111]. To minimize infection, systematic antibiotics are administered 2 to 14 days post-surgery with additional oral prophylaxis [111–112]. When there is severe trauma, implant placement may be delayed to allow clearance of any contaminants introduced in the wound [113].
When infection is present, it is often necessary to supplement antibiotic therapy with implant removal and elimination of infected bone [114–117]. For arthroplasties, optimum treatment includes removal of the infected component and debridement of surrounding bone to remove additional sources of bacteria that have been detected in the tissue around orthopaedic implants, catheters, and other implants [118–119]. When the two-stage procedure is used, an antibiotic-eluting spacer is implanted, and, after treatment periods of ~6 weeks, assuming a good outcome, a new implant can be inserted into the bone [65, 120–121]. Even with these measures, re-infection rates can be high, which again emphasizes the possibility that contamination from the surrounding tissue can re-seed the implant and cause active infection. For the most difficult cases, arthrodesis or amputation may be the only options [114]. This propensity for re-infection underscores the importance of preventing the initial bacterial colonization of the implant.
Because the higher antibiotic doses associated with the antibiotic-eluting spacers can eradicate most infections, systems have been proposed to deliver antimicrobials at high doses from the implant itself to obviate the need for a two-stage procedure. Porous materials, such as cancellous bone [122], collagen sponges and PMMA [123] have been loaded with antibiotics. In common with the antibiotic-bearing cements, the goal of these elution systems is to keep the implant surface sterile while eradicating bacterial contamination of the surrounding tissue [124–128]. Newer systems have used biodegradable implant coatings to facilitate and control antibiotic release [129–133]. A detailed synopsis of controlled-release systems is outside the scope of this review.
Controlled release systems are powerful therapeutic tools and effectively eradicate bacterial contamination. However, they have a number of shortcomings. Firstly, high local antibiotic concentrations are only achieved over the short term. Specifically, in an allograft system, independent of initial concentrations and time of impregnation, ~75 % of the adsorbed vancomycin and ~99% of netilmicin elutes within 120 hours [134]. Likewise within a six week time period, the concentration of antibiotics from spacers has significantly decreased [111], [135]. While these levels may initially approach supratherapeutic levels, bacteria may still be able to evade the antimicrobial agents by adoption of a metabolic state that allows them to “persist,” by biofilm formation, and by dissemination into the surrounding tissues where antibiotic penetrance will be low. Thus, while controlled release of antibiotics provide a highly effective modality for treatment of acute infection, at late treatment times, when antibiotics levels fall to sub-therapeutic levels, surviving bacteria can slowly re-establish a biofilm which will again serve as a nidus for bacterial dissemination [66–67, 136]. This high dissemination rate is in keeping with the relatively high re-infection rates after revision for an infected component. Secondly, the drop in antibiotic levels exposes the surviving bacteria to sub-inhibitory concentrations of antibiotics. This metabolic pressure increases the risk of fostering development of resistant strains of bacteria [77, 137]. Resistant bacterial sub-populations with extremely high MICs were detected after use of gentamicin beads during two-stage exchange arthroplasty [137], supporting the need for integrated systems which ensure that conditions that foster resistance are not established. Thirdly, high levels of antibiotics can cause local tissue toxicity, compromising bone regrowth, immune system surveillance and implant osseointegration.
Silver-impregnated surfaces have enjoyed success as wound healing dressings and appear to decrease levels of both adherent bacteria and bacteria adjacent to the implant [12–15, 138]. Elution times tend to be longer than conventional controlled release systems giving the surfaces an additional advantage. Questions remain as to implant efficacy, long-term tissue toxicity as well as acquisition of silver-resistance.
In the search for complementary systems, we and others have explored the possibility that antibiotics, and in a few cases antimicrobial peptides, covalently attached to an implant surface, may provide protection long after controlled release of antibiotics has waned. The advantage of such a system is firstly, unlike elution systems, the surface could remain anti-bacterial over the lifetime of the coating. While this coating is present, the implant should display antimicrobials, unlike controlled release systems where the antibiotic-depleted surface becomes susceptible to bacterial colonization. [134, 139–141] [142–143]. The long-term antibacterial coverage would have greatest impact in cases of established infection where re-contamination of the implant from the surrounding tissue is always a possibility.
Secondly, because the antibiotics are tethered, no bulk tissue toxicity would be anticipated. If toxicity were experience, it would be expected to be limited to the sub-micron tissue-implant interface. Thirdly, it is likely that the probability of fostering of antibiotic resistance with use of these attachment systems would be low. Indeed, the amounts of antibiotic or other tethered antimicrobial attached to the surface is very low compared to either the MIC of the agent or to controlled release systems. On metal surfaces, based on previous measurements [144–146] we would expect quantities of antibiotic that are in the nanogram range, a million-fold less than the amounts immobilized in the controlled release systems. At these concentrations, even if catastrophic release were to occur, the amount of antibiotic would be too low to foster resistance. Whether resistance will occur and the frequency of its occurrence is, so far, outside the limits of our test. Our resistance experiments to date have been solely based on metabolic pressure exerted by vancomycin-tethered Ti rods on S. aureus under laboratory conditions [144]. Similarly, the strains isolated from our animal experiments, based on limited testing, remain vancomycin sensitive. Thus, the question about the surface’s effects on antibacterial resistance remains open.
Although there are very obvious advantages for non-eluting systems, they also have their weaknesses. Coatings tend to be fragile—a problem both with controlled release as well as tethered systems. For all systems, the fragility is associated with the significant forces that are often applied to orthopaedic hardware during insertion—forces that are sufficient to cause scraping of the metal which would be certain to score the coating. Bare surfaces then re-acquire the original problem, i.e., that they are prone to bacterial contamination and biofilm formation. Furthermore on the tethered surface, such scoring could cause increased instability of the otherwise stable SAM due to its disruption and subsequent hydrolysis. Again, exposure of the underlying surface would occur, giving rise to the same weakness as the spent elution system.
An additional weakness is linked to the role of tissue-associated bacteria in the propagation of infection. By necessity, a tethered antibiotic is only active in the space immediately adjacent to the implant. Thus, the antibiotic would not be able to interact with those bacteria that are but a few microns distant from the surface. For this reason, the best use of such systems would be in combination treatments that include systemic or locally delivered antibiotics.
Orthopaedic implants are highly engineered to display textures or surfaces that either foster bone ingrowth, or in the case of fracture fixation nails, stability without osseointegration. While we have shown that we can tether antibiotics with retention of topography [175], the addition of the SAM, linkers, and the antibiotic to the surface readily allows osteoblast adhesion and maturation. On the one hand, such enhanced cell adhesion would support the osteoblast’s ability to win the race for the surface; on the other hand, application of these surfaces to components that need to be removed might prove problematic.
VI. Surface Tethering Of Antibiotics
Based on the overall advantages of the surface-tethered implant, we developed a strategy to prevent bacterial colonization of implants with two key objectives: 1. Prevent bacterial adhesion and biofilm formation. 2. Avoid therapies likely to foster pathogen resistance. For this purpose, the most efficacious approach is to create an implant surface that integrates a controlled release system (days to weeks) with a permanent, covalently-tethered antibacterial layer that resists colonization over the long-term (months to years). We reasoned that (1) non-adherent planktonic bacteria can be eradicated by local antibiotic release or by the immune system and (2) that minimizing bacterial adhesion through implant coating would prevent biofilm formation and effectively remove the implant as a reservoir of bacteria. Our research findings support the power of these hybrid antibacterial surfaces to combating bacterial adhesion and colonization [147–148]. In this manuscript, the remainder of the discussion is focused on the value of the antibacterial surface.
Bonding of molecules to surfaces has an extensive literature. The cell adhesion peptide RGD has been immobilized to glass [149], quartz [150–152], gold [153], silicone [154], silica [155] and titanium [156–158]. Different linkages have been used on titanium surfaces including diphosphonic acids [159–160], plasma amination [161], silanization to reveal active amines [145, 156, 162], surface photopolymerization of PEG-acrylamide groups [163], reaction with p-nitrophenyl chloroformate to activate the surface [164] and interestingly, titanium-binding peptides to allow PEGylation [165]. Antimicrobial peptides have also been grafted onto surfaces with successful bacterial killing for the cationic LL-37 on Ti [166], and the pore forming toxin maiganin I on thiol-gold [167]. Covalent modification of titanium surfaces with antibiotics has focused to date predominantly on vancomycin [163, 168–170], with one report on daptomycin [171]. In our studies, we have tethered vancomycin to commercially pure titanium [169]; vancomycin [145, 172] gentamicin, ceftriaxone, kanamycin, tetracycline, and doxycycline (unpublished data) to titanium alloy (Ti90Al6V4); and vancomycin [173] and doxycycline to bone allograft. These surfaces showed antibacterial activity with retention of antibiotic specificity.
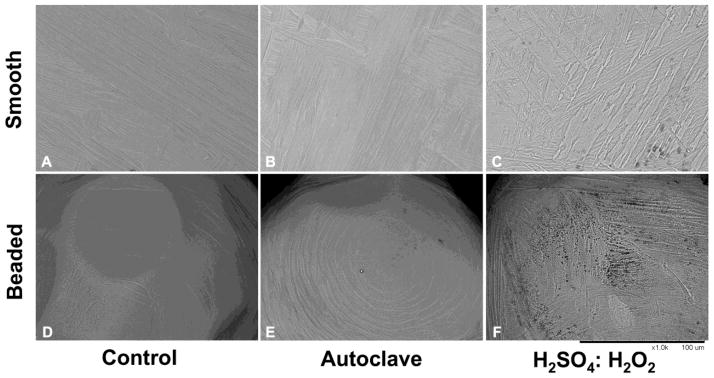
The immobilization strategy that we have used to facilitate chemical coupling of linkers and antibiotics to metal surfaces relies on initial formation of an amine-rich, reactive surface. Our strategy is to generate and maintain the Ti-O linkage which can then be used to form bonds with other agents, in particular aminopropyltriethoxy silane (APTES). APTES forms three siloxy bonds to the titanium oxide; this layer is further stabilized by cross-linking to generate a self-assembled monolayer (SAM) [174]. In this reaction scheme, a fresh oxide layer is formed (i.e., passivation) through use of oxidizing solutions (e.g., chromic acid (H2SO4/HNO3) or piranha acid (H2O2/H2SO4)[161]) that etch the metal or through use of hydrothermal aging [169, 173, 175]. An important consideration is retention of the topography of the highly engineered implant surface. Thus, while piranha acid increased surface pitting, hydrothermal aging appeared to retain surface features (Figure 1 [173]).
Figure 1. SEM imaging reveals preservation of topography.
Microtopographical assessment of smooth and beaded surfaces was performed by scanning electron microscopy (SEM) imaging (1000×). (A) Control titanium alloy surfaces showed normal, shallow machining lines across the surface. (B) Control, commercially pure titanium (cpTi) beaded surfaces showed concentric shallow machining lines. (C) Hydrothermally-aged Ti alloy appeared similar to control surfaces (D) Hydrothermally-aged, beaded cpTi surfaces were also similar to control surfaces. (E) The H2SO4:H2O2 treated Ti alloy surface disc showed obvious etching and deepening of the natural crevices of the metal. Dark areas indicate pitting. (F) Similarly, the etched, beaded cpTi exhibited extensive pitting. (Magnification: 1000X, bar = 100 μm). Figure reproduced from [176] with permission of Springer.
The presence of the SAM that is covalently grafted on the surface of the Ti metal and displays primary amines provides a convenient first step for subsequent tethering reactions. We have confirmed the formation of the amine-bearing SAM (1) through reaction with fluorescamine and detection of fluorescence by confocal laser scanning microscopy [173, 176] and (2) through colorimetric detection of primary amines using the ninhydrin reaction [145, 169].
To enhance the entry of the tethered antibiotic into the bacterium, we sequentially coupled two membrane-soluble Fmoc-[2-(2-amino-ethoxy)-ethoxy]-acetic acid (Fmoc-AEEA) linkers to the APTES surface in the presence of O-(7-azabenzo-triazole-1-yl)-1,1,3,3-tetramethyluronium hexa-fluorophosphate (HATU). After deprotection of the second linker, we coupled the Ti-APTES-(AEEA)2 product with vancomycin in the presence of HATU [169]. The predicted structure would give vancomycin an ~30–40 Å arm for inserting into the bacterium [172].
Vancomycin coverage was visualized through indirect immunofluorescence. Coverage was also estimated through recovery of fluorescent antibodies which suggested that ~29 ng of vancomycin was immobilized on a 1 × 10 mm pin [144–145, 176–177]. Using acid cleavage of the siloxy bonds, the cleaved material was analyzed with MALDI-TOF mass spectroscopy (MALDI-TOF MS). The array of molecular ions detected were consistent with the predicted structure [169, 172], confirming that the desired Ti-APTES-(AEEA)2-vancomycin product was formed.
Vancomycin, the antibiotic used in these studies, interferes with cell wall synthesis in two ways: inhibiting synthesis of the polymeric glycan molecules and blocking polymer cross-linking by reversible binding to the peptidoglycan Lys-D-Ala-D-Ala. The carboxylic acid that is used for tethering of the vancomycin to the implant has been used to make other adducts of vancomycin with retention of activity [178]. The reversibility of the bonding allows the surface to be regenerated after exposure to bacteria. Specifically, because vancomycin has a relatively low affinity for its substrate, bacterial fragments can be released from the surface, even after they have interacted with the vancomycin. We have tested these surfaces in small (rat [148]) and large (sheep (Schaer, Hickok, et al., unpublished data)) animal models. If efficacy were lost due to the presence of either dead bacteria or bacterial fragments on the surface, we would expect a small lag in the establishment of infection, i.e., the animals with coated implants would still establish infection after a lag period. However, we have not observed establishment of a fulminating infection; indeed, these VAN-tethered implants have been surprisingly successful. With respect to other antibiotics, especially those that contain the β-lactam ring, we would expect them to irreversibly bind to the cell wall of the bacterium. While these surfaces would be active initially, we would expect them to loose active antibiotic with repeated exposure, in keeping with out preliminary results (Hickok, et al., unpublished data).
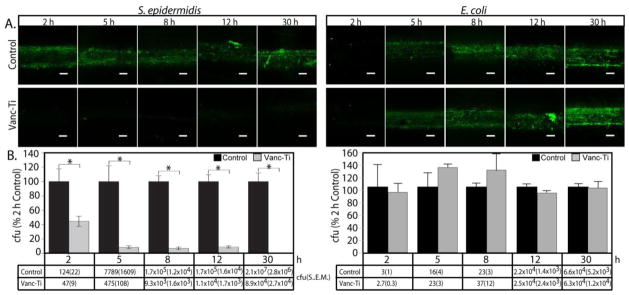
The vancomycin-tethered surface showed a time dependent inhibition of bacterial colonization for the Gram-positive organisms S. aureus [145] and S. epidermidis [177]. The vancomycin surfaces clearly decreased bacterial colonization, but did not eradicate all adherent bacteria. Therefore in studies of vancomycin tethered to bone allograft, we asked the utility of the surfaces against different starting bacterial inocula. At 103–104 cfu bacteria (6 h exposure), the vancomycin-allograft surfaces were most effective, with increased colonization with inocula above 104 cfu [179]. In keeping with the known activity of vancomycin against Gram-positive organisms and as a control, the Gram-negative organism Escherichia coli (E. coli) readily adhered to vancomycin-modified surfaces (Figure 2, [177]). Importantly, the vancomycin-tethered surface could inhibit biofilm formation by S. epidermidis (Figure 3 [177]). While these studies addressed the short-term activity of the surface, they did not assess conservation of biocidal activity for a long time period (months, even year after implant placement). For these studies, vancomycin coverage was assayed in the presence of S. aureus in tryptic soy broth. Both vancomycin coverage and activity appeared to be maintained for dry pins or pins maintained in PBS for times out to 2 years [145, 177]. A similar modification of native allograft bone was stable for at least 10 months by antibody staining and at least two months on the basis of activity determinations [173]. The longevity of polymerized VAN on surfaces has not been published [163, 170, 180], nor is there information on the long-term activity of the antimicrobial peptide surfaces. However, if these surfaces are indeed stable, then they too should be capable of preventing the establishment of bacterial colonization over the long-term—the major advantage over the controlled release systems. Longevity and efficacy will need to be measured in vivo to assess their success.
Figure 2.
Bacterial colonization of vancomycin-tethered Ti (Vanc-Ti) surfaces. A. Control or vancomycin-tethered surfaces were challenged (initial concentration ~1×104 cfu/ml) with S. epidermidis (Gram-positive), which should be sensitive to vancomycin or E. coli (Gram-negative) which does not have vancomycin sensitivity. Colonization of control surfaces were robust, as indicated by green fluorescence, for both S. epidermidis and E. coli. Only E. coli could show any significant colonization of the Vanc-Ti surface, in keeping with the Gram-positive spectrum of activity of vancomycin. B. Numbers of adherent bacteria recovered at each time point for the two surfaces, expressed as a percent of the two hour controls in the histograms and given as colony forming units (cfu) in the tables below the histograms. Values are shown are cfu ± SEM, where * denotes p < 0.5. Magnification: bar = 200 μm. Figure reproduced from [177]with permission of Elsevier.
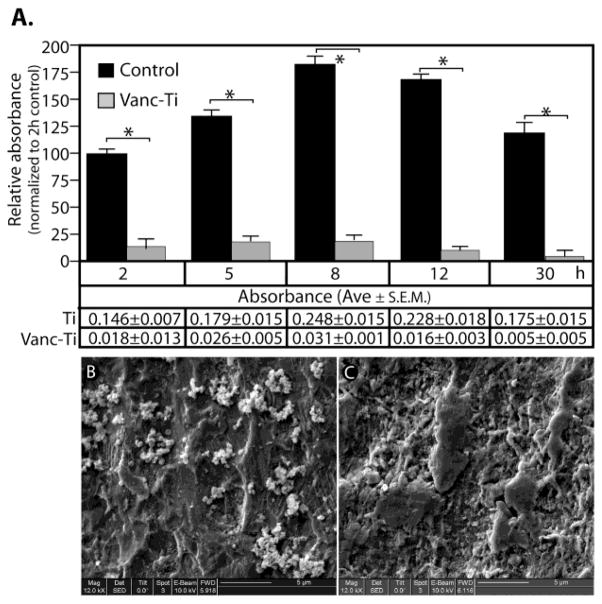
Figure 3.
A. Biofilm formation by S. epidermidis was assessed on control or vancomycin-tethered Ti (Vanc-Ti) surfaces by detection of crystal violet incorporation. Biofilm formation was expressed relative to the absorbance of the 2 h control for both control and Vanc-Ti surfaces, with absorbance values presented in tabular form below the histogram. B, C.. The presence of S. epidermidis on control (B) and Vanc-Ti (C) surfaces was visualized by SEM. The characteristic clusters of grape-like S. epidermidis are apparent on control surfaces whereas on the Vanc-Ti surface, few if any S. epidermidis are visible. Magnification: bar = 5 μm. Figure reproduced from [177]with permission of Elsevier
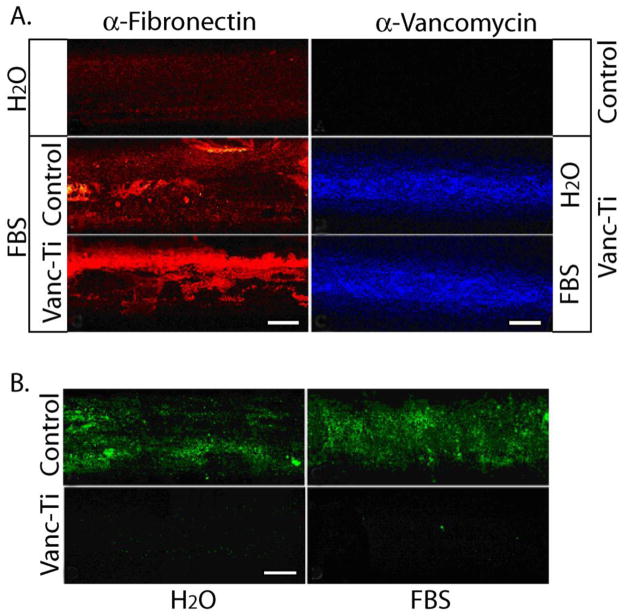
If the surfaces described above are to have clinical utility, they must retain their antibacterial character in a physiological environment and withstand multiple challenges by bacteria. The vancomycin-coupled surfaces were tested for activity in the presence of serum proteins. Firstly, antibody binding and reactivity of the tethered vancomycin was retained in the presence of these proteins [145, 177]. Secondly, the tethered vancomycin continued to inhibit bacterial adhesion, even in the presence of serum proteins (Figure 4 [177]). To determine if these surfaces withstand multiple bacterial challenges, the antibiotic tethered pins were repeatedly challenged for 24 h with S. epidermidis, followed by removal of the adherent bacteria, washing and re-challenge [145]. By the fifth re-challenge, based on Live/Dead staining, some variability appeared not only in colonization of the vancomycin tethered pin, but also in the ability of the bacteria to colonize the control pins probably due to the presence of residual detergent [177]. Overall, however, the tethered vancomycin appeared to endow the implant with the ability to resist bacterial colonization, not only for the first bacterial assault, but for several subsequent challenges.
Figure 4. Antimicrobial activity of the tethered vancomycin (Vanc-Ti) surface in the presence of serum.
(A) Fibronectin adsorption and vancomycin fluorescence. (Left) Control or Vanc-Ti rods were incubated with FBS for 24 h and fibronectin adsorption detected by immunofluorescence analysis. Both surfaces showed abundant fibronectin (red stain), compared to the rods incubated in H2O. (Right) Following incubation with serum proteins, rods were incubated with an antibody against vancomycin and visualized by immunofluorescence. Despite protein adsorption, vancomycin fluorescence (blue stain) was clearly detectable on the Vanc-Ti rods, with no staining on control surfaces. (B) Antimicrobial activity. Vanc-Ti rods that had been treated with serum proteins were challenged with S. epidermidus (initial concentration=~1 × 104 cfu/ml) for 24 h and live, adherent bacteria stained with the Live/Dead kit (green). Fluorescence detected from the serum-treated Vanc-Ti rods was very low and similar to the fluorescence detected from the H2O-incubated Vanc-Ti rods. In both cases, this fluorescence was much less intense than the fluorescent yield generated by bacteria that had colonized the control rods. Magnification: bar = 200 μm. reproduced from [177]with permission of Elsevier.
Importantly, recovery of adherent bacteria from challenges such as those described above and from challenges maintained for several months, indicated that there was no evidence of acquisition or fostering of resistance [144]. Of course, selection for resistance has only been measured under conditions of metabolic pressure. As the bulk of the bacteria (i.e., those not in contact with the surface) will not be experiencing a metabolic pressure, an incidental resistant organism, which is typically slow-growing [181–182], will not have a selective growth advantage and is likely to lose out in the struggle for nutrients. In addition to metabolic pressure, resistance arises in mixed populations of bacteria, especially in biofilms, where transfer of plasmid elements can cause transfer of traits [181–182]. Importantly, as biofilm formation appears to be retarded on these surfaces, the ability to horizontally transfer plasmids is limited, which would minimize the possibility of resistance. Finally, acquisition of antibiotic resistance is rare. Thus, on an implant where the numbers of surface bacteria are limited, the probability of development of resistance is small. While all of these predictions suggest that resistance due to surface-tethered antibiotics will be infrequent, only the simple conditions have been tested and further more detailed studies need to be performed.
VII. Effects Of Tethered Vancomycin on Osteoblast-like Cells
While the tethered antibiotic surfaces are promising with respect to inhibiting bacterial colonization, it is important to characterize them with respect to biocompatibility. As a first measure of this complex phenomenon, which should ultimately address its effects on the immune system as well as the surrounding bone tissue, we have focused on effects of the tethered vancomycin-Ti surface on osteoblast-like cells. As discussed earlier, antibiotics can block osseointegration by down-regulating pre-osteoblast recruitment, proliferation, differentiation, and maturation. Indeed, when osteoblast-like cells are cultured on either control or tethered vancomycin surfaces, we have found that there is little change in morphology, size and density of adherent cells (Figure 5 [144]). In other studies, we have found no significant differences in number, viability, or levels of maturation markers associated with maintaining osteoblasts on vancomycin-modified allograft surfaces [173, 179]. Based on these studies, we would predict that in vivo the surfaces would preserve cell commitment, differentiation and proliferative status while maintaining the osseointegrative properties of the modified implant. Fortunately, as the antibiotic surfaces limit bacterial adherence, biofilm formation and the deleterious effects of colonization in the “race for the surface” would be minimized.
Figure 5.
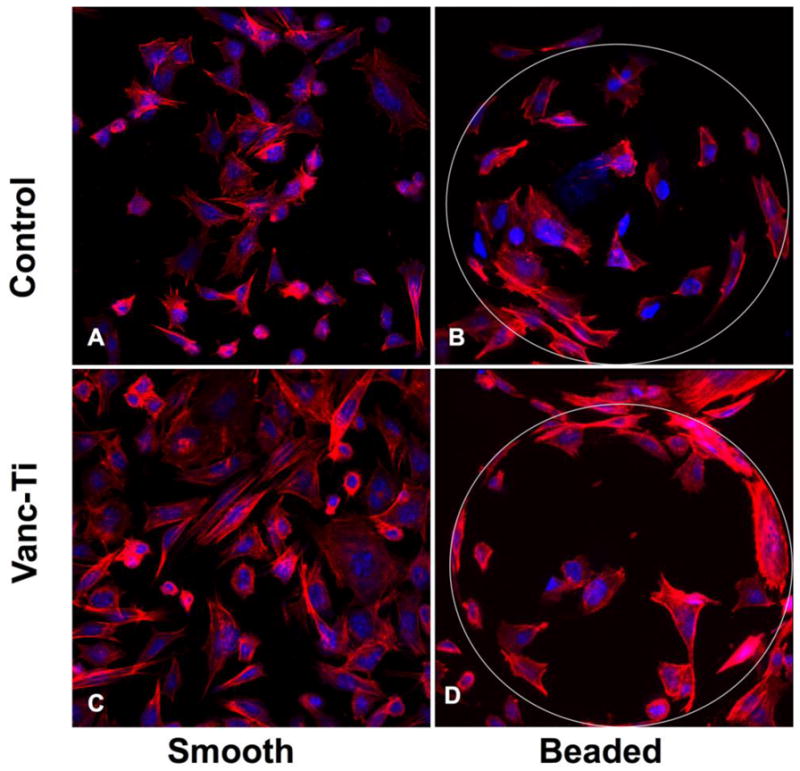
Vancomycin-tethered titanium surfaces support normal cellular morphology. Preosteocyte-like MLO-A5 cells (initial inoculum: ~30,000 cells) were assessed for morphology and cytoskeletal architecture on the different surfaces. (A) MLO-A5 cells readily adhered to smooth, control surfaces, with a number of cells exhibiting an array of actin stress fibers; other cells showed cellular extensions that are characteristic of cells undergoing shape or size changes. (B) MLO-A5 cells readily colonized the VAN-hTi surface, with cell shapes ranging from small to more trapezoidal shapes. Actin stress fibers were apparent throughout, as were cells bearing microspikes, presumably a sign of cell spreading. (C) Cells on control, beaded surfaces appeared well-spread, with abundant actin staining. Some stress fibers were apparent as were short actin bundles. Morphology was within that normally observed for the MLO-A5 cells. (D) Cells seeded on beaded surfaces VAN-hTi surfaces showed abundant cellular colonization, with a normal actin cytoskeletal network. Cell shape appeared normal. (Stain: Actin cytoskeleton: Alexafluor 488 conjugated phalloidin – Red; Nuclei: Propidium Iodide - Blue; Original Magnification: 40X). figure reproduced from [176] with permission of Springer.
We have performed preliminary testing of vancomycin-modified titanium alloy (VAN-Ti) rods in our rodent model of osteomyelitis [148]. After three weeks in uninfected femora, the difficulty of removal of VAN-Ti rods suggested bone ongrowth and osseointegration. As assessed by microCT analysis, when control titanium alloy rods were present in infected femora, bone lysis was apparent; in those femora that contained VAN-Ti rods, bone structure remained close to normal (Figure 6 [161]). This preliminary study thus supports the contention that removal of the implant as a nidus of infection allows the rat’s robust immune system to clear the infection.
Figure 6.
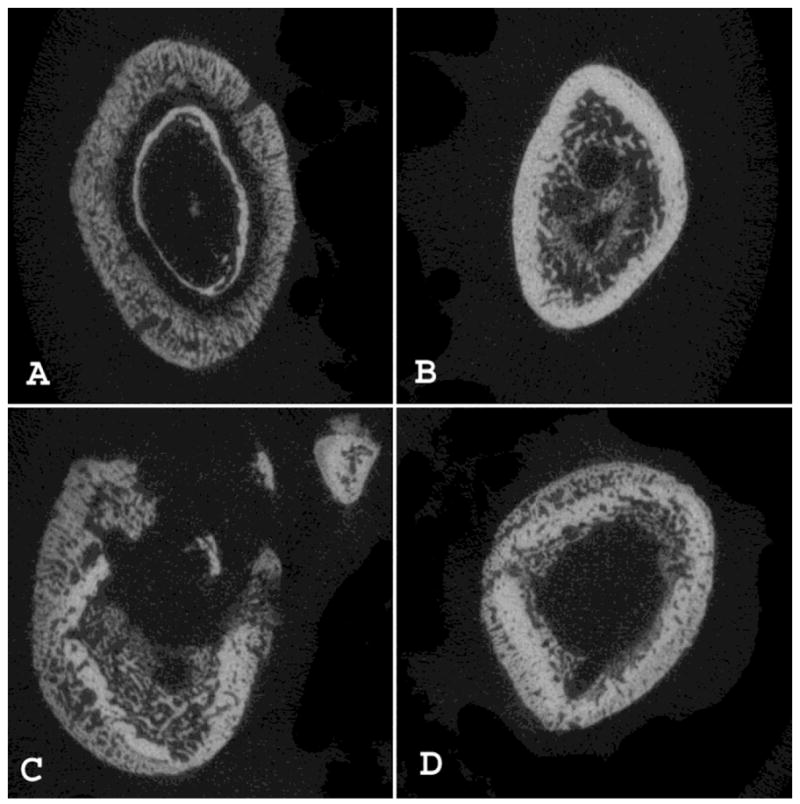
MicroCT cross-sectional analysis of the control and vancomycin-modified titanium rods based on animals sacrificed at Day 14. (A) Cortical mid-shaft cross-sections from the control infected side are compared to (B) sections from the side receiving the modified rod, where severe changes in bone anatomy are observed. (C) In the control femur of another animal, aggressive remodeling with cortical bone penetration and destruction, abundance of cysts, severe periosteal reaction and reorganization, and enlargement of the entire femoral bone are readily apparent in distal diaphyseal cross-sections when compared to (D) sections from the side receiving a vancomycin-modified titanium rod. Figure reproduced from [148]with permission of Wolters Kluwer Health.
Thus, these antibiotic-coupled surfaces, whether constrained to classical antibiotics or embracing new antimicrobials and antimicrobial peptides, appear to overcome many of the problems associated with controlled release systems, i.e., finite lifespan, tissue toxicity, and release of sub-therapeutic levels of antibiotics. Our in vitro and in vivo studies suggest that these antimicrobial-tethered implants decrease bacterial colonization and biofilm formation [148]. Based on the results of these studies and available knowledge concerning organism adherence and biofilm organization, we are of the opinion that this antimicrobial implant surface is ready for pre-clinical and clinical evaluation. Finally, the organometallic chemistry that has been employed to generate the hybrid surface can be adapted to couple a host of other agents to solid surfaces. These surface-bound agents can be tailored to maintain an implant that resists bacterial colonization. Importantly, these surfaces require long-term testing in animal and clinical models to determine if their activity is stable and to test if tissue healing and resistance to infection leads to decreased pain, suffering, and disability due to orthopaedic infection.
Acknowledgments
The authors thank Christopher S. Adams, Valentin Antoci, Jr., Theresa A. Freeman, and Javad Parvizi, along with countless other colleagues who have been instrumental in furthering this work. The authors thank the NIH (grants DE-13319, DE-10875, AR-051303, DE-019901, and HD-061053) and the Department of Defense (grant DAMD17-03-1-0713) for research support. Results presented are not the statement or policy of the funding agencies.
ABBREVIATIONS
- APTES
aminopropyltriethoxysilane
- Cfu
colony forming units, referring to bacterial numbers
- cpTi
commercially pure titanium
- ECM
extracellular matrix
- Fmoc-AEEA
Fmoc-[2-(2-amino-ethoxy)-ethoxy]-acetic acid
- HATU
O-(7-azabenzo-triazole-1-yl)-1,1,3,3-tetramethyluronium hexa- fluorophosphate
- MALDI-TOF MS
Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectroscopy
- MIC
Minimum Inhibitory Concentration
- PBS
phosphate buffered saline
- SAM
self-assembled monolayer
- SEM
scanning electron microscopy
- TSB
BHL Tryptic Soy Broth
- VAN-Ti
Ti alloy displaying covalently tethered vancomycin
Footnotes
Conflict of Interest: Noreen J. Hickok is a founder of SmartTech, Inc., a commercial entity that holds the license for this technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Rel Res. 2001:15–23. [PubMed] [Google Scholar]
- 3.Sanderson PJ. Infection in orthopaedic implants. J Hosp Inf. 1991;18:367–75. doi: 10.1016/0195-6701(91)90043-8. [DOI] [PubMed] [Google Scholar]
- 4.Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Inf Dis. 2003;36:1284–9. doi: 10.1086/374842. [DOI] [PubMed] [Google Scholar]
- 5.Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006;37:S3–S14. doi: 10.1016/j.injury.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Anwar H, Dasgupta MK, Costerton JW. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990;34:2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect. 1995;44:305–313. [PubMed] [Google Scholar]
- 8.Fish DN, Hoffman HM, Danziger LH. Antibiotic-impregnated cement use in U.S. hospitals. Am J Hosp Pharm. 1992;49:2469–2474. [PubMed] [Google Scholar]
- 9.Garvin KL. Two-stage reimplantation of the infected hip. Sem Arthrop. 1994;5:142–146. [PubMed] [Google Scholar]
- 10.Steinbrink K. The case for revision arthroplasty using antibiotic-loaded acrylic cement. Clin Orthop Rel Res. 1990:19–22. [PubMed] [Google Scholar]
- 11.Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32:5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Furkert FH, Sorensen JH, Arnoldi J, Robioneck B, Steckel H. Antimicrobial efficacy of surface-coated external fixation pins. Curr Microbiol. 2011;62:1743–1751. doi: 10.1007/s00284-011-9923-3. [DOI] [PubMed] [Google Scholar]
- 13.Juan L, Zhimin Z, Anchun M, Lei L, Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomedicine. 2010;5:261–267. doi: 10.2147/ijn.s8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 15.Masse A, Bruno A, Bosetti M, Biasibetti A, Cannas M, Gallinaro P. Prevention of pin track infection in external fixation with silver coated pins: clinical and microbiological results. J Biomed Mater Res. 2000;53:600–604. doi: 10.1002/1097-4636(200009)53:5<600::aid-jbm21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exper Cell Res. 1997;235:305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 17.Valappil SP, Knowles JC, Wilson M. Effect of silver-doped phosphate-based glasses on bacterial biofilm growth. Appl Environ Microbiol. 2008;74:5228–5230. doi: 10.1128/AEM.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassler J, Barnett J. A rehabilitation hospital’s experience with ionic silver Foley catheters. Urol Nurs. 2008;28:97–99. [PubMed] [Google Scholar]
- 19.Coughlan A, Boyd D, Douglas CW, Towler MR. Antibacterial coatings for medical devices based on glass polyalkenoate cement chemistry. J Mater Sci Mater Med. 2008;19:3555–3560. doi: 10.1007/s10856-008-3519-x. [DOI] [PubMed] [Google Scholar]
- 20.De Bastiani G, Aldegheri R, Renzi Brivio L. Dynamic axial fixation. A rational alternative for the external fixation of fractures. Int Orthop. 1986;10:95–99. [PubMed] [Google Scholar]
- 21.Thakur AJ, Patankar J. Open tibial fractures. Treatment by uniplanar external fixation and early bone grafting. J Bone Joint Surg Br. 1991;73:448–451. doi: 10.1302/0301-620X.73B3.1670447. [DOI] [PubMed] [Google Scholar]
- 22.Schroder HA, Christoffersen H, Sorensen TS, Lindequist S. Fractures of the shaft of the tibia treated with Hoffmann external fixation. Arch Orthop Trauma Surg. 1986;105:28–30. doi: 10.1007/BF00625656. [DOI] [PubMed] [Google Scholar]
- 23.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 24.C. Centers for Disease and Prevention. Update: allograft-associated bacterial infections--United States, 2002. MMWR Morbid Mortal Weekly Report. 2002;51:207–210. [PubMed] [Google Scholar]
- 25.Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P, Berendt T, Fairbank J. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445–450. doi: 10.1007/s00586-007-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein MA, McCabe JP, Cammisa FP., Jr Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422–426. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Cierny G, 3rd, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;403:23–28. [PubMed] [Google Scholar]
- 28.Duggan JM, Georgiadis GM, Kleshinski JF. Management of prosthetic joint infections. Infect Med. 2001;18:534–541. [Google Scholar]
- 29.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis MJ. Periprosthetic Total Hip Infection: Outcomes using a staging system. Clin Orthop Rel Res. 2002;403:8–15. [PubMed] [Google Scholar]
- 30.Nair SP, Williams RJ, Henderson B. Advances in our understanding of the bone and joint pathology caused by Staphylococcus aureus infection. Rheumatology. 2000;39:821–834. doi: 10.1093/rheumatology/39.8.821. [DOI] [PubMed] [Google Scholar]
- 31.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. An analysis of prosthetic joint infections in their database of 9245 patients. Increased risk factors include morbid obesity, urinary tract infections, among others. most common organisms are Staphylococcus aureus and Staphylococcus epidermidus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows SE, Zuckerman JD, Koval KJ. Posttraumatic tibial osteomyelitis: diagnosis, classification, and treatment. Bull Hosp Joint Dis. 1993;52:11–16. [PubMed] [Google Scholar]
- 33.Mody RM, Zapor M, Hartzell JD, Robben PM, Waterman P, Wood-Morris R, Trotta R, Andersen RC, Wortmann G. Infectious complications of damage control orthopedics in war trauma. J Trauma. 2009;67:758–761. doi: 10.1097/TA.0b013e3181af6aa6. [DOI] [PubMed] [Google Scholar]
- 34.Moriarty TF, Schlegel U, Perren S, Richards RG. Infection in fracture fixation: can we influence infection rates through implant design? J Mater Sci Mater Med. 2010;21:1031–1035. doi: 10.1007/s10856-009-3907-x. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, Nair SP. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun. 2001;69:2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauck CR, Ohlsen K. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr Opin Microbiol. 2006;9:5–11. doi: 10.1016/j.mib.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Piroth L, Que YA, Widmer E, Panchaud A, Piu S, Entenza JM, Moreillon P. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect Immun. 2008;76:3824–3831. doi: 10.1128/IAI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins D, Basaraba R, Hohnbaum A, Lee E, Grainger D, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009;175:161–170. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holt D, Chamberlain L, Grainger D. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–9394. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerli W, Sendi P. Pathogenesis of implant-associated infection: the role of the host. Semin Immunopathol. 2011;33:295–306. doi: 10.1007/s00281-011-0275-7. A review article that concentrates on the complex systems that are set into motion upon implantation of the foreign body, with the role of the immune system and the inflammatory response particularly well delineated. [DOI] [PubMed] [Google Scholar]
- 41.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28:1062–1068. doi: 10.1177/039139880502801103. A review article that takes the reader through the development of the biofilm and delineates the important signaling events that occur, with special reference to medical implants, during its development and maturation. [DOI] [PubMed] [Google Scholar]
- 42.Delmi M, Vaudaux P, Lew DP, Vasey H. Role of fibronectin in staphylococcal adhesion to metallic surfaces used as models of orthopaedic devices. J Orthop Res. 1994;12:432–438. doi: 10.1002/jor.1100120316. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt JE, Poston SM, Noble WC. Adherence of Staphylococcus aureus to cell monolayers. J Appl Bacteriol. 1990;69:834–844. doi: 10.1111/j.1365-2672.1990.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 44.Pizarro-Cerda J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Isberg RR, Hamburger Z, Dersch P. Signaling and invasin-promoted uptake via integrin receptors. Microb Infect. 2000;2:793–801. doi: 10.1016/s1286-4579(00)90364-2. [DOI] [PubMed] [Google Scholar]
- 46.Hauck CR. Cell adhesion receptors - signaling capacity and exploitation by bacterial pathogens. Medical Microbiol Immunol. 2002;191:55–62. doi: 10.1007/s00430-002-0119-0. [DOI] [PubMed] [Google Scholar]
- 47.Ellington JK, Harris M, Hudson MC, Vishin S, Webb LX, Sherertz R. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res. 2006;24:87–93. doi: 10.1002/jor.20003. [DOI] [PubMed] [Google Scholar]
- 48.Sinha B, Herrmann M. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb Haemost. 2005;94:266–277. doi: 10.1160/TH05-04-0235. [DOI] [PubMed] [Google Scholar]
- 49.Baltch AL, Bopp LH, Smith RP, Michelsen PB, Ritz WJ. Antibacterial activities of gemifloxacin, levofloxacin, gatifloxacin, moxifloxacin and erythromycin against intracellular Legionella pneumophila and Legionella micdadei in human monocytes. J Antimicrob Chemother. 2005;56:104–109. doi: 10.1093/jac/dki186. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan EL, Chhatwal GS, Rohde M. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin Infect Dis. 2006;43:1398–1406. doi: 10.1086/508773. [see comment] [DOI] [PubMed] [Google Scholar]
- 51.Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 52.Alexander EH, Hudson MC. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl Microbiol Biotechnol. 2001;56:361–366. doi: 10.1007/s002530100703. [DOI] [PubMed] [Google Scholar]
- 53.Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, Hudson MC. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 54.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br. 2003;85:918–921. An original article that examines the in vitro internalization of Staphylococcus aureus, with the conclusion that the bacteria can persist intracellularly and escape the dying osteoblast to infect neighboring cells. [PubMed] [Google Scholar]
- 55.Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 56.Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Krut O, Sommer H, Kronke M. Antibiotic-induced persistence of cytotoxic Staphylococcus aureus in non-phagocytic cells. J Antimicrob Chemother. 2004;53:167–173. doi: 10.1093/jac/dkh076. [DOI] [PubMed] [Google Scholar]
- 58.Gosden PE, MacGowan AP, Bannister GC. Importance of air quality and related factors in the prevention of infection in orthopaedic implant surgery. J Hosp Infect. 1998;39:173–180. doi: 10.1016/s0195-6701(98)90255-9. [DOI] [PubMed] [Google Scholar]
- 59.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 60.Subbiahdoss G, Kuijer R, Grijpma D, van der Mei H, Busscher H. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomaterialia. 2009;5:1399–1404. doi: 10.1016/j.actbio.2008.12.011. The first of several original articles that explores the "race for the surface" using a flow system. Throughout this series, Subbiahdoss et al explore how this competition fares on different materials and the effect of macrophages on the competition between microbes and cells. [DOI] [PubMed] [Google Scholar]
- 61.Subbiahdoss G, Grijpma D, van der Mei H, Busscher H, Kuijer R. Microbial biofilm growth versus tissue integration on biomaterials with different wettabilities and a polymer-brush coating. J Biomed Mater Res A. 2010;94A:533–538. doi: 10.1002/jbm.a.32731. [DOI] [PubMed] [Google Scholar]
- 62.Subbiahdoss G, Kiujer R, Busscher H, van der Mei H. Mammalian cell growth versus biofilm formation on biomaterial surfaces in an in vitro post-operative contamination model. Microbiology. 2010;156:3073–3078. doi: 10.1099/mic.0.040378-0. [DOI] [PubMed] [Google Scholar]
- 63.Franz S, Rammelt S, Scharnweber D, Simon J. Immune responses to implants-a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Gromov K, Soballe K, Puzas JE, O’Keefe RJ, Awad H, Drissi H, Schwarz EM. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008;26:96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fink B, Grossmann A, Fuerst M, Schafer P, Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467:1848–1858. doi: 10.1007/s11999-008-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broekhuizen CA, Schultz MJ, van der Wal AC, Boszhard L, deBoer L, Vandenbroucke-Grauls CM, Zaat SA. Tissue around catheters is a niche for bacteria associated with medical device infection. Crit Care Med. 2008;36:2395–2402. doi: 10.1097/CCM.0b013e3181818268. These original articles show that the tissue surrounding an implant does in fact harbor pathogens, in agreement with the many studies that suggest that cellular localization of bacteria can be a cause of indolent infection. [DOI] [PubMed] [Google Scholar]
- 67.Broekhuizen CA, Sta M, Vandenbroucke-Grauls CM, Zaat SA. Microscopic detection of viable Staphylococcus epidermidis in peri-implant tissue in experimental biomaterial-associated infection, identified by bromodeoxyuridine incorporation. Infect Immun. 2010;78:954–962. doi: 10.1128/IAI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaat S, Broekhuizen C, Riool M. Host tissue as a niche for biomaterial-associated infection. Future Microbiol. 2010;5:1149–1151. doi: 10.2217/fmb.10.89. [DOI] [PubMed] [Google Scholar]
- 69.Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man: A study of the problems of wound infection. Br J Exp Pathol. 1957;38:573–586. [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Topics Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. A review which delineates differences between antimicrobial resistance vs. tolerance and the role of biofilms and persistor cells in the propagation of the bacterial colony in the host environment. [DOI] [PubMed] [Google Scholar]
- 71.Aslam S. Effect of antibacterials on biofilms. Am J Infect Control Am J Infect Control. 2008;36:S175.e9–11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 73.Stewart PS, Davison WM, Steenbergen JN. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 2009;53:3505–3507. doi: 10.1128/AAC.01728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith K, Perez A, Ramage G, Gemmell CG, Lang S. Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int J Antimicrob Agents. 2009;33:374–378. doi: 10.1016/j.ijantimicag.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 75.Jain A, Gupta Y, Agrawal R, Khare P, Jain SK. Biofilms--a microbial life perspective: a critical review. Crit Rev Ther Drug Carrier Syst. 2007;24:393–443. doi: 10.1615/critrevtherdrugcarriersyst.v24.i5.10. [DOI] [PubMed] [Google Scholar]
- 76.Palmer J, Flint S, Brooks J. Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- 77.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005;437:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Bonazzi M, Cossart P. Bacterial entry into cells: a role for the endocytic machinery. FEBS Lett. 2006;580:2962–2967. doi: 10.1016/j.febslet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Courtney HS, Hasty DL, Dale JB. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med. 2002;34:77–87. doi: 10.1080/07853890252953464. [DOI] [PubMed] [Google Scholar]
- 80.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 81.Khalil H, Williams RJ, Stenbeck G, Henderson B, Meghji S, Nair SP. Invasion of bone cells by Staphylococcus epidermidis. Microb Infecti. 2007;9:460–465. doi: 10.1016/j.micinf.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Melcher GA, Claudi B, Schlegel U, Perren SM, Printzen G, Munzinger J. Influence of type of medullary nail on the development of local infection. An experimental study of solid and slotted nails in rabbits. J Bone Joint Surg Br. 1994;76:955–959. [PubMed] [Google Scholar]
- 83.Arens S, Schlegel U, Printzen G, Ziegler WJ, Perren SM, Hansis M. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647–651. [PubMed] [Google Scholar]
- 84.Yoshinari M, Oda Y, Kato T, Okuda K, Hirayama A. Influence of surface modification to titanium on oral bacterial adhesion in vitro. J Biomed Mat Res. 2000;52:388–394. doi: 10.1002/1097-4636(200011)52:2<388::aid-jbm20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 85.Duarte PM, Reis AF, DeFreitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009;80:1824–1832. doi: 10.1902/jop.2009.090273. [DOI] [PubMed] [Google Scholar]
- 86.Harris LG, Richards RG. Staphylococcus aureus adhesion to different treated titanium surfaces. J Mat Sci Mat Med. 2004;15:311–314. doi: 10.1023/b:jmsm.0000021093.84680.bb. [DOI] [PubMed] [Google Scholar]
- 87.Kouidhi B, Zmantar T, Hentati H, Bakhrouf A. Cell surface hydrophobicity, biofilm formation, adhesives, properties and molecular detection of adhesins genes in Staphylococcus aureus associated to dental caries. Microb Pathog. 2010;49:14–22. doi: 10.1016/j.micpath.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Boks NP, Norde W, van der Mei HC, Bussher HJ. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology. 2008;154:3122–3133. doi: 10.1099/mic.0.2008/018622-0. [DOI] [PubMed] [Google Scholar]
- 89.Boks NP, Kaper HJ, Norde W, Van der Mei HC, Busscher HJ. Mobile and immobile adhesion of staphylococcal strains to hydrophilic and hydrophobic surfaes. J Colloid Interface Sci. 2009;331:60–64. doi: 10.1016/j.jcis.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 90.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mat Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 91.Galante JO, Jacobs J. Clinical performances of ingrowth surfaces. Clin Orthop Relat Res. 1992;276:41–49. [PubMed] [Google Scholar]
- 92.Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19:2219–2232. doi: 10.1016/s0142-9612(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 93.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 94.Ducheyne P, Hench LL, Kagan A, 2nd, Martens M, Bursens A, Mulier JC. Effect of hydroxyapatite impregnation on skeletal bonding of porous coated implants. J Biomed Mat Res. 1980;14:225–237. doi: 10.1002/jbm.820140305. [DOI] [PubMed] [Google Scholar]
- 95.Ducheyne P, Beight J, Cuckler J, Evans B, Radin S. Effect of calcium phosphate coating characteristics on early post-operative bone tissue ingrowth. Biomaterials. 1990;11:531–540. doi: 10.1016/0142-9612(90)90073-y. [DOI] [PubMed] [Google Scholar]
- 96.Block MS, Finger IM, Fontenot MG, Kent JN. Loaded hydroxylapatite-coated and grit-blasted titanium implants in dogs. Int J Oral Maxillofac Implants. 1989;4:219–225. [PubMed] [Google Scholar]
- 97.Bloebaum RD, Beeks D, Dorr LD, Savory CG, DuPont JA, Hofmann AA. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop Relat Res. 1994;298:19–26. [PubMed] [Google Scholar]
- 98.Raulio M, Jarn M, Ahola J, Peltonen J, Rosenholm JB, Tervakangas S, Kolehmainen J, Ruokolainen T, Narko P, Salkinoja-Salonen M. Microbe repelling coated stainless steel analysed by field emission scanning electron microscopy and physicochemical methods. J Ind Microb Biotechnol. 2008;35:751–760. doi: 10.1007/s10295-008-0343-8. [DOI] [PubMed] [Google Scholar]
- 99.Riedewald F. Bacterial adhesion to surfaces: the influence of surface roughness. PDA J Pharm Sci Tech. 2006;60:164–171. [PubMed] [Google Scholar]
- 100.Oga M, Arizono T, Sugioka Y. Bacterial adherence to bioinert and bioactive materials studied in vitro. Acta Orthop Scand. 1993;64:273–276. doi: 10.3109/17453679308993623. [DOI] [PubMed] [Google Scholar]
- 101.Arciola CR, Montanaro L, Moroni A, Giordano M, Pizzoferrato A, Donati ME. Hydroxyapatite-coated orthopaedic screws as infection resistant materials: in vitro study. Biomaterials. 1999;20:323–327. doi: 10.1016/s0142-9612(98)00168-9. [DOI] [PubMed] [Google Scholar]
- 102.Buranapanitkit B, Srinilta V, Ingviga N, Oungbho K, Geater A, Ovatlarnporn C. The efficacy of a hydroxyapatite composite as a biodegradable antibiotic delivery system. Clin Orthop Relat Res. 2004;424:244–252. doi: 10.1097/01.blo.0000130268.27024.c1. [DOI] [PubMed] [Google Scholar]
- 103.Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26:2677–2684. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 104.Shirtliff ME, Calhoun JH, Mader JT. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clin Orthop Relat Res. 2002;401:239–247. doi: 10.1097/00003086-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 105.Baldwin CL, Goenka R. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol. 2006;26:407–442. doi: 10.1615/critrevimmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 106.Anderson JA, Sculco PK, Heitkemper S, Mayman DJ, Bostrom MP, Sculco TP. An articulating spacer to treat and mobilize patients with infected total knee arthroplasty. J Arthroplasty. 2009;24:631–635. doi: 10.1016/j.arth.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31:706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 108.Ivanova EP, Truong VK, Wang JY, Berndt CC, Jones RT, Yusuf, Peake I, Schmidt HW, Fluke C, Barnes D, Crawford RJ. Impact of nanoscale roughness of titanium thin film surfaces on bacterial retention. Langmuir. 2010;26:1973–1982. doi: 10.1021/la902623c. [DOI] [PubMed] [Google Scholar]
- 109.Truong VK, Lapovok R, Estrin YS, Rundell S, Wang JY, Fluke CJ, Crawford RJ, Ivanova EP. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials. 2010;31:3674–3683. doi: 10.1016/j.biomaterials.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 110.Chung KK, Schumacher JF, Sampson EM, Burne RA, Antonelli PJ, Brennan AB. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases. 2007;2:89–94. doi: 10.1116/1.2751405. [DOI] [PubMed] [Google Scholar]
- 111.Lord C, Gebhardt M, Tomford W, Mankin H. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. [PubMed] [Google Scholar]
- 112.Mankin HJ, Doppelt S, Tomford WW. Clinical experience with allograft implantation. The first ten years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 113.Buttaro MA, Morandi A, Rivello HG, Piccaluga F. Histology of vancomycin-supplemented impacted bone allografts in revision total hip arthroplasty. J Bone Joint Surg Br. 2005;87:1684–1687. doi: 10.1302/0301-620X.87B12.16781. [DOI] [PubMed] [Google Scholar]
- 114.Hilpert K, Elliott M, Jenssen H, Kindrachuk J, Fjell CD, Korner J, Winkler DF, Weaver LL, Henklein P, Ulrich AS, Chiang SH, Farmer SW, Pante N, Volkmer R, Hancock RE. Screening and characterization of surface-tethered cationic peptides for antimicrobial activity. Chem Biol. 2009;16:58–69. doi: 10.1016/j.chembiol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 115.Spivak JM, Petrizzo AM. Revision of a lumbar disc arthroplasty following late infection. Eur Spine J. 2010;19:677–681. doi: 10.1007/s00586-009-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sierra-Hoffman M, Jinadatha C, Carpenter JL, Rahm M. Postoperative instrumented spine infections: a retrospective review. South Med J. 2010;103:25–30. doi: 10.1097/SMJ.0b013e3181c4e00b. [DOI] [PubMed] [Google Scholar]
- 117.Kim JI, Suh KT, Kim SJ, Lee JS. Implant removal for the management of infection after instrumented spinal fusion. J Spinal Disord Tech. 2010;23:258–265. doi: 10.1097/BSD.0b013e3181a9452c. [DOI] [PubMed] [Google Scholar]
- 118.Betsch BY, Eggli S, Siebenrock KA, Tauber MG, Muhlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis. 2008;46:1221–1226. doi: 10.1086/529436. [DOI] [PubMed] [Google Scholar]
- 119.Hanssen AD. Managing the infected knee: as good as it gets. J Arthroplasty. 2002;17:98–101. doi: 10.1054/arth.2002.32458. [DOI] [PubMed] [Google Scholar]
- 120.Sukeik m, Haddad FS. Two-stage procedure in the treatment of late chronic hip infections--spacer implantation. Int J Med Sci. 2009;6:253–257. doi: 10.7150/ijms.6.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lazennec JY, Fourniols E, Lenoir T, Aubry A, Pissonnier ML, Issartel B, Rousseau MA. Infections in the operated spine: Update on risk management and therapeutic strategies. Orthop Traumatol Surg Res. 2011;97:373–380. doi: 10.1016/j.otsr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 122.De Grood MP. Pathology, diagnosis and treatment of subdural empyema. Arch Chir Neerl. 1951;3:128–138. [PubMed] [Google Scholar]
- 123.Buttaro MA, Pusso R, Piccaluga F. Vancomycin-supplemented impacted bone allografts in infected hip arthroplasty: two-stage revision results. J Bone Joint Surg Br. 2005;87-B:314–319. doi: 10.1302/0301-620x.87b3.14788. [DOI] [PubMed] [Google Scholar]
- 124.Humphrey JS, Mehta S, Seaber AV, Vail TP. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Relat Res. 1998;349:218–224. doi: 10.1097/00003086-199804000-00027. [DOI] [PubMed] [Google Scholar]
- 125.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000;59:1223–1232. doi: 10.2165/00003495-200059060-00003. [DOI] [PubMed] [Google Scholar]
- 126.Rushton N. Applications of local antibiotic therapy. Eur J Surg - Suppl. 1997;578:27–30. [PubMed] [Google Scholar]
- 127.Suh H, Suh S, Min B. Anti-infection treatment of a transcutaneous device by a collagen-rifampicin composite. ASAIO J. 1994;40:M406–411. doi: 10.1097/00002480-199407000-00031. [DOI] [PubMed] [Google Scholar]
- 128.Wachol-Drewek Z, Pfeiffer M, Scholl E. Comparative investigation of drug delivery of collagen implants saturated in antibiotic solutions and a sponge containing gentamicin. Biomaterials. 1996;17:1733–1738. doi: 10.1016/0142-9612(96)87654-x. [DOI] [PubMed] [Google Scholar]
- 129.Ambrose CG, Clyburn TA, Louden K, Joseph J, Wright J, Gulati P, Gogola GR, Mikos AG. Effective treatment of osteomyelitis with biodegradable microspheres in a rabbit model. Clin Orthop Relat Res. 2004;421:293–299. doi: 10.1097/01.blo.0000126303.41711.a2. [DOI] [PubMed] [Google Scholar]
- 130.Billon A, Chabaud L, Gouyette A, Bouler JM, Merle C. Vancomycin biodegradable poly(lactide-co-glycolide) microparticles for bone implantation. Influence of the formulation parameters on the size, morphology, drug loading and in vitro release. J Microencapsul. 2005;22:841–852. doi: 10.1080/02652040500162790. [DOI] [PubMed] [Google Scholar]
- 131.Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop Relat Res. 1997;341:206–214. [PubMed] [Google Scholar]
- 132.Kalicke T, Schierholz J, Schlegel U, Frangen TM, Koller M, Printzen G, Seybold D, Klockner S, Muhr G, Arens S. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J Orthop Res. 2006;24:1622–1640. doi: 10.1002/jor.20193. [DOI] [PubMed] [Google Scholar]
- 133.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg. 2002;10:53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 134.Witsø E, Persen L, Benum P, Bergh K. Release of netilmicin and vancomycin from cancellous bone. Acta Orthop Scand. 2002;73:199–205. doi: 10.1080/000164702753671812. [DOI] [PubMed] [Google Scholar]
- 135.Tomford W, Thongphasuk J, Mankin H, Ferraro M. Frozen musculoskeletal allografts. A study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am. 1990;72:1137–1143. [PubMed] [Google Scholar]
- 136.Kuijpers AJ, Engbers GH, van Wachem PB, Krijgsveld J, Zaat SA, Dankert J, Feijen J. Controlled delivery of antibacterial proteins from biodegradable matrices. J Control Release. 1998;53:235–247. doi: 10.1016/s0168-3659(97)00257-5. [DOI] [PubMed] [Google Scholar]
- 137.Neut D, van de Belt H, Stokroos I, van Horn JR, van der Mei HC, Busscher HJ. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 2001;47:885–891. doi: 10.1093/jac/47.6.885. [DOI] [PubMed] [Google Scholar]
- 138.Fu J, Ji J, Fan D, Shen J. Construction of antibacterial multilayer films containing nanosilver via layer-by-layer assembly of heparin and chitosan-silver ions complex. J Biomed Mat Res A. 2006;79:665–674. doi: 10.1002/jbm.a.30819. [DOI] [PubMed] [Google Scholar]
- 139.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res. 2007;462:200–206. doi: 10.1097/BLO.0b013e31811ff866. [DOI] [PubMed] [Google Scholar]
- 140.Witso E, Persen L, Benum P, Bergh K. Cortical allograft as a vehicle for antibiotic delivery. Acta Orthop. 2005;76:481–486. doi: 10.1080/17453670510041457. [DOI] [PubMed] [Google Scholar]
- 141.Gray JC, Elves MW. Osteogenesis in bone grafts after short-term storage and topical antibiotic treatment. An experimental study in rats. J Bone Joint Surg Br. 1981;63:441–445. doi: 10.1302/0301-620X.63B3.7021562. [DOI] [PubMed] [Google Scholar]
- 142.Isefuku S, Joyner CJ, Simpson AH. Toxic effect of rifampicin on human osteoblast-like cells. J Orthop Res. 2001;19:950–954. doi: 10.1016/S0736-0266(01)00022-5. [DOI] [PubMed] [Google Scholar]
- 143.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Rel Res. 1996;333:245–251. [PubMed] [Google Scholar]
- 144.Antoci V, Jr, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 2007;461:81–87. doi: 10.1097/BLO.0b013e3181123a50. [DOI] [PubMed] [Google Scholar]
- 145.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–866. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 146.Fiorilli S, Rivolo P, Descrovi E, Riccardi C, Pasquardisi L, Lunelli L, Vanzetto l, Perzoolli C, Onida B, Garron E. Vapor phase self-assembled monolayers of aminosilane on plasma activated silicon substrate. J Colloid Interface Sci. 2008;321:235–241. doi: 10.1016/j.jcis.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 147.Adams CS, Antoci V, Jr, Harrison G, Patal P, Freeman TA, Shapiro IM, Parvizi J, Hickok NJ, Radin S, Ducheyne P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthop Res. 2009;27:701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- 148.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- 149.Massia SP, Hubbell JA. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990;187:292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- 150.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J Biomed Mat Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 151.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 152.Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mat Res. 2000;52:595–600. doi: 10.1002/1097-4636(20001215)52:4<595::aid-jbm3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 153.Derda R, Wherritt DJ, Kiessling LL. Solid-phase synthesis of alkanethiols for the preparation of self-assembled monolayers. Langmuir. 2007;23:11164–11167. doi: 10.1021/la701386v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cavalcanti-Adam EA, Shapiro IM, Composto RJ, Macarak EJ, Adams CS. RGD Peptides Immobilized on a Mechanically Deformable Surface Promote Osteoblast Differentiation. J Bone Miner Res. 2002;17:2130–2140. doi: 10.1359/jbmr.2002.17.12.2130. [DOI] [PubMed] [Google Scholar]
- 155.El-Ghannam AR, Ducheyne P, Risbud M, Adams CS, Shapiro IM, Castner D, Golledge S, Composto RJ. Model surfaces engineered with nanoscale roughness and RGD tripeptides promote osteoblast activity. J Biomed Mater Res A. 2004;68:615–627. doi: 10.1002/jbm.a.20051. [DOI] [PubMed] [Google Scholar]
- 156.Secchi AG, Grigoriou V, Shapiro IM, Cavalcanti-Adam EA, Composto RJ, Ducheyne P, Adams CS. RGDS peptides immobilized on titanium alloy stimulate bone cell attachment, differentiation and confer resistance to apoptosis. J Biomed Mater Res A. 2007;83:577–584. doi: 10.1002/jbm.a.31007. [DOI] [PubMed] [Google Scholar]
- 157.Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20:2323–2331. doi: 10.1016/s0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 158.Okamoto K, Matsuura T, Hosokawa R, Akagawa Y. RGD peptides regulate the specific adhesion scheme of osteoblasts to hydroxyapatite but not to titanium. J Dent Res. 1998;77:481–487. doi: 10.1177/00220345980770030701. [DOI] [PubMed] [Google Scholar]
- 159.Danahy MP, Avaltroni MJ, Midwood KS, Schwarbauer JE, Schwartz J. Self-assembled monolayers of alpha, omega-diphosphonic acids on Ti enable complete or spatially controlled surface derivatization. Langmuir. 2004;20:5333–5337. doi: 10.1021/la036084h. [DOI] [PubMed] [Google Scholar]
- 160.Heijink A, Schwartz J, Zobitz ME, Nicole Crowder K, Lutz GE, Sibonga JD. Self-assembled monolayer films of phosphonates for bonding RGD to titanium. Clin Orthop Rel Res. 2008;466:977–984. doi: 10.1007/s11999-008-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4) on titanium alloy. Biomaterials. 2002;23:2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 162.Wojcik SM, Puleo DA. Biochemical surface modification of Ti-6Al-4V for the delivery of protein to the cell-biomaterial interface. Biomed Sci Instrum. 1997;33:166–171. [PubMed] [Google Scholar]
- 163.Lawson MC, Bowman CN, Anseth KS. Vancomycin derivative photopolymerized to titanium kills S. epidermidis. Clin Orthop Relat Res. 2007;461:96–105. doi: 10.1097/BLO.0b013e3180986706. [DOI] [PubMed] [Google Scholar]
- 164.Mikulec LJ, Puleo DA. Use of p-nitrophenyl chloroformate chemistry to immobilize protein on orthopedic biomaterials. J Biomed Mat Res. 1996;32:203–208. doi: 10.1002/(SICI)1097-4636(199610)32:2<203::AID-JBM8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 165.Khoo X, Hamilton P, O’Toole GA, Snyder BD, Kenan DJ, Grinstaff MW. Directed assembly of PEGylated-peptide coatings for infection-resistant titanium metal. J Am Chem Soc. 2009;131:10992–10997. doi: 10.1021/ja9020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gabriel M, Nazmi K, Veerman EC, Nieuw Amerongen AV, Zentner A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug Chem. 2006;17:548–50. doi: 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- 167.Humblot V, Yala JF, Thebault P, Boukerma K, Hequet A, Berjeaud JM, Pradier CM. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials. 2009;30:3503–3512. doi: 10.1016/j.biomaterials.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Parvizi J, Wickstrom E, Zeiger AR, Adams CS, Shapiro IM, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH, Hickok NJ. Frank Stinchfield Award. Titanium surface with biologic activity against infection. Clin Orthop Relat Res. 2004;429:33–38. doi: 10.1097/01.blo.0000150116.65231.45. [DOI] [PubMed] [Google Scholar]
- 169.Jose B, Antoci V, Jr, Zeiger AR, Wickstrom E, Hickok NJ. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol. 2005;12:1041–8. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 170.Lawson MC, Hoth KC, Deforest CA, Bowman CN, Anseth KS. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin Orthop Relat Res. 2010;468:2081–2091. doi: 10.1007/s11999-010-1266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen CP, Wickstrom E. Self-protecting bactericidal titanium alloy surface formed by covalent bonding of daptomycin bisphosphonates. Bioconjug Chem. 2010;21:1978–86. doi: 10.1021/bc100136e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Edupuganti OP, Antoci V, Jr, King SB, Jose B, Adams CS, Parvizi J, Shapiro IM, Zeiger AR, Hickok NJ, Wickstrom E. Covalent bonding of vancomycin to Ti6Al4V alloy pins provides long-term inhibition of Staphylococcus aureus colonization. Bioorg Med Chem Lett. 2007;17:2692–2696. doi: 10.1016/j.bmcl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Ketonis C, Adams CS, Barr S, Aiyer A, Shapiro IM, Parvizi J, Hickok NJ. Antibiotic modification of native grafts: improving upon nature’s scaffolds. Tissue Eng Part A. 2010;16:2041–2049. doi: 10.1089/ten.tea.2009.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee MH, Brass DA, Morris R, Composto RJ, Ducheyne P. The effect of non-specific interactions on cellular adhesion using model surfaces. Biomaterials. 2005;26:1721–1730. doi: 10.1016/j.biomaterials.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 175.Puleo DA. Retention of enzymatic activity immobilized on silanized Co-Cr-Mo and Ti-6Al-4V. J Biomed Mat Res. 1997;37:222–228. doi: 10.1002/(sici)1097-4636(199711)37:2<222::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 176.Ketonis C, Parvizi J, Adams CS, Shapiro IM, Hickok NJ. Topographic features retained after antibiotic modification of Ti alloy surfaces. Clin Orthop Rel Res. 2009;467:1678–1687. doi: 10.1007/s11999-009-0828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Antoci V, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Zeiger AR, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. Vancomycin-modified Ti alloy inhibits S. epidermidis biofilm formation: Implications for treatment of periprosthetic infection. Biomaterials. 2008;29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Loll PJ, Axelsen PH. The structural biology of molecular recognition by vancomycin. Annu Rev Biophys Biomol Struct. 2000;29:265–289. doi: 10.1146/annurev.biophys.29.1.265. A review that focuses on vancomycin, its structure, and the activity of the various derivatives of this important antibiotic. [DOI] [PubMed] [Google Scholar]
- 179.Ketonis C, Barr S, Adams CS, Shapiro IM, Parvizi J, Hickok NJ. Vancomycin bonded to bone grafts prevents bacterial colonization. Antimicrob Agents Chemother. 2011;55:487–494. doi: 10.1128/AAC.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lawson MC, Shoemaker R, Hoth KB, Bowman CN, Anseth KS. Polymerizable vancomycin derivatives for bactericidal biomaterial surface modification: structure-function evaluation. Biomacromolecules. 2009;10:2221–2234. doi: 10.1021/bm900410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sykes RB. The 2009 Garrod lecture: the evolution of antimicrobial resistance: a Darwinian perspective. J Antimicrob Chemother. 2010;65:1842–1852. doi: 10.1093/jac/dkq217. [DOI] [PubMed] [Google Scholar]
- 182.Wimpenny J. Microbial metropolis. Adv Microb Physiol. 2009;56:29–84. doi: 10.1016/S0065-2911(09)05602-1. [DOI] [PubMed] [Google Scholar]