Abstract
Although pediatric functional abdominal pain (FAP) has been linked to abdominal pain later in life, childhood predictors of long-term outcomes have not been identified. This study evaluated whether distinct FAP profiles based on patterns of pain and adaptation in childhood could be identified and whether these profiles predicted differences in clinical outcomes and central sensitization (wind-up) on average 9 years later. In 843 pediatric FAP patients, cluster analysis was used to identify subgroups at initial FAP evaluation based on profiles of pain severity, gastrointestinal (GI) and non-GI symptoms, pain threat appraisal, pain coping efficacy, catastrophizing, negative affect, and activity impairment. Three profiles were identified: High Pain Dysfunctional, High Pain Adaptive, and Low Pain Adaptive. Logistic regression analyses controlling for age and sex showed that, compared to pediatric patients with the Low Pain Adaptive profile, those with the High Pain Dysfunctional profile were significantly more likely at long-term follow-up to meet criteria for pain-related functional gastrointestinal disorder (FGID) (OR: 3.45; CI: 1.95–6.11), FGID with comorbid non-abdominal chronic pain (OR: 2.6; CI:1.45–4.66), and FGID with comorbid anxiety or depressive psychiatric disorder (OR: 2.84; CI: 1.35–6.00). Pediatric patients with the High Pain Adaptive profile had baseline pain severity comparable to the High Pain Dysfunctional profile, but had outcomes as favorable as the Low Pain Adaptive profile. In laboratory pain testing at follow-up, High Pain Dysfunctional patients exhibited significantly greater thermal wind-up than Low Pain Adaptive patients, suggesting that a subgroup of FAP patients has outcomes consistent with widespread effects of heightened central sensitization.
Keywords: functional gastrointestinal disorder, comorbid, chronic pain, anxiety, depression, central sensitization, wind-up, cluster analysis, pediatric, prospective, prediction
Abdominal pain accounts for 2–4% of pediatric clinic visits [57] and becomes chronic or recurrent in many youth [27]. Medical evaluations rarely identify an explanatory biochemical or structural abnormality for the pain [17]; hence, most cases are considered medically unexplained or “functional” abdominal pain (FAP).
Gastroenterologists developed the “Rome” criteria for functional gastrointestinal disorders (FGIDs) to classify patients into subgroups based on their gastrointestinal (GI) symptoms [20,47]. Most FAP patients meet diagnostic criteria for an FGID associated with abdominal pain, such as irritable bowel syndrome [3,48,52,53]. With its exclusive focus on GI symptoms, however, the Rome taxonomy does not capture important aspects of FAP patients’ pain experience.
Cluster analysis has been used with adults to develop taxonomies of chronic pain that can predict differential health outcomes [4,5,15,25,29,58]. Rather than individual risk factors, cluster analysis identifies risk profiles – combinations of variables characterizing patient subgroups that may differ in pathophysiology, clinical course, and outcomes This study sought to develop a taxonomy of pediatric FAP patients for the ultimate purpose of informing the design of interventions which, rather than treating FAP patients as a single homogeneous group, could be tailored to meet the unique needs of FAP subgroups.
The biopsychosocial model of pain as a multidimensional phenomenon [26,70] and studies of patient characteristics associated with differential outcomes of chronic pain [33] guided our selection of variables to include in the cluster analysis. Previous cluster analytic studies of pediatric chronic pain have identified patient subgroups using measures of psychological adjustment [52,61] and pain coping style [11,62]. In contrast, we included measures of sensory, cognitive, affective, and behavioral dimensions of pain. These measures, administered to patients at the initial tertiary care medical evaluation of FAP, were subjected to cluster analysis to identify patient profiles. The construct validity of the three resulting profiles was evaluated by comparing them on baseline parent-report measures not used in the cluster analysis and by prospectively examining the relation of the profiles to health outcomes in adolescence and young adulthood.
Previous studies of long-term outcomes in FAP patients have documented the persistence of abdominal pain in many patients [27], but have not assessed whether the pain reached clinical significance as defined by the Rome criteria for FGIDs [20]. Moreover, they have not systematically evaluated important concurrent health outcomes such as non-abdominal chronic pain and psychiatric disorders that are common in adults with FGIDs [2,14,21,69]. A central aim of the current study was to evaluate the prospective relation of FAP profiles identified in childhood to three outcomes in adolescence and young adulthood: (a) Rome III criteria for FGIDs associated with abdominal pain; (b) comorbidity of FGID with non-abdominal chronic pain; and (c) comorbidity of FGID with psychiatric disorders. A second aim, pursued in the subset of participants who completed a laboratory assessment at follow-up, was to evaluate the relation of the FAP baseline profiles to subsequent central pain sensitization (reflected in “wind-up”), an index of increased spinal nociceptive responding that has been linked to chronic pain in adults [60,70,71].
Method
Participants
Baseline
Participants were drawn from a database of cohorts of consecutive new patients evaluated for abdominal pain in 1993 – 1995, [67], 1996–1999, [64], and 2001 – 2007 [3]. Eligibility criteria for those studies included abdominal pain of at least 3 months duration, no chronic illness or disability, and no organic disease diagnosis for abdominal pain from the referring primary care physician. Patients whose medical evaluation at Vanderbilt yielded evidence of significant organic disease (e.g., ulcerative colitis) were excluded from the present study. The parent who accompanied the child to the clinic also participated in baseline assessment. Consent/assent for participation in the baseline assessment and for contacting about future studies was obtained. The sample for cluster analysis of baseline characteristics consisted of 843 FAP patients.
Participants in follow-up interviews
Participants in the baseline cluster analytic study were eligible for the long-term follow-up study if, during the three years that data were collected for the follow-up study, they reached 12 years of age or older and at least four years elapsed since the baseline evaluation associated with initial study enrollment. Of the FAP patients who participated in the baseline study, 760 met these eligibility criteria for the FU study. Of those who met eligibility criteria, 261 (34%) could not be reached, 60 (8%) declined to participate, and 40 (5%) indicated interest in participation but did not keep their appointment or could not be scheduled during the study period. Finally, 3 were excluded because of self-reported onset of chronic disease during the follow-up interval (inflammatory bowel disease, celiac disease, multiple sclerosis). Thus, of the 760 former patients who met eligibility criteria, 379 (50%) participated in the follow-up interviews. (For participants under age 18 at follow-up (n = 66), the parent who participated at baseline also completed an interview at follow-up.)
Participants in follow-up laboratory pain assessment
All participants in the follow-up study also were invited to the Vanderbilt Pediatric Clinical Research Center to participate in a laboratory pain assessment. Of the 379 participants who completed the interview portion of the follow-up study, 211 (56%) participated in the laboratory portion of the study.
Procedure
Baseline
An interviewer administered questionnaires to pediatric patients in a private room at the clinic prior to the medical evaluation. Parents completed questionnaires independently at the same time. Medical records were reviewed for results of the medical evaluation. Details regarding baseline assessment procedures are presented elsewhere [64,67].
Follow-up interviews
The presence of FGIDs and chronic pain at follow-up was assessed by structured telephone interview. Psychiatric diagnostic interviews were conducted either in person or by telephone. For participants under age 18, both parent and child were interviewed about the child’s psychiatric symptoms. Interviewers were unaware of participants’ group status.
Follow-up laboratory pain assessment
The laboratory pain assessment was conducted at the Vanderbilt Pediatric Clinical Research Center. Standard instructions were provided for all participants prior to beginning the protocol. Experimenters were unaware of participants’ group status. As an index of central sensitization, thermal wind-up was assessed with a computer-controlled Medoc Thermal NeuroSensory Analyzer (TSA-II, Medoc, Inc., Ramat, Israel). This device applied heat stimuli to the non-dominant ventral forearm using a 30×30mm Peltier thermistor probe as reported in prior studies (e.g., [10,23]). Wind-up trials were conducted using commercially-available software (TPS-CoVAS v3.19, Medoc Inc.) that administered a standardized oscillating thermal stimulation protocol designed specifically to assess C-fiber mediated temporal summation (cf. [10,23,24]). During wind-up assessment, two sequences of 10 heat pulses each were applied to the ventral forearm, with the thermode in a fixed position throughout each sequence. The thermode was moved to a different non-overlapping site on the same arm for the subsequent sequence of trials, with a 1-minute interval between sequences (for details, see [16]). Two sequences using different stimulus intensities (47°C and 48°C) were employed to maximize the likelihood of producing measurable results [24]. At the peak of every heat pulse within each sequence, participants were asked to provide a verbal numeric pain intensity rating using a 0 – 100 scale (anchored with 0 = “No Pain” and 100 = “Worst Possible Pain”). Participants were instructed that the procedure would be stopped if they reported a score of 100 or if they expressed a desire to stop before all 10 heat pulses were administered. The slopes of the lines (determined for each individual by using within-subject regressions) fitted to the series of 10 pain ratings at each stimulus intensity (47°C and 48°C) were used as indices of wind-up, as in our prior work (see [10,16]). These slopes were used as the dependent variable for all wind-up analyses.
Consent/assent was obtained separately for follow-up interviews and laboratory pain testing. All procedures were approved by the Vanderbilt Institutional Review Board.
Measures
Baseline Measures
Abdominal pain severity
The Abdominal Pain Index (API, [67]) assesses abdominal pain frequency, duration, and intensity during the previous two weeks. Responses to 4 questions are converted to 6-point scales ranging from 0 to 5 and averaged to yield the scale score that was retained and then converted to a 5-point scale. Child and parent-report versions were administered. Chronbach’s alpha coefficient was .75 for child-report and .76 for parent-report.
Gastrointestinal and non-gastrointestinal symptoms
The Children’s Somatization Inventory (CSI, [63]) assesses the severity of 35 somatic symptoms (e.g., headaches, low energy, dizziness, chest pain). For each item, participants rate “How much were you bothered by (symptom)?” during the past two weeks using a 5-point scale ranging from “not at all” (0) to “a whole lot” (4). Subscale scores are computed for gastrointestinal (GI) symptoms (9 items, e.g., abdominal pain, nausea, constipation, diarrhea, bloating) and non-GI symptoms (26 items, e.g., dizziness, back pain, headaches, sore muscles) by averaging the relevant items for each subscale. Both subscales had good internal consistency with Cronbach alpha coefficients of .78 and .82 for the GI and non-GI symptom subscales, respectively. A parent report form of the CSI was administered to parents regarding their child’s somatic symptoms. Alpha reliabilities for the parent report version were .70 and .82 for the GI and non-GI symptom subscales, respectively.
Pain Beliefs
The Pain Beliefs Questionnaire (PBQ, [66]) was developed to measure children’s pain appraisals (cf. [38,55]). The PBQ contains 32 items that assess children’s pain beliefs. Primary pain appraisal (20 items) refers to perceived pain threat (e.g. “My stomach aches mean I have a serious illness”). Secondary pain appraisal refers to perceived coping efficacy and is assessed with two 6-item subscales -- problem-focused coping efficacy (e.g., “When I have a bad stomach ache, there are ways I can get it to stop”) and emotion-focused coping efficacy (e.g., “I know I can handle it no matter how bad my stomach hurts”). Respondents indicate how true each statement is about their abdominal pain using a 5-point rating scale ranging from “not at all true” (0) to “very true” (4). All three subscales were computed by averaging the items relevant to that subscale. Parents completed a parent-report version of the PBQ rating their perceptions of their children’s pain threat and pain coping efficacy (e.g. “When my child has a bad stomach ache, he/she just can’t take it”). Reliability, validity, and sensitivity to treatment have been documented for the PBQ scales [1,37,39,41,62,66]. In this study, alpha reliabilities for the subscales were .88, .80, and .76 for child-report Pain Threat, Problem-Focused Coping Efficacy, and Emotion-Focused Coping Efficacy, respectively. Alpha reliabilities for the corresponding parent-report subscales were .83, .66, and .68.
Pain Catastrophizing
Patients’ catastrophizing about their abdominal pain was assessed with the 5-item Catastrophizing subscale of the Pain Response Inventory [67]. The stem for each item is, “When you have a bad stomachache, how often do you…?” followed by a statement such as, “think it’s never going to stop.” Response categories range from never (0), to always (4). The items were averaged to compute this scale. Alpha reliability for the Catastrophizing subscale was .82.
Negative affect
The self-report Children’s Depression Inventory (CDI, [34]) was used to assess the severity of negative affect. For each of 26 items, participants are given three statements and asked to select the one that best described how they felt during the two weeks preceding the medical evaluation. The items were averaged, and the resulting scale score was converted to a 0 to 4 scale for the purposes of this study. Alpha reliability was .86.
Functioning
The Functional Disability Inventory (FDI [12,65]) assesses self-reported difficulty in physical and psychosocial functioning due to physical health during the past 2 weeks. Responses to each of the 15 items are scored on a 5-point scale, ranging from (0) no trouble to (4) impossible. Items were averaged to compute this scale. Alpha reliability of the FDI was .90.
Demographic variables
Patients’ parents indicated their marital status, racial and ethnic group identification, education, and occupation. Family socioeconomic status (SES) was estimated using the Hollingshead Index [31].
Follow-up Measures
Functional gastrointestinal disorders
The Rome III Diagnostic Questionnaire for Functional Gastrointestinal Disorders (FGID; [20]) was developed by the Rome Foundation Board to assess symptoms associated with the diagnostic criteria for FGIDs. We administered 24 items that assessed symptom criteria for FGIDs associated with abdominal pain, including irritable bowel syndrome, functional dyspepsia, abdominal migraine, and functional abdominal pain. Participants’ responses were scored according to the pediatric Rome criteria (for participants under 18 years of age) or the adult Rome criteria (for participants 18 years and older).
Chronic pain
The Persistent Pain Questionnaire (PPQ; [7]) was designed to provide a structured assessment of history and location of any chronic pain. The PPQ lists the standard 9 body locations described by the International Association for the Study of Pain, and asks the respondent to indicate whether he/she has ever had pain in that location “daily or almost every day that continued for 3 months or longer.” The PPQ was modified for this study to assess current chronic pain. For each site of current chronic pain, respondents rated the intensity of their pain on a 0 to 100 scale (anchored from “no pain at all” to “the worst pain possible”). The presence of non-abdominal chronic pain at follow-up was defined as endorsement of any (non-abdominal) current chronic pain rated 30 or higher. For participants under 18 years of age, parents completed the PPQ indicating their child’s current chronic pain and chronic pain history across all 9 body locations.
Psychiatric disorders
Current and lifetime psychopathology were assessed with the Anxiety Disorders Interview Schedule-IV: Adult Lifetime and Child and Parent Versions (ADIS; [19,54]), a semi-structured psychiatric diagnostic interview that assesses the frequency, intensity, and associated impairment of psychiatric symptoms from which DSM-IV diagnoses can be made. The ADIS was designed to focus on anxiety disorders [51] but also includes modules evaluating other disorders. The adult version was used for participants aged 18 and older. The child version, which incorporates information from both parent- and child-report interviews to yield DSM-IV diagnoses for the child, was used for the 66 participants under 18 years. To evaluate inter-reliability of diagnoses assigned to participants, two diagnosticians independently listened to and rated a randomly selected 24 audiotapes (20%) of the interviews [42]. Kappa coefficient regarding presence/absence of disorder was k = .76 for anxiety disorders, and k = 1.0 for other disorders.
Job loss due to illness
Participants were asked to report whether they had ever quit or lost a job because illness interfered with their ability to work. Participants who had never held a job (n = 43; 11.3% of those who completed the FU interview) were coded as “not applicable” and excluded from analyses related to job loss.
Data Analysis Overview
Identification of baseline profiles
Patient profiles were based on measures obtained at the initial assessment including abdominal pain severity, gastrointestinal and non-gastrointestinal symptoms, beliefs regarding pain threat and pain coping efficacy, pain catastrophizing, negative affect, and functional disability. The measure of negative affect was recoded on a 0–4 scale to be consistent with the other clustering variables which were already on a 0–4 scale and to provide standard scaling across variables for cluster analysis.
In the first stage of data analysis, we used cluster analysis of these baseline assessment data to identify patient profile types. Using a procedure similar to that of Gironda and Clark [29], we randomly split the baseline sample (n = 843) into two subsamples. For each of the two subsamples, we conducted hierarchical cluster analysis using Ward’s method with squared Euclidian distance measures and clustering at the patient level. We examined the viability of solutions ranging from two to five clusters in each sample. Examination of the resulting dendrograms and group mean profiles indicated that a three cluster solution was most appropriate in both samples. To assess the level of agreement between the cluster solutions for the two samples, the three profiles were first visually matched across samples. Next, Cohen’s coefficient of pattern similarity, rc, which is an index of the congruence of elevation and shape between two profiles [13], was computed for each of the matched pairs and, for comparison, for each of the unmatched pairs. The results indicated a high level of agreement between the two solutions for all three matched pairs (mean rc = .99, range = .98 – .99) as compared to the level of agreement between the two solutions for unmatched pairs (Mean rc = .42, range = −.08 – .83). Given the high level of agreement between the solutions for the two subsamples, the total sample was used to generate the final cluster solution. As expected, the resulting three cluster solution replicated that of the previous analyses of the split halves. Cluster assignments were retained for further data analysis. ANOVAs were conducted to evaluate differences among the three clusters on each of the variables used in the cluster analyses as well as on parent-report variables not used in the cluster analysis (for validation purposes).
Relation of baseline cluster assignment to long-term outcomes
The second stage of data analysis was based on participants (n = 379) who completed the interview portion of the follow-up study. A series of logistic regression analyses controlling for age and sex compared the baseline cluster assignment (independent variable) on dichotomous outcomes at follow-up (dependent variables) including the presence of FGID without comorbidities at follow-up, FGID with comorbid non-abdominal chronic pain at follow-up, and FGID with comorbid anxiety or depressive psychiatric disorder at follow-up. Analyses of thermal wind-up were conducted for those who participated in the laboratory portion of the follow-up (n = 211, 56% of those who completed the follow-up interview). Planned post-hoc comparisons using the LSD test examined differences among the profile groups.
Results
Baseline patient profiles
Figure 1 illustrates, for each of the three profile groups, the mean score on variables used in the cluster analysis. For descriptive purposes, Table 1 presents results of univariate ANOVAs comparing the three profiles groups on the variables used in the cluster analysis. We generated descriptive names for each profile based on the patterns of high and low scores within the profile itself and in comparison to the other profiles, as described below.
Figure 1. Mean scores on clustering variables by profile.
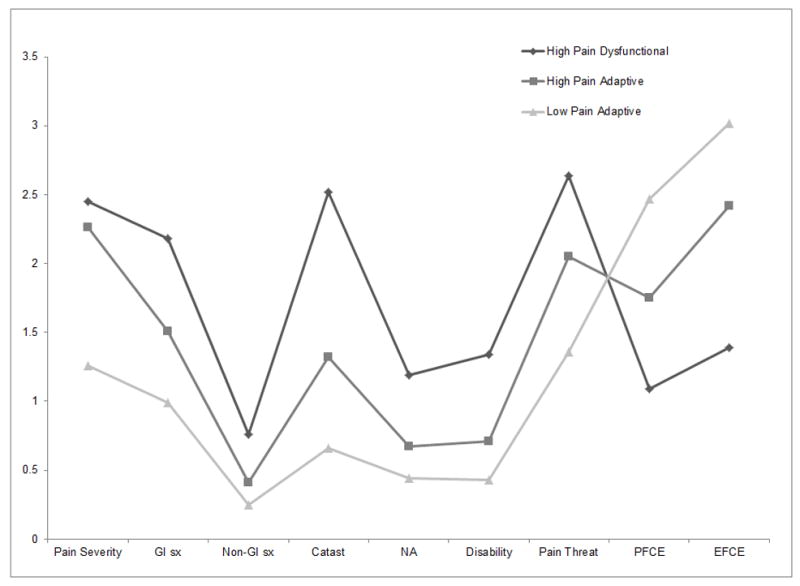
Note. GI sx = gastrointestinal symptoms; Non-GI sx = non-gastrointestinal symptoms; Catast = catastrophizing; NA = negative affect; PFCE = problem focused coping efficacy; EFCE = emotion focused coping efficacy
Table 1.
Pain characteristic means and standard deviations at baseline by patient profile
| Pain related measures | Total (n = 843) | F | High Pain Dysfunctional (n = 190) | High Pain Adaptive (n = 340) | Low Pain Adaptive (n = 313) |
|---|---|---|---|---|---|
| Abdominal Pain Index | 1.93 (.86) | 239.01*** | 2.45 (.77) a | 2.26 (.66) b | 1.26 (.67) c |
| GI symptoms | 1.47 (.80) | 193.91*** | 2.18 (.76) a | 1.51 (.63) b | 0.99 (.63) c |
| Non-GI symptoms | 0.43 (.40) | 125.46*** | 0.76 (.52) a | 0.41 (.32) b | 0.25 (.25) c |
| Catastrophizing | 1.35 (.95) | 500.37*** | 2.52 (.80) a | 1.32 (.61) b | 0.66 (.55) c |
| Depression | 0.70 (.53) | 172.17*** | 1.19 (.59) a | 0.67 (.40) b | 0.44 (.38) c |
| Functional disability | 0.75 (.62) | 180.16*** | 1.34 (.75) a | 0.71 (.45) b | 0.43 (.41) c |
| Pain threat appraisal | 1.92 (.71) | 389.10*** | 2.64 (.57)a | 2.05 (.49) b | 1.35 (.49) c |
| Problem focused coping efficacy | 1.87 (.95) | 186.56*** | 1.09 (.74) a | 1.75 (.86) b | 2.47 (.74) c |
| Emotion focused coping efficacy | 2.41 (.91) | 354.57*** | 1.39 (.72) a | 2.42 (.70) b | 3.02 (.59) c |
p < .001;
Note. Within rows, means with different superscripts differ significantly at p < .01.
Cluster 1: High Pain Dysfunctional Profile (n = 190)
Nearly one quarter (23%) of the 843 participants in the baseline sample were classified into a cluster that we named the High Pain Dysfunctional Profile. Compared to patients with other profiles, these pediatric FAP patients reported significantly higher levels of abdominal pain, gastrointestinal, and nongastrointestinal symptoms. They viewed their abdominal pain as more threatening, believed they had little ability to cope with their pain, and reported extremely high levels of pain catastrophizing. Finally, patients in this group had significantly higher levels of negative affect and health-related impairment in their activities compared to the other groups. This profile was predominantly female (70%) and averaged 12.16 years (SD=2.68).
Cluster 2: High Pain Adaptive Profile (n = 340)
The largest cluster (40% of the baseline sample) reported levels of abdominal pain nearly as high as the High Pain Dysfunctional profile. We named this second cluster the High Pain Adaptive Profile because, compared to those with the High Pain Dysfunctional profile, participants with the High Pain Adaptive profile viewed their pain as significantly less threatening, had greater confidence in their ability to cope with the pain, and engaged in less pain catastrophizing. They also had relatively low levels of negative affect and health-related impairment in activities. The majority of this profile was female (63%) and averaged 11.83 years (SD=2.43).
Cluster 3: Low Pain Adaptive Profile (n = 313)
The third cluster accounted for about a third (37%) of the baseline pediatric sample and was labeled the Low Pain Adaptive Profile. Patients with this profile had low levels of abdominal pain and appraised their pain as considerably less threatening compared to both High Pain profiles. Patients with the Low Pain Adaptive profile were highly confident of their ability to cope with their pain and showed little negative affect or health-related impairment of activities. Patients with this profile were slightly younger than those with other profiles (M=11.14, SD=2.27; F(2,839) = 11.87, p < .001) and were evenly split between males and females.
External validation of baseline FAP profiles using baseline parent-report measures
As a means of validating baseline differences among the three clusters, we compared the three profiles on parent reports of the child’s abdominal pain severity, GI symptoms, non-GI symptoms, as well as parents’ appraisal of pain threat (related to their child’s abdominal pain) and beliefs about their child’s emotion focused and problem focused pain coping efficacy (Table 2). Consistent with their children’s self-reports, parents of patients with High Pain Dysfunctional and High Pain Adaptive profiles rated their children’s pain above the midpoint on the pain severity scale, with both ratings significantly higher than the pain ratings made by parents of children with the Low Pain Adaptive profile. According to the parents’ perspective, children with the High Pain Dysfunctional and High Pain Adaptive profiles displayed comparable levels of GI symptoms, non-GI symptoms, and pain threat appraisal, but children with the High Pain Adaptive profile had significantly higher emotion-focused coping efficacy than those with the High Pain Dysfunctional profile. Finally, consistent with the child-report, parents reported that children with the High Pain Dysfunctional and the High Pain Adaptive profiles displayed significantly higher abdominal pain, GI symptoms, non-GI symptoms, and pain threat as well as significantly lower emotion and problem focused coping efficacy compared to patients with the Low Pain Adaptive profile. Thus, parent report data supported the validity of the three profiles.
Table 2.
Parent report pain characteristic means and standard deviations by patient profile
| Pain related measures | Total (n range = 288–799) | F | High Pain Dysfunctional (n range = 56–181) | High Pain Adaptive (n range = 128–133) | Low Pain Adaptive (n range = 104–296) |
|---|---|---|---|---|---|
| Abdominal Pain Index | 2.29 (.88) | 25.02*** | 2.61 (.80) a | 2.50 (.87) a | 1.84 (.74) b |
| GI symptoms | 1.69 (.80) | 9.15*** | 1.92 (.74) a | 1.79 (.83) a | 1.43 (.73) b |
| Non-GI symptoms | 0.32 (.35) | 5.99** | 0.39 (.39) a | 0.37 (.37) a | 0.23 (.29) b |
| Primary appraisal | 2.16 (.58) | 42.62*** | 2.32 (.52)a | 2.28 (.60) a | 1.92 (.52) b |
| Problem focused coping efficacy | 1.46 (.81) | 10.36*** | 1.33 (.80) a | 1.39 (.81) a | 1.63 (.79) b |
| Emotion focused coping efficacy | 2.25 (.82) | 16.43*** | 2.02 (.82) a | 2.19 (.82) b | 2.44 (.80) c |
p < .01;
p < .001;
Note. Within rows, means with different superscripts differ significantly at p < .05.
Predictive validity of Baseline FAP Profiles in the Prospective Study of Health Outcomes
Characteristics of the follow-up sample
Participants in the follow-up had a mean age at baseline of 12 years (SD=2.8) and a mean age at follow-up of 21 years (SD=3.9). They were predominantly female (65%). Mean duration of the follow-up interval was 9.11 years (SD = 3.52). Among participants in the follow-up, 22.5% (n=88) had a High Pain Dysfunctional baseline profile, 40.2% (n=157) had a High Pain Adaptive baseline profile, and 34.3% (n=134) had a Low Pain Adaptive baseline profile. Participants and nonparticipants in the follow-up interviews did not differ significantly on baseline age, baseline pain severity, or baseline profile cluster (p’s >.10). However, of baseline study participants who were eligible to complete the follow-up study, a slightly greater proportion of eligible females chose to participate in the follow-up study than eligible males, p < .05. Participant and non-participants in the laboratory portion of the follow-up study did not differ significantly on sex, age, baseline pain severity, or baseline profile.
Functional gastrointestinal disorders (FGIDs) at Follow-Up
As shown in Figure 2, the majority (63.6%) of FAP patients with the High Pain Dysfunctional baseline profile met Rome III symptom criteria for FGID at follow-up, as compared to approximately one third of those with the High Pain Adaptive (35.7%) and Low Pain Adaptive baseline profiles (32.8%). Controlling for sex and age at follow-up, the odds for those with the High Pain Dysfunctional baseline profile to meet criteria for a FGID at follow-up were 3.45 times greater than for those with the Low Pain Adaptive baseline profile (OR: 3.45; 95% Confidence Interval [CI]: 1.95–6.11; p < .001). (Table 3). In contrast, despite their high level of baseline pain severity, the odds of FGID at follow-up for those with the High Pain Adaptive baseline profile did not differ significantly from the odds for those with the Low Pain Adaptive baseline profile (OR: 1.12; 95% CI: .68–1.83; p > .05).
Figure 2. Percent of each FAP profile meeting criteria for various outcomes at follow-up.
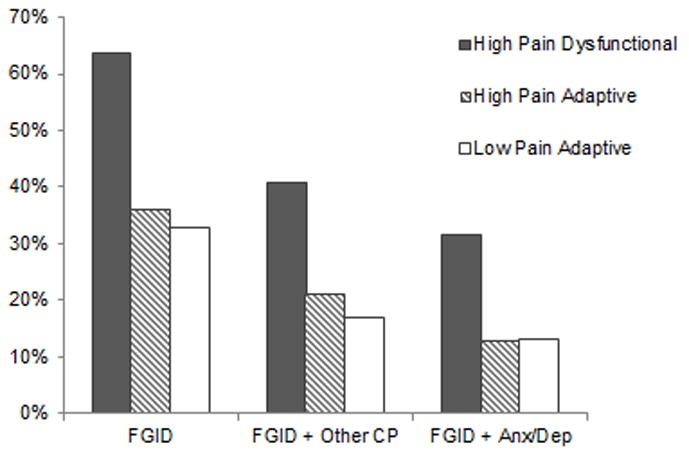
Note. FGID = Functional gastrointestinal disorder w/abdominal pain; CP = chronic pain; Anx/Dep = DSM-IV criteria for anxiety or depressive disorder.
Table 3.
Odds ratios and confidence intervals comparing the profiles on outcomes
| FGID | FGID + Other Chronic Pain | FGID + Anx/Dep | |
|---|---|---|---|
| Low Pain Adaptive | REFERENT | REFERENT | REFERENT |
| High Pain Adaptive | 1.1 CI (0.7, 1.8) | 0.8 CI (0.5, 1.6) | 0.9 CI (0.4, 1.9) |
| High Pain Dysfunctional | 3.4 CI (1.9, 6.1)** | 2.6 CI (1.5, 4.7)** | 2.8 CI (1.3, 6.0)** |
p < .01;
Note. Analyses adjusted for sex and age at follow-up. FGID = Functional Gastrointestinal Disorder; Anx/Dep = Anxiety or Depression; CI = 95% Confidence Interval
FGID comorbid with non-abdominal chronic pain at follow-up
Among patients with the High Pain Dysfunctional baseline profile, 41% had both FGID and non-abdominal chronic pain at follow-up, compared to 11% with the High Pain Adaptive baseline profile and 17% with the Low Pain Adaptive baseline profile. (Figure 2). Controlling for age and sex, the odds for those with the High Pain Dysfunctional baseline profile to have FGID with concurrent non-abdominal chronic pain at follow-up was 2.6 times greater than the odds for the Low Pain Adaptive baseline profile (OR: 2.60; 95% CI: 1.45–4.66, p < .01), whereas the odds for those with the High Pain Adaptive baseline profile did not differ significantly from the odds for those with the Low Pain Adaptive baseline profile (OR: 0.85; 95% CI: .46–1.56, p >.05).
FGID comorbid with anxiety or depressive disorder at follow-up
Approximately one third (32%) of those with the High Pain Dysfunctional baseline profile met criteria for both FGID and a concurrent anxiety or depressive disorder at follow-up, compared to 13% of those with the High Pain Adaptive baseline profile and 13% of those with the Low Pain Adaptive baseline profile (see Figure 2). Controlling for sex and age at follow-up, the odds for those with the High Pain Dysfunctional baseline profile to have FGID with concurrent anxiety or depressive disorder at follow-up was 2.84 times greater than the odds for those with the Low Pain Adaptive baseline profile (OR: 2.84; 95% CI: 1.35–6.00, p <.01), whereas the odds for those with the High Pain Adaptive baseline profile did not differ significantly from the odds for those with the Low Pain Adaptive baseline profile (OR: 0.89; 95% CI: .42–1.90, p > .05).
Job loss secondary to illness
Of those with the High Pain Dysfunctional baseline profile, 19.2% reported at follow-up that they had lost a job due to illness, compared to 12% of those with the High Pain Adaptive baseline profile and 5.8% of those with the Low Pain Adaptive baseline profile. Controlling for sex and age at follow-up, the odds for those with the High Pain Dysfunctional baseline profile to report at follow-up that they had lost a job due to illness was 2.85 times greater than the odds for those with the Low Pain Adaptive baseline profile (OR: 2.85; 95% CI: 1.05–7.71, p <.05). In contrast, the odds for those with the High Pain Adaptive baseline profile to report illness-related job loss did not differ significantly from the odds for those with the Low Pain Adaptive baseline profile (OR: 1.79; 95% CI: .70–4.62, p > .05).
Central sensitization to thermal pain at follow-up
Laboratory assessment of thermal pain wind-up was conducted with two series of trials, the first at a temperature of 47 °C and the second at a temperature of 48°C. For these trials, sample sizes were 50, 86, and 75 for the High Pain Dysfunctional, High Pain Adaptive, and Low Pain Adaptive baseline profiles, respectively. In the trials at 48°C, individuals with the High Pain Dysfunctional baseline profile demonstrated significantly greater wind-up to heat stimuli (M = 1.38, SD = 1.74) compared to those with the Low Pain Adaptive baseline profile [(M = 0.75, SD = .99), t(70.25) = 2.32, p < .05; See Figure 3]. There was a nonsignificant trend for greater wind-up in those with the High Pain Dysfunctional baseline profile compared to those with the High Pain Adaptive baseline profile [(M = .82, SD = 1.64), t(134) = 1.87, p = .06]. Those with High Pain Adaptive and Low Pain Adaptive baseline profiles did not differ significantly on wind-up at follow-up, t(142.3)= −.33, ns. No significant differences in wind-up were seen between the FAP profile groups in the trials at 47°C.
Figure 3. Mean wind-up slopes for 47° and 48° by FAP profile.
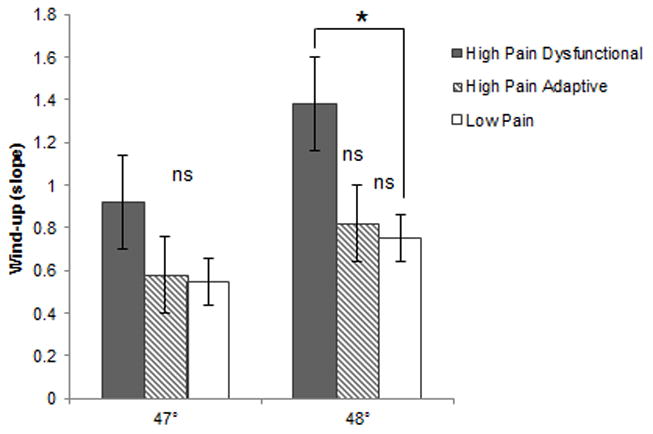
Note. * p < .05
Discussion
The purpose of this study was to evaluate whether distinct profiles of pediatric FAP based on multidimensional assessment of pain in childhood could be identified and whether these profiles could predict differences in clinical outcomes at a follow-up evaluation conducted in adolescence and young adulthood. Using cluster analysis, three distinct patient profiles were identified and labeled according to their characteristics as High Pain Dysfunctional, High Pain Adaptive, and Low Pain Adaptive. Validity was demonstrated by profile differences on parent-report measures and by the ability of the profiles to differentially predict long-term outcomes including FGIDs, non-abdominal chronic pain, psychiatric disorders, and illness-related job loss in adolescence and young adulthood. In addition, the baseline profiles differentially predicted temporal summation of thermal pain stimuli (“wind-up”) among participants who completed pain testing in the laboratory at follow-up.
Two baseline patient profiles had high levels of abdominal pain -- above the midpoint on both child- and parent-reports on a composite measure of pain frequency, duration, and intensity obtained at the initial tertiary care medical evaluation for FAP. Patients with one of these baseline profiles, labeled High Pain Dysfunctional, were characterized by low perceived pain coping efficacy and high levels of negative affect, pain catastrophizing, and functional disability. Patients with the other high pain profile, labeled High Pain Adaptive, were characterized by moderate perceived pain coping efficacy and low levels of negative affect, pain catastrophizing, and disability. The third baseline profile, labeled Low Pain Adaptive, had significantly lower baseline pain severity than the other profiles and was characterized by high perceived pain efficacy, very low negative affect, and minimal disability.
Based on pain severity alone, one might expect both high pain profiles in childhood to predict poor outcomes in adolescence and young adulthood. However, nearly two-thirds of those with the High Pain Dysfunctional baseline profile met criteria for FGID at follow-up compared to only one-third of those with the High Pain Adaptive baseline profile. Differences in the cognitive, affective, and behavioral dimensions of their pain may have contributed to these significant differences between the two high pain profiles in long-term health outcomes.
Because FAP and FGIDs in general often occur concurrently with psychiatric disorders and other chronic pain complaints, we examined these comorbidities in the assessment of long-term outcomes. At follow-up, patients with the High Pain Dysfunctional baseline profile had more than double the rates of FGID with comorbid chronic pain and psychiatric disorder compared to patients with the High Pain Adaptive and Low Pain Adaptive baseline profiles. This finding has important clinical and public health significance, as it suggests that allocation of intensive specialized treatments in childhood following the medical evaluation for FAP should be directed at the subgroup of patients, approximately 23% in this cohort, who fit the High Pain Dysfunctional profile and are at significantly higher risk for poor health outcomes compared to other FAP patients.
Perhaps our most intriguing finding was that FAP patients with the High Pain Dysfunctional baseline profile showed laboratory evidence of elevated central sensitization to pain an average of nine years later. That is, in laboratory pain testing at follow-up, patients with the High Pain Dysfunctional baseline profile exhibited enhanced wind-up to heat pain consistent with elevated central sensitization to painful stimuli. This finding is consistent with literature linking chronic pain to central sensitization in adults [43,72] and suggests that FAP patients with the High Pain Dysfunctional profile may share characteristics with what have been termed “central pain syndromes” [70], a group of disorders (e.g., fibromyalgia, non-cardiac chest pain, headache) that often are comorbid with each other, have high rates of concurrent psychiatric disorder, and are associated with dysfunctional central processing of pain [71].
The finding of central sensitization at long-term follow-up in those with the High Pain Dysfunctional baseline profile raises the question of whether patients with this profile already had elevated pain sensitivity at the time of their initial tertiary care evaluation for FAP. If so, interactions among maladaptive psychological (catastrophizing, negative affect) and physiological (central sensitization) characteristics might potentially have contributed to long-term chronic pain comorbidities. For example, high negative affect and low perceived coping potential associated with the High Pain Dysfunctional profile might play a role in elevated pain-related fear, which, in combination with physiologically-based central pain hypersensitivity, might increase avoidance of activities previously associated with pain. Such avoidance, in turn, might lead to deconditioning and social isolation that have the potential to exacerbate pain and disability. Of course, it also is possible that patients with the other FAP profiles had elevated pain sensitivity in childhood that resolved by the time of the follow-up study.
Future research should evaluate central pain modulation, including both central sensitization (wind-up) and descending pain inhibitory function [45], in FAP patients at the initial subspecialty medical evaluation that rules out significant organic disease. Comorbidities that are common in FGIDs, such as non-abdominal pain and psychiatric disorders, also should be investigated at the time of initial FAP evaluation in childhood. Longitudinal designs with multiple assessments over time [32] are needed to understand how a trajectory of long-term chronic pain may develop early in life in some FAP patients.
The cluster analytic approach used in this study was helpful in elucidating psychological processes that might underlie pain outcomes. Specifically, the constellation of characteristics associated with the High Pain Dysfunctional profile are consistent with models of chronic pain that emphasize high threat appraisal, low perceived pain efficacy, and avoidant coping as mechanisms contributing to the persistence of pain and disability [59,66]. In addition to the psychological characteristics included in the profiles described here, genetic [8,18,49,60] and social contextual factors [9,36,40,68] may play a role in FAP and merit inclusion in future investigations.
The present study is limited by the absence of baseline assessment of psychiatric disorders and non-abdominal chronic pain. It is possible that these comorbidities already were overrepresented among FAP patients with the High Pain Dysfunctional profile at initial evaluation. The study also is limited by loss of some participants to follow-up. However, those who participated in the follow-up did not differ from non-participants on baseline pain characteristics, suggesting that results are representative of the cohort under study. It also should be noted that results of cluster analysis may be sample dependent; this issue was mitigated here by split sample replication of the cluster solution. Nonetheless, the profiles should be replicated in FAP and other pediatric pain conditions. Finally, additional external validation of the profiles should include objective measures such as school absence.
In a recent review of prognostic studies of pediatric chronic abdominal pain, Gieteling and colleagues [28] found insufficient evidence that either baseline pain severity or children’s psychosocial characteristics predicted the persistence of abdominal pain. The reviewed studies had methodological limitations including unvalidated measures, retrospective identification of research participants by medical chart review, and limited evaluation of outcomes at follow-up. Moreover, these and other [30] studies of FAP outcomes to date have examined child characteristics individually as predictors of abdominal pain outcomes. In contrast, using cluster analysis to capture the multidimensional nature of chronic pain, the present study provided strong evidence that baseline pain severity in conjunction with pain-specific psychological characteristics do indeed predict long-term outcomes of pediatric FAP.
Strengths of this study included the large sample comprised of consecutive new pediatric patients who underwent medical evaluation for FAP to rule out organic disease, a multi-year prospective design, and evaluation of multiple outcomes using established diagnostic criteria. The Rome III criteria for FGIDs were applied in evaluating FAP outcomes; this is an important advance because abdominal pain complaints are common in the general population and do not necessarily reflect a clinically significant disorder [50,56]. Moreover, use of a structured psychiatric diagnostic interview allowed us to identify clinically significant psychiatric disorders at follow-up. Finally, while several population based studies have linked internalizing emotional symptoms in childhood to the persistence of various types of pediatric pain [6,22,35,44,46,56], this is the first study to prospectively link pain-specific psychological characteristics such as pain catastrophizing and perceived pain coping efficacy in childhood to the persistence of pain into adolescence and young adulthood.
Classification of FGIDs based on patterns of GI symptoms is helpful in understanding and managing GI symptoms associated with FAP. The taxonomy of FAP profiles developed here reflects patterns of pain-related psychological characteristics that may significantly affect long-term clinical outcomes and also are potential targets for intervention. Further research is needed to determine the extent to which these FAP profiles are associated with different underlying etiologies and unique treatment needs that may benefit from tailored interventions.
Acknowledgments
This research was supported by R01 HD23264 from the National Institute on Child Health and Development and does not necessarily represent the official views of the National Institute on Child Health and Development or the National Institutes of Health. Support also was provided by the Vanderbilt Kennedy Center (P30 HD15052), the Vanderbilt Digestive Disease Research Center (DK058404), and the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health.
Footnotes
None of the authors have a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JL, Acra S, Bruehl S, Walker LS. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47(3):309–315. doi: 10.1097/MPG.0b013e3181653a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azpiroz F, Dapoigny M, Pace F, Muller-Lissner S, Coremans G, Whorwell P, Stockbrugger RW, Smout A. Nongastrointestinal disorders in the irritable bowel syndrome. Digestion. 2000;62(1):66–72. doi: 10.1159/000007780. [DOI] [PubMed] [Google Scholar]
- 3.Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47(3):299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergstrom C, Hagberg J, Bodin L, Jensen I, Bergstrom G. Using a psychosocial subgroup assignment to predict sickness absence in a working population with neck and back pain. BMC Musculoskelet Disord. 2011;12:81. doi: 10.1186/1471-2474-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boersma K, Linton SJ. Psychological processes underlying the development of a chronic pain problem: a prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin J Pain. 2006;22(2):160–166. doi: 10.1097/01.ajp.0000159582.37750.39. [DOI] [PubMed] [Google Scholar]
- 6.Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain. 2004;8(3):187–199. doi: 10.1016/j.ejpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, France CR, France J, Harju A, al’Absi M. How accurate are parental chronic pain histories provided by offspring? Pain. 2005;115(3):390–397. doi: 10.1016/j.pain.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Buonavolontà R, Coccorullo P, Turco R, Boccia G, Greco L, Staiano A. Familial aggregation in children affected by functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2010:1. doi: 10.1097/MPG.0b013e3181b182ef. [DOI] [PubMed] [Google Scholar]
- 9.Campo JV, Bridge J, Lucas A, Savorelli S, Walker L, Di Lorenzo C, Iyengar S, Brent DA. Physical and emotional health of mothers of youth with functional abdominal pain. Arch Pediatr Adolesc Med. 2007;161(2):131–137. doi: 10.1001/archpedi.161.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Chung OY, Bruehl S, Diedrich L, Diedrich A. The impact of blood pressure and baroreflex sensitivity on wind-up. Anesth Analg. 2008;107(3):1018–1025. doi: 10.1213/ane.0b013e31817f8dfe. [DOI] [PubMed] [Google Scholar]
- 11.Claar RL, Baber KF, Simons LE, Logan DE, Walker LS. Pain coping profiles in adolescents with chronic pain. Pain. 2008;140(2):368–375. doi: 10.1016/j.pain.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1–2):77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J. rc: A profile similarity coefficient invariant over variable reflection. Psychol Bull. 1969;71(4):281–284. doi: 10.1037/h0026865. [DOI] [PubMed] [Google Scholar]
- 14.Creed FH. Relationship between IBS and psychiatric disorder. In: Camilleri M, Spiller R, editors. Irritable Bowel Syndrome: Diagnosis and Treatment. W.B. Saunders; 2002. pp. 45–54. [Google Scholar]
- 15.Davis PJ, Reeves JL, 2nd, Graff-Radford SB, Hastie BA, Naliboff BD. Multidimensional subgroups in migraine: differential treatment outcome to a pain medicine program. Pain Med. 2003;4(3):215–222. doi: 10.1046/j.1526-4637.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 16.Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152(4):802–808. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Jr, Walker LS, Kanda PT. Chronic abdominal pain in children: A technical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40(3):249–261. doi: 10.1097/01.mpg.0000154661.39488.ac. [DOI] [PubMed] [Google Scholar]
- 18.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 19.DiNardo PA, Brown TA, Barlow DH. Client Interview Schedule. Psychological Corporation; 1994. Anxiety Disorders Interview Schedule for DSM-IV. [Google Scholar]
- 20.Drossman DA. Rome III: the functional gastrointestinal disorders. McLean, Va: Degnon Associates; 2006. [Google Scholar]
- 21.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 22.Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: a prospective cohort study. Pain. 2011;152(1):66–73. doi: 10.1016/j.pain.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21(5):387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- 24.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75(1):121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 25.Gatchel RJ, Noe CE, Pulliam C, Robbins H, Deschner M, Gajraj NM, Vakharia AS. A preliminary study of multidimensional pain inventory profile differences in predicting treatment outcome in a heterogeneous cohort of patients with chronic pain. Clin J Pain. 2002;18(3):139–143. doi: 10.1097/00002508-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 27.Gieteling MJ, Bierma-Zeinstra SM, Passchier J, Berger MY. Prognosis of chronic or recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr. 2008;47(3):316–326. doi: 10.1097/MPG.0b013e31815bc1c1. [DOI] [PubMed] [Google Scholar]
- 28.Gieteling MJ, Bierma-Zeinstra SM, van Leeuwen Y, Passchier J, Berger MY. Prognostic factors for persistence of chronic abdominal pain in children. J Pediatr Gastroenterol Nutr. 2011;52(2):154–161. doi: 10.1097/MPG.0b013e3181e82a28. [DOI] [PubMed] [Google Scholar]
- 29.Gironda RJ, Clark ME. Cluster analysis of the pain outcomes questionnaire. Pain Med. 2008;9(7):813–823. doi: 10.1111/j.1526-4637.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 30.Helgeland H, Sandvik L, Mathiesen KS, Kristensen H. Childhood predictors of recurrent abdominal pain in adolescence: A 13-year population-based prospective study. J Psychosom Res. 2010;68(4):359–367. doi: 10.1016/j.jpsychores.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Hollingshead AB. Unpublished Manuscript. 1975. Hollingshead Four Factor Index of Social Status. [Google Scholar]
- 32.Jones GT. Pain in children - a call for more longitudinal research. Pain. 2011;152(10):2202–2203. doi: 10.1016/j.pain.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Psychological aspects of persistent pain: current state of the science. J Pain. 2004;5(4):195–211. doi: 10.1016/j.jpain.2004.02.576. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs M. Children’s Depression Inventory (CDI) New York: Multi-health Systems, Inc; 1992. [Google Scholar]
- 35.Kroner-Herwig B, Gassmann J, van Gessel H, Vath N. Multiple pains in children and adolescents: a risk factor analysis in a longitudinal study. J Pediatr Psychol. 2011;36(4):420–432. doi: 10.1093/jpepsy/jsq099. [DOI] [PubMed] [Google Scholar]
- 36.Langer SL, Romano JM, Levy RL, Walker LS, Whitehead WE. Catastrophizing and parental response to child symptom complaints. Child Health Care. 2009;38(3):169–184. doi: 10.1080/02739610903038750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer SL, Walker LS, Romano JM, Whitehead WE, Feld L, Levy RL. Predictors of maternal responses to child abdominal pain. Child Health Care. 2007;36(1):63–81. [Google Scholar]
- 38.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 39.Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, DuPen MM, Feld AD, Ballard SA, Welsh EM, Jeffery RW, Young M, Coffey MJ, Whitehead WE. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol. 2010;105(4):946–956. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, Christie D. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99(12):2442–2451. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 41.Lipsitz JD, Gur M, Albano AM, Sherman B. A psychological intervention for pediatric chest pain: development and open trial. J Dev Behav Pediatr. 2011;32(2):153–157. doi: 10.1097/DBP.0b013e318206d5aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyneham HJ, Abbott MJ, Rapee RM. Interrater reliability of the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent version. J Am Acad Child Adolesc Psychiatry. 2007;46(6):731–736. doi: 10.1097/chi.0b013e3180465a09. [DOI] [PubMed] [Google Scholar]
- 43.Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, Knott C, Slade GD, Ohrbach R, Diatchenko L, Fillingim RB. Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12(11 Suppl):T75–91. doi: 10.1016/j.jpain.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikkelsson M, El-Metwally A, Kautiainen H, Auvinen A, Macfarlane GJ, Salminen JJ. Onset, prognosis and risk factors for widespread pain in schoolchildren: a prospective 4-year follow-up study. Pain. 2008;138(3):681–687. doi: 10.1016/j.pain.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 46.Paananen MV, Taimela SP, Auvinen JP, Tammelin TH, Kantomaa MT, Ebeling HE, Taanila AM, Zitting PJ, Karppinen JI. Risk factors for persistence of multiple musculoskeletal pains in adolescence: a 2-year follow-up study. Eur J Pain. 2010;14(10):1026–1032. doi: 10.1016/j.ejpain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robins PM, Glutting JJ, Shaffer S, Proujansky R, Mehta D. Are there psychosocial differences in diagnostic subgroups of children with recurrent abdominal pain? J Pediatr Gastroenterol Nutr. 2005;41(2):216–220. doi: 10.1097/01.mpg.0000170601.88263.50. [DOI] [PubMed] [Google Scholar]
- 49.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138(4):1276–1285. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154(3):322–326. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 51.Schniering CA, Hudson JL, Rapee RM. Issues in the diagnosis and assessment of anxiety disorders in children and adolescents. Clin Psychol Rev. 2000;20(4):453–478. doi: 10.1016/s0272-7358(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 52.Schurman JV, Danda CE, Friesen CA, Hyman PE, Simon SD, Cocjin JT. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings. 2008;15(3):241–251. doi: 10.1007/s10880-008-9120-0. [DOI] [PubMed] [Google Scholar]
- 53.Schurman JV, Friesen CA, Danda CE, Andre L, Welchert E, Lavenbarg T, Cocjin JT, Hyman PE. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41(3):291–295. doi: 10.1097/01.mpg.0000178438.64675.c4. [DOI] [PubMed] [Google Scholar]
- 54.Silverman WK, Albano AM. Book The Anxiety Disorders Interview Schedule for Children for DSM-IV: Child and parent versions. City: Psychological Corporation; 1996. The Anxiety Disorders Interview Schedule for Children for DSM-IV: Child and parent versions. [Google Scholar]
- 55.Smith CA, Lazarus RS. Emotion and adaptation. In: Pervin LA, editor. Handbook of Personality Theory and Research. New York: Guilford; 1990. pp. 609–637. [Google Scholar]
- 56.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138(1):11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 57.Starfield B, Gross E, Wood M, Pantell R, Allen C, Gordon IB, Moffatt P, Drachman R, Katz H. Psychosocial and psychosomatic diagnoses in primary care of children. Pediatrics. 1980;66(2):159–167. [PubMed] [Google Scholar]
- 58.Turk DC, Sist TC, Okifuji A, Miner MF, Florio G, Harrison P, Massey J, Lema ML, Zevon MA. Adaptation to metastatic cancer pain, regional/local cancer pain and non-cancer pain: role of psychological and behavioral factors. Pain. 1998;74(2–3):247–256. doi: 10.1016/s0304-3959(97)00187-5. [DOI] [PubMed] [Google Scholar]
- 59.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 60.von Baeyer CL, Champion GD. Commentary: Multiple pains as functional pain syndromes. J Pediatr Psychol. 2011;36(4):433–437. doi: 10.1093/jpepsy/jsq123. [DOI] [PubMed] [Google Scholar]
- 61.Vowles KE, Jordan A, Eccleston C. Toward a taxonomy of adolescents with chronic pain: exploratory cluster and discriminant analyses of the bath adolescent pain questionnaire. Eur J Pain. 2010;14(2):214–221. doi: 10.1016/j.ejpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137(2):266–275. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker LS, Beck JE, Garber J, Lambert W. Children’s Somatization Inventory: psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34(4):430–440. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker LS, Garber J, Smith CA, Van Slyke DA, Claar RL. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J Consult Clin Psychol. 2001;69(1):85–91. [PMC free article] [PubMed] [Google Scholar]
- 65.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 66.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24(4):364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the Pain Response Inventory for Children. Psychol Assess. 1997;9(4):392–405. [Google Scholar]
- 68.Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain. 2006;122(1–2):43–52. doi: 10.1016/j.pain.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 70.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10(8):777–791. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3, Supplement):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Q, Price DD, Callam CS, Woodruff MA, Verne GN. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J Pain. 2011;12(2):297–303. doi: 10.1016/j.jpain.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


