Abstract
Background
(±)-Modafinil has piqued interest as a treatment for ADHD and stimulant dependence. The R-enantiomer of modafinil may have unique pharmacological properties that should be further investigated.
Methods
(±)-Modafinil and its R-(−)- and S-(+)-enantiomers were synthesized and tested for inhibition of [3H]DA uptake and [3H]WIN 35,428 binding in hDAT WT and mutants with altered conformational equilibria. Data were compared to cocaine and the atypical dopamine uptake inhibitor, JHW 007. R- and S-modafinil were also evaluated in microdialysis studies in the mouse NAc shell and in a cocaine discrimination procedure.
Results
(±)-, R- and S-Modafinil bind to the DAT and inhibit dopamine uptake less potently than cocaine, with R-modafinil having ~3-fold higher affinity than its S-enantiomer. Molecular docking studies revealed subtle differences in binding modes for the enantiomers. R-modafinil was significantly less potent in the DAT Y156F mutant compared to wild-type DAT, whereas S-modafinil was affected less. Studies with the Y335A DAT mutant showed that the R- and S-enantiomers tolerated the inward facing conformation better than cocaine, which was further supported by MTSET reactivity on the DAT E2C I159C. Microdialysis studies demonstrated that both R- and S-modafinil produced increases in extracellular DA concentrations in the NAc shell less efficaciously than cocaine, and with a longer duration of action. Both enantiomers fully substituted in mice trained to discriminate cocaine from saline.
Conclusions
R-modafinil displays an in vitro profile different from cocaine. Future trials with R-modafinil as a substitute therapy with the potential benefit of cognitive enhancement for psychostimulant addiction are warranted.
Keywords: dopamine transporter, cocaine, methamphetamine, addiction, microdialysis, abuse liability
The development of medications to treat stimulant abuse disorders remains an unmet medical need despite decades of research (1–3). One approach to this challenge is using “reverse translation” of clinically available medications that may have mechanisms of action that are related to those associated with addictive drugs, such as inhibition of dopamine (DA) reuptake via the dopamine transporter (DAT). By further investigating these agents at the molecular level and relating these observations to behavior, a rationale for testing these agents in humans addicted to psychostimulants may be provided. Herein we compare cocaine to the clinically available (±) modafinil, its R-enantiomer (armodafinil) and S-modafinil at the DAT.
Modafinil (Fig. 1) is used as a wake-promoting agent for the treatment of narcolepsy and other sleep disorders (4). Modafinil has been described as a psychostimulant but is not amphetamine-like in chemical structure, pharmacological profile, or mechanism of action. As such it has sparked interest for the treatment of cognitive dysfunction in disorders such as attention deficit hyperactivity disorder (ADHD) (4, 5). Modafinil has also attracted attention for the treatment of cocaine (6) and methamphetamine dependence (7, 8). In addition, the emerging emphasis on cognitive impairment in neuropsychiatric disorders, including addiction (9), has stimulated investigations into the potential pro-cognitive effects of modafinil in this population (10–12).
Figure 1. Chemical structures of drugs used in the study.
The mechanisms of action underlying modafinil’s therapeutic actions have been debated. Modafinil modulates the activity of hypocretin, histamine, α-adrenergic, γ-aminobutyric acid (GABA) and/or glutamate receptors (4, 13). However, its ability to bind to the DAT and block DA reuptake, though with low affinity compared to cocaine, has received the most attention (14–17). Positron emission tomography (PET) studies in human subjects have demonstrated that modafinil binds to the DAT at therapeutic doses leading to alerts regarding its abuse potential (18). Although, preclinical data have suggested that modafinil is like cocaine or may reinstate cocaine taking (19–23), the preponderance of clinical literature indicates a low abuse liability (24–26).
Modafinil is comprised of R-(−)- and S-(+)-enantiomers (Fig. 1) and was originally prescribed as the racemate (Provigil) (27). However, more recent human studies suggest that R-(−)-modafinil is the more metabolically stable and longer-acting enantiomer (28–31). We recently prepared the R- and S-modafinil enantiomers, along with a series of analogues, and showed that the R-enantiomer had ~3-fold-higher affinity for the DAT than the S-enantiomer in rat brain tissue (17). Further, although the (±)-, R- and S-enantiomers all stimulated locomotor activity in mice, they were less effective and less potent than cocaine. The reduced efficacy and unusual structure of modafinil suggested that it might bind to the DAT in a different mode than cocaine. Indeed, the biphenyl ring system resembles that of the benztropine class of DAT inhibitors, exemplified by JHW 007 (Fig. 1) that has been extensively characterized as being “atypical” with potential for development as a medication to treat cocaine addiction (32, 33).
We previously compared the binding and DA uptake inhibition of a series of tropane-based DAT inhibitors in several DAT mutants that were designed to shift the conformational equilibrium toward either an outward- or inward-facing state (34, 35). In that study we discovered that the cocaine-like compounds (e.g. WIN 35,428) preferred an outward-facing conformation of the DAT, whereas the benztropines (e.g. JHW 007) preferred a more occluded conformation. Remarkably these data correlated with effectiveness in producing cocaine-like effects in rats (36). In subsequent studies, these differences in binding modes were supported and further highlighted different DAT binding interactions between these structurally distinct classes of DAT inhibitors (37, 38).
Based on those studies, we hypothesized that R- and S-modafinil might also bind the DAT differently from cocaine, contributing to their in vivo pharmacological profiles. Hence in the present study, we compare the binding of the enantiomers and their potency for inhibition of DA uptake in hDAT transfected COS-7 cells to those of cocaine. We then tested the enantiomers in DAT mutants biased towards inward- or outward-facing conformations to investigate the induction of specific conformations of the DAT by modafinil binding. In addition DA concentrations in the mouse NAc shell were assessed in vivo using microdialysis procedures. This brain area has been suggested to play a significant role in mediating the reinforcing effects of abused drugs (39–41). Finally, we tested modafinil and its enantiomers in a cocaine-discrimination procedure to determine if the enantiomers substituted for the discriminative-stimulus effects of cocaine, as (±)-modafinil has been reported to do in other species (19, 22, 23).
Methods
In vitro studies
[3H]DA uptake and [3H]WIN 35,428 binding experiments were carried out using standard methods on transiently transfected COS-7 cells expressing the human DAT WT or mutants as described previously (35) and in detail in S.I. The [2-(trimethylammonium)ethyl]-methanethiosulfonate (MTSET) labeling experiments were performed essentially as before (36) and described in detail in S.I. In short, the ligands dissolved in uptake buffer (UB) were added to the intact cells expressing either DAT E2C or DAT E2C I159C in the following concentrations: (±)-modafinil: 100 μM, R-(−)-modafinil: 100 μM, S-(+)-modafinil: 100 μM, DA: 100 μM, cocaine: 30 μM and JHW 007: 5 μM. The concentration of inhibitor was chosen as the highest possible concentration that could be washed away to allow subsequent [3H]DA analysis. MTSET was added at a final concentration of 0.5 mM and the cells were incubated at room temperature for 10 min. The preincubation was stopped and [3H]DA uptake initiated to determine the degree of transport inactivation by MTSET.
Modeling of DAT/ligand complexes
The complexes between DAT and modafinil enantiomers were modeled similar to that described previously, (38) using a well established induced-fit docking (IFD) protocol (42) and are described in detail in S.I.
Microdialysis
Methods have been described in detail elsewhere (43) and provided in S.I. Briefly, following approximately 45 hours after the surgical procedures and starting at 9.00 a.m. microdialysis sessions were initiated with probes connected via swivels and perfused with Ringer’s solution at a constant flow rate of 1 μl/min. Dialysate sampling (10 μl/10 min) started after about 30 minutes. Mice received cocaine, (±)-, S- or R-modafinil or vehicle injections only when stable DA values were obtained. Sample collection continued for 360 min, but after 2 hours occurred every 20 min. Dialysate samples were immediately injected without purification into a high-performance liquid chromatography coupled with a ESA 5200 coulochem detector to quantify DA. Assay sensitivity for DA was 2 fmoles per sample.
Cocaine Discrimination
Experimental details are essentially identical to those described previously (44) and provided in S.I.. In brief, subjects were placed in operant-conditioning chambers with overall illumination, two response levers, and pairs of green and yellow lights above each lever. Mice were trained with food reinforcement to press both levers, and eventually trained to press one after cocaine (10 mg/kg) and the other after saline i.p. injections, on a double-alternation daily schedule. The ratio of responses to food pellets was ultimately 10 (fixed-ratio or FR 10). Experimental sessions started after a 5-min period in darkness during which responses had no consequences. Following this period lights were turned on until the completion of the FR requirement and the presentation of food. Sessions ended after 20 food presentations or 15 min, whichever occurred first, and were conducted 5 days per week. Testing with different doses of cocaine or modafinil was initiated after subjects met the training criteria (see S.I.). Test sessions were identical to training sessions with the exception that 10 responses on either lever were reinforced.
Results
Assessment of the affinity for (±)-modafinil and its R- and S-enantiomers to the DAT
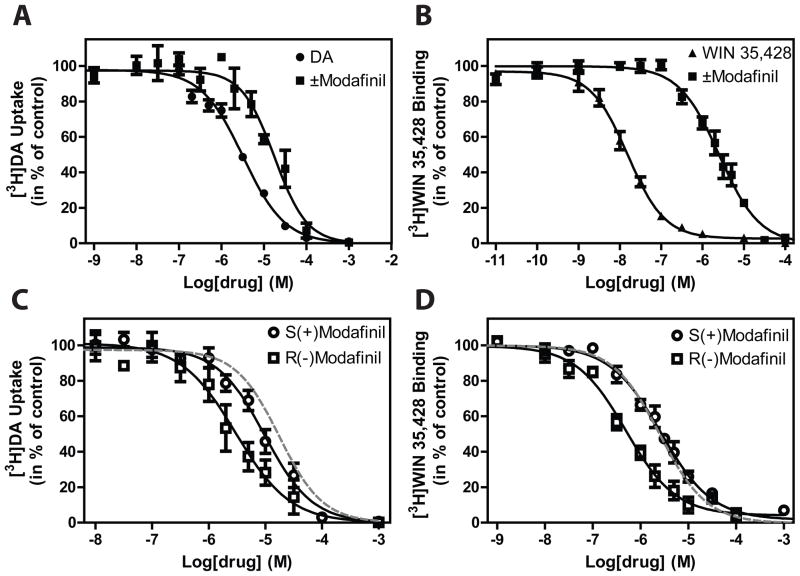
(±)-Modafinil and its enantiomers were tested for inhibition of [3H]DA uptake and displacement of [3H]WIN 35,428 (Fig. 2). (±)-Modafinil inhibited [3H]DA uptake with a potency that was more than 7-fold lower than observed for DA (inhibition potency for DA and (±)-modafinil for the DAT was 1.7 and 13 μM, respectively, Fig. 2A and Table 1). Inhibition of [3H]WIN 35,428 binding by (±)-modafinil revealed lower Ki values as compared to [3H]DA uptake inhibition (Ki = 2.3 μM, Fig. 2B and Table 1) and comparable to our previously published values in rat brain tissue (17). Interestingly, (±)-modafinil was less potent in inhibiting [3H]DA uptake than both the R- and S- enantiomers (Fig. 2C and Table 1). However, for the inhibition of [3H]WIN 35,428 binding, the observed affinity for the S-enantiomer was almost indistinguishable from the affinity for the racemate, whereas the R-enantiomer had a higher affinity (Table 1). The discrepancy between inhibition potencies of DA transport and Ki values in the binding assay could be due to the fact that [3H]DA uptake inhibition is not performed under equilibrium conditions, as the binding experiments are.
Figure 2. Characterization of (±)-, R- and S-modafinil binding to the DAT.
Inhibition of (A+C) [3H]DA uptake and (B+D) [3H]WIN 35,428 binding in COS-7 cells transiently expressing DAT WT by (●)DA, (▲) WIN 35,428, (■) (±)-modafinil, (○) S-modafinil, and (□) R-modafinil. The grey punctured lines in (C) and (D) are the observed IC50 values for (±)-modafinil from (A) and (B), respectively, shown for comparison. The observed IC50 value for a compound is basis for the calculated inhibition potencies and Ki values in Table 1. Data are means ± S.E. of 6 to10 experiments performed in triplicate.
Table 1.
[3H]DA uptake inhibition and [3H]WIN 35,428 binding data in DAT WT and mutants
| hDAT Mutants | [3H]DA Uptake (N) | IC50 (μM) [SE interval] | [3H]WIN 35,428 Binding (N) | Kd (or Ki; μM) [SE interval] | |
|---|---|---|---|---|---|
| hDAT | DA | N=6 | 1.7[1.5;2.1] | ||
| cocaine | N=12 | 0.23 [0.19;0.26] a | N=3 | 0.45 [0.34;0.59]b | |
| WIN 35,428 | N=10 | 0.013[0.012;0.014] | |||
| (±)-modafinil | N=6 | 13[10;16] | N=7 | 2.3[1.9;2.6] | |
| R-modafinil | N=9 | 4.0[2.6;6.4] | N=7 | 0.78[0.67;0.90] | |
| S-modafinil | N=9 | 8.7[7.5;10] | N=8 | 2.5 [2.2;2.9] | |
| hDAT Y156F | cocaine | N=5 | 0.35 [0.28;0.45]b | ||
| WIN 35,428 | N=6 | 0.013[0.0096;0.017] | |||
| (±)-modafinil | N=5 | 8.0[6.4;9.9] | |||
| R-modafinil | N=3 | 11[7.5;15] | |||
| S-modafinil | N=5 | 5.5[4.7;6.4] | |||
| hDAT Y335A | DA | N=3 | 0.99[0.60;1.7] | ||
| cocaine | N=12 | 24[20;30]a | |||
| (±)-modafinil | N=4 | 83[48;143] | |||
| R-modafinil | N=3 | 43[26;71] | |||
| S-modafinil | N=3 | 129[97;170] |
The inhibition potency for [3H]dopamine uptake, and the Kd or Ki for [3H]WIN 35,428 binding were calculated from non-linear regression analysis of uptake and binding data, respectively, performed on COS7 cells transiently transfected with hDAT WT or the indicated mutant. The IC50 values used in the estimation of Ki and Kd values were calculated from means of pIC50 values and the SE interval from the pIC50 ±SE (See Materials and Methods, S.I.). nd, not determined.
Data are taken from Loland et al. (36);
Data are taken from Beuming et al. (37).
We were not able to measure any significant binding or transport inhibition of the R- and S-enantiomers to either of the homologous transporters for norepinephrine (NET) or serotonin (SERT), assessed by their ability to inhibit [3H]DA uptake or [3H]nisoxetine binding at the NET and [3H]5-HT at the SERT (IC50 > 100 μM for all experiments, N=3, data not shown). These observations are also in accord with binding experiments performed in native rat brain tissue (17).
The modafinil binding site in DAT overlaps with the central binding site for substrate (S1)
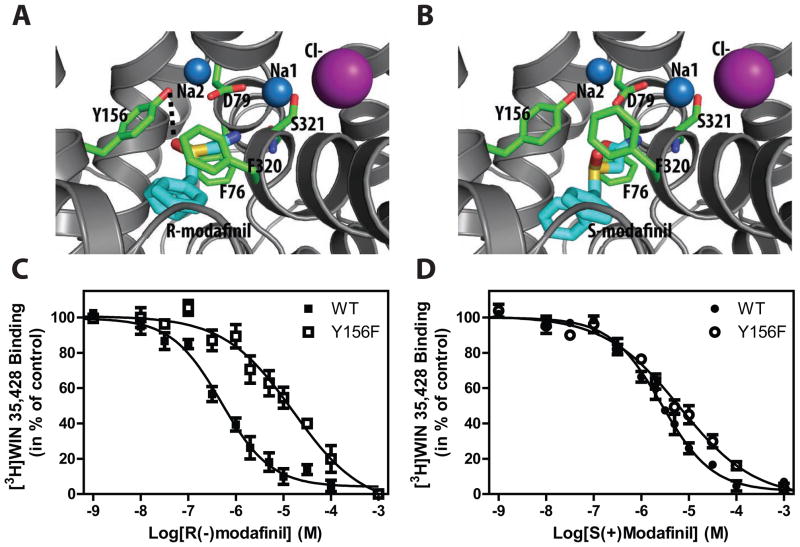
To characterize the modafinil binding site in the DAT, we carried out a docking study of the two enantiomers with DAT models described previously (37, 38) based on the crystal structure of the bacterial homologue, LeuT (Fig. 3A and B). The modafinil enantiomers were docked in the primary binding pocket (S1) in the center of the protein that is also the binding site for DA and cocaine (37) as well as for the atypical DAT inhibitor JHW 007 and its analogues (38). The top-ranked binding poses revealed a significant similarity between the binding modes of the enantiomers, however, with unique interactions. One distinctive structural feature of modafinil, compared to either cocaine or JHW 007, is that it lacks a charged pyramidal nitrogen. Thus, while the orientations of the biphenyl ring systems in the binding modes of both modafinil enantiomers may be similar to those of the benztropine derivatives (38), there is no direct interaction with Asp79. In contrast, the terminal amide moiety of modafinil tends to stack with the phenyl ring of Phe76 and H-bond to the backbone of Ser321. These restraints result in different positioning of the chiral S=O in the R- and S-enantiomers. Although both enantiomers are in close vicinity to Tyr156, the S=O of R-modafinil interacts with the -OH group of the Tyr156 residue, whereas this interaction does not occur with S-modafinil.
Figure 3. Localization of the binding sites for R- and S-modafinil in the DAT.
(A) and (B) The predicted binding modes of R-modafinil and S-modafinil in the central binding site of DAT, respectively. Note the amide bond of both modafinils are stacked with the phenyl ring of Phe76 and carboxyl group of Asp79, while only the sulfinyl group of R-modafinil interacts with the hydroxyl group of Tyr156. The modafinil enantiomers are in thicker stick presentation with the carbon colored in cyan. (C) Removal of the hydroxyl group on Tyr156 in DAT (Y156F. open symbols) results in a >14-fold decrease in IC50 for R-modafinil as compared to the DAT WT (solid symbols). (D) For S-modafinil, the Y156F mutation caused a 2-fold decrease in the IC50-value. All data in C+D are assessed by displacement of [3H]WIN 35,428 binding by the indicated modafinil enantiomer performed on intact COS-7 cells transiently transfected with DAT WT or mutant. Data are means ± S.E. of 3 to 8 experiments performed in triplicate.
Thus, we sought to experimentally validate the docking results and hypothesized that the removal of the OH-group of Tyr156 by changing it to a phenylalanine (Y156F) could disrupt the interaction with R-modafinil, but have little effect on the S-modafinil binding interaction. Indeed R-modafinil showed a marked decrease in affinity for the DAT Y156F mutant as compared to DAT WT protein (Fig. 3C) with a 14-fold change (Table 1, compare WT and Y156F DAT binding), whereas only a 2-fold difference in affinity was observed for S-modafinil by the Y156F mutation (Fig. 3D, Table 1). These data are in agreement with the docking models and suggest that only R-modafinil interacts with Tyr156 in the primary binding pocket.
R- and S-modafinil preferentially bind to a different DAT conformation than cocaine
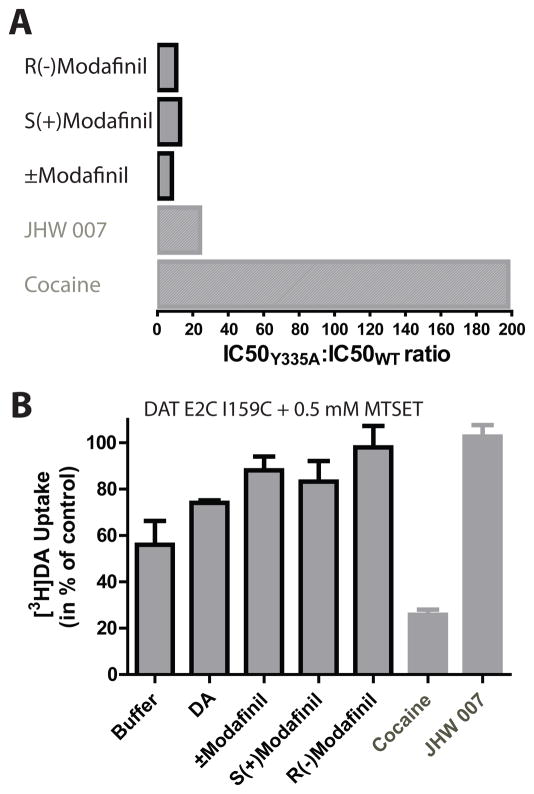
To identify the preferred DAT conformational state induced by R- and S-modafinil, two previously established methods (36) that assess the conformational state of the DAT upon binding of different ligands were employed: (i) The shift in IC50 for [3H]DA uptake inhibition by the modafinil enantiomers between the WT and a Tyr335 to alanine mutation, and (ii) the accessibility of a cysteine inserted in TM3 to the cysteine-reactive compound [2-(trimethylammonium)ethyl]-methanethiosulfonate (MTSET). We have previously shown that Tyr335 is critical for regulating conformational isomerization in the transport cycle (34, 35, 45) and that mutation of this residue (Y335A) shifts the conformational equilibrium towards an inward facing conformation. This suggestion has recently been supported by the crystallization of the inward facing conformation of LeuT using the cognate mutation (46). Thus, the Y335A mutation can be used as a tool to probe whether a drug favors an inward-facing or outward-facing conformation (36). To assess this for the modafinil enantiomers, we investigated their [3H]DA uptake inhibition potency in the DAT Y335A mutant and compared it to WT (Fig. 4A). As previously determined, DAT inhibitors such as cocaine bind preferentially to the outward-facing conformation of the DAT. This results in a large decrease in potency for [3H]DA uptake inhibition between DAT WT and the Y335A mutant as determined in Fig. 4A for cocaine, resulting in an almost 200-fold change in IC50. On the contrary, the non-stimulant atypical DAT inhibitor JHW 007 shows only a minor, ~20-fold, change in IC50, suggesting that it binds to a conformation that differs from that which is preferred by cocaine (Fig. 4A). Performing the same experiments on the R- and S-modafinil enantiomers gave Y335A:WT IC50 ratios (10.6 and 14.8, respectively) that approximate that for JHW 007 (Fig. 4A). This suggests that these DA uptake inhibitors bind to a DAT conformation distinct from the cocaine-induced conformation and closer to the one observed for JHW 007.
Figure 4. R- and S-modafinil bind to a DAT conformation that differs from cocaine.
(A) Effect of the Y335A mutation on IC50 values for inhibition potency of [3H]DA uptake by the (±)-modafinil and enantiomers, JHW007 and cocaine compared to the DAT WT. The calculated difference in inhibition potency (IC50) of [3H]DA uptake by (±)-modafinil and enantiomers in the DAT Y335A mutant relative to WT is displayed as an IC50Y335A:IC50WT ratio. The data for JHW007 and cocaine are shown in grey for comparison and are in agreement with previously determinaed data (36). (B) Effect of (±)-modafinil and enantiomers on MTSET (0.5 mM) inhibition of [3H]DA uptake on the DAT E2C I159C (a mutant in which two endogenous cysteines, Cys90 and Cys306, has been changed to alanines rendering it insensitive to MTSET (36), data not shown). Data are shown as mean±SEM of the effect of preincubating with the indicated drug on the MTSET reactivity. 100% activity is set as the preincubation of drug alone followed by vehicle only. All experiments are performed on COS7 cells transiently expressing DAT WT or mutant of at least three experiments performed in triplicate.
The second assay is based on the reactivity of a cysteine inserted into position 159 in TM3 of the DAT located in the vicinity of the ligand binding site on the extracellular side. Previous observations in DAT (35, 36, 47, 48), NET and SERT (49) have suggested that the accessibility of this position is dependent on the conformational state of the transporter: it is accessible to the extracellular environment when the DAT is in the outward-facing conformation and inaccessible in the closed or inward-facing conformation. Importantly, reaction of an inserted cysteine in this position (I159C) with MTSET results in inactivation of the DAT allowing the use of DA uptake as a functional read-out for I159C reactivity (36). The I159C mutant was generated in a MTSET insensitive DAT background (E2C) in which the two external endogenous cysteines were mutated to alanines (C90A-C306A). Incubation of 0.5 mM MTSET for 10 min in buffer resulted in an inactivation to about 60% of the initial [3H]DA transport capacity. The addition of cocaine (30 μM) together with MTSET resulted in a marked increase in inactivation to about only 20% remaining transport capacity, whereas DA (100 μM) and, in agreement with previous results (36), JHW 007 (5 μM) caused a protection from the MTSET reactivity (Fig 4B). The addition of the R- or S-modafinil enantiomers (100 μM) caused a similar protection of Cys159 from MTSET reactivity as observed for JHW 007 (98±9 % and 83±9 % for R- and S-modafinil, respectively, compared to 102±5 % for JHW 007, N=4–8, Fig. 4B). This further suggests that both modafinil enantiomers induce a conformation of the DAT in which the extracellular vestibule is closed, thus protecting Cys159 from reacting with the added MTSET.
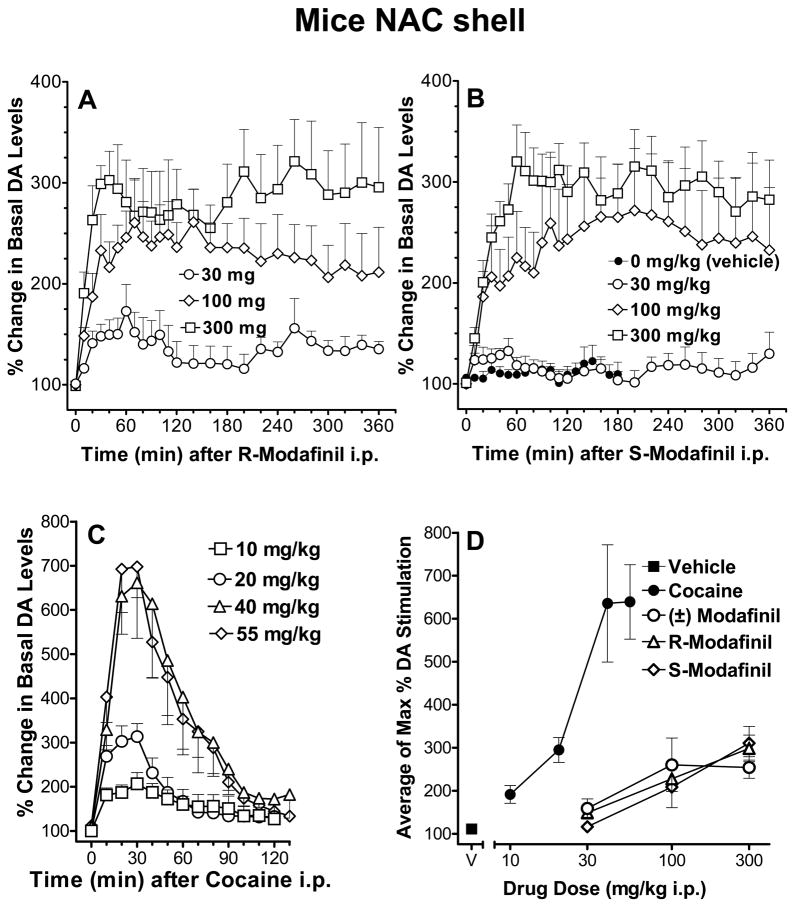
Microdialysis studies with cocaine, (±)-, R- and S-modafinil
To investigate the comparative pharmacology of modafinil and cocaine, we measured extracellular DA concentrations in the mouse NAc shell (see Fig. 5 for statistical analyses). R-modafinil, (30, 100, 300 mg/kg i.p.), significantly stimulated DA levels to approximately 300% of DA basal levels at 40–60 min after injection. These levels were maintained throughout the 6 hours of measurement (Fig. 5A). Similar effects were obtained with S-modafinil (Fig. 5B). As in previous reports (43, 50), cocaine (10 – 55 mg/kg) significantly stimulated the extracellular levels of DA, with rapid onsets and offsets of action. DA reached a maximum of approximately 700% of basal levels at ~30 min after cocaine injection (Fig 5C). The maximal increases in DA levels were strongly related to the dose of cocaine, whereas the slopes of the dose-effect curves for modafinil and its enantiomers were much more shallow (Fig. 5D), indicating a limited dose-dependency in effects of modafinil and a lower level of maximal stimulation of DA compared to cocaine (43).
Figure 5.
Panels A and B show the effects of R- and S-modafinil, respectively, on extracellular levels of DA in dialysates from the NAc shell in mice. For panels A–C ordinates represent the change in extracellular DA concentration as a percentage of basal values. Abscissae represent the time after drug administration. Drugs were administered at doses of 30, 100 and 300 mg/kg i.p. Basal DA values were 50.4±3.9 (n=5), 50.3±3.8 (n=6), and 40.4±4.6 (n=5) fmoles/sample for respective doses of R-modafinil; and 70.0±9.4 (n=5), 52.6±4.8 (n=7), and 44.1±5.4 (n=5) fmoles/sample for S-modafinil. Two-way ANOVA indicated main effects of R-modafinil dose (F3,16=8.652, p<0.01), time (F24,384=7.776, p<0.001), and their interaction (F72,384=2.212, p<0.001). An ANOVA for S-modafinil indicated main effects of dose (F3,17=5.259, p<0.01), time (F24,408=6.441, p<0.001) and their interaction (F72,408=2.385, p<0.001). Panel C shows the dose-dependent effects of acute administration of cocaine on extracellular DA levels in the NAc shell in mice at doses of 10 to 55 mg/kg. Group size, n = 5 for all groups; basal DA values were 48.5±8.1, 57.4±12.2, 29.8±6.3, and 34.2±6.0 fmoles/sample, for respective doses of cocaine. A two-way ANOVA indicated main effects of cocaine dose (F3,17=6.636, p<0.01), time (F12,204=39.189, p<0.001), and their interaction (F12,36=5.376, p<0.001). Each point represents the mean DA levels in 10-min dialysate samples, expressed as a percentage of basal values. For panel D ordinates represent the change in extracellular DA concentration as a percentage of basal values during the 30-min period after drug administration in which maximal stimulation of DA was observed. Abscissae, dose of drug in milligrams per kilogram, log scale. V=Vehicle, 10% DMSO + 15% Tween 80 in sterile water. Vertical bars in all panels represent SEM.
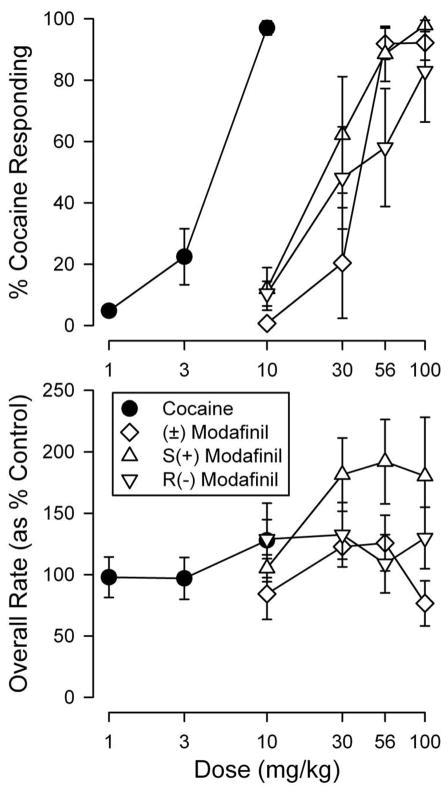
Cocaine Drug Discrimination
Cocaine produced a dose-related increase in the percentage of drug-appropriate responses in mice trained to discriminate cocaine (10 mg/kg) from saline injections (Fig. 6, filled symbols). The (±)-modafinil and both enantiomers fully substituted for cocaine, though with approximately one tenth the potency of cocaine on a molar basis (Table 2). There were no significant differences in potency between the enantiomers of modafinil, similar to the observations in the microdialysis studies.
Figure 6.
Effects of various doses of cocaine and modafinil enantiomers in mice trained to discriminate injections of cocaine (10 mg/kg) from saline. Cocaine was administered 5 min before and the modafinil enantiomers were administered 60 min before testing. Ordinates for the top panel indicate percentage of responses on the cocaine-appropriate key, and ordinates for the bottom panel indicate the rates at which responses were emitted (as a percentage of response rates after saline administration). Abscissae: drug dose in mg/kg (log scale). Each point represents average effects of six mice, with the exception of the highest dose of R-modafinil, which was examined in only five subjects. Note the dose-effect curve for R-modafinil has been “nudged” to the left to ensure that it can be discerned in the graphic presentation of the data.
Table 2.
Comparisons of ED50 values and potencies relative to cocaine of the enantiomers of modafinil in substituting for cocaine in mice trained to discriminate cocaine from saline injections. Values in parentheses are 95% confidence limits.
| Compound | ED50 Value (μmol/kg) | Potency Relative to Cocaine |
|---|---|---|
| cocaine | 11.3* (9.60 – 13.5) | — |
| (±)-modafinil | 125* (99.2 – 157) | 0.0896* (0.0677 – 0.120) |
| S-modafinil | 89.0 (64.0 – 115) | 0.127† (0.0881 – 0.191) |
| R-modafinil | 132 (77.9 – 226) | 0.0883 (0.0513 – 0.159) |
Significant deviation from linearity
Significant effect of preparations
Discussion
(±)-Modafinil and its R- and S-enantiomers bind with relatively low affinity to the hDAT and inhibit DA uptake in COS7 cells, with the R- slightly more potent than the S-enantiomer. As reported previously in rat brain tissue (17), neither enantiomer showed measurable binding to SERT or NET.
Both the R- and S-enantiomers docked at a common DAT binding pocket significantly overlapping with the S1 binding site for DA and cocaine (37), as well as for the atypical DAT inhibitors, e.g. JHW 007 (38). Although a significant overlap in binding of the modafinil enantiomers was apparent, a unique residue Tyr156 that coordinated differently with the R- and S-enantiomers was identified (Fig. 3A and B). In order to verify these models, we investigated the interaction of the enantiomers with Tyr156, through a single point mutation. According to the model, both enantiomers interact with Tyr156, but only the R-enantiomer interacts with the Tyr-OH group. Thus, we hypothesized that removal of the OH-group with mutation of Tyr156 to a phenylalanine would disrupt R-modafinil but have little effect on S-modafinil binding. Indeed R-modafinil showed a 14-fold decrease in affinity for the DAT Y156F mutant as compared to DAT WT protein (Fig. 3C; Table 1), whereas only a minor difference in affinities were obtained with S-modafinil. Moreover the removal of the OH-group disrupts a hydrogen bond to Asp79 in TM1, which presumably alters the orientation of the residue and therefore only slightly affects coordination to the S-modafinil.
We have previously provided evidence that DAT Tyr335 is critical for regulating conformational isomerization in the transport cycle (34, 35, 45). Tyr335 is located in the third intracellular loop, and is 100% conserved throughout the family of Neurotransmitter:Sodium Symporter (NSS) proteins (51). Mutation of this residue changes the conformational equilibrium of the DAT resulting in a transporter residing preferentially in an inward-facing conformation (52). The crystal structure of LeuT, a bacterial homolog of DAT, supports the suggestion of Tyr335 as part of an intracellular gate (46, 53). Our experiments revealed that the R- and S-modafinil enantiomers gave Y335A:WT IC50 ratios that were similar to those for JHW 007 and in contrast to cocaine. This suggests that these DAT inhibitors bind differently from the cocaine-induced conformation and closer to the one observed for JHW 007.
Previous observations of DAT (36), NET and SERT (49) have suggested that Cys159 is accessible to the extracellular environment when the transporter is in an outward-facing conformation, but becomes less accessible when the extracellular gate closes and the DAT isomerizes toward an inward facing conformation. Importantly, reaction of Cys159 with the sulfhydryl reactive MTSET, results in inactivation of the transporter allowing the use of DA uptake as a functional read-out for I159C reactivity (36). In this experiment, both R- and S-modafinil protected Cys159 from reacting with MTSET, further supporting their binding mode as preferring a more extracellular occluded conformation of the DAT, unlike cocaine (Fig. 4B). These findings are consistent with the recently reported study of W84L and D313N DAT mutants wherein (±)-modafinil displayed WT/mutant ratios different from cocaine and more like those of the DAT inhibitors benztropine, GBR 12909 and bupropion (54).
Although these in vitro experiments suggested that the modafinil enantiomers may be more like atypical DAT inhibitors, several reports of (±)-modafinil in models of psychostimulant abuse suggest a cocaine-like pharmacological profile (4–6). Consistent with the literature, cocaine, modafinil, and its enantiomers stimulated DA levels in the NAc shell, suggesting that these drugs might produce reinforcing effects like those produced by other abused drugs (39–41). However, cocaine administration showed a time- and dose-related stimulation of DA levels that differed from that produced by modafinil and its enantiomers. Because a temporal contingency between drug-injection and drug-effects is an important feature of the reinforcing effects of drugs, this predicts that modafinil and its enantiomers will have lower liability for abuse in humans. Moreover, the highest doses of modafinil and its enantiomers reached similarly lower maximal effects on DA levels than those with cocaine, suggesting that though they block DA reuptake, they do so differently than cocaine. Also, at variance with cocaine, the DA elevations produced by modafinil and its enantiomers were obtained at doses 10–15 times higher than effective cocaine doses. Taken together these data suggest a low abuse liability for (±)-modafinil and its enantiomers in humans.
Finally, although (±)-modafinil has been evaluated in several species, including humans, the cocaine-like discriminative-stimulus effects of the enantiomers have not previously been described. We found that both the R- and S-enantiomers of modafinil fully substituted for cocaine in mice, at a pretreatment time of 60 min. and were ~8- to 11-fold less potent than cocaine. In addition, as with the microdialysates, no enantioselectivity was observed.
There may be several explanations for the differences we observe in the computational/in vitro studies and behavior in mice, as compared to the benztropine-like DAT inhibitors. First, the modafinil enantiomers bind with relatively low affinity to the DAT and their binding affinities at NET and SERT were too low to quantify, although NET binding has been reported previously (15). Although the modafinil enantiomers share the diphenyl moiety of the benztropines, previously reported structure-activity relationships suggest that the modafinil analogues may bind somewhat differently at the DAT (55). Further, the modeling studies described herein clearly show that R-modafinil interacts with the Tyr156–OH, and this interaction likely causes this molecule to prefer a more occluded conformation of the DAT, as opposed to cocaine, wherein the 2-carbomethoxy group prevents a H-bond from forming between Asp79 and Tyr 156 and thus keeps this “gate” open (37). Finally, the modafinil enantiomers are non-amine DAT inhibitors (56, 57) in that they have no basic nitrogen to interact with the Asp79, as do most DAT inhibitors. Thus, modafinil and its enantiomers may not be “atypical” defined as having high affinity for the DAT with a preference for a more occluded conformation and devoid of significant cocaine-like behaviors. However, they are certainly different from cocaine in both binding mode and pharmacological effects, and show strong preference for a DAT conformation different from cocaine and more similar to the benztropines.
The present data are comparable to reported discriminative stimulus effects of (±)-modafinil in rats (22) and primates (23). Using self-administration procedures, Gold and Balster (19) found reinforcing effects of modafinil in rhesus monkeys trained to self-administer cocaine. In contrast, Deroche-Gamonet et al. (58) failed to find reinforcing effects of (±)-modafinil in rats without a history of cocaine self-administration. Interestingly, one study found chronic administration of (±)-modafinil to decrease cocaine self-administration (23), supporting its use as a potential substitute therapy in human cocaine abusers.
(±)-Modafinil and its R-enantiomer (armodafinil) are clinically available and the racemate is currently being evaluated for treatment of ADHD, cocaine, and methamphetamine addiction (59). Despite clinical availability, there are no reports of their abuse, and the literature for the racemate predicts a low abuse liability (24, 25, 60). Importantly, (±)-modafinil did not serve as a reinforcer in cocaine abusers, making it an attractive candidate for treatment of this population (61). R-modafinil does not appear to be significantly different from the racemate in preclinical studies, but reports indicate an improved pharmacokinetic profile and duration of action in humans (28, 31, 62, 63). Thus, we suggest that R-modafinil might be a promising candidate for a well-designed and compliance controlled clinical trial (8, 64) for cocaine or methamphetamine addiction. The therapeutic dose for R-modafinil is typically 50–250 mg p.o. per day (65), which is lower than (±)-modafinil (200–600 mg, p.o. per day), and may translate into an improved side-effect profile. Further, the recently reported cognitive-enhancing actions of (±)-modafinil, especially in methamphetamine abusers (12) suggest that additional benefit of R-modafinil might be realized in this patient population.
Supplementary Material
Acknowledgments
Support for this research was provided to AHN, OMO, JC, TK, MM, JLK and GT by the NIDA Intramural Research Program. CJL is supported by the Lundbeck Foundation and the Danish Council for Independent Research Sapere Aude programme. We thank Dawn French- Evans, Lone Rosenquist, Pia Elsman and Sonia Mazier for excellent technical assistance.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of Behavioral and Pharmacological Treatments for Addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson NE. Translational research in addiction: Toward a framework for the development of novel therapeutics. Biochem Pharmacol. 2011;81:1388–1407. doi: 10.1016/j.bcp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nat Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiat. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 5.Minzenberg MJ, Carter CS. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacol. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Raga J, Knecht C, Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr Drug Abuse Rev. 2008;1:213–221. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- 7.Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: Clinical evidence. Pharmacol Biochem Behav. 2011;99:285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 11.Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacol. 2010;35:2101–2109. doi: 10.1038/npp.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of Modafinil on Learning and Task-Related Brain Activity in Methamphetamine-Dependent and Healthy Individuals. Neuropsychopharmacol. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007;3:349–364. [PMC free article] [PubMed] [Google Scholar]
- 14.Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 15.Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 16.Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao JJ, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, et al. SARs at the Monoamine Transporters for a Novel Series of Modafinil Analogues. ACS Med Chem Lett. 2011;2:48–52. doi: 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of Modafinil on Dopamine and Dopamine Transporters in the Male Human Brain Clinical Implications. J Am Med Assoc. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold LH, Balster RL. Evaluation of the cocaine like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology. 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- 20.Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [H-3]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi RE, Lewis JR, Lattal KM, Berger SP. Modafinil reinstates a cocaine conditioned place preference following extinction in rats. Behav Brain Res. 2009;204:250–253. doi: 10.1016/j.bbr.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson NE, Fedolak A, Olivier B, Hanania T, Ghavami A, Caldarone B. Psychostimulant-like discriminative stimulus and locomotor sensitization properties of the wake-promoting agent modafinil in rodents. Pharmacol Biochem Behav. 2010;95:449–456. doi: 10.1016/j.pbb.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral Evaluation of Modafinil and the Abuse-Related Effects of Cocaine in Rhesus Monkeys. Exp Clin Psychopharm. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- 25.Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance--a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- 26.Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan JL, Malcolm RJ, Markowitz JS, DeVane CL. Chiral analysis of d- and l-modafinil in human serum: application to human pharmacokinetic studies. Ther Drug Monit. 2003;25:197–202. doi: 10.1097/00007691-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Wong YN, Simcoe D, Hartman LN, Laughton WB, King SP, McCormick GC, et al. A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. J Clin Pharmacol. 1999;39:30–40. doi: 10.1177/00912709922007534. [DOI] [PubMed] [Google Scholar]
- 29.Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–137. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Dinges DF, Arora S, Darwish M, Niebler GE. Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Curr Med Res Opin. 2006;22:159–167. doi: 10.1185/030079906X80378. [DOI] [PubMed] [Google Scholar]
- 31.Garnock-Jones KP, Dhillon S, Scott LJ. Armodafinil. CNS drugs. 2009;23:793–803. doi: 10.2165/11203290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Newman AH, Katz JL. Atypical dopamine uptake inhibitors that provide clues about cocaine’s mechanism at the dopamine transporter. Top Med Chem. 2009;4:95–129. [Google Scholar]
- 33.Tanda G, Newman AH, Katz JL. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol. 2009;57:253–289. doi: 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn2+ switch in the dopamine transporter: Mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. P Natl Acad Sci USA. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loland CJ, Granas C, Javitch JA, Gether U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J Biol Chem. 2004;279:3228–3238. doi: 10.1074/jbc.M304755200. [DOI] [PubMed] [Google Scholar]
- 36.Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, et al. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- 37.Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisgaard H, Larsen MAB, Mazier S, Beuming T, Newman AH, Weinstein H, et al. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology. 2011;60:182–190. doi: 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pontieri FE, Tanda G, DiChiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. P Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pontieri FE, Tanda G, Orzi F, DiChiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 41.Tanda G, Pontieri FE, DiChiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu 1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 42.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 43.Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, et al. Combinations of Cocaine with Other Dopamine Uptake Inhibitors: Assessment of Additivity. J Pharmacol Exp Ther. 2009;330:802–809. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li SM, Kopajtic TA, O’Callaghan MJ, Agoston GE, Cao JJ, Newman AH, et al. N-Substituted Benztropine Analogs: Selective Dopamine Transporter Ligands with a Fast Onset of Action and Minimal Cocaine-Like Behavioral Effects. J Pharmacol Exp Ther. 2011;336:575–585. doi: 10.1124/jpet.110.173260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem. 2008;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrer JV, Javitch JA. Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. P Natl Acad Sci USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reith MEA, Berfield JL, Wang LC, Ferrer JV, Javitch JA. The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem. 2001;276:29012–29018. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- 49.Chen JG, Rudnick G. Permeation and gating residues in serotonin transporter. P Natl Acad Sci USA. 2000;97:1044–1049. doi: 10.1073/pnas.97.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha BA, Goulding EH, O’Dell LE, Mead AN, Coufal NG, Parsons LH, et al. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci. 2002;22:10039–10045. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- 52.Forrest LR, Rudnick G. The Rocking Bundle: A Mechanism for Ion-Coupled Solute Flux by Symmetrical Transporters. Physiology. 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt KC, Reith MEA. The Atypical Stimulant and Nootropic Modafinil Interacts with the Dopamine Transporter in a Different Manner than Classical Cocaine-Like Inhibitors. Plos One. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. Novel 4′-Substituted and 4′,4″-Disubstituted 3-Alpha-(Diphenylmethoxy)Tropane Analogs as Potent and Selective Dopamine Uptake Inhibitors. J Med Chem. 1995;38:3933–3940. doi: 10.1021/jm00020a006. [DOI] [PubMed] [Google Scholar]
- 56.Goulet M, Miller GM, Bendor J, Liu SH, Meltzer PC, Madras BK. Non-amines, drugs without an amine nitrogen, potently block serotonin transport: Novel antidepressant candidates? Synapse. 2001;42:129–140. doi: 10.1002/syn.1108. [DOI] [PubMed] [Google Scholar]
- 57.Madras BK, Fahey MA, Miller GM, De La Garza R, Goulet M, Spealman RD, et al. Non-amine-based dopamine transporter (reuptake) inhibitors retain properties of amine-based progenitors. Eur J Pharmacol. 2003;479:41–51. doi: 10.1016/j.ejphar.2003.08.055. [DOI] [PubMed] [Google Scholar]
- 58.Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- 59.http://www.clinicaltrials.gov
- 60.Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Stoops WW, Lile JA, Fillmore MT, Glaser PEA, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 62.Darwish M, Kirby M, Hellriegel ET, Robertson P. Armodafinil and Modafinil Have Substantially Different Pharmacokinetic Profiles Despite Having the Same Terminal Half-Lives Analysis of Data from Three Randomized, Single-Dose, Pharmacokinetic Studies. Clin Drug Invest. 2009;29:613–623. doi: 10.2165/11315280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Darwish M, Kirby M, D’Andrea DM, Yang RH, Hellriegel ET, Robertson P. Pharmacokinetics of Armodafinil and Modafinil After Single and Multiple Doses in Patients With Excessive Sleepiness Associated With Treated Obstructive Sleep Apnea: A Randomized, Open-Label, Crossover Study. Clin Ther. 2010;32:2074–2087. doi: 10.1016/j.clinthera.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Becker RE, Greig NH. Lost in translation: neuropsychiatric drug development. Science Transl Med. 2010;2:61rv66. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.http://www.drugs.com/pro/nuvigil.html (2000–2011) Nuvigil.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.