Abstract
Dendritic cells (DCs) are antigen presenting cells capable of inducing specific immune responses against microbial infections, transplant antigens, or tumors. DCs have been shown to possess a high plasticity showing different phenotypes in response to their microenvironment. For example, tumor-associated DCs can acquire an angiogenic phenotype thus promoting tumor growth. Further, DCs cultured in vitro under different conditions are able to upregulate the expression of endothelial markers and to express angiogenic factors. Indeed, it has been shown that soluble factors such as VEGF of PGE-2, that are present in the microenvironment of several tumors, affect the biology of these cells. We hypothesize that in addition to soluble factors the adhesion to different substrates will also define the phenotype and function of DCs. Herewith we demonstrate that murine myeloid(m) DCs upregulate endothelial markers such as VE-Cadherin, and to a lesser extent TIE-2, and decrease their immune capabilities when cultured on solid surfaces as compared with the same cells cultured on ultra-low binding (ULB) surfaces. On the other hand, the expression of angiogenic molecules at the level of RNA was not different among these cultures. In order to further investigate this phenomenon we used the murine ID8 model of ovarian cancer which can generate solid tumors when cancer cells are injected subcutaneously or a malignant ascites when they are injected intraperitoneally. This model gave us the unique opportunity to investigate DCs in suspension or attached to solid surfaces under the influence of the same tumor cells. We were able to determine that DCs present in solid tumors showed higher levels of expression of endothelial markers and angiogenic molecules but were not able to respond to inflammatory stimuli at the same extent as DCs recovered from ascites. Moreover, mDCs cultured on ULB surfaces in the presence of tumor factors do not expressed endothelial markers. Taking into account all these data we consider that tumor factors might be responsible for inducing angiogenic properties in DCs, but that in some settings the expression of endothelial markers such as VE-Cadherin and TIE-2 might be a function of attachment to solid surfaces and independent of the angiogenic properties of these cells.
Keywords: Dendritic cells, endothelial markers, angiogenesis, antigen presentation, ovarian cancer
INTRODUCTION
Dendritic cells (DCs) are antigen presenting cells (APCs) found in peripheral tissues and in immunological organs (1-3). DCs present in peripheral tissues sample the organism for the presence of antigens, which they take up, process and expose in on their surface in the context of major histocompatibility (MHC) molecules. Antigen-loaded DCs present the processed antigens to T lymphocytes in order to trigger specific immune responses. Effective activation of specific T cells will depend on the levels of expression and the interplay between positive and negative costimulatory molecules in both DCs and T cells. For example, antigen uptake in the absence of inflammatory signals, render phenotypically immature DCs, expressing low levels of MHC-II and costimulatory molecules. Antigen presentation in the absence of effective positive costimulation can lead to T-cell anergy and tolerance (4). These DCs are considered as “tolerogenic” in comparison with “immunogenic” DCs capable of inducing potent specific immune responses. Interestingly, DCs can switch from immunogenic to tolerogenic depending on microenvironment conditions (5). For example, viral infections can differentiate plasmacytoid DCs into T helper(Th)-1-inducing DCs, (6) while IL-3 can promote the differentiation of Th1-inducing DCs into Th-2 inducing DCs (7).
DCs are conspicuous members of the microenvironment of several types of cancer (8-12). Tumor-associated DCs (TA-DCs) showing highly immunosuppressive properties are able to render T cells anergic or tolerised, thus abrogating antitumor immune responses. Treatment of the TA-DCs with inflammatory molecules, render immunogenic DCs with the capability to activate T cells (13). Besides an immune “paralysis”, it has been shown that TA-DCs, or leukocyte expressing DC markers, are able to produce angiogenic factors and can promote angiogenic processes in the tumor microenvironment (9, 10, 14). Further highlighting the plasticity of these cells, we and others have shown that monocytes or DCs can undergo an endothelization process in vitro characterized by the loss of CD14/CD45 and upregulation of endothelial markers such as CD31, CD34, von Willebrand factor, VEGF receptor (VEGFR)-2 and VE-Cadherin (15-17). These cells displayed other characteristics of bonafide endothelium such as LDL uptake, lectin binding or formation of cord-like structures in 3D gels (15-20). In addition, CD45-VE-Cadherin double positive cells were described as promoters of neovascularization in a model of cardiac ischemia (21). DCs with proangiogenic properties have been also shown to participate in choroidal neovascularization (22). Further, it has been shown that under the influence of tumor factors, human DCs are able to express endothelial markers and assemble into endothelial-like structures in vitro (17). Finally, it has been reported that APCs can even acquire functional properties similar to brain microvascular endothelial cells under the appropriate stimuli (16).
We hypothesized that this phenotype shifts might be caused not only by the action of specific cytokines or growth factors, but also by the interaction of these cells with particular surfaces. Herewith, we performed a series of studies in order to determine the relevance of adhesion to solid surfaces on the capability of these cells to express endothelial markers or to induce immune responses.
MATERIAL AND METHODS
Animals
Six to eight week old female C57BL/6 (H-2Kb) and BALB/c (H-2Kd) mice (Charles River Laboratories, Wilmington, MA) were used in protocols approved by the Institutional Animal Care and Use Committee at Ohio University.
In vitro generation and maturation of murine myeloid DCs
Murine DCs were generated from bone marrow precursors recovered from femurs and tibiae of 6–8 week old female C57BL/6 mice by the method of Lutz et al. (23, 24) as we recently described in detail (25). Briefly, bone marrow cells were dispersed by vigorous pipetting and cultured in RPMI-1640 supplemented with penicillin (100 μg/ml), streptomycin (100 U/ml), L-glutamine (2 mM) and 10% heat-inactivated fetal bovine serum (FBS)(all Invitrogen, Carlsbad, CA) in the presence of 20 ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF, 315-03, Peprotech Inc., Rocky Hill, NJ) for 8 days. GM-CSF was replenished on days 3 and 6. In some experiments, DC maturation was induced by culturing the cells in the presence of 5 ng/ml of GM-CSF, 20 ng/ml of mouse tumor necrosis factor alpha (TNF-α, 315-01A, Peprotech) and 100 ng/ml of bacterial ultrapure lipopolysaccharide (LPS), lipoteichoic acid (5 μg/ml), and poly(I:C) (10 μg/ml), CPG (5 μg/ml), (all Invivogen, San Diego, CA); or anti-IL10 receptor/CD210 (10 μg/ml, 1B1.3a) (BD Biosciences, San Diego, CA).
Cell lines and tumors
In the experiments depicted herewith we used the murine ID8-Vegf-A cell line of ovarian cancer (26). The ID8 cell line is a tumor cell line derived from spontaneous in vitro malignant transformation of C57BL/6 mouse ovarian surface epithelial cells originally generated by Roby et al. (27). This line has been engineered to express high levels of VEGF-A (VEGF-164) (28). These cells were maintained in DMEM (Invitrogen, Carlsbad, California) supplemented with 2 mM L-glutamine, 100 μg/ml penicillin, 100 U/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS) (all Invitrogen). Ectopic ID8-Vegf-A solid ovarian tumors were initiated in C57BL/6 mice by subcutaneous (s.c) injection of 7×106 tumor cells (28, 29). Orthotopic tumors were initiated in C57BL/6 mice by intraperitoneal (i.p.) injection of 5×106 tumor cells. In the i.p. model, mice develop a conspicuous ascites in around 30 days. For some experiments, single cell suspensions from solid tumors and ascites were prepared. Solid tumors were mechanically disaggregated as we previously described (18). Briefly, solid tumors were aseptically recovered, minced with scissors in PBS and filtered through 70 μM cell strainers (BD Biosciences, San Jose, CA). Then, filtered cell suspensions were collected and washed in PBS before using them for other studies. Ascites was collected from the peritoneal cavity and centrifuged in order to separate the cell fraction. Then, red blood cells were lysed by hypotonic shock and nucleated cells recovered and washed in PBS as above. In addition, cell-free ascites supernatants were filtered and kept at −70°C until use.
In order to investigate the effect of tumor factors on DCs, we prepared tumor conditioned media. To accomplish this, ID8-Vegf-A cells were cultured until 80% confluence and supernatants recovered, filtered and kept at −70°C until use.
Culture of DCs on different surfaces
The effect of different surfaces on the biology of DCs was studied by culturing these cells on commercially available plates coated with different extracellular matrix components such as fibronectin, collagen I, gelatin, Matrigel (all BD Biosciences), or synthetic surfaces such as polystyrene or ultra-low binding surfaces (Corning Costar, Corning, NY). DCs were seeded on these plates at a concentration of 5 x 105 cells/ml in either RPMI 10 % FBS or endothelial EBM-2 medium with supplements (EGM2-MV BulletKit, Lonza) with the addition of GM-CSF (3 ng/ml). Cells were cultured up to 3 weeks in these conditions and media was replenished once a week. Pictures of live cells were obtained with an inverted microscope attached to a Motic 2000 Camera (Motic, Richmond, British Columbia, Canada).
Purification of CD11c by means of magnetic sorting
CD11c cells were recovered from DC cultures, solid tumor samples or ascites by immunomagnetic sorting. To accomplish this, single cells suspensions were prepared from solid tumors and ascites as described above while cultured DCs were mechanically detached from the culture plates by using cell scrapers, or directly recovered from the supernatants of ultra-low binding surfaces. Dead cells were eliminated by using the immunomagnetic Dead Cell Removal kit following the manufacturer’s instructions (MACS Miltenyi, Auburn, CA). After blocking Fc receptors with anti-CD16/CD32 antibody (Fc block, 2.4G2; BD Biosciences), cells were labeled with anti-CD11c magnetic beads (MACS Miltenyi) and positive cells isolated by using MS paramagnetic columns in an octoMACS magnet (all MACS Miltenyi) following the manufacturer’s instructions. In order to ensure purity, bead-labeled cells were passed through two consecutive columns.
Flow cytometry
Cells were subjected to multi-color cytometry on a FACS Aria flow cytometer using FACSDiva software (Becton Dickinson, San Jose, CA). Non-specific staining was blocked with Fc block in FACS buffer (PBS 2% FBS, 0.05% sodium azide). Fluorochrome-conjugated monoclonal antibodies against CD45 (30-F11), CD11c (HL3), CD80 (16-10A1), CD86 (GL1), MHC-II (KH74), CD54 (3E2), CD11b (M1/70), CD40 (3/23), B7-DC/CD273 (TY25), PDL-1/CD274 (MIH5) (all BD Biosciences); and CD31 (PECAM-1), OX40-L (RM134L), B220 (RA3-6B2), CD107b (ABL-93) and CD137/41BB (17B5) (all eBioscience, San Diego, CA) were used at 1/100 dilution. Biotinylated antibodies against TIE-2 (BAF762), VE-Cadherin (BAF1002) and biotinylated isotype control (all R&D Systems, Minneapolis, MN) were used at 1/50 dilution followed by staining with streptavidin-FITC (BD Pharmingen) at 1/500 dilution.
RT-PCR and Real-Time Quantitative Reverse Transcription-PCR
RNA was isolated with TRIzol (Invitrogen) and then reverse transcribed by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following the manufacturer’s instructions. All RNA samples were treated with DNAse in order to eliminate possible contaminating genomic DNA. For qualitative PCR analysis, the PCR cycling was conducted with Taq polymerase at 94°C (30s), 57°C (30s), and 72°C (20s-1 min depending on product size) for 40 cycles.
Expression of specific molecules was also analyzed at the level of RNA by means of quantitative real time RT-PCR (qPCR) analysis. For qPCR experiments, we used the absolute quantification method by generating standard curves for our genes of interest and housekeeping genes. In these assays we used (PerfeCTa SYBR Green FastMix, Quantas Biosciences) for detection of the PCR reaction. Each amplification experiment was performed in 96-well optical grade PCR plates covered with optical film) in an iCycler iQ5 real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA). Primers are described in Table 1. All the primers were designed with the public web Primer 3 program in order to generate PCR products that cross introns. We have used this program to design the primers described in our previous publications (15, 18, 29, 30). We normalized the cDNA load to mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data were expressed as relative units to GAPDH mRNA molecules.
Table 1.
List of primers used for qualitative and quantitative PCR studies
| Target Gene | Primer Sequence |
|---|---|
| Angiogenin | Forward 5′- CAG CAT GTG GAC CCT CAG GT -3′ Reverse 5′- TGA AGC GTT TGC ACT GGA CA -3′ |
| Annexin-1 | Forward 5′-CGG ATG TTG CTG CCT TGC AC -3′ Reverse 5′-CCT TGA TCT GCT GGC GCT GA -3′ |
| bFGF | Forward 5′-TGT GTG CCA ACC GGT ACC TT-3′ Reverse 5′-TTC CAG TCG TTC AAA GAA GAA ACA -3′ |
| CD31 | Forward 5′-CAG TGT CCC CAG AAG CAA AAT CA -3′ Reverse 5′- AGA GAG CAA TGA TCA CTC CGA TG -3′ |
| CD44 exon6 common |
Reverse 5′ GAT CCA TGA GTC ACA GTG CG -3′ |
| CD44 exon5 | Forward 5′- GCC TAC TGG AGA TCA GGA TG -3′ |
| CD44v10 | Forward 5′- CTA AGA GCG GCG CTA AAG AT -3′ |
| CD44v9 | Forward 5′- CAC AGA GTC ATT CTC AGA AC -3′ |
| CD44v8 | Forward 5′- ATA CAG ACT CCA GTC ATA GT -3′ |
| CD44v7 | Forward 5′- CTT CGG CCC ACA ACA ACC AT -3′ |
| CD44v6 | Forward 5′- CTC CTA ATA GTA CAG CAG AA -3′ |
| CD44v5 | Forward 5′- ATA GAC AGA ATC AGC ACC AG -3′ |
| CD44v4 | Forward 5′- TTG CAA GTA CTC CAC GGG TT -3′ |
| CD44v3 | Forward 5′- GTA CGG AGT CAA ATA CCA AC -3′ |
| CD44v2 | Forward 5′- TGA TGA CCA CCC CTG AAA CA -3′ |
| CD44v1 | Forward 5′- TTG CCT CAA CTG TGC ACT CA -3′ |
| Endoglin | Forward 5′-CTC CAG CTG CGG TGG TGT GT -3′ Reverse 5′- TGA GTA GCA CGG GGC TGC AT-3′ |
| GAPDH | Forward 5'-CCT GCA CCA CCA ACT GCT TA-3' Reverse 5'-CAT GAG TCC TTC CAC GAT ACC A-3' |
| Heparanase | Forward 5'- GGG GCC GGA TGG ATT ACT TT-3' Reverse 5' -CCA TGA AAA ACC CGT CTC CA-3' |
| HGF | Forward 5′-GGG ACG GTA TCC ATC ACT AAG A -3′ Reverse 5′-CTT TAC CGC GAT AGC TCG AA-3′ |
| MMP2 | Forward 5′-GCA TCG CTC AGA TCC GTG GT-3′ Reverse 5′-GAA TGT GGC CAC CAG CAA GG-3′ |
| MMP8 | Forward 5′-GCT TAC AGG GAA CCC AGC ACC T-3′ Reverse 5′-GGG CCC AGT AGG TTG GAT GG-3′ |
| MMP9 | Forward 5′-TAA AGG CCG CTC GGA TGG TT-3′ Reverse 5′-CCA ACT ACG GTC GCG TCC AC -3′ |
| NRPI | Forward 5′- TGT CGC TAT GAC CGG CTG GAT -3′ Reverse 5′- TCC GGC CAG TTT TCT GC -3′ |
| TIE-2 | Forward 5′-ACT TGG AGC CGC GGA CTG AC -3 Reverse 5′- AGG CCC ATG CCC TTC TC -3′ |
| TWEAK | Forward 5′-CCG AGC TAT TGC AGC CCA TT-3′ Reverse 5′-GCC ACT CAC TGT CCC ATC CA-3′ |
| VE-Cadherin | Forward 5′- AGC CCA GGC GGG TGT CAG -3′ Reverse 5′- CCG CCT CCG CAG GAT GAT-3′ |
| VEGF-A | Forward 5′- GCC AGC ACA TAG AGA GAA TGA GC-3′ Reverse 5′- CAA GGC TCA CAG TGA TTT TCT GG-3′ |
PCR arrays
For these studies, RNA was pooled from CD11c cells purified from 3-4 independent experiments involving either solid tumors or ascites. The expression of angiogenic factors was analyzed using the RT2 Profiler PCR Array System for mouse angiogenesis (SABiosciences, PAMM-024A, Frederick, Maryland). RNA was reverse transcribed to cDNA using the RT2 First Strand Kit (SABiosciences, Frederick, Maryland) according to manufacturer’s specifications. cDNA was loaded to preformed wells of the RT2 Profiler PCR Array System according to manufacturer’s specifications with an iCycler iQ real-time PCR instrument (Bio-Rad Laboratories, Hercules, California). Resulting data was analyzed using RT2 Profiler PCR Array Data Analysis software (SABiosystems, Frederick, Maryland).
Immunohistochemistry
Solid tumor samples were snapped-frozen in OCT medium (Tissue Tek, Sakura, Torrance, CA) and sections were prepared using a Leica CM1950 Cryostat (Leica Microsystems, Bannockburn, IL). Sections were fixed in cold acetone for 10 minutes, pretreated with 3% H2O2 for 20 min to block endogenous peroxidase activity and blocked in normal horse serum (Vector Laboratories). Biotinylated rat anti-mouse CD11c (HL3) and biotinylated hamster isotype control (both BD Pharmingen) were used at 1:50 dilution for these studies. Then, the Vectastain ABC kit was applied as described by the manufacturer (Vector Laboratories). Sections were counterstained with Gill’s hematoxylin (Vector Laboratories). Images were acquired through a Micropublisher 5.0 Digital CCD Color Camera (Qimaging, Surrey, BC Canada).
ELISA analysis
The concentration of different cytokines in culture supernatants was quantified by antigen capture ELISA. We used the following purified antibodies for capture: anti-mouse IL-6 (MP5-20F3) (eBioscience, San Diego, CA) and anti-mouse-VEGF (BAF493, R&D Systems). For detection we used biotin anti-mouse IL-6 (MP5-32C11) and biotin anti-mouse VEGF (AF-493-NA, R&D Systems) at 1 μg/well. Standard curves were constructed using recombinant murine IL-6 (216-16) and VEGF (450-32) (all Peprotech). Each dilution of recombinant standard or sample was assayed in duplicate. The reaction was developed by using streptavidin-horseradish peroxidase (554066, BD Pharmingen) and the 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate(6)] (ABTS) substrate system (Roche Diagnostics GmbH, Mannheim, Germany). The blue-green color produced by enzymatic activity was quantified at 405 nm in an ELISA microplate reader (Multiskan RC, ThermoLabsystems).
Proliferation assay
Murine myeloid C57BL/6 DCs were recovered from long-term cultures; and CD11c positive cells purified by magnetic sorting and reseeded in polystyrenes surfaces (96-well round-bottom plates) at a concentration of 1x105/well in RPMI containing 10% FBS. Cultures were treated for 48 h with an inflammatory cocktail as described above. Spleens were resected from healthy BALB/c mice and minced in a sterile fashion to yield a single cell suspension and erythrocytes were eliminated by hypotonic shock. Then untouched CD3 T cells were purified from this suspension by magnetic sorting using the Pan T Cell Isolation Kit (MACS Miltenyi) following the manufacturer’s instructions. T cells were labeled with CFSE as previously described (31) and were incubated at a concentration of 1x105 cells/well with the recovered DCs for 5 days. CFSE dilution, an indication of cell proliferation, was assessed by flow cytometry analysis of gated T cells.
Statistical analysis
For multiple comparisons we performed ANOVA analysis with post-analysis comparisons by the Tukey-Kramer multiple comparisons test. A value of p<0.05 was considered significant. Data are expressed as mean ± SD. Data was analyzed by using the Graph Pad Instat software (GraphPad Software, Inc., San Diego, CA).
RESULTS
Myeloid DCs show higher level of maturation markers when not attached to solid surfaces
Murine bone marrow-derived (myeloid) DCs have been extensively used as models for determining the efficacy and improvement of DC-based vaccines; investigating DC:T cell interactions or DC development; and determining DC role in pathological conditions such cancer or infectious diseases (32-39). We have previously reported that these cells exhibit high plasticity, being capable of acquiring angiogenic properties in vivo under pathological conditions (15). We hypothesized that this might be caused not only by the presence of specific cytokines or growth factors, but also by the interaction with different extracellular matrix (ECM) components as we have recently demonstrated (40). Taking into account this, we decided to determine the relevance of substrate adherence on the biology of myeloid(m) DCs.
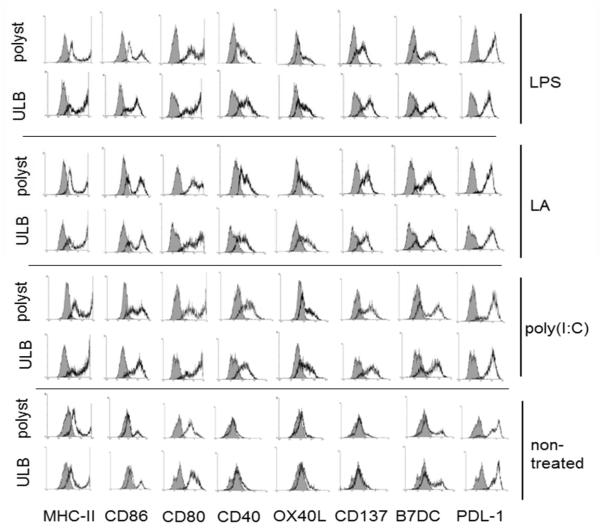
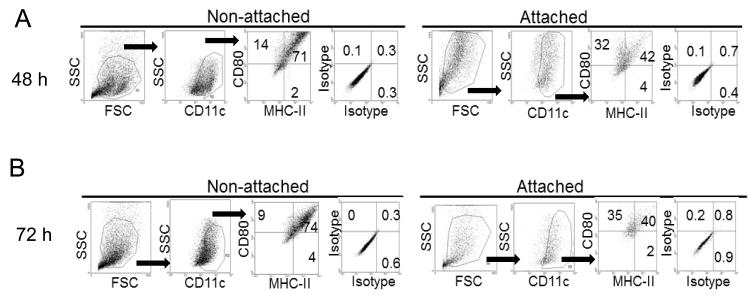
In a first series of studies we investigated the expression of MHC-II and members of the costimulatory B7/CD28 (B7-1/2[CD80, CD86], and PDL-1/2) and the TNF/TNF receptor (CD40, OX40L, and CD137) families in mDCs cultured for 48 h on polystyrene or ultralow binding (ULB) surfaces in the presence of different inflammatory stimuli. These molecules can participate either in activation (CD80, CD86, CD40, CD137, and OX40L) or suppression (PDL1/2) of T cell activity (41), being the final effect a result of the interplay between these sets of stimulators and inhibitors. As shown in Fig. 1, all treatments increased the expression of almost every costimulatory molecule on mDCs being the exception PDL-1 which retained similar levels to those observed in non-treated cells. Interestingly, MHC-II, CD86 and CD80 were expressed at higher levels when mDCs were cultured on ULB surfaces. We hypothesized that this difference could be due to the presence of two mDC populations in the polystyrene cultures, one composed by cells not attaching to the surface and other by cells that were attached to it. In order to investigate this, we cultured mDCs for three days in the presence of a typical inflammatory cocktail (TNFα + LPS). Every 24 h cells were recovered from both the supernatants or from the polystyrene surface by using cell scrapers and the expression of MHC-II and CD80 was evaluated by flow cytometry analysis on live cells. As shown in Fig. 2, cells present in the supernatants of the polystyrene cultures showed higher expression of costimulatory molecules in all the days studied.
Figure 1.
Expression of costimulatory molecules by mature mDCs. Flow cytometry analysis of costimulatory molecules on mDCs cultured for 48 h on ULB or polystyrene surfaces in the presence of lipoteichoic acid (TLR-2 ligand), poly(I:C) (TLR-3 ligand), ultrapure LPS (TLR-4 ligand) or left untreated. Grey histograms represent isotype controls. An experiment representative of 4 independent experiments is depicted.
Figure 2.
Analysis of costimulatory molecules in attached and non-attached DCs. Expression of DC markers and costimulatory molecules was investigated in attached and non-attached mDCs upon 48 (A) and 72 h (B) culture on polystyrene in the presence of a typical inflammatory cocktail (LPS + TNFα). An experiment representative of 4 independent experiments is shown.
mDCs express endothelial markers upon culture on solid surfaces
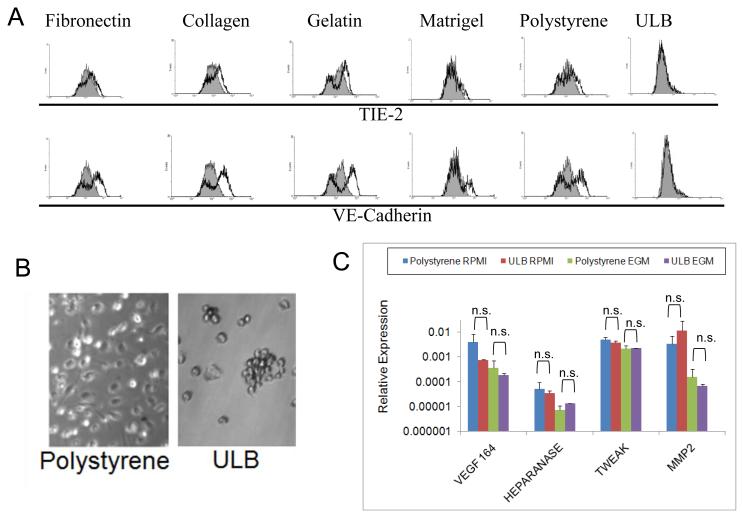
In our first series of studies we were able to determine that mDCs not attached to solid surfaces have a more immunostimulatory profile. As described above, it has been shown that DCs are able to express endothelial markers when cultured in the presence of angiogenic factors. Taking into account this, we decided to investigate if DCs cultured on ULB surfaces in the presence of angiogenic factors were able to express endothelial markers. To accomplish this, mDCs were cultured in the presence of endothelial cell growth medium (EGM). The rationale being that in previous studies endothelial media conditions seemed to skew the cells farther away from a typical immunostimulatory DC phenotype (42, 43). This medium is supplemented with 10% FBS together with several growth factors human (h)EGF, hydrocortisone, hVEGF, hFGF-B, hIGF-1, ascorbic acid) that support endothelial cell proliferation. In addition to ULB surfaces, we also cultured mDCs on different solid surfaces. For this purpose we used plates coated with fibronectin, collagen I, gelatin, Matrigel, and polystyrene. Upon 1 week of culture in different conditions mDCs were recovered and analyzed for the expression of VE-Cadherin and TIE-2, markers characteristically associated with angiogenic APCs (15, 18, 43, 44). As shown in Fig. 3A, attached mDCs cultured for a week with EGM expressed variable level of these molecules at the level of protein. This was particular evident for VE-Cadherin, although some level of TIE-2 expression was observed in repeated experiments in cells cultured on collagen I, and polystyrene. On the other hand, we were not able to detect expression of these molecules in cells cultured on ULB surfaces.
Figure 3.
Expression of endothelial markers and angiogenic molecules on mDC cultures. (A) mDCs were cultured for 1 week on different surfaces with EGM media. Cells were recovered from different cultures and analyzed by flow cytometry. Analysis was performed on CD11c gated cells. Grey histograms represent isotype controls. An experiment representative of 2 independent experiments is shown. (B) Microphotograph of mDCs after 3 week of culture on polystyrene or ULB surfaces (20X magnification). An experiment representative of 4 independent experiments is shown. (C) Expression of angiogenic molecules by 3-week DC cultures. mDCs were recovered from different cultures after 3 weeks in EGM or RPMI, RNA extracted and reverse-transcribed. Then, quantitative real-time PCR was performed to analyze several angiogenic molecules in these cells. Data were analyzed by ANOVA followed by Tukey-Kramer Multiple Comparisons post-test. Samples were run in duplicate in each experiment and further analyzed in duplicate by qPCR. An experiment representative of 2 independent experiments is shown.
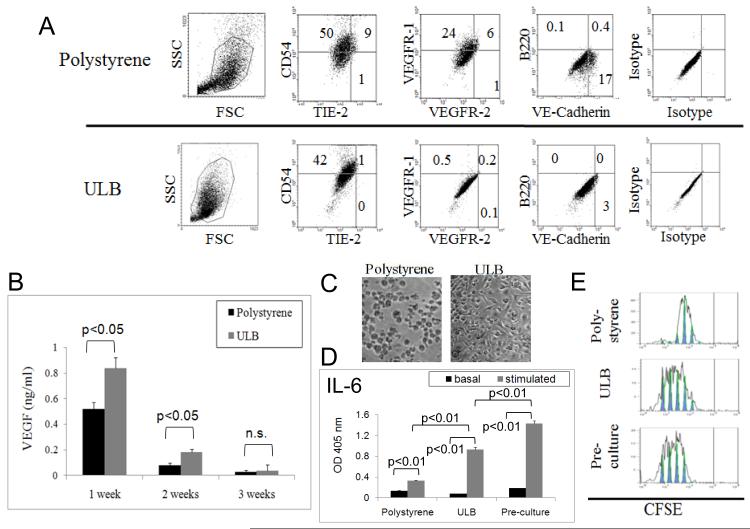
In order to further determine the effect of substrate adherence on the biological properties of mDCs, these cells were cultured for 3 weeks on polystyrene or ULB surfaces. As shown in Fig. 3B, mDCs on ULB surfaces mostly remained in suspension, adopted a spherical shape and tended to aggregate into clumps, while cells on polystyrene remained attached to the surface. We have recently shown that mDCs cultured on different extracellular matrices for 3 weeks expressed angiogenic molecules in the absence of inflammatory stimuli (40). Herewith we decided to investigate if mDCs were also able to express angiogenic molecules when cultured on ULB surfaces. To accomplish this we analyzed, by means of real-time PCR, the expression of several angiogenic molecules previously described in APCs. In particular, we evaluated the expression of VEGF-A 164 (which is the predominant VEGF-A isoform) (45), Heparanase (46), TWEAK (47, 48) and matrix metalloprotease (MMP) 2 (49). As shown in Fig. 3C, mDCs cultured on ULB surfaces expressed these angiogenic molecules at the level of RNA. Interestingly we were not able to detect significant differences in the levels of these molecules among ULB and polystyrene cultures. Further, when these cells were analyzed for the expression of endothelial markers by qualitative flow cytometry analysis, we were able to detect the expression of VEGFR1, VE-Cadherin and TIE-2 in a proportion of cells cultured on polystyrene (Fig. 4A). On the other hand, these endothelial markers were not detected in cells cultured on ULB surfaces. Interestingly, VE-Cadherin and TIE-2 were not detected in these cells before culture (not shown). In addition, we were not able to detect expression of B220, a marker of murine plasmacytoid DCs when the cells were cultured on either surface, indicating that our treatments did not induce a phenotypic shift towards this immunosuppressive phenotype. Finally, we evaluated the expression of VEGF in supernatants of mDCs cultured on polystyrene or ULB surfaces for up to 3 weeks. It is noteworthy to comment that VEGF is a paradigmatic angiogenic molecule (45, 50, 51). As shown in Fig. 4B, ULB cultures showed higher levels of VEGF during the first two weeks of study, but no differences we observed at 3 weeks of culture, mirroring the behavior of VEGF expression at the level of RNA in the same week. Altogether these data indicated that the expression of endothelial markers and the angiogenic capability of mDCs do not strictly correlate. mDCs preserve their immunological properties when cultured on ULB surfaces
Figure 4.
Expression of angiogenic markers and immune functions of 3-weeks mDC cultures. (A) mDCs were recovered from polystyrene and ULB surfaces after 3 weeks in EGM and analyzed by flow cytometry. Analysis was performed on CD11c gated cells. Quadrants were defined by using isotype controls. (B) VEGF was detected by ELISA analysis on mDC culture supernatants. The ELISA assay specifically recognized murine VEGF. Significant differences were determined by ANOVA analysis followed by Tukey-Kramer Multiple Comparisons post-test. An experiment representative of 2 independent experiments is shown. (C) Microphotograph of reattached DCs. Myeloid DCs cultured for 3 weeks on different surfaces with EGM were detached, purified using CD11c magnetic beads and cultured on polystyrene for 24 h (20X magnification). An experiment representative of 2 independent experiments is shown. (D) IL-6 was detected by ELISA analysis on reseeded mDC cultures after 48 h stimulation with TNFα and LPS. Data was analyzed by ANOVA followed by Tukey-Kramer Multiple Comparisons post-Test. An experiment representative of 2 independent experiments is shown. (E). CFSE dilution analysis. Proliferation of CFSE-stained allogeneic BALB/c lymphocytes was determined after 5 day co-culture with the same mDCs as in (D). An experiment representative of 2 independent experiments is shown.
As shown in Fig. 4A, DCs cultured for 3 weeks on different surfaces and EGM expressed high levels of CD54. This molecule has a crucial role in the clustering of DCs with lymphocytes by interacting with LFA-1 on the surface of T cells (52). This indicated that the cells were able to interact with T cells even after 3 weeks of culture with EGM. Thus, we considered relevant to investigate the immunological properties of these cells. To accomplish this, we isolated CD11c positive cells from the cultures by means of magnetic sorting and cultured equal amounts of live cells in regular culture plastic plates (polystyrene) for 24 h (Fig. 4C) before inducing maturation. After 3 weeks in suspension ULB cultured cells were able to attach to polystyrene. To induce maturation, cultures were treated with a typical inflammatory cocktail (100 ng/ml of LPS + 20 ng/ml of TNFα) for 48 h. We used fresh DCs as a control for these studies (Pre-culture). As shown in Fig. 4D, we were able to detect different levels of inflammatory cytokine IL-6 in our cultures in response to stimulation. We have recently shown that IL-6 is a reliable marker of DC maturation (25, 40). In particular, low levels of those molecules were produced by CD11c cells recovered from polystyrene cultures, while CD11c recovered from ULB cultures produced cytokine levels closer to those generated by fresh DCs. In addition, we analyzed the expression of CD80, a typical costimulatory molecule. Upon stimulation we observed that CD11c cells recovered from ULB, but not polystyrene cultures upregulated the expression of this molecule, albeit at lower levels than those expressed by Pre-culture DCs (not shown). Finally, after stimulation with an inflammatory cocktail we cocultured these cells with CFSE-stained allogeneic BALB/c T lymphocytes. Consistent with the ELISA and FACS data, CD11c cells recovered from ULB cultures induced higher levels of proliferation of allogeneic T cells than DCs recovered from polystyrene cultures, almost mirroring what was observed when stimulated Pre-culture cells were used (Fig. 4E). Altogether, these data indicate that lack of adherence to a surface can preserve the immunological properties of mDCs on long-term cultures.
DCs present in ovarian cancer solid tumors or ascites have different angiogenic and immunological capabilities
In order to investigate the relevance of adhesion to a substrate in an in vivo pathological setting, we decided to use the ID8-Vegf-A model of ovarian cancer. ID8 is a cell line derived from spontaneous in vitro malignant transformation of C57BL/6 mouse ovarian surface epithelial cells (27) that was engineered to express mouse VEGF-A (26). Our published data support that this model mimics the pathophysiology of human ovarian cancer which expresses levels of VEGF-A similar to our model. ID8-Vegf-A tumor cells are able to generate solid tumor or ascites when injected into syngeneic C57BL/6 mice subcutaneously or via the intraperitoneally route respectively. This model gave us the unique opportunity to compare back-to-back DCs attached to solid surfaces (solid tumors) and DCs in suspensions (ascites) under the influence of the same tumor cells in vivo.
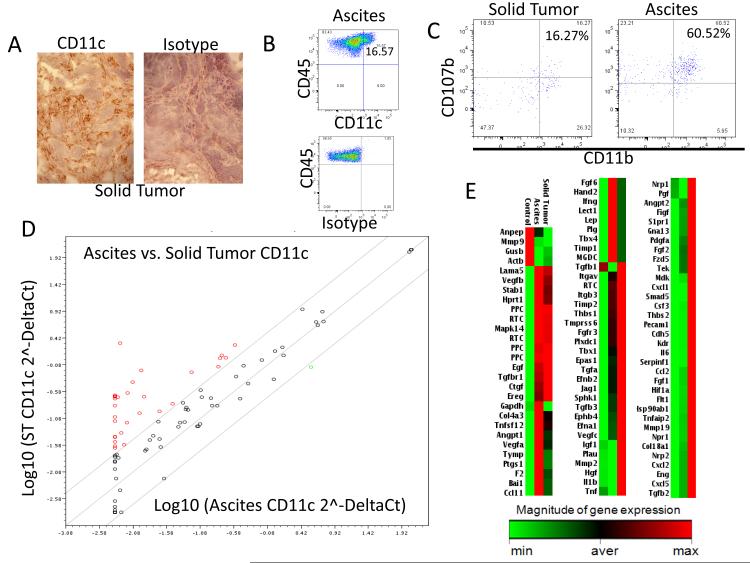
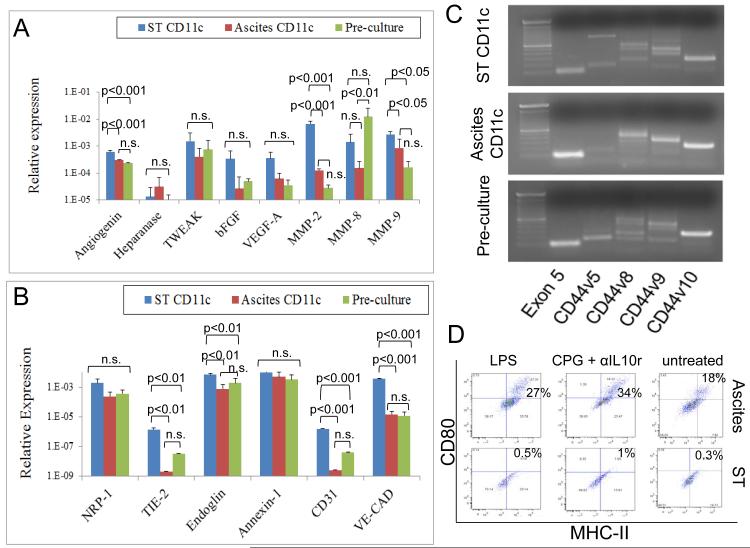
In order to identify DCs in mouse tumors we used CD11c, a typical murine myeloid DC marker. As shown in Figs. 5A and B, CD11c cells were present both in solid tumors and ascites samples in these models as determined by immunohistochemistry and flow cytometry analysis respectively. In addition, as shown in Fig. 5C, we were able to determine that a subpopulation of TA-DCs (CD45+CD11c+) recovered from ascites and solid tumors coexpressed CD11b and CD107b markers. These are markers of inflammatory DCs as previously described (53). It is noteworthy to comment that bone marrow-derived DCs raised in the presence of GM-CSF are postulated as the in vitro counterpart of naturally occurring inflammatory DCs (53). By means of immunomagnetic purification we isolated CD11c cells from ascites or single cell suspensions prepared by mechanically disaggregating solid tumors. Then, RNA was extracted from these cells and equal amounts of RNA from 4 independent experiments were pooled in order to analyze the expression of several angiogenesis-related molecules by means of PCR array technology. Firstly, the levels of expression of these molecules were compared between ascites and solid tumors CD11c cells using a Scatter plot analysis. As shown in Fig. 5D, we were able to observe higher expression of many angiogenic molecules, at the level of RNA, in CD11c cells recovered from solid tumors. This was also evident, when the samples were displayed in a clustergram (Fig. 5E). Altogether these data indicated that mDCs present in ascites or solid tumors have a different pattern of expression of angiogenic molecules. To further investigate this we analyzed by means of real time quantitative PCR the expression of several endothelial markers and angiogenic molecules in CD11c cells recovered from solid tumors, ascites or myeloid DCs generated in vitro from bone marrow cells. The latter are the same type of cells used in our previous studies, where we named them Pre-culture cells. As shown in Fig 6A, we observed significant higher levels of expression of MMP-2, MMP-9 and angiogenin in CD11c cells recovered from solid tumors as compared with their ascites counterparts. We also observed a trend to the expression of higher levels of VEGF and bFGF in the same cells. In addition, we were able to detect a higher expression of endothelial markers such as endoglin, CD31 and more importantly VE-Cadherin and TIE-2 in CD11c cells recovered from solid tumors. As described above, these two molecules have been reported in angiogenic APCs. This is consistent with our previous data showing, by means of flow cytometry analysis, that in human ovarian cancer, solid tumor-associated DCs expressed higher levels of endothelial markers than ascites-associated DCs (18, 54).
Figure 5.
Characteristics of CD11c cells recovered form ovarian cancer solid tumor and ascites. (A) Staining of mouse ovarian tumors with CD11c shows heavy DC infiltration. Immunohistochemistry analysis (200X magnification). (B) Similarly, CD45-CD11c positive cells were detected in murine ovarian cancer ascites by means of flow cytometry analysis. (C) Inflammatory DCs in murine ovarian cancer tumors. Single cells suspensions prepared from solid tumor or ascites were analyzed by flow cytometry gating on the CD45+CD11c+ population. A subpopulation of cells expressing CD11b and CD107b, markers of inflammatory DCs was detected in both tumor models. (D) Scatter plot of gene expression of angiogenic factors (2-ΔCt) at the RNA level for solid tumor CD11c cells versus ascites CD11c cells. Diagonals delimit a 95% confidence interval. Results were obtained using the RT2 Profiler PCR Array Data Analysis software (SABiosystems). Red dots indicate molecules upregulated in the solid tumor CD11c cells. (E) Clustergram of the magnitude of gene expression for all genes analyzed by the RT2 Profiler PCR Array System for mouse angiogenesis (SABiosciences, PAMM-024A). Light green represents minimal gene expression, and red indicated maximum gene expression as indicated by the legend. The clustergram shows all genes of interest and the magnitude each is expressed in Pre-culture mDCs (control), solid tumor CD11c cells and ascites CD11c cells. RT2 Profiler PCR Array Data Analysis software (SABiosystems) was used to construct this figure. RNA pooled form 4 independent experiments were used for these studies.
Figure 6.
Angiogenic and immunological properties of solid tumor and ascites CD11c cells. Solid tumor and ascites CD11c cells were isolated by immunomagnetic purification, and RNA was extracted and reverse-transcribed. Then, quantitative real-time PCR was performed to analyze the expression of several angiogenic molecules (A) and endothelial markers (B) in these cells. Data were analyzed by ANOVA followed by followed by Tukey-Kramer Multiple Comparisons post-Test. An experiment representative of 2 independent experiments is shown. (C) Expression of different CD44 variants by the same cells was analyzed by qualitative PCR analysis. (D) CD11c cells isolated as above from solid tumor and ovarian cancer ascites were cultured for 1 week in RPMI 10% FBS containing LPS; CPG plus anti-IL10 receptor; or left untreated. Then expression of CD80 and MHC-II was analyzed in these cells by flow cytometry. Live CD11c cells isolated from 4 independent experiments were pooled and run in quadruplicate for this study.
In order to further define the characteristics of CD11c cells present in solid tumor and ascites, we investigated the expression of different CD44 variants in these cells. CD44 is transmembrane glycoprotein that is involved in diverse cellular processes, such as regulation of growth, survival, differentiation and migration (55). CD44 possess several transcripts variants due to alternative splicing, generating proteins with different lengths (55). Altered expression of CD44 has been observed in tumor cells and is also associated with different pathological conditions (55). Taking into account that CD44 is present in mDCs as we have recently shown (40) and that this molecule is involved in adhesion to hyaluronan, collagen, laminin and fibronectin we considered interesting to define the profile of CD44 variant expression in CD11c cells recovered from solid tumor and ascites. It is noteworthy to comment that most splicing variants in this molecule occur between exons 5 and 6, giving rise to at least 10 splicing variants (v1-10). We decided to investigate the expression of these splicing variants by qualitative PCR analysis. To accomplish this, we used previously described primers (56), consisting of a reverse complement primer for exon 6 that is used in combination with forward primers for exons 5, v1, v2, v3, v4, v5, v6, v7, v8, v9, and v10. The short CD44 splicing variant, which eliminates the sequences between exons 5 and 6, is the one present in most cells (55). As shown in Fig. 6C, by using primers designed to visualize PCR products for the short form (exon 5) of CD44, and CD44v5, v8, v9, and v10 we were able to detect in our DC samples different patterns of expression of CD44 variants. This is another indication that solid tumor and ascites DCs possess different properties.
Finally, in order to determine the immunological properties of these cells, we isolated solid tumor and ascites CD11c cells by means of immunomagnetic purification and we cultured equal amounts of live cells for 1 week with RPMI 10% FBS in the presence of stimulatory factors. To accomplish this we used LPS or a combination of CPG plus anti-IL10 receptor, which has been shown to overcome the immune paralysis of tumor-associated DCs (13). As shown in Fig. 6D, solid tumor CD11c cells expressed low to null level of costimulatory molecules upon 1–week culture with inflammatory stimuli. On the contrary CD11c cells recovered from ascites were able to upregulate costimulatory molecules under the same conditions. This agrees with recent reports proposing the use of DCs isolated from ovarian cancer ascites for vaccination purposes (57).
Altogether these data indicate that in these models of ovarian cancer, CD11c cells present in solid tumor and ascites have a different phenotype, expressing different levels of angiogenic molecules and endothelial markers, CD44 variants and the capability to respond to inflammatory stimuli.
Tumor factors are not able to induce expression of endothelial markers in non-attached DCs
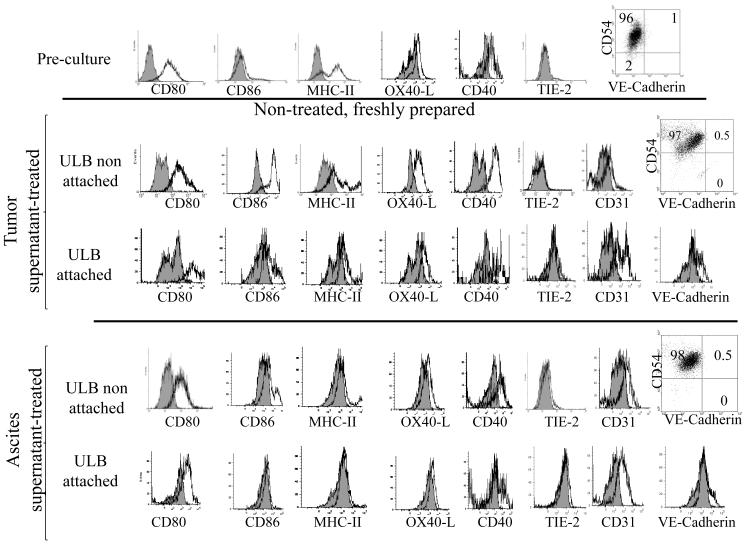
It has been previously shown that DCs treated with tumor factors acquire angiogenic potential (17, 42, 43). As shown above, mDCs cultured on solid surfaces were able to upregulate expression of VE-Cadherin, and to a lower extent, TIE-2 at the level of protein, but this was not observed in cells cultured on ULB surfaces. In order to determine if the presence of soluble tumor factors in the absence of attachment to a solid surface was able to induce the expression of these molecules, we cultured mDCs for 1 week on ULB surfaces with culture medium containing 30% of ID8-Vegf-A tumor conditioned media or cell-free ascites supernatant. Although the majority of cells do not attach to this surface a small proportion of the mDCs remain loosely attached to it. For this series of experiments we recovered both the floating cells and those attached to the surface and we compared the expression of endothelial markers and costimulatory molecules in both populations. As shown in Fig. 7, treatment with tumor factors was not able to induce the expression of TIE-2 or VE-Cadherin in the non-attached cells. On the other hand, we were able to observe that a small population of VE-Cadherin positive cells was generated in the attached cell population. The attached cells also showed higher expression of CD31, another endothelial marker. Again, this indicates that attachment to a substrate and not necessarily the presence of tumor factors, favors the expression of some endothelial markers in these cells.
Figure 7.
Effect of tumor factors on DCs cultured on ULB surfaces. Expression of surface markers on DC cultured for 1 week on ULB surfaces was analyzed by flow cytometry and compared to that of Pre-culture cells. Cells were cultured in the presence of RPMI 10% FBS supplemented with 30% of ID8-VegfA conditioned medium or cell-free ascites. Analysis was performed on CD11c gated cells. Grey histograms represent isotype controls. Quadrants were defined by using isotype controls. Cultured cells showed higher autofluorescence than Pre-culture cells. An experiment representative of 2-4 independent experiments is shown.
DISCUSSION
As described above, DCs are present in the microenvironment of different types of cancer (8-12). Within the tumor microenvironment, cytokines such as VEGF, IL-10 and PGE-2 can profoundly affect the nature of APCs. In particular, DCs showing low levels of costimulatory molecules have been detected in tumors expressing high levels of VEGF (58, 59) a paradigmatic angiogenic molecule which has been proposed as a target for antitumor therapies (50, 51, 60). On the contrary, highlighting the importance of VEGF in this process, cancer patients treated with anti-VEGF antibody showed a decrease in the levels of immunosuppressive DCs (61). In the same way, it has been shown that the endothelial cell-produced antiangiogenic cytokine vascular endothelial growth inhibitor induces DC maturation in cancer patients (62). Thus, soluble tumor factors are able to define the immunological functions of DCs. But in addition to immunological functions, DCs can also participate in angiogenic process.
Tumors require blood supply for expansive growth, thus inducing the formation of neovessels (63). These are different from vessels of normal tissues at the morphologic and molecular level (64, 65). We and others have described the capability of APCs such as DCs or macrophages, to collaborate with neoangiogenesis in ovarian and other human cancers and in different mouse tumor models (9, 10, 14, 19). Interestingly, depletion of these angiogenic DCs from the tumor microenvironment reduced tumor growth in a mouse model of ovarian carcinoma (54). Data from the late Dr. J. Folkman’s lab highlighted the contribution of DCs to angiogenesis in the peritoneal Lewis lung carcinoma tumor model (19). Also, the same group showed that immature DCs participate in angiogenic processes in non-tumor settings (22).
As described above, DCs are able to express endothelial markers both in vivo and in vitro under the influence of tumor factors, although it is not clear if there is a correlation between the expression of these markers and the capability of the cells to promote angiogenesis. We have recently reported that the interaction with extracellular matrix components define different immunological and angiogenic properties in murine mDCs (40). Building on that data, herewith we showed that attachment to a solid surface like polystyrene is able to induce expression of VE-Cadherin, and to a lesser extent TIE-2, in murine mDCs. This was not observed when cells were cultured on ULB surfaces. Moreover, cells attached to polystyrene showed lower levels of maturation markers than those on suspension when treated with inflammatory stimuli. On the other hand, we did not observe a difference on the expression of angiogenic molecules between the two types of cultures. Further, we were able to detect higher expression of VEGF in supernatants of ULB cultures when compared to polystyrene cultures. We have recently shown that mDCs are able to generate VEGF when cultured on different substrates (40). Taking into account that murine mDCs express VEGF receptors as we have recently shown (40), we hypothesize that this molecule might act as a paracrine survival signal for cells in suspension in order to prevent anoikis. Finally, when these cells were cultured for 3 weeks on ULB or polystyrene surfaces, mDCs recovered from ULB cultures showed immunological properties similar to those present in the same cells before culture.
Altogether these data suggest that lack of adherence to a substrate can preserve the immunological properties of mDCs, that adherence to a substrate might induce the expression of some endothelial markers in these cells, and that the expression of these markers might not correlate with the angiogenic properties of the cells.
In order to investigate the relevance of substrate adherence on the biology of DCs we decided to use the ID8-Vegf-A model of ovarian carcinoma that we have previously used in different publications (26, 29, 66). The ID8 model of mouse ovarian carcinoma, originally generated by Roby et al. (27), gives us the unique opportunity to investigate DCs attached to substrates (solid tumors) and in suspension (ascites) under the influence of the same tumor cells. We have previously demonstrated in human ovarian cancer that DCs present in solid tumor and ascites have different properties as determined by flow cytometry analysis (18, 67). It has been postulated that in the steady state murine DCs only originate from DC precursors, while during inflammatory or pathological settings they might also arise from monocytes and colonize lymphoid organs or non-immune tissues (68-72). These “inflammatory DCs” are characterized by the expression of CD11c, CD11b and CD107b(MAC3) (53). Indeed, murine bone marrow-derived DCs generated in the presence of GM-CSF are considered the in vitro counterpart of the naturally occurring “inflammatory DCs”. Herewith, we were able to detect co-expression of CD11b and CD107b in CD11c leukocytes present in ovarian cancer ascites and solid tumors indicating that part of the tumor-associated DCs in these models are inflammatory DCs. In this context, data obtained using in vitro GM-CSF generated DCs can help interpret the characteristics of tumor-associated DCs in these cancer models. In the studies described here we showed that solid tumor CD11c cells expressed higher levels of angiogenic molecules than CD11c cells present in ascites which in turn express higher levels of angiogenic molecules than in vitro generated mDCs. Interestingly, we were able to detect higher levels of expression of VE-Cadherin and TIE-2 in solid tumor CD11c cells compared to ascites CD11c cells. Further, the levels of these molecules were not significantly different among ascites CD11c cells and freshly in vitro generated mDCs. Upregulation of costimulatory molecules was only observed in ascites CD11c cells. Finally, when mDCs were cultured on ULB surfaces in the presence of tumor factors we were not able to detect expression of VE-Cadherin and TIE-2 in the non-attached cells.
Taking into account all these data we consider that tumor factors might be responsible for inducing angiogenic properties in DCs, but that in some settings the expression of endothelial markers such as VE-Cadherin and TIE-2 might be a function of attachment to solid surfaces, and independent of the angiogenic properties of these cells. In this way we consider that the combination of soluble factors together with adhesion surfaces will determine a particular DC profile. Finally, in order to design treatments focused on changing the phenotype of DCs associated with diseases, such as cancer (73-75) or atherosclerosis (76, 77) among others, it might not be sufficient to specifically target these cells but must be also necessary to modify the microenvironment in which these cells are present.
ACKNOWLEDGEMENTS
This work supported in part by the NIH under Grant R15 CA137499-01 (F.B.) and a startup fund from Ohio University (F.B.). The FACSAria flow cytometer was obtained through the NSF grant MRI: Acquisition of a Fluorescence Activated Cell Sorter for Research and Training at Ohio University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Bonasio R, von Andrian UH. Generation, migration and function of circulating dendritic cells. Curr Opin Immunol. 2006;18:503. doi: 10.1016/j.coi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. 2001. p. 291. [DOI] [PubMed]
- 4.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 5.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 7.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 8.Baleeiro RB, Anselmo LB, Soares FA, Pinto CA, Ramos O, Gross JL, Haddad F, Younes RN, Tomiyoshi MY, Bergami-Santos PC, Barbuto JA. High frequency of immature dendritic cells and altered in situ production of interleukin-4 and tumor necrosis factor-alpha in lung cancer. Cancer Immunol Immunother. 2008;57:1335. doi: 10.1007/s00262-008-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Sozzani S, Locati M, Schioppa T, Saccani A, Allavena P, Sica A. Infiltration of tumours by macrophages and dendritic cells: tumour-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Novartis Found Symp. 2004;256:137. [PubMed] [Google Scholar]
- 11.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 12.Whiteside TL. The role of immune cells in the tumor microenvironment. Cancer Treat Res. 2006;130:103. doi: 10.1007/0-387-26283-0_5. [DOI] [PubMed] [Google Scholar]
- 13.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O’Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 16.Glod J, Kobiler D, Noel M, Koneru R, Lehrer S, Medina D, Maric D, Fine HA. Monocytes form a vascular barrier and participate in vessel repair after brain injury. Blood. 2006;107:940. doi: 10.1182/blood-2004-11-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottfried E, Kreutz M, Haffner S, Holler E, Iacobelli M, Andreesen R, Eissner G. Differentiation of human tumour-associated dendritic cells into endothelial-like cells: an alternative pathway of tumour angiogenesis. Scand J Immunol. 2007;65:329. doi: 10.1111/j.1365-3083.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 18.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 19.Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L, Nakai K, Pravda E, Hornstein MD, D’Amato RJ, Folkman J. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. Faseb J. 2008;22:522. doi: 10.1096/fj.07-9034com. [DOI] [PubMed] [Google Scholar]
- 20.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kogata N, Arai Y, Pearson JT, Hashimoto K, Hidaka K, Koyama T, Somekawa S, Nakaoka Y, Ogawa M, Adams RH, Okada M, Mochizuki N. Cardiac ischemia activates vascular endothelial cadherin promoter in both preexisting vascular cells and bone marrow cells involved in neovascularization. Circ Res. 2006;98:897. doi: 10.1161/01.RES.0000218193.51136.ad. [DOI] [PubMed] [Google Scholar]
- 22.Nakai K, Fainaru O, Bazinet L, Pakneshan P, Benny O, Pravda E, Folkman J, D’Amato RJ. Dendritic cells augment choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:3666. doi: 10.1167/iovs.07-1640. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Schnare M, Menges M, Rossner S, Rollinghoff M, Schuler G, Gessner A. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 25.Muccioli M, Pate M, Omosebi O, Benencia F. Generation and Labeling of Murine Bone Marrow-derived Dendritic Cells with Qdot Nanocrystals for Tracking Studies. J Vis Exp. 2011 doi: 10.3791/2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, Allman D, Coukos G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161:2295. doi: 10.1016/s0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Conejo-Garcia JR, Yang N, Huang W, Mohamed-Hadley A, Yao W, Benencia F, Coukos G. Different effects of glucose starvation on expression and stability of VEGF mRNA isoforms in murine ovarian cancer cells. Biochem Biophys Res Commun. 2002;292:860. doi: 10.1006/bbrc.2002.6710. [DOI] [PubMed] [Google Scholar]
- 29.Benencia F, Courreges MC, Conejo-Garcia JR, Mohamed-Hadley A, Zhang L, Buckanovich RJ, Carroll R, Fraser N, Coukos G. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther. 2005;12:789. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Benencia F, Courreges MC, Fraser NW, Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther. 2008;7:1194. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- 31.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 32.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 33.Grolleau-Julius A, Abernathy L, Harning E, Yung RL. Mechanisms of murine dendritic cell antitumor dysfunction in aging. Cancer Immunol Immunother. 2009;58:1935. doi: 10.1007/s00262-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macary PA, Too CT, Dai X. Targeting tumours by adoptive transfer of immune cells. Clin Exp Pharmacol Physiol. 2006;33:569. doi: 10.1111/j.1440-1681.2006.04409.x. [DOI] [PubMed] [Google Scholar]
- 35.Bianco NR, Kim SH, Morelli AE, Robbins PD. Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol Biol. 2007;380:443. doi: 10.1007/978-1-59745-395-0_28. [DOI] [PubMed] [Google Scholar]
- 36.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 37.Simon JC, Hara H, Denfeld RW, Martin S. UVB-irradiated dendritic cells induce nonproliferating, regulatory type T cells. Skin Pharmacol Appl Skin Physiol. 2002;15:330. doi: 10.1159/000064537. [DOI] [PubMed] [Google Scholar]
- 38.Yamagami S, Usui T, Amano S, Ebihara N. Bone marrow-derived cells in mouse and human cornea. Cornea. 2005;24:S71. doi: 10.1097/01.ico.0000178732.42921.05. [DOI] [PubMed] [Google Scholar]
- 39.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol. 2000;27:313. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 40.Sprague L, Muccioli M, Pate M, Meles E, McGinty J, Nandigam H, Venkatesh AK, Gu MY, Mansfield K, Rutowski A, Omosebi O, Courreges MC, Benencia F. The interplay between surfaces and soluble factors define the immunologic and angiogenic properties of myeloid dendritic cells. BMC Immunol. 2011;12:35. doi: 10.1186/1471-2172-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pujol B. Fernandez, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 43.Pujol B. Fernandez, Lucibello FC, Zuzarte M, Lutjens P, Muller R, Havemann K. Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol. 2001;80:99. doi: 10.1078/0171-9335-00136. [DOI] [PubMed] [Google Scholar]
- 44.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 46.Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536. doi: 10.1074/jbc.M400554200. [DOI] [PubMed] [Google Scholar]
- 47.Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, Hla T, Williams MS, Winkles JA. TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol. 2003;23:594. doi: 10.1161/01.ATV.0000062883.93715.37. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama M, Harada N, Okumura K, Yagita H. Characterization of murine TWEAK and its receptor (Fn14) by monoclonal antibodies. Biochem Biophys Res Commun. 2003;306:819. doi: 10.1016/s0006-291x(03)01051-9. [DOI] [PubMed] [Google Scholar]
- 49.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 51.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 52.Scheeren RA, Koopman G, Van der Baan S, Meijer CJ, Pals ST. Adhesion receptors involved in clustering of blood dendritic cells and T lymphocytes. Eur J Immunol. 1991:1101. doi: 10.1002/eji.1830210503. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 54.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, Sentman CL, Conejo-Garcia JR. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 56.Yu Q, Toole BP. Common pattern of CD44 isoforms is expressed in morphogenetically active epithelia. Dev Dyn. 1997;208:1. doi: 10.1002/(SICI)1097-0177(199701)208:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 57.Adams S, Facciabene A, McCann G, Jean S, Peng X, Gamerman V, Gimotty P, Coukos G. Intraperitoneal injection of ascites-derived antigen-presenting cells following ex vivo treatment with Toll-like receptor agonists confers significant protection against tumor challenge. 2010. p. S156.
- 58.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 59.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963. [PubMed] [Google Scholar]
- 60.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian F, Grimaldo S, Fujita M, Cutts J, Vujanovic NL, Li LY. The endothelial cell-produced antiangiogenic cytokine vascular endothelial growth inhibitor induces dendritic cell maturation. J Immunol. 2007;179:3742. doi: 10.4049/jimmunol.179.6.3742. [DOI] [PubMed] [Google Scholar]
- 63.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 64.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 65.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 66.Benencia F, Courreges MC, Conejo-Garcia JR, Buckanovich RJ, Zhang L, Carroll RH, Morgan MA, Coukos G. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16:765. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 67.Huarte E, Tirapu I, Arina A, Vera M, Alfaro C, Murillo O, Palencia B, Busto V, Marin V, Mazzolini G, Melero I. Intratumoural administration of dendritic cells: hostile environment and help by gene therapy. Expert Opin Biol Ther. 2005;5:7. doi: 10.1517/14712598.5.1.7. [DOI] [PubMed] [Google Scholar]
- 68.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 69.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 70.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gutgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 73.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, Sentman CL, Kedl R, Conejo-Garcia JR. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cubillos-Ruiz JR, Fiering S, Conejo-Garcia JR. Nanomolecular targeting of dendritic cells for ovarian cancer therapy. Future Oncol. 2009;5:1189. doi: 10.2217/fon.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bobryshev YV, Tran D, Killingsworth MC, Buckland M, Lord RV. Dendritic cell-associated immune inflammation of cardiac mucosa: a possible factor in the formation of Barrett’s esophagus. J Gastrointest Surg. 2009;13:442. doi: 10.1007/s11605-008-0746-x. [DOI] [PubMed] [Google Scholar]
- 76.Bobryshev YV, Lord RS. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res. 1998;37:799. doi: 10.1016/s0008-6363(97)00229-0. [DOI] [PubMed] [Google Scholar]
- 77.Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]