Abstract
Objective:
To assess efficacy and safety of once-daily 8 or 12 mg perampanel, a noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor antagonist, when added to concomitant antiepileptic drugs (AEDs) in the treatment of drug-resistant partial-onset seizures.
Methods:
This was a multicenter, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier: NCT00699972). Patients (≥12 years, with ongoing seizures despite 1–3 AEDs) were randomized (1:1:1) to once-daily perampanel 8 mg, 12 mg, or placebo. Following baseline (6 weeks), patients entered a 19-week double-blind phase: 6-week titration (2 mg/week increments to target dose) followed by a 13-week maintenance period. Percent change in seizure frequency was the primary endpoint; 50% responder rate was the primary endpoint for EU registration.
Results:
Of 388 patients randomized and treated, 387 provided seizure frequency data. Using this intent-to-treat population over the double-blind phase, the median percent change in seizure frequency was −21.0%, −26.3%, and −34.5% for placebo and perampanel 8 and 12 mg, respectively (p = 0.0261 and p = 0.0158 for 8 and 12 mg vs placebo, respectively). Fifty percent responder rates during the maintenance period were 26.4%, 37.6%, and 36.1%, respectively, for placebo, perampanel 8 mg, and perampanel 12 mg; these differences were not statistically significant for 8 mg (p = 0.0760) or 12 mg (p = 0.0914). Sixty-eight (17.5%) patients discontinued, including 40 (10.3%) for adverse events. Most frequent treatment-emergent adverse events were dizziness, somnolence, irritability, headache, fall, and ataxia.
Conclusions:
This trial demonstrated that once-daily, adjunctive perampanel at doses of 8 or 12 mg improved seizure control in patients with uncontrolled partial-onset seizures. Doses of perampanel 8 and 12 mg were safe, and tolerability was acceptable.
Classification of evidence:
This study provides Class I evidence that once-daily 8 and 12 mg doses of adjunctive perampanel are effective in patients with uncontrolled partial-onset seizures.
Perampanel is an orally active, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor antagonist under development for the treatment of epilepsy. AMPA receptors, a class of ionotropic glutamate receptors, are localized at excitatory synapses in the CNS where they serve as the principal mediators of fast excitatory postsynaptic neurotransmission and are critical to the generation and spread of epileptic activity.1 Perampanel selectively inhibits AMPA receptors and to date has not been found to interact with other molecular targets at relevant concentrations.1,2 In particular, perampanel does not inhibit NMDA receptors, a different class of ionotropic glutamate receptors with distinct physiologic roles at excitatory synapses.
Perampanel, like other selective AMPA receptor antagonists, has broad-spectrum activity in animal models used to identify antiepileptic drugs (AEDs), including electroshock, chemoconvulsant, and kindled seizure models.2 AMPA receptor antagonists have not been found to cause the psychotomimetic effects that can occur with some NMDA receptor antagonists.1,3
Two phase II dose-escalation, placebo-controlled studies provided therapeutic proof of concept for perampanel in patients with partial-onset seizures, and suggested the maximum tolerated dose of perampanel.4 Using this information, 3 phase III trials were initiated and have been reported: 1 assessing low/middle once-daily doses (2, 4, and 8 mg)5 and 2 assessing middle/high once-daily doses (8 and 12 mg).6,7 The phase III trial reported here assessed the efficacy and safety of middle and high effective doses (8 and 12 mg) of perampanel when added to 1 to 3 approved AEDs in patients with uncontrolled partial-onset seizures.
METHODS
Classification of evidence.
This interventional study had a >80% completion rate; it provides Class I evidence that once-daily, adjunctive perampanel (8 and 12 mg, respectively) significantly reduces seizure frequency (26.3% [p = 0.0261] and 34.5% [p = 0.0158]) compared with placebo (21.0%), and improves (albeit not statistically significantly) responder rates (37.6% [p = 0.0760] and 36.1% [p = 0.0914]) compared with placebo (26.4%) in patients ≥12 years with uncontrolled partial-onset seizures.
Standard protocol approvals, registration, and patient consents.
This trial (Eisai Inc. protocol E2007-G000-304, ClinicalTrials.gov identifier: NCT00699972), conducted between April 2008 and November 2010 at 68 centers across Argentina, Canada, Chile, Mexico, and the United States, was performed in accordance with the Declaration of Helsinki, Good Clinical Practice ICH-E6 Guideline CPMP/ICH/135/95, European Directive 2001/83/EC, and US Code of Federal Regulations Part 21. Trial protocol, amendments, and informed consent were reviewed by national regulatory authorities in each country and independent ethics committees or institutional review boards for each site. Before participation, all patients gave written informed consent.
Patients.
Eligible patients were ≥12 years of age, diagnosed with partial-onset seizures with or without secondary generalization according to the 1981 International League Against Epilepsy Classification of Epileptic Seizures,8 had failed ≥2 AEDs, had ≥5 partial seizures during baseline, and were taking stable doses of up to 3 approved AEDs. Detailed criteria are available in table e-1 on the Neurology® Web site at www.neurology.org.
Trial design.
This was a multicenter, multinational, randomized, double-blind, placebo-controlled trial consisting of 4 periods: baseline, titration, maintenance, and follow-up or entry into extension trial. Enrolled patients entered a 6-week baseline period to assess pretreatment seizure frequency and determine their eligibility for the double-blind phase. Patients were then randomized (1:1:1) to once-daily treatment with placebo, perampanel 8 mg, or perampanel 12 mg. Randomization was performed using a computer-generated random allocation sequence, approved and locked after review by an independent statistician. Kit numbers were generated for blinded study drug/kit dispensing by the investigator to each patient at each visit. Treatment codes remained blinded throughout the study. The 19-week double-blind treatment phase consisted of 6-week titration and 13-week maintenance periods. Seizure frequency data were captured in a diary maintained by the patient or caregiver. A 4-week follow-up phase at the end of the trial collected safety and efficacy data without treatment. Patients completing the maintenance period had the option to enter a perampanel open-label extension trial (study 307, NCT00735397).
During titration, perampanel patients had weekly 2 mg increments up to the randomized dose. Patients experiencing intolerable adverse events (AEs) could defer up-titration or have their dose reduced. Consecutive 2 mg down-titration was discouraged and doses could be increased when tolerability improved. Patients not tolerating at least 2 mg of perampanel or placebo by the end of titration were withdrawn from the trial.
During maintenance, patients continued the dose achieved during titration. Patients who discontinued treatment during maintenance, or who did not participate in the extension trial, entered the follow-up phase.
Measurement of efficacy.
Efficacy assessments included seizure counts from patient diaries, Clinical and Patient Global Impression of Change (CGIC/PGIC), and the Quality of Life in Epilepsy questionnaire (QOLIE-31-P). The protocol-defined percent change in seizure frequency per 28 days for the intent-to-treat (ITT) population (all patients receiving at least 1 dose of study medication in the double-blind phase and providing any seizure outcome data) in the 19-week double-blind phase was compared with baseline as the primary endpoint. The responder rate (percentage of patients who experienced a ≥50% reduction in seizure frequency in the maintenance period relative to baseline) was also designated as a primary endpoint for registration in the European Union (EU) according to EU guidelines.
Measurement of safety.
Safety assessments included prior and concomitant medication use, AEs, withdrawals, clinical laboratory parameters, vital signs, ECGs, physical and neurologic examinations, photosensitivity, and withdrawal questionnaires.
Statistical analysis.
Sample size determination was based on the mean reduction in seizure frequency in perampanel dose-ranging studies.4 A sample of 125 patients in each treatment group was considered sufficient for >80% power to detect a treatment difference of 22% (assuming common SD of 56%) in percentage change in seizure frequency between placebo and each active treatment arm. This sample size had 90% power to detect a treatment difference of 16% in responder rate.
Initially, the analyses for percent change and ≥50% responder rate were to be done over the maintenance period for all patients who received at least 1 dose of study medication and who provided at least 2 weeks of seizure data during the double-blind phase. The analysis for percent change in seizure frequency was modified to include all patients who received at least 1 dose of study medication in the double-blind phase and who provided any seizure outcome data (ITT analysis set); the responder rate analysis was modified to include all ITT patients. These are consistent with the Food and Drug Administration and European Medicines Agency guidelines. Other secondary efficacy endpoints included percent change in the frequency of complex partial seizures plus secondary generalized seizures in the double-blind phase relative to baseline, and a dose-response analysis of percent change in seizure frequency during maintenance. Available seizure data during the double-blind phase were converted to seizure frequency per 28 days. For the analysis of percent change in seizure frequency, the baseline seizure frequency per 28 days and the percent change per 28 days during treatment were rank transformed separately prior to regression analysis due to the skewed distribution of the seizure frequency data. An analysis of covariance (ANCOVA) was then conducted on the rank-transformed data, with treatment and pooled countries as factors and the ranked baseline seizure frequency per 28 days as a covariate. Log transformation-based ANCOVA was conducted to assess the robustness of the results. Dose-response analysis of the percent change in seizure frequency was performed via a linear contrast using the ranked ANCOVA model described above. These analyses were prespecified in the protocol.
Responder rates were analyzed using the Cochran-Mantel-Haenszel test adjusting for pooled countries, with last observation carried forward for patients with less than 8 weeks of maintenance seizure data.
A closed, sequential testing procedure (8 mg then 12 mg treatment group vs placebo) was employed to control the family-wise type I error rate for analyses of the primary efficacy endpoint for different dose groups.
RESULTS
Patient allocation and demographics.
Between August 2008 and November 2010, 534 patients were screened; 388 were randomized and received once-daily treatment (placebo = 121, 8 mg = 133, and 12 mg = 134). Of these, 228 were from North America (227 in ITT analysis set), and 160 from Central/South America. A total of 320 (82.5%) patients completed the trial and 68 (17.5%) discontinued early, including 40 (10.3%) due to AEs. The proportions of patients achieving their randomized dose were 103 (77.4%) and 78 (58.7%) for 8 and 12 mg perampanel, respectively. Patient disposition is outlined in figure 1.
Figure 1. Patient flow.
a Patients who signed informed consent forms. b Includes 3 patients who were screen failures and were inappropriately randomized to a treatment group. c One patient was treated for 1 day and did not complete a seizure diary that day. Admin = administrative; INC/EXC = inclusion/exclusion; ITT = intent-to-treat.
Study groups were evenly distributed for demographic and epilepsy characteristics (table 1). Patients taking 1, 2, and 3 AEDs were 15.5%, 55.7%, and 28.9%, respectively. Mean number ± SD of prior (last 5 years) and concomitant AEDs at baseline across all groups was 2.8 ± 1.2. The median number of seizures ranged from 12.0 to 14.3 per 28 days during baseline.
Table 1.
Baseline patient demographics and clinical characteristics (safety population)

Abbreviations: AED = antiepileptic drug; BMI = body mass index.
Data shown for the intent-to-treat population.
No. of patients (%) taking a perampanel clearance-inducing AED (carbamazepine, oxcarbazepine, or phenytoin).
AEDs used in ≥10% of all patients.
Efficacy.
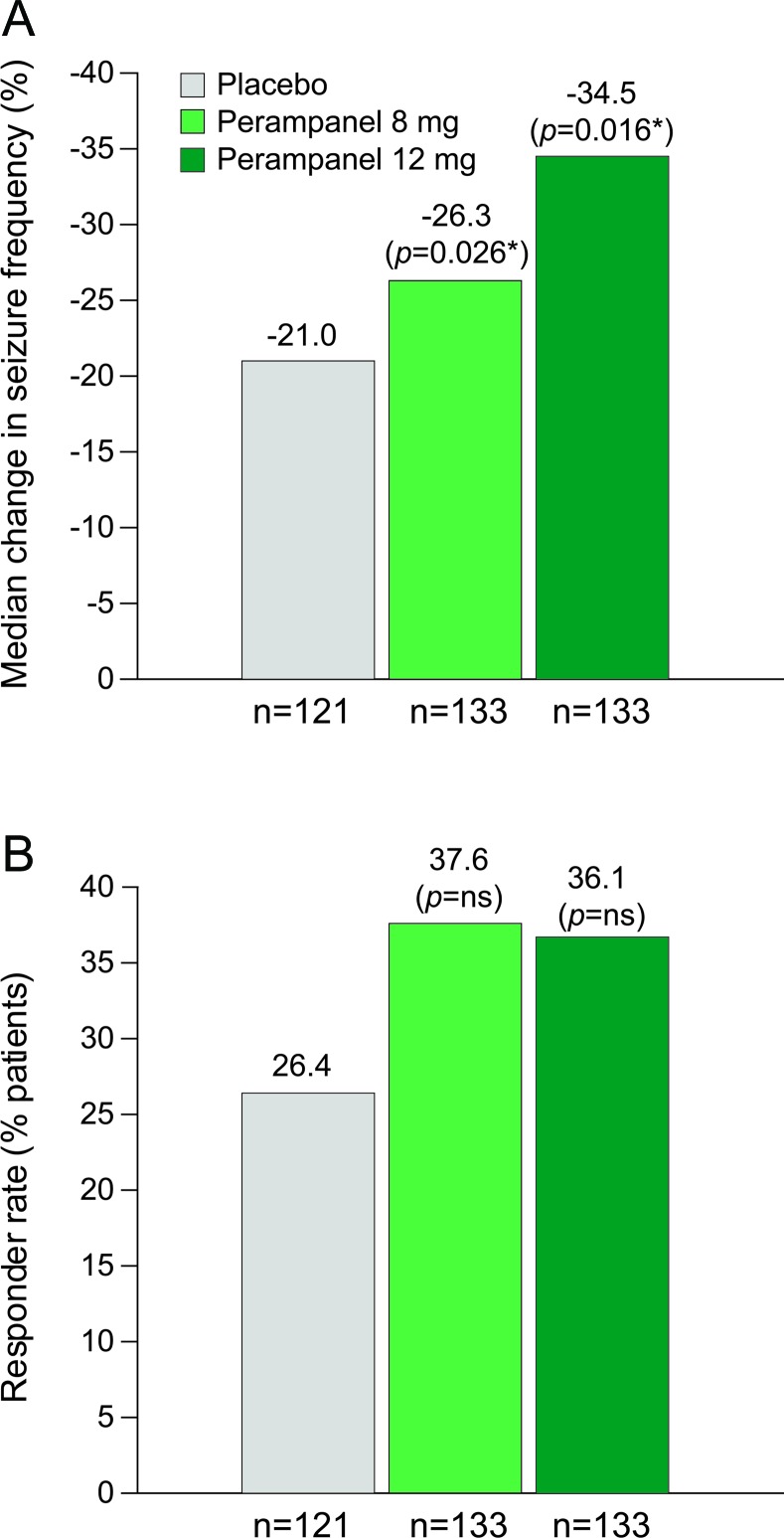
The median percent changes in seizure frequency over the double-blind phase were −26.3% (p = 0.0261), −34.5% (p = 0.0158), and −21.0% for 8 mg, 12 mg, and placebo, respectively, based on the rank ANCOVA (figure 2A). Log transformation-based ANCOVA p values were p = 0.0044 and p = 0.0184, respectively. Median (95% confidence interval [CI]) differences compared with placebo were −13.5% (−26.2, −1.9) and −14.2% (−25.0, −2.7) for 8 and 12 mg, respectively. There was a positive dose response (p = 0.0262) for all seizure types during the maintenance period using linear contrast in the rank ANCOVA.
Figure 2. Median percentage change and responder rates.
(A) Median percentage change in seizure frequency per 28 days (ITT population). (B) Responder rates (percentage of patients achieving a ≥50% reduction in seizure frequency, ITT population). *vs placebo. ITT = intent-to-treat; NS = not significant.
For percent change in complex partial plus secondary generalized seizures, the results were −33.0% (p = 0.0020) for 8 mg and −33.1% (p = 0.0081) for 12 mg vs −17.9% for placebo.
Fifty percent responder rates (95% CI) were 37.6% (29.4, 45.8) for 8 mg (p = 0.0760) and 36.1% (27.9, 44.3) for 12 mg (p = 0.0914) vs 26.4% (18.6, 34.3) for placebo (figure 2B). In a subanalysis of responder rates by region, the North American (n = 227) responder rates significantly differed from placebo (21.9%, p < 0.05 for both) for perampanel 8 mg (40.5%) and 12 mg (40.0%), whereas the Central and South American (n = 160) responder rate showed no difference vs placebo (33.3%) for either perampanel 8 mg (33.9%) or 12 mg (30.2%).
The numbers needed to treat were 9 and 10 patients for a responder, and the absolute risks (95% CI) were 11.2% (−0.2, 22.5) and 9.7% (−1.7, 21.0), respectively, for 8 and 12 mg. Mean compliance was >98% in all groups.
Categorical seizure reductions at 75%–100% were 18.8% (p = 0.001) and 17.3% (p = 0.001) for 8 and 12 mg, respectively, vs 5.0% for placebo (p values were not adjusted for multiplicity). Seizure-free rates during the entire maintenance period were 2.2% (8 mg) and 1.5% (12 mg) vs 0.0% (placebo), conforming to the preferred “pragmatic ITT” suggested by Gazzola et al.9 Seizure-free rates using the maintenance period excluding dropouts showed similar results with 2.6% (8 mg) and 2.0% (12 mg) vs 0.0% (placebo).
The CGIC at the end of treatment showed “much” or “very much” improvement with perampanel treatment: 43.2% (p = 0.0122) for 8 mg and 35.7% (p = 0.2545) for 12 mg vs 27.4% for placebo. The PGIC showed “much” or “very much” improvement with perampanel treatment: 51.6% (p = 0.0533) for 8 mg and 46.5% (p = 0.2706) for 12 mg vs 38.5% for placebo. Responses given in the QOLIE-31-P showed that changes in quality of life were similar between groups.
Safety.
Treatment-emergent AEs (TEAEs) are summarized in table 2. The majority of TEAEs were rated mild or moderate (83.9% for perampanel vs 95.0% for placebo). The most common TEAEs occurred in the CNS (table 2). Worsening seizures, defined as a >50% increase in seizures compared with baseline, occurred in 9% (8 mg) and 9% (12 mg) of patients vs 14% (placebo).
Table 2.
Incidence of treatment-emergent adverse events (safety population)a

Abbreviations: AE = adverse event; TEAE = treatment-emergent adverse event.
TEAEs were defined as AEs that emerged during treatment or that worsened in severity during treatment. Any AE that occurred up to 30 days after a patient's last treatment was considered to be a TEAE.
Study/treatment discontinuation.
TEAEs more frequent with perampanel (occurring in ≥1%) and leading to discontinuation were dizziness (3.0% vs 0%), ataxia (2.6% vs 0%), aggression (1.9% vs 0% [mild n = 1, moderate n = 3, severe n = 1]), vertigo (1.9% vs 0%), dysarthria (1.1% vs 0%), somnolence (1.1% vs 0%), and blurred vision (1.1% vs 0%). Study drug interruptions or dose reductions with perampanel (occurring in ≥2%) were dizziness (13.5%), somnolence (5.2%), ataxia (2.2%), headache (2.2%), hypersomnia (2.2%), and vertigo (2.2%).
There were no cases of sudden unexpected death in epilepsy. One patient was found dead after a convulsion during baseline. Six patients in the placebo group had a total of 8 serious AEs (SAEs), 8 patients in the 8 mg group had a total of 9 SAEs, and 9 patients in the 12 mg group had a total of 15 SAEs. These events led to discontinuation of study treatment in 2, 2, and 6 patients, respectively. The only relevant SAEs (occurring in ≥1 patient in any group) were those related to epilepsy (e.g., convulsion, grand mal convulsion, and status epilepticus) in 1 (<1%) patient in the placebo group, 2 (1.5%) in the 8 mg group, and 4 (3.0%) in the 12 mg group. There were also 2 SAEs of staphylococcal wound infection in the 8 mg group. Psychiatric SAEs overall had a higher rate in the 12 mg group (3.7%) than the 8 mg (<1%) or placebo (1.7%) groups. There were no reports of abuse or diversion of perampanel. One placebo patient and 1 perampanel 8 mg patient each had a TEAE related to suicidality (suicidal ideation).
No clinically important mean changes in laboratory values, ECGs, vital signs, or physical or neurologic examinations were observed. The large majority of patients with markedly abnormal laboratory values were taking concomitant carbamazepine, oxcarbazepine, or valproic acid. Weight increase >7% was observed in 19.2% of perampanel-treated patients without any apparent dose effect, vs 8.3% in the placebo group. The mean weight changes from baseline were 1.6 kg (8 mg) and 1.9 kg (12 mg) vs 0.6 kg (placebo). There were 3 reported overdoses each in the placebo and perampanel 12 mg treatment groups; all were errors associated with drug blister pack configuration except for 1 patient in the 12 mg group (nonstudy drug overdose). Two patients received 14 mg of perampanel instead of 12 mg.
Over 80% of patients did not complete the post-baseline withdrawal questionnaire (continued treatment in the extension study); however, of those who did, most rated each symptom as none or mild. For the photosensitivity questionnaire, 1 patient in both the placebo and 8 mg groups and 3 patients in the 12 mg group recorded positive responses to skin rash/reaction/change in pigmentation/skin complaint.
DISCUSSION
Perampanel is the first AED with a specific action on glutamate-mediated excitatory neurotransmission reporting phase III clinical trials. The present study demonstrated that the frequency of partial-onset seizures in patients with drug-resistant epilepsy is reduced by adjunctive administration of once-daily 8 mg or 12 mg doses of perampanel.
Only patients with highly treatment-resistant partial-onset seizures were enrolled into this study. The impact of perampanel in less refractory patients and other seizure types and syndromes is currently unknown. While the specific mechanism of perampanel remains to be fully explored, no other activity has been detected1,2 against other molecular targets. Therefore, this phase III study, in conjunction with a separate phase III study of lower daily doses,5 provides support for the clinical utility of targeting AMPA receptors for the treatment of partial-onset seizures.
The exploratory efficacy endpoints were based on variables related to seizure frequency, such as number of seizure-free days, and on more subjective measurements, such as the PGIC and CGIC. Results showed general improvement associated with perampanel, further supporting the findings of primary and secondary efficacy endpoints.
When analyzed according to percent change in seizure frequency, both doses of perampanel produced a significantly greater reduction in median seizure frequency than observed with placebo. The magnitude of seizure reduction was comparable to that reported in clinical efficacy trials of other recently approved AEDs. In addition, the proportion of patients experiencing a ≥50% reduction in seizure frequency was greater with 8 and 12 mg perampanel compared with placebo; however, the magnitude of reduction was not significantly different vs placebo.
The difference between outcomes for seizure frequency and responder rate may be that responder rate is a single point in an entirety of response and not a continuous variable. Thus, 50% responder rate provides less information from all possible responses; it may be less sensitive. Increasing the gauge of responder rate to 75% showed greater improvements compared with placebo.
In North American patients, the 50% responder rate for both perampanel 8 mg and 12 mg differed significantly from placebo. In contrast, Central and South American patients showed no difference between groups. The North American results here are consistent with those from a previous study conducted in central Europe and Asia.5 The basis for the regional difference in responses is unexplained, but the observation that the placebo responder rate was substantially higher than expected in Central and South American patients implies that patient selection or study conduct may have been an issue.
Once-daily 8 mg perampanel was relatively well tolerated. Although dizziness and somnolence did occur at this dose, the rate of withdrawal due to TEAEs was similar to that with placebo (6.8% vs 6.6%, respectively). No safety issues associated with psychotomimetic effects were seen with perampanel, as predicted by previous studies with AMPA antagonists.1,3 Weight gain occurred but with only modest mean increases over the 19 weeks of treatment. A modestly greater frequency of AEs occurred with 12 mg; however, the large majority of AEs were mild/moderate in severity with both doses. Although the maintenance period was twice as long as the titration period, AEs were less frequent during maintenance than titration, suggesting they were (to some extent) transient in nature and possibly related to the fixed titration schedule. It is noteworthy that the dose-dependent increase in AE frequency was accompanied by greater efficacy (percent reduction in seizures) at the higher dose. Rapid titration to the high dose, as required by clinical trial design, may exacerbate AEs which could be eliminated in clinical practice by more gradual titration. For certain patients, for example those with more refractory epilepsy, it is expected that the 12 mg dose will provide enhanced efficacy with acceptable tolerability. Furthermore, once-daily dosing of perampanel may offer a practical benefit, potentially increasing its attractiveness to some patients.
Along with an identically designed trial performed primarily in Europe, South Africa, and the United States,6,7 this study will support the regulatory approval of perampanel for partial-onset seizures. The data presented here indicate that perampanel at a daily dose of 8 or 12 mg is efficacious with acceptable tolerability.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Deborah McGregor, PhD, of Complete Medical Communications for assistance with editing the manuscript for nonintellectual content.
GLOSSARY
- AE
adverse event
- AED
antiepileptic drug
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- ANCOVA
analysis of covariance
- CGIC
Clinical Global Impression of Change
- CI
confidence interval
- EU
European Union
- ITT
intent-to-treat
- PGIC
Patient Global Impression of Change
- QOLIE-31-P
Quality of Life in Epilepsy questionnaire
- SAE
serious adverse event
- TEAE
treatment-emergent adverse event
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
J. French: conceptualization of the study, analysis of the data, drafting or revising the manuscript for intellectual content. G.L. Krauss: conceptualization of the study, analysis of the data, drafting or revising the manuscript for intellectual content. V. Biton: conceptualization of the study, analysis of the data, drafting or revising the manuscript for intellectual content. D. Squillacote: conceptualization of the study, analysis of the data, drafting or revising the manuscript for intellectual content. H. Yang: analysis of the data, drafting or revising the manuscript for intellectual content. A. Laurenza: analysis of the data, drafting or revising the manuscript for intellectual content. D. Kumar: conceptualization of the study, analysis of the data, drafting or revising the manuscript for intellectual content. M.A. Rogawski: drafting or revising the manuscript for intellectual content.
DISCLOSURE
J. French serves as the President of The Epilepsy Consortium, a non-profit organization. Dr. French receives 25% salary support from The Epilepsy Study Consortium. The consortium receives funding from Eisai Inc. for work performed (National PI, advisory board, FDA submission, consulting) by Dr. French on behalf of The Epilepsy Study Consortium, as well as many other companies with antiepileptic drugs in development or marketed antiepileptic drugs. G.L. Krauss is an investigator and consultant for Eisai Inc., UCB, and Novartis, and is an investigator for Neuronex and Sunovian. V. Biton is currently an investigator for Eisai Inc., Elan/Janssen, King, Medivation, Pfizer, Sepracor/Sunovion, UCB, Xenoport, Lundbeck, Schering-Plough/Merck, Upsher-Smith, SK Life Sciences, and Wyeth. Dr Biton has recently been an investigator for Marinus, Schwartz, Johnson & Johnson, Vernalis, Valeant, Forest, Genzyme, Icagen, Supernus, Vertex, and Myriad. He is currently a consultant for Upsher-Smith and UCB. D. Squillacote is an employee of Eisai Inc. H. Yang is an employee of Eisai Inc. A. Laurenza is an employee of Eisai Inc. D. Kumar is an employee of Eisai Inc. M.A. Rogawski has served as a consultant and has received research support from Eisai Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr 2011; 11: 56– 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanada T, Hashizume Y, Tokuhara N, et al. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 2011; 52: 1331– 1340 . [DOI] [PubMed] [Google Scholar]
- 3. Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007; 4: 18– 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krauss GL, Bar M, Biton V, et al. Tolerability and safety of perampanel: two randomized dose-escalation studies. Acta Neurol Scand 2012; 125: 8– 15 . [DOI] [PubMed] [Google Scholar]
- 5. Krauss GL, Serratosa JM, Villanueva VE, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 2012; 78: 1408– 1415 . [DOI] [PubMed] [Google Scholar]
- 6. French J, Elger C, Goldberg-Stern H, et al. Global phase III trial of perampanel, a selective, non-competitive AMPA receptor antagonist, as adjunctive therapy in patients with refractory partial-onset seizures. Presented at the 63rd annual meeting of the American Academy of Neurology, Honolulu, HI, April 9–16, 2011 Abstract 002. [Google Scholar]
- 7. French J, Elger C, Goldberg-Stern H, et al. Use of perampanel, a selective, non-competitive AMPA receptor antagonist, as adjunctive therapy in patients with refractory partial-onset seizures: results of a global phase III study. Presented at the 29th International Epilepsy Congress, Rome, Italy, August 28–September 1, 2011 Abstract 020. [Google Scholar]
- 8. International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures: from the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981; 22: 489– 501 . [DOI] [PubMed] [Google Scholar]
- 9. Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia 2007; 48: 1303– 1307 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.