Abstract
Objectives:
To compare the risk of epileptic seizures in adults during conservative management or following invasive treatment for a brain arteriovenous malformation (AVM).
Methods:
We used annual general practitioner follow-up, patient questionnaires, and medical records surveillance to quantify the 5-year risk of seizures and the chances of achieving 2-year seizure freedom for adults undergoing AVM treatment compared to adults managed conservatively in a prospective, population-based observational study of adults in Scotland, newly diagnosed with an AVM in 1999–2003.
Results:
We identified 229 adults with a new diagnosis of an AVM, of whom two-thirds received AVM treatment (154/229; 67%) during 1,862 person-years of follow-up (median completeness of follow-up 97%). There was no significant difference in the proportions with a first or recurrent seizure over 5 years following AVM treatment, compared to the first 5 years following clinical presentation in conservatively managed adults, in analyses stratified by mode of presentation (intracerebral hemorrhage, 35% vs 26%, p = 0.5; seizure, 67% vs 72%, p = 0.6; incidental, 21% vs 10%, p = 0.4). For patients with epilepsy, the chances of achieving 2-year seizure freedom during 5-year follow-up were similar following AVM treatment (n = 39; 52%, 95% confidence interval [CI] 36% to 68%) or conservative management (n = 21; 57%, 95% CI 35% to 79%; p = 0.7).
Conclusions:
In this observational study, there was no difference in the 5-year risk of seizures with AVM treatment or conservative management, irrespective of whether the AVM had presented with hemorrhage or epileptic seizures.
Adults with a brain arteriovenous malformation (AVM) are at risk of epileptic seizures, especially when the AVM is supratentorial (in the temporal lobe in particular) and after intracerebral hemorrhage (ICH) has occurred.1 The main aim of AVM treatment is to reduce the risk of AVM-related ICH, but invasive procedures might also reduce the risk of seizures by obliterating epileptogenic foci. Conversely, surgical excision, endovascular embolization, and stereotactic radiosurgery could also raise the risk of seizures.
Case series have reported conflicting results about seizure control following AVM treatment: surgery has been associated with better,2–7 unchanged, or worse seizure control8–10; the effect of stereotactic radiosurgery has been promising,11–21 although a delayed increase in seizure frequency has been reported22; the effects of embolization have been mixed23–27; and the effects of multimodality AVM treatment have been promising.28,29 Only 1 observational study compared the risk of de novo seizures following AVM surgery to the risk during conservative management, finding that the risk was greater following surgery, but the study was retrospective and based on clinical practice between 1941 and 1984.30 There have been no randomized controlled trials to compare seizure outcomes following AVM treatment vs conservative management.31
Therefore, we conducted a prospective, population-based, observational cohort study and analyzed it to compare the risk of a first seizure, the risk of epilepsy, and the chances of achieving 2-year seizure freedom, for adults undergoing AVM treatment or conservative management.
METHODS
Inclusion criteria.
The Scottish Intracranial Vascular Malformation (IVM) Study (SIVMS) is a prospective, population-based study that uses anonymized data extracts from a National Health Service clinical audit of adults aged ≥16 years resident in Scotland (population 5.1 million) with a new diagnosis of an IVM during 1999–2003 and 2006–2010 (The Scottish Audit of Intracranial Vascular Malformations; www.saivms.scot.nhs.uk). We identified patients using multiple overlapping sources of case ascertainment that included a Scotland-wide collaborative network of neurologists, neurosurgeons, stroke physicians, radiologists, and pathologists and central registers of hospital discharges and death certificates.32 We restricted this analysis to adults with AVMs first diagnosed in 1999–2003 so that we could accrue at least 5 years of follow-up data for each patient. We confirmed AVM diagnosis on brain imaging studies (reviewed by our 2 study neuroradiologists J.J.B. and R.J.S.) or pathologic examination.
First presentation (inception).
We classified a patient's first presentation as when they developed symptoms, relevant or not, that led to an investigation that diagnosed an AVM. We defined presentation with ICH as a symptomatic event that was associated with evidence of intracranial blood on brain imaging, CSF, or postmortem examination. We defined an incidental presentation as one that could not be related to the underlying AVM. We classified the initial presentation as epileptic seizure if it was not symptomatic of a concomitant ICH (seizures symptomatic of acute ICH were those that were witnessed and occurred within 24 hours of ICH onset). We reviewed all patient records, neuroimaging, and pathology reports and attributed seizures to the AVM if there was no better explanation for them. We reviewed patient records to identify prior episodes of symptomatic ICH and seizures. We calculated hematoma volume using axial brain imaging and the ABC/2 method and measured maximum AVM nidus diameter on MRI or catheter angiography (pial arteriovenous fistulae without a nidus were scored as 0 cm).
Follow-up.
Prospective follow-up started from the date of first presentation (defined as the date on which the patient developed symptoms that led to the diagnosis of an AVM) for conservatively managed patients and from the date of first intervention for the treated group. We used annual surveillance of general (family) practitioner and hospital medical records, as well as annual questionnaires to general practitioners and consenting patients, to establish patients' medical histories, mode of clinical presentation, events during follow-up, and antiepileptic drug (AED) prescriptions. We evaluated completeness of follow-up by comparing all follow-up successfully obtained to the maximum amount of follow-up that was potentially available using these multiple sources.33 If an adult had no history of epileptic seizures prior to presentation, then their first-ever seizure was the initial one that occurred at presentation (unprovoked by ICH) or during prospective follow-up, and we determined their development of epilepsy as when they had their next seizure during prospective follow-up. If the exact day or month of a seizure was not available, we imputed the date as the midpoint of the month or year, respectively.
Statistical analysis.
We used parametric statistics to compare demographic, clinical, and radiologic characteristics between the groups when the data were normally distributed and nonparametric statistics when they were not. We performed survival analysis using life tables and Kaplan-Meier statistics. We censored patients on the date of death, last available follow-up, or at the end of 5-year follow-up if an event of interest did not occur. We evaluated time to first seizure during prospective follow-up during conservative management (follow-up started at clinical presentation) vs after AVM treatment (follow-up started at the time of first intervention) according to their mode of presentation (ICH, seizure, or incidental). We subdivided patients according to the type of intervention received when analyzing time to next seizure in adults presenting with a seizure (because there were sufficient numbers of patients and outcome events to enable us to do this). We hypothesized that early seizure risk after AVM treatment would be greatest in patients who underwent surgery (due to the craniotomy and the potential impact on the surrounding brain tissue), followed by embolization, and then stereotactic radiosurgery. Therefore, if a patient underwent multimodality AVM treatment, we classified them in the surgery category if they ever underwent surgical excision, in the embolization category if they never underwent surgery (irrespective of whether they received stereotactic radiosurgery), and in the stereotactic radiosurgery category if they underwent this procedure but never had surgery or embolization. We evaluated time to 2-year seizure freedom in patients who presented with seizures and epilepsy (there were too few outcome events to permit these analyses for adults with other modes of initial presentation). We performed univariable comparisons using the log-rank test and multivariable Cox regression analyses only when the proportional hazards assumption was met,34 and prespecified the inclusion of AVM treatment, hematoma volume, and the occurrence of symptomatic seizures at the time of ICH1 in a multivariable analysis of factors that may increase the risk of seizures for adults who presented with ICH. We used a sensitivity analysis to evaluate our decision to include adults with both a single seizure and established epilepsy before AVM diagnosis in the time to 2-year seizure freedom analysis. All statistical tests were 2-tailed (α = 0.05) and performed using SPSS (version 14.0).
Standard protocol approvals, registrations, and patient consents.
We performed analyses on anonymized extracts of The Scottish Audit of Intracranial Vascular Malformations dataset. The Multicenter Research Ethics Committee for Scotland (MREC/98/0/48) and the Fife and Forth Valley Research Ethics Committee (08/S0501/76) approved SIVMS and the conduct of postal questionnaires.
RESULTS
We identified 229 adults first diagnosed in 1999–2003 with a definite AVM for whom there was a total of 1,862 person-years of follow-up (median 9 years per person, interquartile range [IQR] 7 to 10 years) with a median completeness of follow-up of 97% (IQR 96% to 100%) on February 18, 2011.35 Half of these adults (n = 115) presented with intracranial hemorrhage, 60 (26%) presented with epileptic seizures, 44 (19%) AVMs were incidental discoveries, and the remaining 10 (5%) (not analyzed further in this article) presented with a focal neurologic deficit in the absence of intracranial hemorrhage. Two-thirds of the adults included in our analyses (149/219; 68%) had AVM treatment.
Effect of AVM treatment on risk of seizures for adults presenting with ICH.
Most patients with ICH (91/115; 79%) underwent AVM treatment. All patients undergoing hematoma evacuation underwent concurrent AVM resection or embolization. Adults in the treated group were younger (p = 0.001) but otherwise demographic characteristics did not significantly differ between groups (table 1). AVM treatment obliterated two-thirds of AVMs (table 1).
Table 1.
Clinical characteristics of patients undergoing conservative management or invasive treatment following intracranial hemorrhage due to an arteriovenous malformation (AVM)a
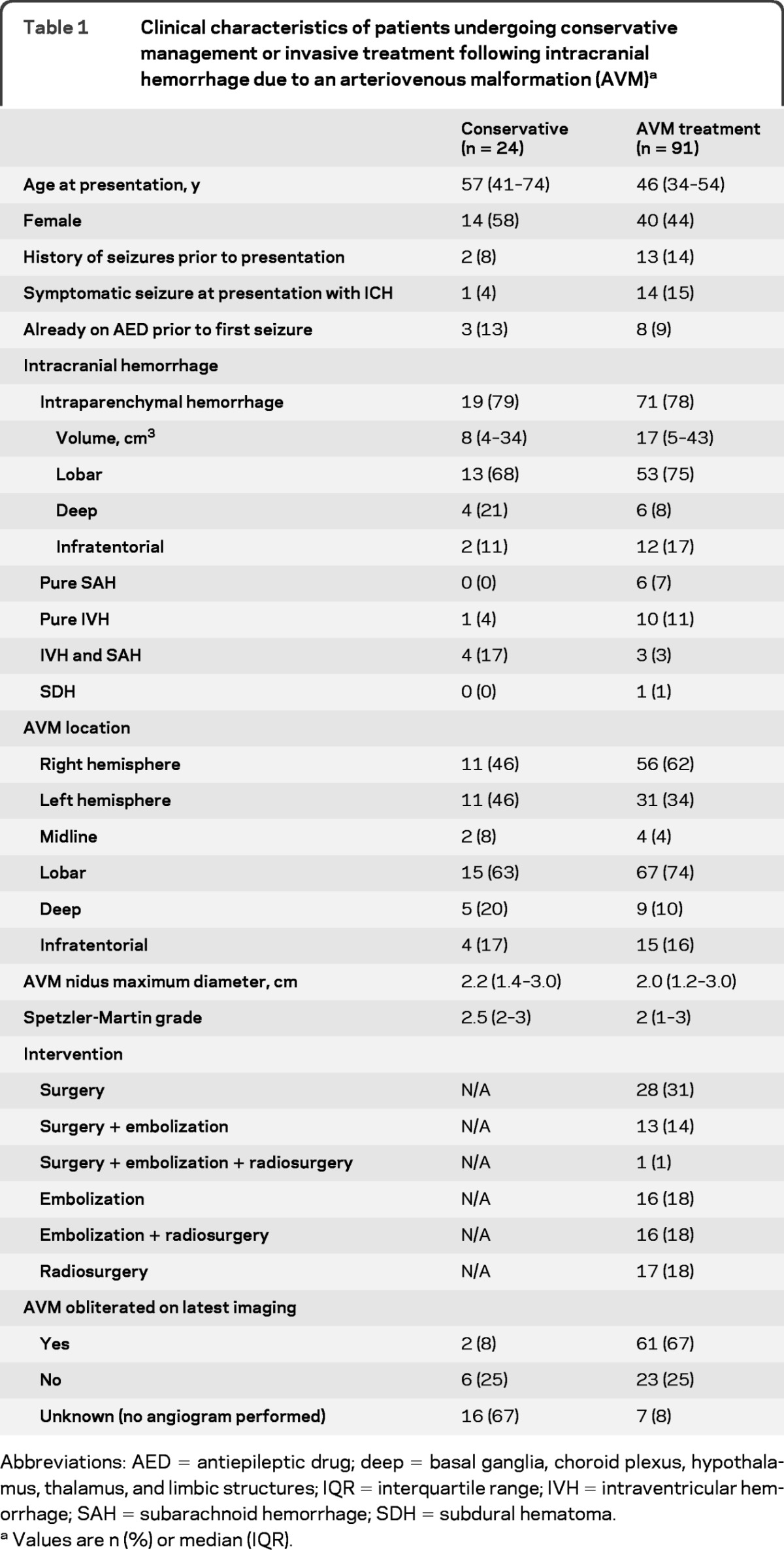
Abbreviations: AED = antiepileptic drug; deep = basal ganglia, choroid plexus, hypothalamus, thalamus, and limbic structures; IQR = interquartile range; IVH = intraventricular hemorrhage; SAH = subarachnoid hemorrhage; SDH = subdural hematoma.
Values are n (%) or median (IQR).
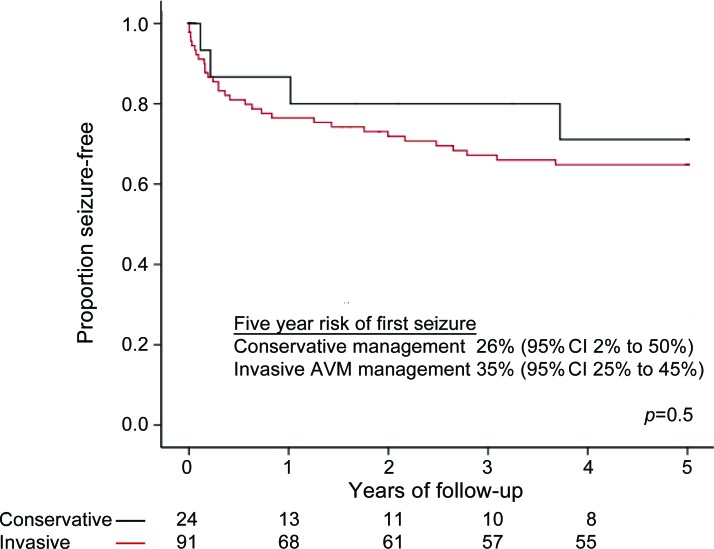
During 560 person-years of follow-up (median 5.6 years per person, IQR 0.4 to 8 years), the 5-year risk of a first unprovoked seizure was not significantly different during conservative management (n = 24; 26% [95% CI 2% to 50%]) or following first AVM treatment (n = 91; 35% [95% CI 25% to 45%], p = 0.5; figure 1). All adults with a temporal lobe AVM (n = 8) who presented with ICH received AVM treatment. Temporal lobe AVMs have been associated with an increased risk of seizures in this cohort,1 but in a sensitivity analysis in which we removed the 8 adults with a temporal lobe AVM from the analysis there remained no significant difference in the 5-year risk of a first-ever unprovoked seizure between adults receiving conservative management or AVM treatment (p = 0.6). The 5-year risk of a first unprovoked seizure was not greater in those whose treatment resulted in incomplete AVM obliteration (p = 0.14).
Figure 1. The 5-year risk of a first-ever unprovoked seizure after intracranial hemorrhage.
The 5-year risk of a first-ever unprovoked seizure after presentation for adults with an arteriovenous malformation (AVM) who presented with intracranial hemorrhage and were managed conservatively (black line; follow-up started at initial clinical presentation) or with AVM treatment (red line; follow-up started at first invasive procedure). CI = confidence interval.
Because age at presentation was the only definite baseline difference between the 2 groups (table 1), and because there were 36 outcomes, we included this variable with our 3 prespecified variables in a Cox proportional hazards analysis of predictors of seizures for adults presenting with ICH. Only hematoma volume on the first CT scan following presentation with symptomatic ICH significantly influenced the 5-year risk of a first seizure during follow-up (hazard ratio [HR] = 1.02 [95% CI 1.01 to 1.04]; p < 0.001); the occurrence of a symptomatic seizure at ICH onset (HR = 2.4 [95% CI 0.9 to 6.2]; p = 0.07), receipt of AVM treatment (HR = 3.0 [95% CI 0.4 to 24]; p = 0.3), and age at presentation (HR = 1.0 [95% CI 0.98 to 1.03]; p = 0.8) did not independently influence this risk.
Effect of AVM treatment on risk of recurrent seizures in adults presenting with a seizure.
Most adults who presented with an unprovoked seizure underwent AVM treatment, and these adults were younger (p = 0.005), a larger proportion was already on an AED at the start of prospective follow-up (p < 0.001), and they more frequently had left hemispheric AVMs (p = 0.03; table 2). The proportion of adults with a temporal lobe AVM was similar in the conservatively managed group (7/21 [33%]) and after AVM treatment (13/39 [33%]; p = 0.6). AVM treatment obliterated almost three-quarters of AVMs (table 2).
Table 2.
Clinical characteristics of patients undergoing conservative management or invasive treatment following presentation with a seizure related to an arteriovenous malformation (AVM)a
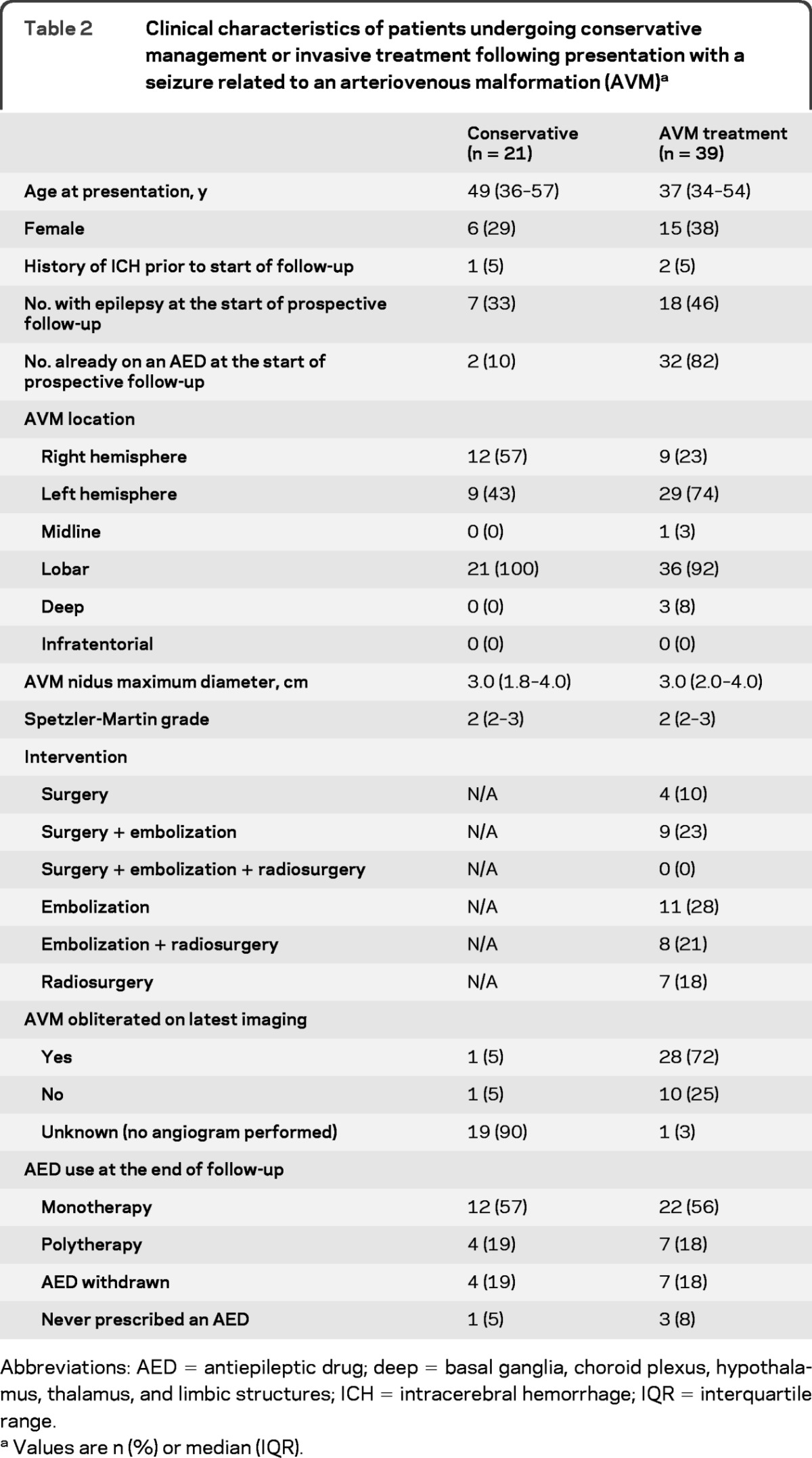
Abbreviations: AED = antiepileptic drug; deep = basal ganglia, choroid plexus, hypothalamus, thalamus, and limbic structures; ICH = intracerebral hemorrhage; IQR = interquartile range.
Values are n (%) or median (IQR).
During 170 person-years of follow-up (median 0.7 years per person, IQR 0.2 to 6 years, in view of the large number of outcomes in the first year), the 5-year risk of a recurrent unprovoked seizure during conservative management (72% [95% CI 52% to 92%]) was not significantly different from that following first AVM treatment (67% [95% CI 51% to 83%], p = 0.6; figure e-1 on the Neurology® Web site at www.neurology.org). The 5-year risk of a recurrent unprovoked seizure did not differ according to the type of AVM treatment (figure e-2) or according to whether the AVM was obliterated following treatment (p = 0.6).
Effect of AVM treatment on the chance of 2-year seizure freedom in adults presenting with a seizure.
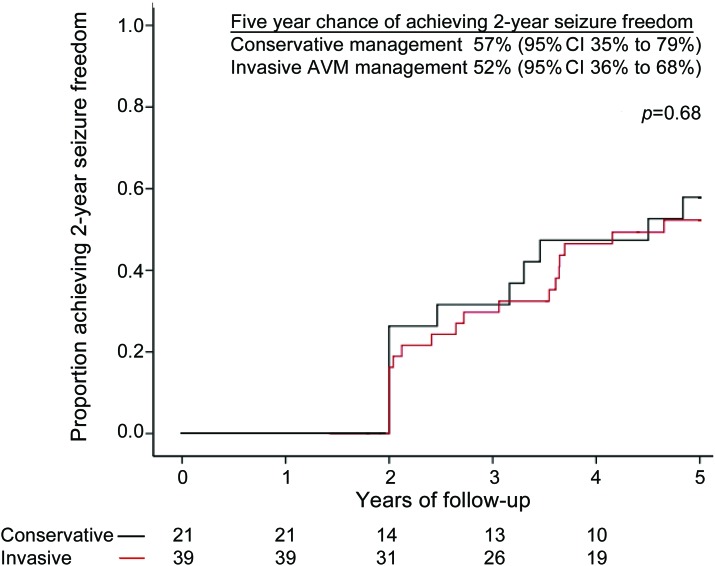
Of the 60 adults who first presented with seizures due to their AVM, by 2 years 76% (16/21) of the conservatively managed group and 77% (30/39) of the AVM treatment group developed epilepsy. The chance of achieving 2-year seizure freedom over 5 years of prospective follow-up did not differ between the 21 conservatively managed adults (57% [95% CI 35% to 79%]) and the 39 adults undergoing AVM treatment (52% [95% CI 36% to 68%], p = 0.7; figure 2). Similar proportions of adults in both the conservatively managed and AVM treatment groups were on AED therapy at the end of follow-up (p = 0.3; table 2). The chance of achieving 2-year seizure freedom remained similar between groups in a sensitivity analysis involving only those with established epilepsy at the time of initial presentation (47% [95% CI 23% to 71%] during conservative management vs 61% [95% CI 39% to 83%] following first AVM treatment, p = 0.3).
Figure 2. Chance of achieving 2-year seizure freedom for adults over 5 years follow-up.
The 5-year chance of achieving 2-year seizure freedom in adults with an arteriovenous malformation (AVM) who presented with a seizure and were managed conservatively (black line; follow-up started at initial clinical presentation) or with AVM treatment (red line; follow-up started at first invasive procedure). CI = confidence interval.
Effect of AVM treatment on risk of first-ever seizure for adults with an incidentally discovered AVM.
Among adults with an AVM diagnosed despite having no symptoms or unrelated symptoms, those who were conservatively managed were older than those undergoing AVM treatment (p = 0.002), but baseline characteristics did not differ otherwise (table 3). AVM treatment obliterated nearly four-fifths of AVMs. During 250 person-years of follow-up (median 7 years per person, IQR 1 to 8 years), the 5-year risk of a first unprovoked seizure was not significantly different between conservatively managed adults and those undergoing AVM treatment (figure e-3). The 5-year risk of a first unprovoked seizure was not greater in those whose treatment resulted in incomplete AVM obliteration (p = 0.5).
Table 3.
Clinical characteristics of patients undergoing conservative management or invasive treatment following presentation with symptoms incidental to their arteriovenous malformation (AVM)a
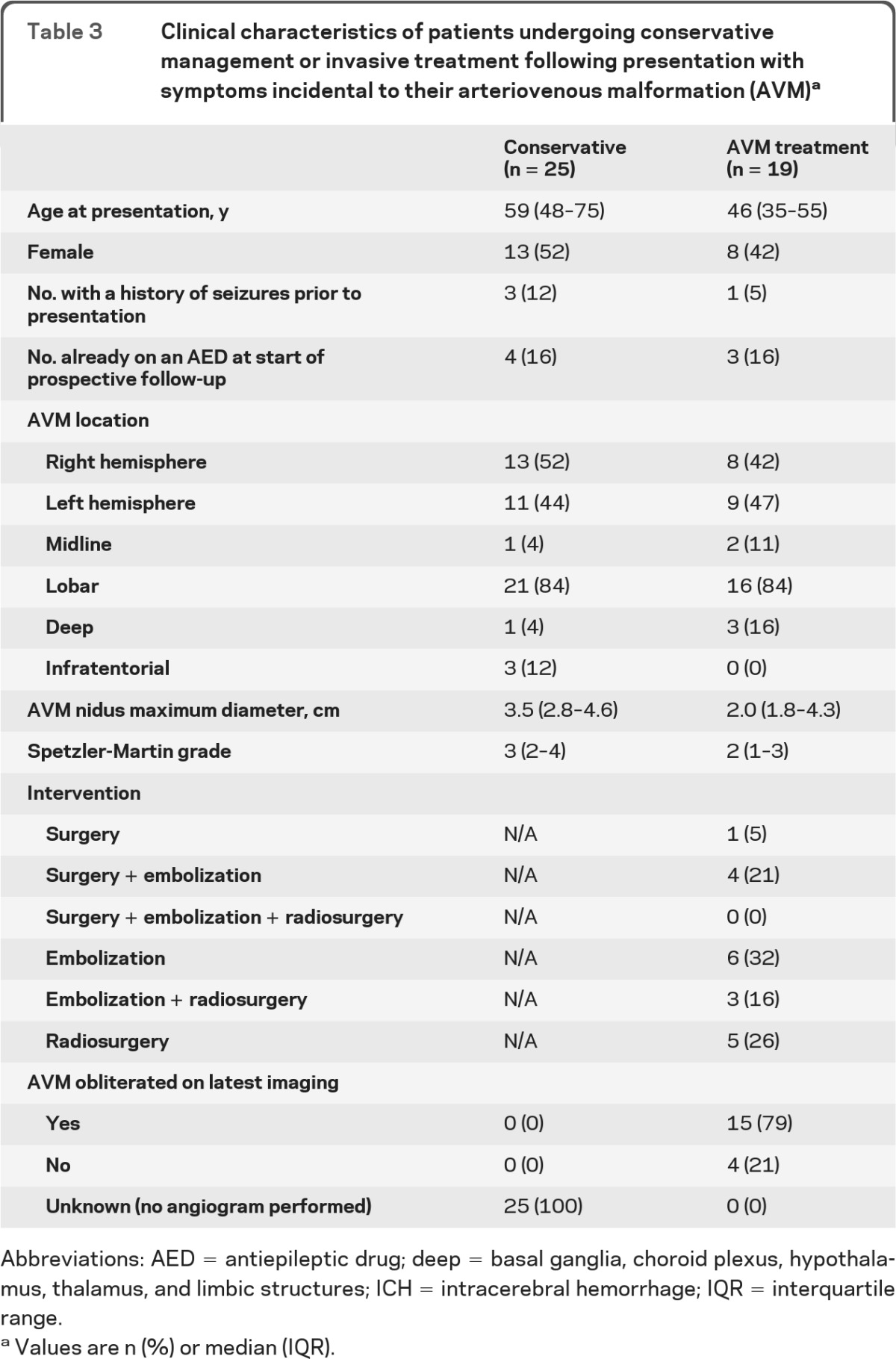
Abbreviations: AED = antiepileptic drug; deep = basal ganglia, choroid plexus, hypothalamus, thalamus, and limbic structures; ICH = intracerebral hemorrhage; IQR = interquartile range.
Values are n (%) or median (IQR).
DISCUSSION
In this prospective, population-based observational study, the risk of first-ever or recurrent seizures following AVM treatment did not differ from that seen in conservatively managed adults, irrespective of their mode of presentation. The only variable associated with a greater prospective risk of seizures was increasing hematoma volume in adults who had presented with ICH.
AVM treatment did not influence either the 5-year risk of a recurrent seizure or the chance of achieving 2-year seizure freedom in patients who had presented with an unprovoked seizure. Significantly more of the adults in the treatment group were already being treated with an AED at the start of follow-up, but by the end of follow-up similar proportions were on AEDs (table 2). The risk of a first-ever seizure following AVM treatment for unruptured incidental AVMs appeared higher than with conservative management although this difference was not statistically significant (figure e-3), which is consistent with our previous finding that AVM treatment independently worsens short-term outcome for adults with unruptured AVMs.36
Our study has benefited from multiple overlapping sources of case ascertainment and a median completeness of follow-up of 97%. The crude detection rate of AVMs in the first 2 years of our study in Scotland was not significantly different from the pooled detection rate in a recent meta-analysis.37,38 The population-based design of our study sought to avoid referral and selection biases. Largely because of the logistical and financial constraints involved with studying a geographically dispersed population, we relied on clinicians' evaluations in patients' medical records as well as questionnaire data to patients and their family practitioners, rather than regularly scheduled study visits. The use of 2-year seizure freedom (rather than 1-year seizure freedom) should limit reporting bias that may exist in patients with a long-standing history of epilepsy who may be less inclined to present to medical attention as a result of a seizure, and this outcome measure is directly relevant to clinical practice since AEDs tend not to be withdrawn until a patient is at least 2 years seizure-free.39
Due to the complexities of AVM treatment, we simplified the analysis by starting follow-up from the time of first-ever intervention, and allocating a single mode of intervention to those undergoing multimodality treatment (and consequently the time to first seizure in the surgery group may have been overestimated among patients who received presurgical embolization). AVM treatment obliterated approximately three-quarters of the AVMs, which is similar to the findings of everyday practice at other institutions. The 5-year risk of a seizure did not differ according to whether treatment resulted in complete or incomplete AVM obliteration. We could not perform a multivariable analysis of factors that contribute to the 5-year risk of a recurrent seizure or the chances of achieving 2-year seizure freedom in adults presenting with unprovoked seizures due to the fact that the survival curves did not satisfy the assumptions of the Cox proportional hazards model. However, receipt of AVM treatment did not appear to affect the risk of recurrent seizure or chance of 2-year seizure freedom in adults with AVM-related epilepsy. The effect of AVM treatment may have been modified by the greater proportion of adults on an AED at the start of follow-up (because immediate AED use does in general delay time to first and second seizure, and appears to reduce time to 2-year seizure freedom40) and such an imbalance would have been expected to favor the AVM treatment group. However, equivalent proportions of adults were on AED by the end of follow-up (table 2). Finally, the lack of statistically significant differences may be due to a type II error on account of sample size, and we have recruited a second 5-year cohort to address this in future analyses.
While AVM treatment may reduce the risk of rebleeding, our observational study could not demonstrate a difference between AVM treatment and conservative management on the clinical course of epileptic seizures. We cannot, however, rule out the influence of confounding in a nonrandomized study. Although the differences were not statistically significant, adults undergoing AVM treatment had higher frequencies of seizures prior to presentation, symptomatic seizures at ICH onset, temporal lobe AVM location, and these adults may have had a higher prospective risk of seizures. Further recruitment and follow-up will improve the precision of our estimates of seizure risk and allow us to expand our multivariable analyses of factors that might influence the development of de novo seizures and predict seizure control. Randomized controlled trials, such as A Randomized trial of Unruptured Brain Arteriovenous Malformations (ARUBA, www.arubastudy.org, ISRCTN 44013133), are required to confirm or refute our findings.
Supplementary Material
ACKNOWLEDGMENT
The authors thank their collaborators (see reference 32 for a collaborator listing; updates at www.saivms.scot.nhs.uk) and Rosemary Anderson and Aidan Hutchinson for their support with study coordination and programming respectively. They also thank the Royal College of Physicians International Sponsorship Scheme.
GLOSSARY
- AED
antiepileptic drug
- AVM
arteriovenous malformation
- CI
confidence interval
- HR
hazard ratio
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- SIVMS
Scottish Intracranial Vascular Malformation Study.
Footnotes
Editorial, page 492
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.B.J., J.J.B., C.E.C., V.P., V.R., R.R., R.S., C.P.W., and R.A.-S.S. contributed to the design and C.B.J., C.E.C., C.P.W., and R.A.-S.S. all contributed to the drafting and revising of the manuscript. C.B.J., J.J.B., C.E.C., V.P., V.R., R.R., R.S., C.P.W., and R.A.-S.S. all agree to the publication of this version of the manuscript.
DISCLOSURE
C. Josephson and J. Bhattacharya report no disclosures. C. Counsell has received research support as a principal investigator from the Chief Scientist Office of the Scottish Government (grant number CZG/2/419), Parkinson's UK, and the Dystonia Society. He has received research support as a coapplicant from National Institute of Health Research (grant number RP-PG-0707–10124). V. Papanastassiou and V. Ritchie report no disclosures. R. Roberts has received honoraria from UCB, Eisai, Janssen Cilag, GSK, Pfizer, and Cyberonics for advisory boards, lectures, and funding to attend academic meetings. R. Sellar and C. Warlow report no disclosures. R. Al-Shahi Salman has received research support through the Medical Research Council (Clinical Training Fellowship G84/5176, Clinician Scientist Fellowship G108/613, and Senior Clinical Fellowship G1002605); the Chief Scientist Office of the Scottish Government (grant numbers K/MRS/50/C2704, CZB/4/35, CZG/2/265); a Project Grant from the United Kingdom Stroke Association (grant number TSA04/01), and Chest Heart and Stroke Scotland. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Josephson CB, Leach JP, Duncan R, Roberts RC, Counsell CE, Al-Shahi Salman R. Seizure risk from cavernous or arteriovenous malformations: prospective population-based study. Neurology 2011;76:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piepgras DG, Sundt TM, Jr, Ragoowansi AT, Stevens L. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg 1993;78:5–11. [DOI] [PubMed] [Google Scholar]
- 3. Yeh HS, Kashiwagi S, Tew JM, Jr, Berger TS. Surgical management of epilepsy associated with cerebral arteriovenous malformations. J Neurosurg 1990;72:216–223. [DOI] [PubMed] [Google Scholar]
- 4. Yeh HS, Tew JM, Jr, Gartner M. Seizure control after surgery on cerebral arteriovenous malformations. J Neurosurg 1993;78:12–18. [DOI] [PubMed] [Google Scholar]
- 5. Thorpe ML, Cordato DJ, Morgan MK, Herkes GK. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J Clin Neurosci 2000;7:107–111. [DOI] [PubMed] [Google Scholar]
- 6. Korosue K, Hara Y, Tamaki N, Heros RC. Long-term prognosis of seizures after complete surgical resection of AVMs of the brain. Jpn J Neurosurg 1994;3:10–17. [Google Scholar]
- 7. Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery 1990;26:570–577. [DOI] [PubMed] [Google Scholar]
- 8. Parkinson D, Bachers G. Arteriovenous malformations: summary of 100 consecutive supratentorial cases. J Neurosurg 1980;53:285–299. [DOI] [PubMed] [Google Scholar]
- 9. Forster DM, Steiner L, Hakanson S. Arteriovenous malformations of the brain: a long-term clinical study. J Neurosurg 1972;37:562–570. [DOI] [PubMed] [Google Scholar]
- 10. Murphy MJ. Long-term follow-up of seizures associated with cerebral arteriovenous malformations: results of therapy. Arch Neurol 1985;42:477–479. [DOI] [PubMed] [Google Scholar]
- 11. Kida Y, Kobayashi T, Tanaka T, Mori Y, Hasegawa T, Kondoh T. Seizure control after radiosurgery on cerebral arteriovenous malformations. J Clin Neurosci 2000;7:6–9. [DOI] [PubMed] [Google Scholar]
- 12. Kurita H, Kawamoto S, Suzuki I, et al. Control of epilepsy associated with cerebral arteriovenous malformations after radiosurgery. J Neurol Neurosurg Psychiatry 1998;65:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schauble B, Cascino GD, Pollock BE, et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology 2004;63:24. [DOI] [PubMed] [Google Scholar]
- 14. Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med 1990;323:96–101. [DOI] [PubMed] [Google Scholar]
- 15. Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg 1992;77:1–8. [DOI] [PubMed] [Google Scholar]
- 16. Eisenschenk S, Gilmore RL, Friedman WA, Henchey RA. The effect of LINAC stereotactic radiosurgery on epilepsy associated with arteriovenous malformations. Stereotact Funct Neurosurg 1998;71:51–61. [DOI] [PubMed] [Google Scholar]
- 17. Lim YJ, Lee CY, Koh JS, Kim TS, Kim GK, Rhee BA. Seizure control of Gamma Knife radiosurgery for non-hemorrhagic arteriovenous malformations. Acta Neurochir Suppl 2006;99:97–101. [DOI] [PubMed] [Google Scholar]
- 18. Heikkinen ER, Konnov B, Melnikov L, et al. Relief of epilepsy by radiosurgery of cerebral arteriovenous malformations. Stereotact Funct Neurosurg 1989;53:157–166. [DOI] [PubMed] [Google Scholar]
- 19. Falkson CB, Chakrabarti KB, Doughty D, Plowman PN. Stereotactic multiple arc radiotherapy: III: influence of treatment of arteriovenous malformations on associated epilepsy. Br J Neurosurg 1997;11:12–15. [DOI] [PubMed] [Google Scholar]
- 20. Sutcliffe JC, Forster DM, Walton L, Dias PS, Kemeny AA. Untoward clinical effects after stereotactic radiosurgery for intracranial arteriovenous malformations. Br J Neurosurg 1992;6:177–185. [DOI] [PubMed] [Google Scholar]
- 21. Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 1991;75:512–524. [DOI] [PubMed] [Google Scholar]
- 22. Izawa M, Hayashi M, Chernov M, et al. Long-term complications after gamma knife surgery for arteriovenous malformations. J Neurosurg 2005;102(suppl):34–37. [DOI] [PubMed] [Google Scholar]
- 23. Lv X, Li Y, Jiiang C, Yang X, Wu Z. Brain arteriovenous malformations and endovascular treatment: effect on seizures. Interv Neuroradiol 2010;16:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin G, Gao F, Hu S. [Endovascular treatment of brain arteriovenous malformation.] Zhonghua Wai Ke Za Zhi 1997;35:117–118. [PubMed] [Google Scholar]
- 25. Lundqvist C, Wikholm G, Svendsen P. Embolization of cerebral arteriovenous malformations: part II: aspects of complications and late outcome. Neurosurgery 1996;39:460–467. [DOI] [PubMed] [Google Scholar]
- 26. Wolpert SM, Barnett FJ, Prager RJ. Benefits of embolization without surgery for cerebral arteriovenous malformations. AJR Am J Roentgenol 1982;138:99–102. [DOI] [PubMed] [Google Scholar]
- 27. Fournier D, TerBrugge KG, Willinsky R, Lasjaunias P, Montanera W. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg 1991;75:228–233. [DOI] [PubMed] [Google Scholar]
- 28. Hoh BL, Chapman PH, Loeffler JS, Carter BS, Ogilvy CS. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery 2002;51:303–309. [PubMed] [Google Scholar]
- 29. Pasqualin A, Scienza R, Cioffi F, et al. Treatment of cerebral arteriovenous malformations with a combination of preoperative embolization and surgery. Neurosurgery 1991;29:358–368. [DOI] [PubMed] [Google Scholar]
- 30. Crawford PM, West CR, Shaw MD, Chadwick DW. Cerebral arteriovenous malformations and epilepsy: factors in the development of epilepsy. Epilepsia 1986;27:270–275. [DOI] [PubMed] [Google Scholar]
- 31. Ross J, Al-Shahi Salman R. Interventions for treating brain arteriovenous malformations in adults. Cochrane Database Syst Rev 2010;CD003436. [DOI] [PubMed] [Google Scholar]
- 32. Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Scottish Intracranial Vascular Malformation Study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort. Stroke 2003;34:1156–1162. [DOI] [PubMed] [Google Scholar]
- 33. Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–1310. [DOI] [PubMed] [Google Scholar]
- 34. Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part III: multivariate data analysis: choosing a model and assessing its adequacy and fit. Br J Cancer 2003;89:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–1310. [DOI] [PubMed] [Google Scholar]
- 36. Wedderburn CJ, van BJ, Bhattacharya JJ, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population-based cohort study. Lancet Neurol 2008;7:223–230. [DOI] [PubMed] [Google Scholar]
- 37. Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003;34:1163–1169. [DOI] [PubMed] [Google Scholar]
- 38. Gabriel RA, Kim H, Sidney S, et al. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke 2010;41:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure-free patients: summary statement: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1996;47:600–602. [DOI] [PubMed] [Google Scholar]
- 40. Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005;365:2007–2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.