Abstract
Objective:
Activated microglia are thought to play a major role in cortical gray matter (GM) demyelination in multiple sclerosis (MS). Our objective was to evaluate microglial activation in cortical GM of patients with MS in vivo and to explore its relationship to measures of disability.
Methods:
Using PET and optimized modeling and segmentation procedures, we investigated cortical 11C-PK11195 (PK11195) binding in patients with relapsing-remitting MS (RRMS), patients with secondary progressive MS (SPMS), and healthy controls. Disability was assessed with the Expanded Disability Status Scale (EDSS) and Multiple Sclerosis Impact Scale (MSIS-29).
Results:
Patients with MS showed increased cortical GM PK11195 binding relative to controls, which was multifocal and highest in the postcentral, middle frontal, anterior orbital, fusiform, and parahippocampal gyri. Patients with SPMS also showed additional increases in precentral, superior parietal, lingual and anterior superior, medial and inferior temporal gyri. Total cortical GM PK11195 binding correlated with EDSS scores, with a stronger correlation for the subgroup of patients with SPMS. In patients with SPMS, PK11195 binding also correlated with MSIS-29 scores. No correlation with disability measures was seen for PK11195 binding in white matter. Higher EDSS scores correlated with higher levels of GM PK11195 binding in the postcentral gyrus for patients with RRMS and in precentral gyrus for those with SPMS.
Conclusions:
Microglial activation in cortical GM of patients with MS can be assessed in vivo. The distribution is not uniform and shows a relationship to clinical disability. We speculate that the increased PK11195 binding corresponds to enhanced microglial activation described in postmortem SPMS cortical GM.
Multiple sclerosis (MS) has traditionally been regarded as a white matter (WM) disease, but neuropathologic1–6 and magnetic resonance spectroscopic (MRS) and MRI studies7–9 have demonstrated substantial damage in the cortical gray matter (GM), which plays a major role in the progression of disability.6,10 A characteristic feature of cortical MS lesions is the relative lack of peripheral immune cell infiltrates2 with prominent microglial activation.6 However, the consequences of microglial activation in cortical GM in MS are uncertain, and could be a consequence of tissue damage or a major mechanism of cell injury that directly or indirectly cause neuronal dysfunction.1,6,11–15
Microglial activation can be visualized in vivo using 11C-PK11195 (PK11195) PET,16 but studies have been limited in scope and have characterized WM changes only.17–19 PK11195 has been demonstrated to detect activated macrophages and microglia in postmortem tissue17 by specifically binding to the translocator protein 18KDa (TSPO) that is upregulated with activation.16,20
However, until recently,21–23 accurate measurements of TSPO binding in the brains of patients with MS have been difficult because of the lack of an anatomically consistent reference region and the limitations of image registration and segmentation procedures due to variable ventricular enlargement and brain atrophy.
Here we have applied improved image-processing techniques and PK11195 PET to characterize cortical microglial activation in a cross-sectional study of patients with MS with a range of disabilities in order to test the hypotheses that PK11195 can detect areas of cortical pathology and that increased binding is associated with greater disability.
METHODS
Subjects and clinical evaluation.
Ten patients with active relapsing-remitting MS (RRMS) and 8 patients with secondary progressive MS (SPMS) were studied (table 1). Disability was assessed using the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Impact Scale (MSIS-29). Eight healthy subjects, with no history of neurologic or psychiatric illness and not taking medication, served as the control group (5 F; age = 32.9 ± 4.6 years, mean ± SD).
Table 1.
Clinical characteristics of patients with MS
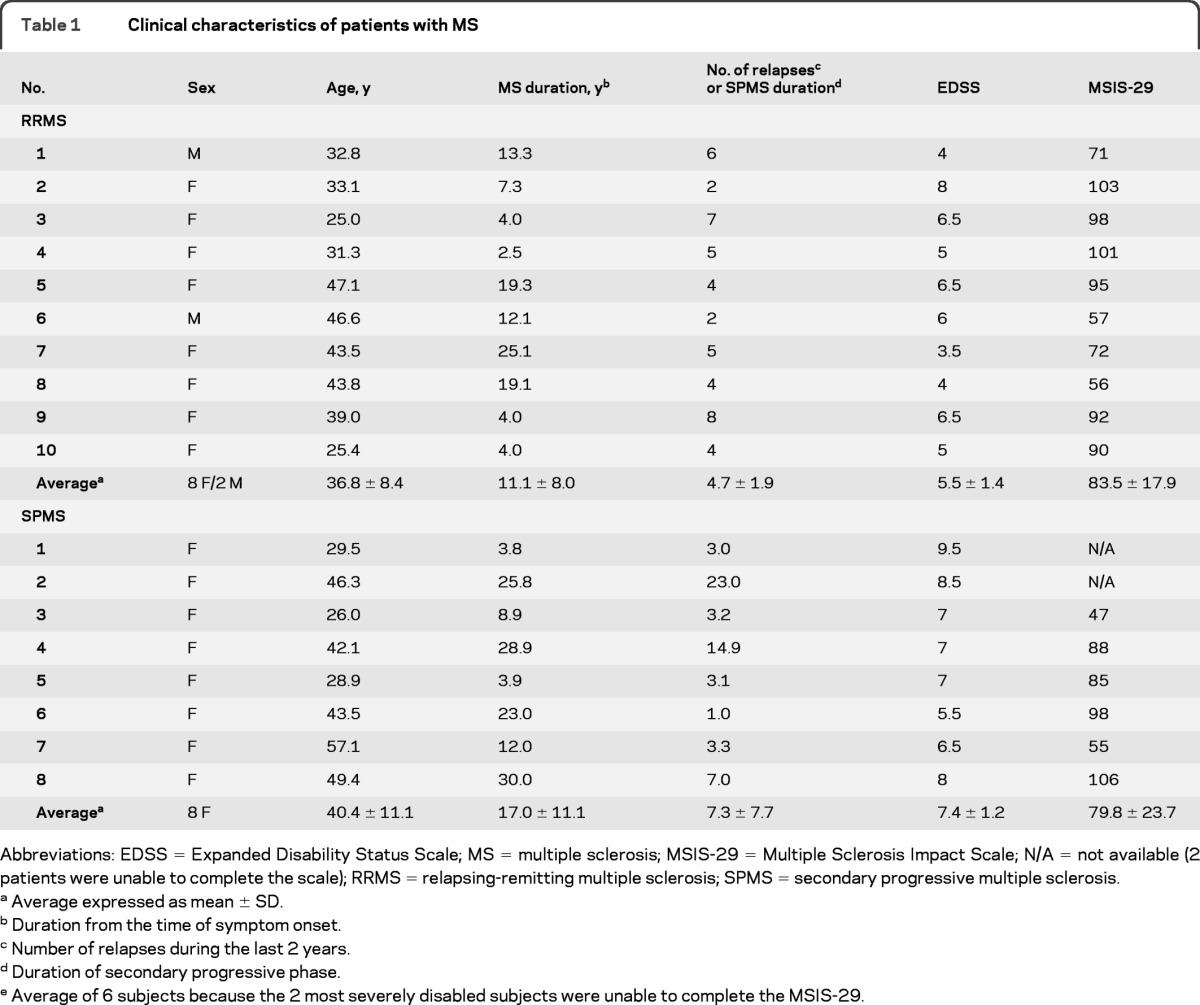
Abbreviations: EDSS = Expanded Disability Status Scale; MS = multiple sclerosis; MSIS-29 = Multiple Sclerosis Impact Scale; N/A = not available (2 patients were unable to complete the scale); RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
Average expressed as mean ± SD.
Duration from the time of symptom onset.
Number of relapses during the last 2 years.
Duration of secondary progressive phase.
Average of 6 subjects because the 2 most severely disabled subjects were unable to complete the MSIS-29.
Standard protocol approvals, registrations, and patient consents.
Ethical approval was received from the Hammersmith and Queen Charlotte's Research Ethics Committee and permission to administer PK11195 was obtained from the Administration of Radioactive Substances Advisory Committee (ARSAC) of the Department of Health, UK. Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Scanning procedures.
Subjects received PK11195 PET and MRI no more than 7 days apart. The PK11195 PET scan was performed using a GE Discovery RX PET/CT scanner.24 The mean injected dose for PK11195 was 360 MBq and scanning began 30 seconds before tracer infusion as an IV bolus, generating 18 time frames of tissue data over 60 minutes.
Subjects were positioned supine with their transaxial planes parallel to the line intersecting the anterior-posterior commissure (AC–PC) line. Head position was maintained with the help of individualized foam holders and monitored by video. Subjects were scanned at rest in a quiet room with low light. Smoking and consumption of alcohol, coffee, and other caffeinated beverages were not allowed for 12 hours before scanning. Eating and drinking were not allowed for 8 hours before PET scanning.
MRI was performed using a clinical 1.5-Tesla MRI system (Siemens Magnetom Avanto). T2-weighted sequences (axial T2-spin echo: repetition time [TR] = 4,540 msec, echo time [TE] = 97 msec, 5 mm slice thickness; axial fluid-attenuated inversion recovery: TR = 9,000 msec, TE = 114 msec, inversion time [TI] = 2,500 msec, 5-mm slice thickness) and volumetric T1 sequences (coronal and axial T1-spin echo: TR = 635 msec, TE = 17 msec, 5 mm slice thickness; T1 volumetric magnetization-prepared rapid gradient echo: TR = 1,900 msec, TE = 3.53 msec, TI = 1,100 msec, flip angle 15, 1 mm isotropic voxels) pre-gadolinium (Gd) and post-Gd chelate IV administration (7.5 mmol gadobutrol) were acquired for image registration, for brain volume measurement, and to define regions of interest (ROI) and the MRI-visible lesion load.
MRI analyses.
MRI were reorientated with the horizontal line defined by the AC–PC line and the sagittal planes parallel to the midline. MRI scans were automatically segmented using the multi-atlas propagation with enhanced registration (MAPER) approach23 into 83 anatomic regions.25–27 This robust technique improves the quality of multi-atlas based automatic whole-brain segmentations,28 allows the automatic segmentation of PET data in anatomic regional volumes, and is applicable even to subjects with ventriculomegaly. The MRIs were then further segmented into GM and WM tissue classes using Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience, UCL) implemented in Matlab 6.5 (The Mathworks Inc.).
PK11195 PET analyses.
Following reconstruction of the dynamic PK11195 image volume, a summed image volume was created from the entire dynamic dataset. The input function was derived using the SUPERPK software package (Imperial Innovations).21,22 In brief, the supervised clustering algorithm models the time-activity curves (TAC) of each pixel as the sum of the kinetics of 4 predefined tissues (normal GM and WM, and the 2 sources of specific binding, activated microglia and the vasculature) obtained from a database of control subjects and patients. Additional signals from the skull and extracerebral muscle tissue are excluded by elimination of nonbrain voxels with the use of the coregistered GM and WM components extracted from the individual MRIs. The reference kinetic is obtained by averaging on the whole brain, whereas each pixel TAC is weighted by its normal GM index. The reference TAC is then used as input for a simplified reference region modeling approach29 which additionally incorporates the specific (but of no interest) binding of PK11195 to the vasculature30 and is applied to the PET dynamic volume to generate a pixel-by-pixel map of PK11195 binding potential of the specifically bound radioligand relative to the nondisplaceable radioligand in tissue (BPND).
PK11195 parametric images of each subject were coregistered to their respective MRI scans using the mutual information registration algorithm in the SPM2 software package. This same transformation was applied to the thresholded (probability of 0.5) GM and WM masks and to the 83 ROIs generated with the MAPER approach. Multiplication across these coregistered images and masks created BP maps, the patients' individual 83 ROIs in which then were sampled with the ANALYZE medical imaging software (version 8.1, Mayo Foundation) (for example, see figure e-1 on the Neurology® Web site at www.neurology.org).
Statistical analyses.
Statistical analyses were performed using SPSS software package (version 16, SPSS Inc.) for Macintosh. For all comparisons, variance homogeneity and Gaussianity were tested with Bartlett and Kolmogorov-Smirnov tests. GM volume and PK11195 BPND values are reported (median, range, and percentage difference of the mean) for total cortex and the bilaterally averaged cortical gyri. We tested for statistical differences of the group means by using analysis of variance corrected for multiple comparisons (Tukey-Kramer or Dunn). Repeated-measures analysis of covariance was used to interrogate the association between cortical GM PK11195 BPND with clinical parameters (duration of MS symptoms, duration of confirmed MS diagnosis, duration of secondary progressive phase, EDSS and MSIS-29 scores), where regional PK11195 BPND was the repeated measure.31 If a significant interaction was found between a clinical variable and the repeated-measure variable ROI, univariate tests were then carried out on the individual ROIs and resulting p values corrected for multiple comparisons. The test level α was set at p < 0.05, corrected.
RESULTS
General MRI analysis.
T2-hyperintense lesion numbers and volumes in the WM were not significantly different between the patients with RRMS and patients with SPMS. The median number of T2 lesions was 31 (range 2–73) in the patients with RRMS and 21 (range 2–47) in the patients with SPMS. T2 lesions occupied median brain volumes of 7,916 mm3 in patients with RRMS (range 90–45,048 mm3) and 22,908 mm3 in patients with SPMS (range 127–42,800 mm3). However, the patients with RRMS had significantly greater volume of T1 Gd-enhancing lesions (median 144 mm3; range 30–886 mm3) compared to patients with SPMS (median 89 mm3; range 37–117 mm3) (p < 0.001). The mean total cortical GM volume was 24% lower in the patients with SPMS (median 432 cm3; range 359–511 cm3; p < 0.01) and 11% lower in the patients with RRMS (median 489 cm3; range 386–607 cm3; p > 0.05) than in healthy controls (median 560 cm3; range 454–702 cm3).
PK11195 binding in cortical gray matter.
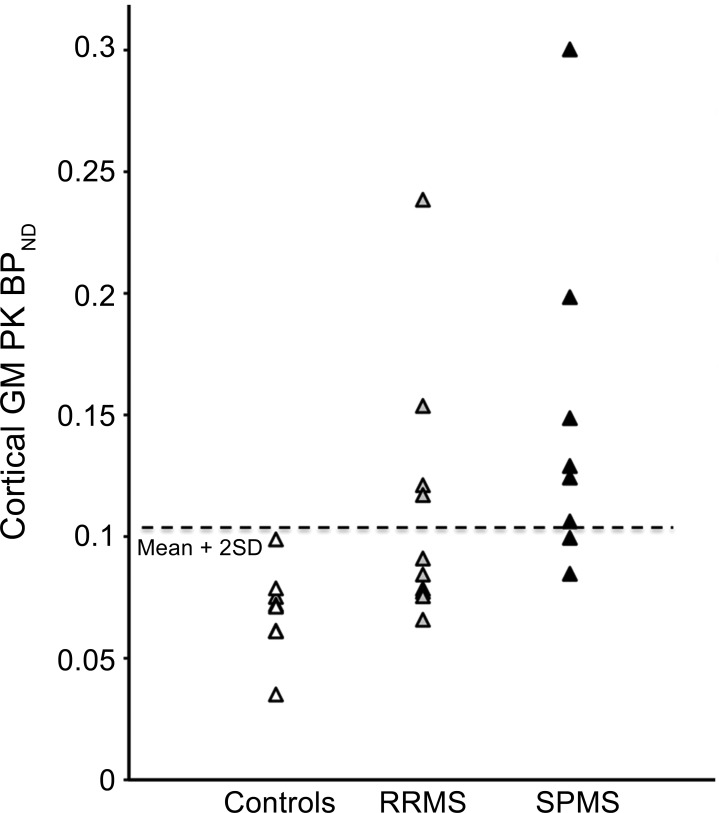
Mean total cortical GM PK11195 BPND was increased by over 100% in patients with SPMS (median 0.127 BPND; range 0.085–0.300 BPND; p < 0.05) and by almost 60% in patients with RRMS (median 0.088 BPND; range 0.066–0.238 BPND; p > 0.05) compared to healthy controls (median 0.072 BPND; range 0.035–0.099 BPND) (figure e-1 and figure 1). The proportion of patients with abnormally high total cortical GM PK11195 BPND (>2 SD above total cortical GM PK11195 mean BPND for healthy controls) was greater for the patients with SPMS (6/8 patients) than for the patients with RRMS (4/10) (figure 2). The 2 patients with RRMS with the highest total cortical GM PK11195 BPND values (patient 3: BPND = 0.153; patient 9: BPND = 0.238) had experienced the greatest number of relapses during the previous 2 years (table 1).
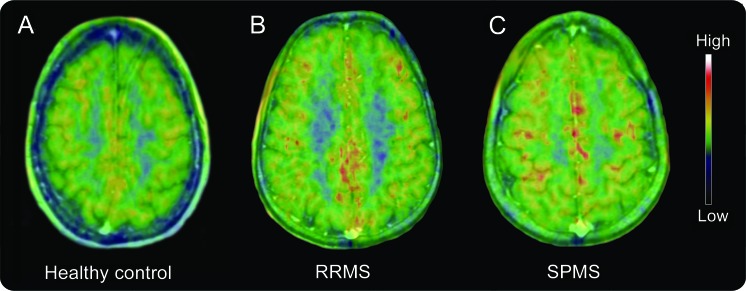
Figure 1. Increased PK11195 binding in the cortical gray matter (GM) of patients with multiple sclerosis (MS).
Summed PK11195 PET images coregistered and fused with 1.5 Tesla MRI at the cortical level for (A) a healthy 40-year-old woman (cortical GM PK BPND is 0.061); (B) a 46-year-old man with a 12-year history of MS (10 years of confirmed diagnosis) experiencing an average of 1 relapse per year (Expanded Disability Status Scale [EDSS] of 6 and Multiple Sclerosis Impact Scale [MSIS-29] of 57) (cortical GM PK BPND is 0.121); and (C) a 46-year-old woman with secondary progressive multiple sclerosis (SPMS) and a 26-year history of MS (25 years of confirmed diagnosis) who developed progressive disease after 3 relapses in the first 2 years of symptoms (EDSS of 8.5, MSIS-29 could not be completed, cortical GM PK BPND is 0.198). RRMS = relapsing-remitting multiple sclerosis.
Figure 2. Scatterplot showing individual cortical gray matter (GM) PK11195 BPND values.
Values are from groups of healthy controls (n = 8), patients with relapsing-remitting multiple sclerosis (RRMS) (n = 10), and patients with secondary progressive multiple sclerosis (SPMS) (n = 8). Dotted line represents the healthy control mean + 2 SD.
Distribution of PK11195 binding across the cortical gray matter.
Increased PK11195 BPND was not uniformly distributed across the cortical GM in the patients with MS. Cortical atlas volumes for patients with RRMS with highest PK11195 BPND (threshold: increases greater than 100%) included the postcentral, middle frontal, anterior orbital, fusiform, and parahippocampal gyri. In patients with SPMS, PK11195 BPND additionally exceeded this threshold in the precentral, superior parietal, lingual and anterior superior, medial, and inferior temporal gyri (table e-1 and figure 3). No significant correlations were found between total cortical or individual gyral GM volumes and the associated PK11195 BPND values (p > 0.05).
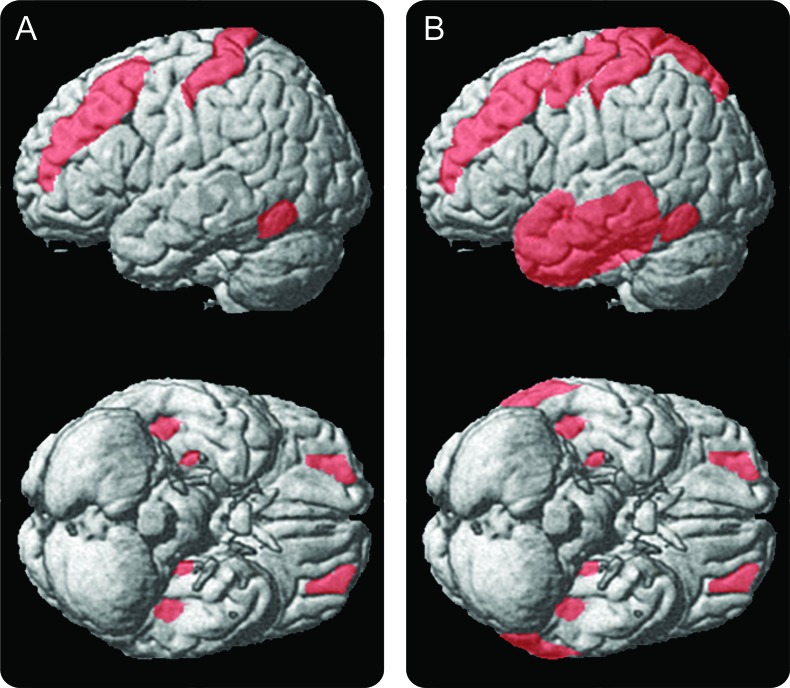
Figure 3. Statistical parametric maps showing significant increases of PK11195 cortical gray matter (GM) binding.
The overlap of significant increases of PK11195 cortical GM binding for (A) patients with relapsing-remitting multiple sclerosis (n = 10) and (B) patients with secondary progressive multiple sclerosis (n = 8) are projected onto a surface-rendered 3-dimensional brain (statistical threshold of p < 0.05, corrected for multiple comparisons; multi-atlas propagation with enhanced registration atlases were used to warp PK11195 BPND images on SPM5). See table e-1 for detailed list of anatomic regions with abnormally increased PK11195 BPND binding.
Clinical correlates of increased cortical gray matter PK11195 BPND.
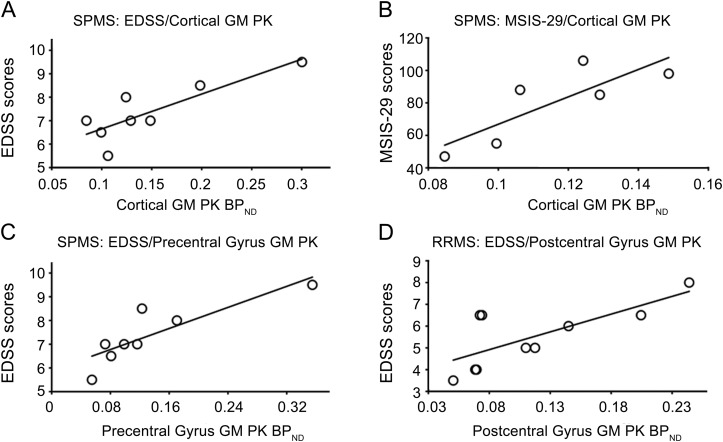
EDSS (r = 0.52, p = 0.026) but not MSIS-29 (r = 0.21, p = 0.44) scores were significantly correlated with total cortical GM PK11195 BPND values for the patients with MS. When we explored the subgroups, we found that this correlation was driven by the relationships for patients with SPMS; strong correlations were observed between total cortical GM PK11195 BPND values and both EDSS (r = 0.84, p = 0.0089) and MSIS-29 (r = 0.82, p = 0.046) scores in patients with SPMS (figure 4, A and B), but not in patients with RRMS. Total cortical GM PK11195 BPND values were not significantly correlated with the duration of MS symptoms or the duration of confirmed MS diagnosis for the whole group or either patient subgroup or with the duration of secondary progressive phase for patients with SPMS.
Figure 4. Correlations between gray matter (GM) PK11195 BPND values and Expanded Disability Status Scale (EDSS) and Multiple Sclerosis Impact Scale (MSIS-29) scores in patients with multiple sclerosis (MS).
Significant correlations were found between PK11195 PET imaging and clinical data. Total cortical GM PK11195 BPND values in patients with secondary progressive multiple sclerosis (SPMS) are significantly correlated with (A) EDSS and (B) MSIS-29 scores. (C) Precentral gyrus GM PK11195 BPND values in patients with SPMS and (D) postcentral gyrus GM PK11195 BPND values in patients with relapsing-remitting multiple sclerosis (RRMS) significantly correlated with EDSS scores. The MSIS-29 correlation includes data from only 6 patients with SPMS, as the remaining 2 in our study subgroup could not complete the scale.
We performed a further analysis to explore whether increased GM PK11195 BPND in specific cortical regions correlated with disability measures. We found that EDSS scores significantly correlated with precentral gyrus GM PK11195 BPND values in the patients with SPMS (r = 0.90, p = 0.0046) and with postcentral gyrus GM PK11195 BPND values in the patients with RRMS (r = 0.73, p = 0.016) (figure 4, C and D).
PK11195 BPND in white matter.
Mean total WM PK11195 BPND was increased by over 130% in patients with SPMS (median 0.122 BPND; range 0.080–0.240 BPND; p < 0.001) and by almost 80% in patients with RRMS (median 0.095 BPND; range 0.060–0.157 BPND; p > 0.05) compared to healthy controls (median 0.050 BPND; range 0.030–0.086 BPND) (figure e-2). However, we found no correlations between WM PK11195 BPND and EDSS and MSIS-29 scores for the whole group of patients with MS or for either of the subgroups (figure e-3).
DISCUSSION
Using noninvasive in vivo PK11195 PET and an optimized method for modeling the signal, we have demonstrated significant increases in TSPO expression, reflecting microglial activation, in the cortical GM of patients with RRMS and patients with SPMS. Microglial activation is a prominent feature of cortical lesions in MS1,6 and our observations demonstrate that this can be assessed in the cortical GM in vivo. We showed that cortical GM PK11195 binding correlated with disability scores in the patients with MS with a stronger correlation for the subgroup of patients with SPMS. Moreover, we showed that the distribution of increased signal is not uniform across the cortical GM. Our results suggest that increased PK11195 BPND in functionally eloquent anatomic regions involved in motor control and somatosensation (precentral gyrus for SPMS and postcentral gyrus for RRMS) may have particular relevance to disability.
Although PK11195 BPND increases were also observed in WM, they did not show correlation with measures of disability, in keeping with recent studies suggesting that accumulating cortical GM pathology plays the major role in progression of both physical and cognitive disability.8 Although it is not possible to have confirmatory postmortem data, we suggest that the distribution of increased PK11195 binding is related to the distribution of areas of enhanced cortical pathology. Previous postmortem tissue studies have shown that PK11195 binding is relatively specific for microglia and macrophages.17
Microglia become activated in response to acute and chronic neuronal and myelin insults and are thought to contribute to tissue repair.32,33 However, chronic microglial activation has also been identified as an important pathologic mechanism involved in neurodegeneration in a variety of neurologic diseases, including MS.34 Microglial activation can be the consequence of tissue damage but can also be responsible for neuronal death.35–37 In chronic cortical GM lesions, these neurodegenerative effects may predominate and lead to progressive loss of neurons and oligodendrocytes associated with increasing disability.6
Previous postmortem tissue studies have shown that increased microglial numbers and increased activation are associated with variable degrees of axonal/neuritic injury, demyelination, and neuronal loss in the cortical GM in the progressive stages of MS.5,6 However, it is as yet unclear how early during the course of MS these degenerative events begin. Future longitudinal in vivo studies relating microglial activation to measures of local cortical atrophy or dysfunction and the progression of disability in individual subjects (particularly in the context of treatments that reverse disability)38 should contribute to improve our understanding of the consequences of cortical pathology at different disease stages.
We observed a striking regional distribution of increased PK111195 binding across the cortex. Neuropathologic studies have highlighted that cortical GM lesions can occur as long ribbons of subpial demyelination and are more commonly found in particular cortical regions.1,3,6 The presence of substantial meningeal inflammation in some cortical sulci is associated with exacerbated cortical GM demyelination and this has led to the suggestion that microglial activation and cortical demyelination may occur in a gradient fashion from the pial surface inwards.5,6,12 Thus, there may be specific anatomic regions with greater potential to initiate, propagate, or sustain an inflammatory cascade in MS.
Our analysis suggested a correlation between higher EDSS scores and increased microglial activation in the precentral gyrus in patients with SPMS. This observation is consistent with a recent postmortem study showing increased density of activated microglia and substantial neuronal loss in GM lesions of the precentral gyrus of SPMS cases exhibiting significant meningeal inflammation.6 Neuronal loss and increased density of activated microglia were associated with both the subpial lesions and the normal-appearing nondemyelinated GM,6 as reflected in the widespread increase in the cortical GM PK signal observed in the present study. Changes in cortical GM PK signal may reflect a combination of direct effects of proinflammatory and cytotoxic mediators derived from the meninges, degenerative changes due to downstream WM lesions, and GM demyelination.
We also found a correlation between higher EDSS scores and increased microglial activation in the postcentral gyrus for the subgroup of patients with RRMS. Previous studies have shown significant GM volume loss in the postcentral gyrus of patients with RRMS.39,40 Although we did not identify any correlation between local GM volume and PK11195 BPND, we believe that further investigations using more precise measures of voxel-wise GM concentration or cortical thickness are necessary to test this further. In addition, increased PK11195 binding was seen in the middle frontal, anterior orbital, fusiform, and parahippocampal gyri in the patients with RRMS and a broader range of cortical regions extending to superior parietal, lingual and anterior superior, medial and inferior temporal gyri in patients with SPMS. These large, highly associative cortical regions are involved to some degree in many cognitive processes. While nonspecific to support this contention directly, higher MSIS-29 scores correlated with increases in total cortical GM microglial activation. It is intriguing to speculate that such widespread cortical microglial activation may contribute to cognitive deficits in MS.e1
A unique feature of our study is the focus on cortical pathology in MS. Microglial activation may be less prominent in GM than in WM lesions,1 which may account in part for why previous studies have reported only on WM changes.17–19 In our study we were able to take advantage of improved modeling and anatomic registration methods that have improved sensitivity for detection of cortical signal. Modeling the signal arising from the specific binding of PK11195 to TSPO has previously proved challenging.e2,e3 The use of SUPERPK allows the quantification of specific binding with robust quantitative implementation at the pixel level.21 In addition, the atrophy and ventriculomegaly observed in MS patient brains confound accurate registration and anatomic segmentation. The MAPER approach has been designed to accurately address this issue.23
Nonetheless, there are limitations of our study. 1) Our small sample is limiting the potential to confidently characterize relationships between PK11195 BPND and disability measures and to extend the correlations with other indices, such as MRI. Also, this is a cross-sectional study and therefore, not able to directly test for temporal changes in microglia activation with the pathologic evolution of MS. 2) PK11195 nonspecifically binds to other glial cells such as astrocytes.e4,e5 3) While a variability of only 10% was found between scans for Alzheimer disease (AD),21 different biological factors could influence the reproducibility of measurements for patients with MS. Test-retest data on patients with MS will be needed for explorations of any applications to decision-making for individual patients or for prospective powering of clinical trials using these measures. 4) PET image analysis and quantification of PK11195 can be confounded by the vascular binding of the tracer and, in patients with AD, blood–brain barrier disruption causes a small reduction in binding.30 However, here by using SUPERPK21,22,30 we have used a reference region and not plasma input. 5) Patients with MS have greater GM atrophy with disability progression, although this should bias toward an apparent reduction in BP due to partial volume effects arising from the limited spatial resolution of the scanner, rather than the greater BP we report with SPMS relative to RRMS. Measurements of BP should be able to be made more accurate in future studies by higher resolution scanning and accounting explicitly for any variations in local gray matter thickness in the kinetic model.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and their families.
GLOSSARY
- AC–PC
anterior-posterior commissure
- EDSS
Expanded Disability Status Scale
- Gd
gadolinium
- GM
gray matter
- MAPER
multi-atlas propagation with enhanced registration
- MRS
magnetic resonance spectroscopy
- MS
multiple sclerosis
- MSIS-29
Multiple Sclerosis Impact Scale
- ROI
region of interest
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- TAC
time-activity curve
- TE
echo time
- TI
inversion time
- TR
repetition time
- WM
white matter
Footnotes
Editorial, page 496
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Marios Politis, Richard Reynolds, Richard Nicholas, Paola Piccini. Drafting the manuscript: Marios Politis. Revising the manuscript: All authors. Acquisition of data: Marios Politis, Paolo Giannetti, Kit Wu. Analysis of data: Marios Politis, Paul Su, Federico Turkheimer, Shiva Keihaninejad. Interpretation of data: Marios Politis, Adam Waldman, Omar Malik, Paul M. Matthews, Richard Reynolds, Richard Nicholas, Paola Piccini. Statistical analysis: Marios Politis.
DISCLOSURE
M. Politis has served in the advisory board and received consultancy fees from Abbott and receives research support from Michael J. Fox Foundation for Parkinson's Research. P. Giannetti receives research support from European federation of neurologic societies. P. Su reports no disclosures. F. Turkheimer receives research support from the PET Methodology program grant from the Medical Research Council. S. Keihaninejad and K. Wu report no disclosures. A. Waldman has received consultancy fees from Bayer Schering. O. Malik has served in the advisory board and received consultancy fees from Biogen/IDEC Plc and Merck-Serono. P. Matthews is a full-time employee of GlaxoSmithKline Ltd and has served in the advisory board and received honoraria from Medical Research Council. Additionally, he serves as an editorial board member for Nature Reviews Neurology, holds stocks and options in GlaxoSmithKline Ltd, receives book royalties from Oxford University Press and MIT Press, and receives research training support from the Medical Research Council, Wellcome Trust, and EU FP7 Marie Curie Programme. R. Reynolds has received consultancy fees from Eisai London Plc and receives research support from the Medical Research Council. R. Nicholas has received consultancy fees and honoraria from Merck Serono, Novartis, and TEVA. P. Piccini has received research support from the Michael J. Fox Foundation for Parkinson's Research, Parkinson's UK, EU FP7, and CHDI. SUPERPK is licensed by Imperial Innovations, Ltd, but licenses are available for academic investigators at no cost. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001; 50: 389– 400 [DOI] [PubMed] [Google Scholar]
- 2. Bø L, Vedeler CA, Nyland H, Trapp BD, Mørk SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 2003; 9: 323– 331 [DOI] [PubMed] [Google Scholar]
- 3. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005; 128: 2705– 2712 [DOI] [PubMed] [Google Scholar]
- 4. Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 2006; 67: 960– 967 [DOI] [PubMed] [Google Scholar]
- 5. Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089– 1104 [DOI] [PubMed] [Google Scholar]
- 6. Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010; 68: 477– 493 [DOI] [PubMed] [Google Scholar]
- 7. Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 2002; 52: 650– 653 [DOI] [PubMed] [Google Scholar]
- 8. Calabrese M, Rocca MA, Atzori M, et al. A three year study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol 2010; 67: 376– 383 [DOI] [PubMed] [Google Scholar]
- 9. Schmierer K, Parkes HG, So PW, et al. High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain 2010; 133: 858– 867 [DOI] [PubMed] [Google Scholar]
- 10. Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol 2008; 7: 841– 851 [DOI] [PubMed] [Google Scholar]
- 11. Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004; 55: 458– 468 [DOI] [PubMed] [Google Scholar]
- 12. Lassmann H. Multiple sclerosis: is there neurodegeneration independent from inflammation? J Neurol Sci 2007; 259: 3– 6 [DOI] [PubMed] [Google Scholar]
- 13. Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 2006; 29: 518– 527 [DOI] [PubMed] [Google Scholar]
- 14. Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 2006; 59: 478– 489 [DOI] [PubMed] [Google Scholar]
- 15. Dal Bianco A, Bradl M, Frischer J, Kutzelnigg A, Jellinger K, Lassmann H. Multiple sclerosis and Alzheimer's disease. Ann Neurol 2008; 63: 174– 183 [DOI] [PubMed] [Google Scholar]
- 16. Banati RB. Visualising microglial activation in vivo. Glia 2002; 40: 206– 217 [DOI] [PubMed] [Google Scholar]
- 17. Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000; 123: 2321– 2337 [DOI] [PubMed] [Google Scholar]
- 18. Debruyne JC, Versijpt J, Van Laere KJ, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol 2003; 10: 257– 264 [DOI] [PubMed] [Google Scholar]
- 19. Versijpt J, Debruyne JC, Van Laere KJ, et al. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler 2005; 11: 127– 134 [DOI] [PubMed] [Google Scholar]
- 20. Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol 2006; 80: 308– 322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turkheimer FE, Edison P, Pavese N, et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 2007; 48: 158– 167 [PubMed] [Google Scholar]
- 22. Boellaard R, Turkheimer FE, Hinz R, et al. Performance of a modified supervised cluster algorithm for extracting reference region input functions from (R)-[C-11]PK11195 brain PET studies. IEEE 2009; IEEE Nuclear Science Symposium/Medical Imaging Conference; 4666– 4668 [Google Scholar]
- 23. Heckemann RA, Keihaninejad S, Aljabar P, Rueckert D, Hajnal JV, Hammers A, Alzheimer's Disease Neuroimaging Initiative Improving intersubject image registration using tissue-class information benefits robustness and accuracy of multi-atlas based anatomical segmentation. Neuroimage 2010; 51: 221– 227 [DOI] [PubMed] [Google Scholar]
- 24. Kemp BJ, Kim C, Williams JJ, Ganin A, Lowe VJ, National Electrical Manufacturers Association (NEMA) NEMA NU 2–2001 performance measurements of an LYSO-based PET/CT system in 2D and 3D acquisition modes. J Nucl Med 2006; 47: 1960– 1967 [PubMed] [Google Scholar]
- 25. Hammers A, Chen CH, Lemieux L, et al. Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Hum Brain Mapp 2007; 28: 34– 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 2003; 19: 224– 247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gousias IS, Rueckert D, Heckemann RA, et al. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage 2008; 40: 672– 684 [DOI] [PubMed] [Google Scholar]
- 28. Heckemann RA, Hajnal JV, Aljabar P, Rueckert D, Hammers A. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. Neuroimage 2006; 33: 115– 126 [DOI] [PubMed] [Google Scholar]
- 29. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6: 279– 287 [DOI] [PubMed] [Google Scholar]
- 30. Tomasi G, Edison P, Bertoldo A, et al. Novel reference region model reveals increased microglial and reduced vascular binding of 11C-(R)-PK11195 in patients with Alzheimer's disease. J Nucl Med 2008; 49: 1249– 1256 [DOI] [PubMed] [Google Scholar]
- 31. McColl JH, Holmes AP, Ford I. Statistical methods in neuroimaging with particular application to emission tomography. Stat Methods Med Res 1994; 3: 63– 86 [DOI] [PubMed] [Google Scholar]
- 32. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752– 758 [DOI] [PubMed] [Google Scholar]
- 33. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308: 1314– 1318 [DOI] [PubMed] [Google Scholar]
- 34. Biber K, Neumann H, Inoue K, Boddeke H. Neuronal on and off signals control microglia. Trends Neurosci 2007; 30: 596– 602 [DOI] [PubMed] [Google Scholar]
- 35. Melton LM, Keith AB, Davis S, Oakley AE, Edwardson JA, Morris CM. Chronic glial activation, neurodegeneration, and APP immunoreactive deposits following acute administration of double-stranded RNA. Glia 2003; 44: 1– 12 [DOI] [PubMed] [Google Scholar]
- 36. Nakanishi H. Microglial functions and proteases. Mol Neurobiol 2003; 27: 163– 176 [DOI] [PubMed] [Google Scholar]
- 37. Taylor DL, Jones F, Kubota E, Pocock JM. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers TNFα-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci 2005; 25: 2952– 2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. CAMMS223 Trial Investigators. Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786– 1801 [DOI] [PubMed] [Google Scholar]
- 39. Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage 2006; 29: 859– 867 [DOI] [PubMed] [Google Scholar]
- 40. Ceccarelli A, Rocca MA, Pagani E, et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 2008; 42: 315– 322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.