Abstract
Sex differences in the spinal processing of somatic and visceral stimuli contribute to greater female sensitivity in many pain disorders. The present study examined spinal mechanisms that contribute to sex differences in visceral sensitivity. The visceromotor response to colorectal distention (CRD) was more robust in normal female rats and following intracolonic mustard oil compared to males. No sex difference was observed in the CRD-evoked response of lumbosacral (LS) and thoracolumbar (TL) colonic afferents in normal and mustard oil-treated rats, but there was a sex difference in spontaneous activity that was exacerbated by intracolonic mustard oil. The response of visceroceptive dorsal horn neurons to CRD was greater in normal females in the LS and TL spinal segments. The effect of intracolonic mustard oil on the CRD-evoked response of different phenotypes of visceroceptive dorsal horn neurons was dependent on sex and segment. The NMDA receptor antagonist APV dose-dependently attenuated the visceromotor response in normal rats with greater effect in males. Correspondingly, there was greater cell membrane expression of the GluN1 subunit in dorsal horn extracts in females. Following intracolonic mustard oil there was no longer a sex difference in the effect of APV nor GluN1 expression in LS segments but greater female expression in TL segments.
These data document a sex difference in spinal processing of nociceptive visceral stimuli from the normal and inflamed colon. Differences in dorsal horn neuronal activity and NMDA receptor expression contribute to the sex differences in the visceral sensitivity observed in awake rats.
Keywords: visceromotor response, hyperalgesia, primary afferent, spinal cord, dorsal horn neuron, visceral pain, gonadal hormone, NMDA receptor
Introduction
In general, women are more sensitive to pain than men and some painful disorders such as irritable bowel syndrome, temporomandibular disorder and fibromyalgia are more prevalent in women [6;13;25;26]. In animals, sex differences in cutaneous nociception depends on strain and species while females are generally more sensitive to noxious stimulation of deep tissue including muscle and common visceral organs [3;16;17;30;37;42]. Following injury sex differences remain or emerge if not apparent in the absence of inflammation [4;10;16;68]. However, mechanisms that underlie sex differences in nociceptive processing are still unclear.
The rat distal colon and rectum are dually innervated by primary afferents projecting in the lumbar splanchnic nerve to the thoracolumbar (TL) spinal segments and the pelvic nerve to the lumbosacral (LS) spinal segments [7;15;46;61]. Pseudoaffective responses such as the visceromotor response (VMR) evoked by colorectal distention (CRD) in normal rats are dependent on pelvic nerve input, while both pelvic and lumbar splanchnic input and the respective segmental spinal processing contribute to the enhanced VMR during colonic irritation/inflammation [48;60;62;64;67].
Mustard oil is an irritant that activates TRPA1 receptors sensitizing primary afferents [8;38;54]. The majority of colonic afferents express TRPA1 and colonic application of mustard oil depolarizes colonic afferents, increases the VMR in adult rats and mice, and sensitizes neonatal animals inducing hypersensitivity in adults [1;12;14;31;60]. Previous observations from our lab suggested quantitative and qualitative sex differences in response to colorectal distention. Colonic inflammation in male rats increased the response of some phenotypes of dorsal horn neurons in the TL and LS spinal segments [66]. Different phenotypes of dorsal horn neurons were modulated by gonadal hormones and/or inflammation in females and estradiol modulated NMDA receptor activity in females [28;31;59]. In the present study we measured reflexive and neuronal responses in male and female rats in normal and hypersensitive states to examine mechanisms that contribute to sex differences in visceral pain.
Methods
Experiments were performed on 245 age-matched adult male and female Sprague-Dawley rats (Harlan) weighing 220–350 g. Rats were housed in same sex pairs with free access to food and water at 25 °C with 12 hr/12 hr alternating light-dark cycle. Experimental protocols were approved by the University of Maryland Dental School Institutional Animal Care and Use Committee and adhered to guidelines for experimental pain in animals published by the International Association for the Study of Pain.
Visceromotor response (VMR)
Rats were sedated with isoflurane. Electromyogram (EMG) electrodes (Teflon-coated 32 gauge stainless steel wire; Cooner Wire Company, Chatsworth, CA) were stitched into the ventrolateral abdominal wall. The electrode leads were tunneled subcutaneously and exteriorized at the back of the neck. During the same surgery an intrathecal catheter (32 g PE tubing, Micor Inc., Allison Park, PA) was inserted through the atlantooccipital membrane to the lumbar enlargement and exteriorized with the EMG electrodes [65]. Rats were individually housed and tested 5–7 days after surgery. Rats were fasted for 18–24 hrs prior to testing. Water was available ad libitum.
On the day of testing rats were briefly sedated with isoflurane, a 5–6 cm balloon attached to Tygon tubing was inserted into descending colon and rectum through the anus. The secured end of the balloon was at least 1 cm proximal to the external anal sphincter. Rats were loosely restrained in Plexiglas tubes and given 30 min to recover from sedation. Rats were distended to 80 mmHg for 3–4 distention trials (each trial consisted of 5 distentions, 20 sec duration, 3 min interstimulus interval) to get a stable baseline visceromotor response (less than 20% variability between the last two trials). Rats were then briefly sedated with isofluorane and mustard oil (2%, 0.25 ml) was injected into the colon with a gavage needle which was slowly withdrawn during the injection. The distention balloon was replaced, rats returned to the Plexiglas tubes and recording commenced 30 minutes later.
In normal rats or following establishment of hyperalgesia, the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (APV; 0.1–200 nmol, i.t.) was injected in a volume of 10 μl followed by a 10 μl saline flush. The VMR was measured 15, 45 and 90 minutes later. Each rat was tested with 1 dose of APV.
The EMG signal was recorded with a CED 1401 plus and analyzed using Spike 2 for windows software (Cambridge Electronic Design, Cambridge, UK). The EMG was rectified and the mean spontaneous EMG over the 20 sec before distention was subtracted from the mean response during the 20 sec distention. The data are shown as mean ± SEM. The pre/post inflammation data were analyzed using a two-way repeated measures (RM) ANOVA with sex and time as the main factors and pre/post colonic inflammation (time) as the repeated measure. The APV data were converted to percent maximum possible effect (%MPE: normalized to the baseline response in normal rats or the post mustard oil response) and analyzed by two-way ANOVA (sex and dose). p<0.05 was considered significant.
Electrophysiology
Rats were anesthetized with Nembutal (50 mg/kg, i.p.). The left jugular vein was catheterized for continuous infusion of Nembutal at a rate of 5–10 mg/kg/hr. The left carotid artery was catheterized for continuous arterial blood pressure monitoring and bolus administration of pancuronium bromide (0.2 mg/kg/hr). A tracheal cannula was inserted for artificial ventilation. End-tidal CO2 was maintained at 3.5–4.5%. Body temperature was maintained with a water-jacket heating pad and overhead lamp. The rat was placed in a head holder and suspended with thoracic vertebral and ischial clamps. The LS (L6-S2) or TL (T13-L2) spinal cord segments were exposed by laminectomy. The dura matter was cut and the spinal cord was bathed in the warm paraffin oil. The distention balloon was placed into the colon and the rat left undisturbed for 1 hour before recording.
Primary afferents
A pair of silver electrodes was used for recording. The dorsal roots were cut close to the root entry zone and the end in continuity with the periphery was carefully split into fine filaments until a single CRD responsive fiber could be isolated. Signals were amplified (model 1800 AC amplifier; A-M systems, Carlsborg, WA) and passed through a dual time and voltage window discriminator (DDIS-1; BAK Electronics, Germantown, MD) to isolate a single unit. Data were collected with a CED micro 1401 and Spike 2 for Windows software for online and offline analysis. The response to graded intensities of CRD (20, 40, 60, 80 mmHg) was recorded.
The response to distention was quantified as the mean discharge frequency during the 20 sec distention minus the mean spontaneous activity in the preceding 20 sec. The data are expressed as mean ± SEM. Data were analyzed using one-way or two-way ANOVA as appropriate; p < 0.05 was considered significant.
Dorsal horn neurons
The surgery was done as described above. Tungsten microelectrodes (1–2 MΩ; Micro probe, Potomac, MD) were used for extracellular single-unit recording in the TL or LS spinal segments (0–1.5 mm lateral to midline, 500–1500 m ventral to spinal cord dorsum). Signals were amplified, isolated and recorded as above.
Cells showing excitatory responses to CRD were classified as Abrupt or Sustained neurons on the basis of their response to 80 mmHg CRD. Abrupt neurons ceased responding within 4 seconds of terminating the stimulus; activity dropping below the mean plus 2 standard deviations of the spontaneous activity (mean of 20 sec prior to distention) for 2 sec. Sustained neurons had an afterdischarge that persisted longer than 4 seconds that ceased when activity dropped below the spontaneous activity plus 2 standard deviations for 2 sec. After identifying a neuron, at least two graded intensity distention trials (20, 40, 60 and 80 mmHg, each distention lasting for 20 sec with a 3 min interstimulus interval) were run to establish the baseline response of the neuron, after which mustard oil (2%, 0.25 ml) was injected into the colon. The response to CRD was recorded at 30, 60 and 90 min after mustard oil injection.
Abrupt unit activity was quantified as the mean discharge frequency during the 20 sec of the CRD stimulus minus the mean spontaneous activity determined in the preceding 20 sec. Sustained unit activity was measured as the mean discharge frequency from the start of distention till the end of the afterdischarge minus the mean spontaneous activity. The data are expressed as mean ± SEM. Data were analyzed by two-way ANOVA or two-way repeated measures (RM) ANOVA as appropriate. Post-hoc comparisons following a significant ANOVA were conducted with a Bonferroni correction for multiple tests. An adjusted p value < 0.05 was considered significant. Only one neuron was studied per rat.
Fos expression
Rats were anesthetized with urethane (1.25 g/kg i.p.) and a distention balloon placed in the descending colon and rectum. Mustard oil (2%, 0.25 ml) was injected with a gavage needle before the distention balloon was put in place. Staring ten minutes after the balloon placement the rats were repetitively distended to 80 mmHg (30 sec on, 90 sec off) for 2 hrs. Immediately after the distention, the rats were overdosed with 1 ml Nembutal (100~150 mg/kg i.p.) and perfused through the heart with saline followed by ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer. The LS and TL spinal cord segments were removed, and placed in fresh fixative overnight then transferred to 30% sucrose for 24–48 hrs. Thirty micron transverse sections were cut on a cryostat.
Sections were labeled for Fos (AB-5, 1:50,000, Oncogene Science, Cambridge, MA) using standard procedures for DAB [63]. Sections were examined and labeled cells plotted using a camera lucida. Data were analyzed using two-way ANOVA; p< 0.05 was considered significant.
Western Blot
Rats were euthanized with CO2 and decapitated. The spinal cord was removed by pressure ejection with 10 ml ice cold saline. LS and TL spinal segments were isolated, snap frozen and kept at −80 °C until use. The dorsal spinal cord tissue was dissected and sonicated in RIPA buffer with Protease Inhibitor Cocktail (Roche, IN). The homogenate was centrifuged at 14000 rpm for 15 min and the supernatant was used as whole cell protein. For extracting the membrane rich protein, the homogenate was centrifuged at 2900 rpm for 20 min at 4°C. The supernatant was further centrifuged at 20,000 rpm for 45 minutes. The pellet (rich in membrane) was resuspended in RIPA buffer and the protein concentration determined using the Bradford method. Fifteen to thirty g protein was separated on a 7.5% SDS-PAGE gel and blotted to a nitrocellulose membrane. The membrane was stained with Ponseau S for 5 min, rinsed in dH2O and the density of each column was measured and used as the loading control. The membrane was then blocked with Startblock blocking buffer (Pierce, IL) and further incubated with the respective primary and secondary antibodies. The immunoreactive band was detected using Enhanced Chemiluminescence (ECL, Pierce). The primary antibodies used were anti-NR1 antibody (1:1000, Santa Cruz, CA) and anti-actin antibody (1:10000, Sigma, MO). Data were normalized to the expression in male rats and analyzed using t-test. p< 0.05 was considered significant.
Results
The visceromotor response to noxious CRD
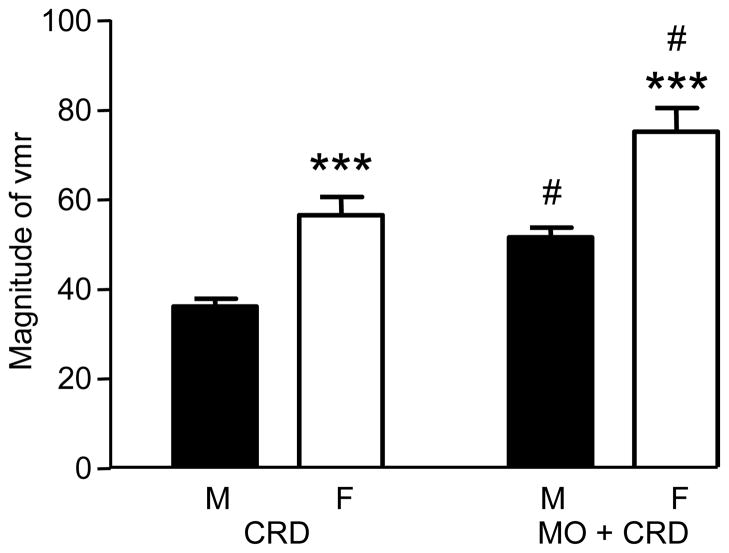
The VMR to 80 mmHg CRD was recorded in awake male (n = 33) and female (n = 23) rats under normal (e.g., non-irritated/non-inflamed) conditions, and following intracolonic mustard oil (Figure 1). The magnitude of the VMR was significantly greater in normal and mustard oil-treated females compared to males (two-way RM ANOVA: sex p<0.0001; treatment p<0.0001). The mean increase in VMR magnitude was greater in females (18.7 ± 1.7) compared to males (14.8 ± 1.0; t-test, p<0.05). These data indicate the magnitude of the VMR in female rats is greater than males and intracolonic mustard oil has a greater effect in females.
Figure 1.
The magnitude of VMR to 80 mmHg CRD in normal (CRD) and mustard oil-treated (MO+CRD) male and female rats. *** p<0.001 vs. male. # p<0.001 vs. same sex CRD.
The response of colonic primary afferent fibers to graded intensities of CRD
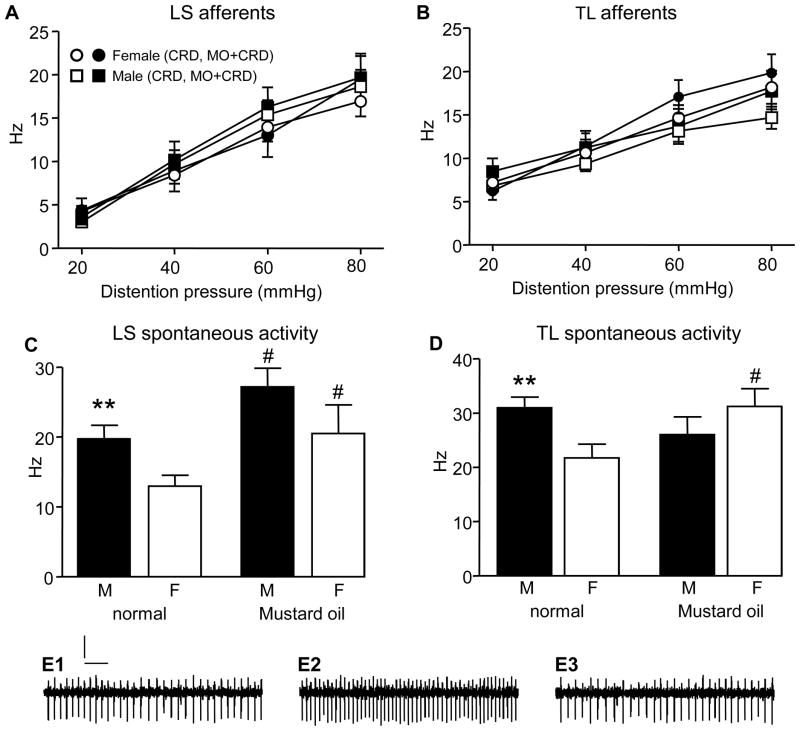
The CRD-evoked response of colonic afferents (LS and TL) was compared between normal and mustard oil-treated male and female rats to determine if sex differences in the VMR could be accounted for by differences in primary afferent responses to CRD. Surprisingly, there was no sex difference in the stimulus-response curves of LS colonic afferents in normal (male n=48 afferents, female n=42 afferents) and mustard oil-treated rats (male n=18, female n=14; two-way ANOVA; Figure 2A), nor was there sensitization of the evoked response to CRD in males or females following intracolonic mustard-oil. Similarly, there was no difference in the response to distention between TL colonic afferents from normal or mustard oil-treated male and female rats (normal: male n=37, female n=35; mustard oil: male n=20, female n=23; two-way ANOVA; Figure 2B). These data indicate that sex differences in the evoked response of colonic afferents to CRD in this model do not contribute to sex differences in the VMR.
Figure 2.
A,B: The response of colonic afferent fibers in the LS and TL dorsal roots to graded intensities of colorectal distention. Legend in A is the same for B. C,D: the mean spontaneous activity in LS and TL colonic afferents recorded from normal rats and following colonic mustard oil. ** p<0.01 vs. female; # p<0.05 vs. same sex (normal). E: trace recording of a primary afferent before (E1), during (E2) and after (E3) CRD. Scale bars: 200 μV, 100 ms.
In contrast to the absence of a sex difference in the evoked response in LS colonic afferents, there was a sex difference in the spontaneous activity (two-way ANOVA: sex p<0.01; treatment p<0.005; Figure 2C). Prior to mustard oil injection, the mean spontaneous activity in male LS colonic afferents was greater than in females (p<0.01). Mustard oil increased the spontaneous activity of colonic afferents in males by 38% (t-test vs. normal males: p<0.05) and females by 58% (t-test: p<0.05) abolishing the sex difference. In TL colonic afferents there was a significant interaction between sex and treatment in spontaneous activity (two-way ANOVA: p<0.01; Figure 2D). Spontaneous activity in normal males was greater than normal females (p<0.005). Mustard oil increased spontaneous activity in females by 44% (t-test: p<0.05), but not males (16% decrease, not significant) and the sex difference in spontaneous activity was lost.
Spinal cord Fos expression in response to CRD
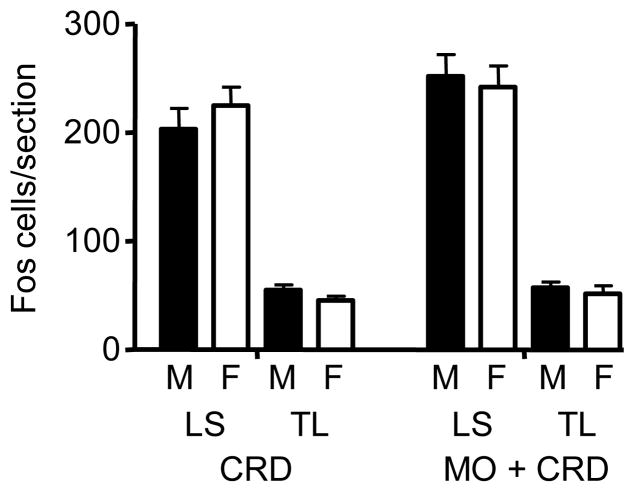
In both the LS and TL spinal cord segments (n=8/group), colonic distention (80 mmHg) induced a comparable number of Fos-immunoreactive neurons per section in male (LS: 203 ± 19; TL: 55 ± 5) and female (LS: 224 ± 17; TL: 45 ± 4) rats (Figure 3). There were a significantly greater number of Fos positive neurons in the LS compared to TL segments in both male and female rats (p< 0.001). Surprisingly, mustard oil plus CRD induced a slight, but non-significant increase in Fos expression in the LS segments (M: 252 ± 20; F: 242 ± 20) and no change in the TL segments (M: 57 ± 6; F: 51 ± 7).
Figure 3.
The number of Fos labeled neurons per section in the LS and TL segments following CRD or mustard oil + CRD.
Response of spinal dorsal horn neurons to graded intensities of CRD
Although there was no sex difference in CRD-induced Fos expression, nor did mustard oil increase the number of visceroceptive neurons, single unit recording was used to determine if there was a sex difference in the magnitude of response of LS and TL dorsal horn neurons to CRD prior to and following treatment with mustard oil. Two phenotypes of dorsal horn neurons with excitatory responses to CRD were examined: Abrupt and Sustained.
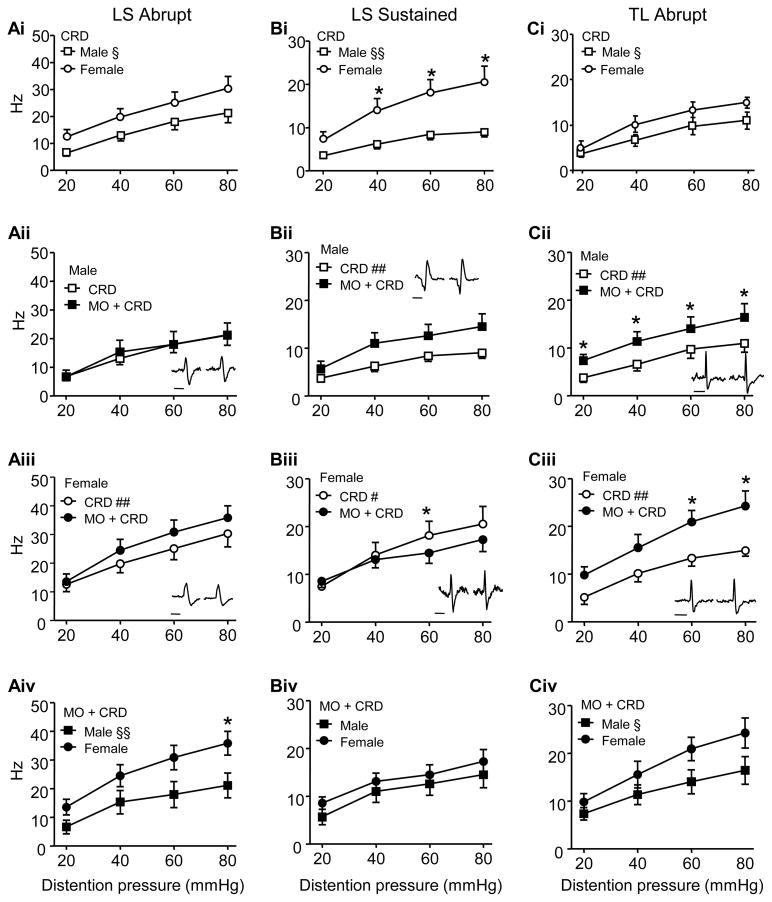
In the LS segments of normal rats, the response of Abrupt neurons was significantly more robust in females (n=11 neurons) compared to males (n=14 neurons; two-way ANOVA: sex, p<0.01; Figure 4Ai), paralleling the sex difference in the VMR of normal rats. Intracolonic mustard oil had no effect on the magnitude of response in males (two-way RM ANOVA: p>0.05; Figure 4Aii), but increased the response to CRD in females (two-way RM ANOVA: treatment, p<0.001; Figure 4Aiii). Thus, following colonic mustard oil the response in females was significantly greater than males (two-way ANOVA: sex, p<0.001; Figure 4Aiv) and the magnitude of the sex difference increased from 49% in normal rats to 72% following mustard oil (comparing the difference in the area under the curve in 4Ai and 4Aiv).
Figure 4.
The response of LS Abrupt (A), LS Sustained (B) and TL Abrupt (C) dorsal horn neurons in normal rats and following intracolonic mustard oil. i) The response of neurons in normal male and female rats. ii) The response of neurons in males before and after intracolonic mustard oil. iii) The response of neurons in females before and after intracolonic mustard oil. iv) The response of neurons in mustard oil-treated males and females. Note that the direction of effect on the response of LS Abrupt and LS Sustained neurons following intracolonic mustard oil differed in males and females. § p<0.01, §§ p<0.001 vs. female; # p<0.05, ## p<0.001 vs. MO + CRD; * p <0.05 in that pressure. ii,iii: inserts show representative examples of spikes recorded before (left) and after (right) mustard oil. Bar = 1 ms. Error bars are shown in one direction for clarity.
LS Sustained neurons were qualitatively different from LS Abrupt neurons. In normal rats, similar to Abrupt neurons, the response of LS Sustained neurons in female rats (n = 9) was greater than males (n = 11; two-way ANOVA: sex, p<0.001; Figure 4Bi). However, contrary to Abrupt neurons, colonic mustard oil increased the response of Sustained neurons in male rats (two-way RM ANOVA: treatment, p<0.001; Figure 4Bii) and decreased the response in female rats (two-way RM ANOVA: treatment, p<0.05; treatment x pressure, p<0.05; Figure 4Biii). This resulted in the loss of the sex difference in Sustained neurons following intracolonic mustard oil (two-way ANOVA: p>0.05; Figure 4Biv) from 120% in normal rats to 49% following mustard oil.
The TL spinal cord is unlikely to contribute to the VMR in normal rats [48;60;67]. Nevertheless, Abrupt neurons were recorded in these segments in order to determine the effect of mustard oil. Prior to mustard oil the response of TL Abrupt neurons was significantly greater in females (n=7) compared to males (n=11; two-way ANOVA: sex, p< 0.01; Figure 4Ci).
Following intracolonic mustard oil the response of Abrupt neurons to CRD in both male (two-way RM ANOVA: treatment, p<0.001; Figure 4Cii) and female rats (two-way RM ANOVA: treatment, p<0.001; Figure 4Ciii) increased, maintaining the direction (two-way ANOVA: sex, p<0.005; Figure 4Civ) and magnitude (40% in normal rats, 43% following mustard oil) of the sex difference. Sustained neurons were rarely encountered in the TL segments of male rats [66] and therefore were not studied.
Sex difference in NMDA receptor activity
NMDA receptors contribute to spinal processing of acute nociceptive visceral stimuli and estradiol increases NMDA receptor expression in the spinal cord of ovariectomized rats increasing the magnitude of the VMR [59]. This prompted us to determine if sex differences in NMDA receptor activity contribute to the sex difference in the VMR.
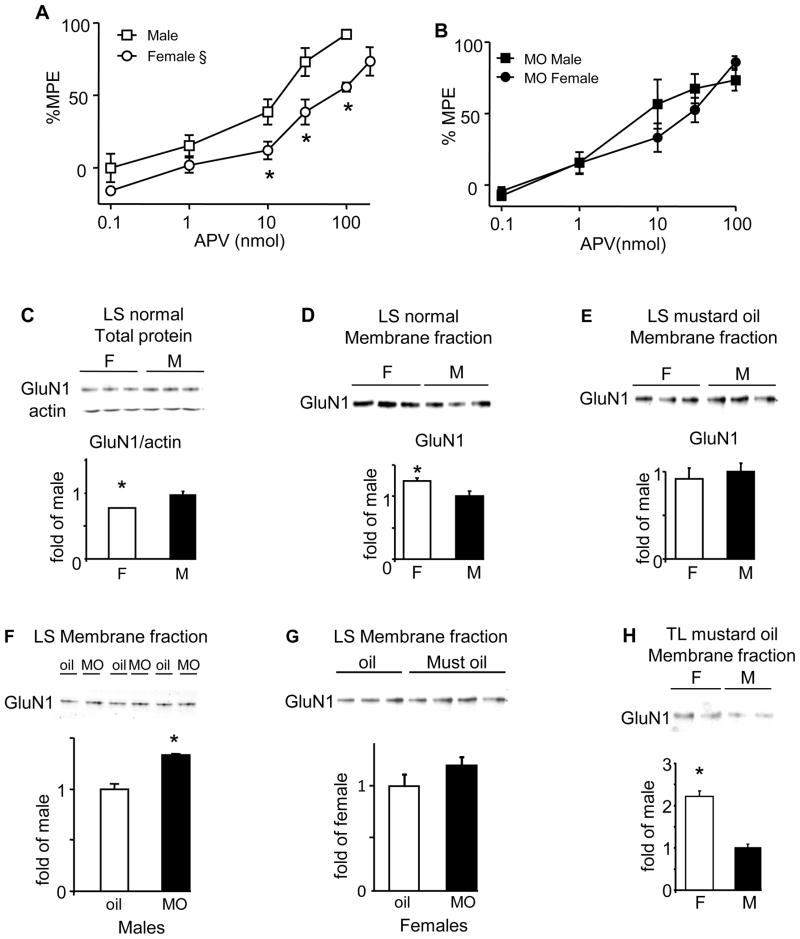
The NMDA receptor antagonist APV dose-dependently attenuated the VMR in normal males and females (Fig 5A). 100 nmol APV produced 92% attenuation of the VMR (%MPE) in males compared to 55% in females (t-test, p<0.001). 200 nmol APV further attenuated the VMR to 74% MPE in females equalizing the %MPE to 100 nmol in males (t-test, p=0.1303). Overall, APV had a greater effect in males (%MPE: two-way ANOVA: sex p<0.0001; APV p<0.0001). Following intracolonic mustard oil there was still a dose-dependent attenuation of the VMR in rats of both sexes, but the sex difference was lost (two-way ANOVA: dose p<0.0001; Figure 5B) as 100 nmol APV attenuated the VMR by 86% in females.
Figure 5.
A, B: dose-response curves to i.t. APV from normal (A) and mustard oil-treated (B) rats. § p<0.0001 vs. male. * p<0.05 in that pressure. C: Western blot and quantification showing total GluN1 from the LS spinal cord of normal males and females. Actin was used as a loading control. D–H: Western blots and quantification of GluN1 from the membrane fraction of: the LS spinal cord from normal rats (D); the LS spinal cord of mustard oil-treated rats (E); the LS spinal cord from male rats ± mustard oil (F); the LS spinal cord from female rats ± mustard oil (G); and the TL spinal cord of mustard oil treated rats (H). * p<0.05. N=3–7/group.
Western blots were used to measure the expression of the obligatory component of the NMDA receptors, GluN1 (formerly NR1), in the LS and TL spinal segments in normal and mustard oil-treated rats. Normal males had more total GluN1 protein in the LS spinal cord compared to females (t-test: p<0.01; Figure 5C), but females had greater GluN1 expression in the membrane fraction (t-test: p<0.05; Figure 5D). In the TL spinal cord, there was no sex difference in the membrane expression of GluN1 (data not shown). Taken together, these data suggest greater NMDA receptor activity in the LS spinal cord in females might underlie the sex difference in the magnitude of the VMR under normal conditions.
Following colonic mustard oil there was no longer a sex difference in membrane expression of GluN1 in the LS spinal cord (t-test: p>0.05; Figure 5E). This was due to an increase in GluN1 in males (t-test, p<0.001; Figure 5F), but not females (Figure 5G). In contrast, there was significantly more GluN1 expression in the membrane fraction in the TL segments of female rats (t-test: p<0.05; Figure 5H).
Discussion
The present study examined mechanisms underlying the sex difference in nociceptive processing from the lower GI tract. Consistent with previous reports [27;30] the CRD-evoked VMR was more robust in female rats compared to males. Intracolonic mustard oil increased the VMR in male and female rats, though slightly more in females, maintaining the sex difference. In the present study we addressed the hypothesis that sex differences in nociceptive processing intrinsic to the spinal cord, at least partially account for sex differences in response to colorectal distention.
The magnitude of the VMR varies with the stage of the estrous cycle, greatest in proestrus and least in met/di-estrus [32]. However, the threshold of the VMR, which is negatively correlated with the magnitude, was greater in males than females in any stage of the estrous cycle [27;56] so it is unlikely that estrous cycle modulation of the VMR accounts for the sex difference in visceral sensitivity. Therefore, it is not surprising that intact female rats, which were likely at different stages of the estrous cycle at the time of testing, had an overall higher magnitude VMR.
The abdominal muscle contraction measured during the VMR in normal rats is dependent upon intact colonic afferent input to the LS spinal cord and a spinobulbospinal loop. It is unlikely the TL spinal cord has any contribution in normal rats since deafferenting the TL spinal cord had no effect on the VMR [48] and deafferenting the LS spinal cord abolished the VMR [60]. Following colonic irritation/inflammation there is an increase in activity in the TL spinal cord contributing to the increased magnitude of the VMR and visceral hypersensitivity [23;45;60;66]. Therefore, sex differences in the VMR could arise from differences in primary afferent input, dorsal horn neuron or supraspinal processing. We examined the contribution of peripheral and spinal processing to the sex difference in the VMR.
Primary afferent activity
In the present study we found no sex difference in the response of LS or TL colonic afferent fibers to CRD. This is consistent with our previous finding that ovariectomy did not alter the response of colonic afferents projecting in the LS dorsal roots [28], suggesting a lack of gonadal hormone modulation of colonic afferents.
Surprisingly, colonic mustard oil did not sensitize colonic afferents to CRD given that colonic afferents express TRPA1 [9;36;40] and a higher concentration of mustard oil (2 ml, 5% [58] vs. the present study 0.25 ml, 2%) increased colonic afferent responses to CRD. However, we did observe an increase in spontaneous activity following mustard oil, consistent with mustard oil evoked depolarization of colonic afferents in vitro [12]. It is possible the lower concentration of mustard oil was insufficient to sensitize the evoked response to CRD, but the increase in spontaneous activity was sufficient to induce central sensitization. The spontaneous activity was greater in males, consistent with greater excitatory amino acid release in the male nucleus caudalis [5]. This suggests that colonic afferent activity is not necessarily correlated with the magnitude of CRD-evoked reflexes and differences in receptor expression/function in the spinal cord could be more important in mediating the sex differences in the VMR.
Spinal cord processing of visceral stimuli
Fos expression in the spinal cord
The immediate early gene product c-Fos is a transcription factor that regulates protein synthesis and its expression is widely used as a marker for neuronal activation. There were a comparable number of CRD-induced Fos-labeled neurons in male and female rats in both the LS [44] and TL segments, and there was no increase in Fos expression following intracolonic mustard oil. This differs from previous observations that Fos expression increased following intracolonic zymosan in male rats [62], suggesting Fos expression may depend on the specific afferent populations stimulated. Furthermore, since mustard oil did not sensitize colonic afferents to CRD, this might be reflected in the absence of an increase in Fos expression. Alternatively, changes in Fos expression in individual neurons may reflect changes in excitability following intracolonic mustard oil. The increase in the response of LS Abrupt neurons and decrease in LS Sustained neurons to CRD in females may mirror an increase/ decrease in Fos expression in those cells, leaving the total number of Fos expressing neurons in the LS spinal cord unchanged. Finally, it cannot be ruled out that Fos expression is modulated at different rates in males and females and measurement at a single time could miss dynamic changes.
Sex differences in dorsal horn neuronal activity
The two most prominent phenotypes of dorsal horn neurons that are excited by CRD are Abrupt and Sustained neurons [2;29;31;34;47;50;66]. The activities of these neurons are differentially modulated by gonadal hormones, neurotransmitters and inflammatory agents, but the functional significance of these two types of neurons to visceroceptive processing is debatable. In the present study the response of Abrupt and Sustained neurons in the LS spinal cord were more robust in normal females compared to males. For Abrupt neurons this may reflect the greater overall plasma estrogen concentration in females [18;19;24] since estradiol facilitates Abrupt neuron responses to CRD [29]. In contrast, hormonal modulation of Sustained neurons is complex: ovariectomy with/without estradiol replacement or estrogen receptor (ER) α activation had no effect on the response of Sustained neurons [28;29;31], although selective ERβ activation inhibited Sustained neurons in female rats [11].
Following colonic mustard oil there was an increase in the response of LS Abrupt neurons in females, but not males, while the response of LS Sustained neurons increased in males, but not females. The response of Abrupt neurons in the TL spinal cord increased in both males and females. The pattern of activity of Abrupt neurons and the VMR are the same; on and off with the stimulus; suggesting the former underlies the later. The more robust response of LS Abrupt neurons in normal female rats and the increase in response of LS and TL Abrupt neurons following mustard oil supports the greater VMR in females under both conditions. The increase in TL Abrupt neuron activity in males likely contributes to the increase in the VMR following mustard oil which is not as great as females. The role of Sustained neurons is unclear, but they may contribute to other aspects of visceral pain.
Sex difference in spinal NMDA receptor activity/expression
Spinal NMDA receptors partially mediate acute or transient visceral pain [33;35;51;65]. It is well acknowledged that NMDA receptors are functionally regulated by estrogen throughout the brain [20;43;49;59;69]. Estrogen increases GluN1 protein expression in the hippocampus of gonadectomized females, increases [(3)H]-glutamate binding in the hippocampus of gonadectomized females but not males, and increases NMDA receptor activity in the hippocampus [21;43;55;57].
At the spinal cord level, estrogen increases NMDA receptor activity through a presynaptic increase in transmitter release and/or postsynaptic increase in excitability, which might account for a sex difference in painful signal processing [52;53]. In DRG neurons, estradiol evokes greater NMDA receptor-mediated currents in females compared to males [41]. In dorsal horn neurons, NMDA receptors colocalize with estrogen receptors and estrogen increased GluN1 protein expression in the spinal cord of gonadectomized females [59]. In the current study, we observed a greater expression of GluN1 in the plasma membrane in the LS spinal cord in female rats compared with males. Accordingly, a greater antinociceptive effect of APV on the VMR was observed in males, suggesting greater NMDA receptor activity in females. Therefore, it is reasonable to conclude that estrogen modulation of NMDA receptor expression in the plasma membrane could contribute to sex differences in nociceptive processing in the absence of colonic mustard oil.
Peripheral tissue inflammation increases spinal GluN1 and GluN2B receptor phosphorylation/expression [22;39;70]. In male rats Abrupt and Sustained neurons respond to NMDA receptor antagonists [33]. In the current study, the loss of the sex difference in the expression of membrane GluN1 in the LS spinal cord following mustard oil is consistent with the loss of the sex difference in the effect of APV and consistent with the differential change in response of LS Abrupt and Sustained neurons in male and female rats. However, the TL spinal cord had more membrane GluN1 expression in mustard oil-treated females compared to males. This finding may partially explain the greater response of TL Abrupt neurons and the VMR in mustard oil-treated females, but contradicts the lack of sex difference in the effect of APV. Since the i.t. catheter was centered at the LS spinal cord, it is possible APV was less effective at the TL spinal cord level, causing the mismatch of receptor expression and response to APV.
In conclusion, the present study demonstrates there are sex related differences in visceral nociceptive processing in rats in the absence and presence of colonic inflammation. The greater magnitude of pseudoaffective responses in female rats may reflect a differential increase in spinal dorsal horn neuronal activity compared to males that is partially dependent on NMDA receptor expression. In combination with previous reports, these findings suggest sex differences in the way the nervous system processes noxious stimuli that could be hormonally dependent.
Summary.
Sex differences to noxious and inflammatory visceral stimuli are dependent on differences in afferent input, dorsal horn neuronal activity and spinal NMDA receptors.
Acknowledgments
Thanks to Sangeeta Pandya for technical assistance. Supported by R01 NS 37424.
Footnotes
The authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 2.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, Pace MC, Chiefari M, Aurilio C, Giamberardino MA. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur J Pain. 2010;14:602–607. doi: 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Bereiter DA, Shen S, Benetti AP. Sex differences in amino acid release from rostral trigeminal subnucleus caudalis after acute injury to the TMJ region. Pain. 2002;98:89–99. doi: 10.1016/s0304-3959(01)00476-6. [DOI] [PubMed] [Google Scholar]
- 6.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 8.Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol. 2011;589:3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns BE, Gazerani P. Sex-related differences in pain. Maturitas. 2009;63:292–296. doi: 10.1016/j.maturitas.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Cao D-Y, Ji Y, Tang B, Traub RJ. Estrogen receptor βactivation is antinociceptive in a model of visceral pain in the rat. Journal of Pain. 2012 doi: 10.1016/j.jpain.2012.04.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 14.Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2005;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 16.Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- 17.Craft RM. Modulation of pain by estrogens. Pain. 2007;132 (Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Dong XD, Mann MK, Sessle BJ, Arendt-Nielsen L, Svensson P, Cairns BE. Sensitivity of rat temporalis muscle afferent fibers to peripheral N-methyl-D-aspartate receptor activation. Neuroscience. 2006;141:939–945. doi: 10.1016/j.neuroscience.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 21.Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76. doi: 10.1186/1744-8069-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington AM, Brierley SM, Isaacs N, Hughes PA, Castro J, Blackshaw LA. Sprouting of colonic afferent central terminals and increased spinal MAP kinase expression in a mouse model of chronic visceral hypersensitivity. J Comp Neurol. 2012 doi: 10.1002/cne.23042. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins RA, Freedman B, Marshall A, Killen E. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. Br J Cancer. 1975;32:179–185. doi: 10.1038/bjc.1975.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- 26.Heitkemper MM, Jarrett M. Gender differences and hormonal modulation in visceral pain. Curr Pain Headache Rep. 2001;5:35–43. doi: 10.1007/s11916-001-0008-z. [DOI] [PubMed] [Google Scholar]
- 27.Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br J Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011:1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 31.Ji Y, Tang B, Traub RJ. Estrogen increases and progesterone decreases behavioral and neuronal responses to colorectal distention following colonic inflammation in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J Neurophysiol. 2001;86:1783–1791. doi: 10.1152/jn.2001.86.4.1783. [DOI] [PubMed] [Google Scholar]
- 34.Ji Y, Traub RJ. Differential effects of spinal CNQX on two populations of dorsal horn neurons responding to colorectal distention in the rat. Pain. 2002;99:217–222. doi: 10.1016/s0304-3959(02)00106-9. [DOI] [PubMed] [Google Scholar]
- 35.Kolhekar R, Gebhart GF. Modulation of spinal visceral nociceptive transmission by NMDA receptor activation in the rat. J Neurophysiol. 1996;75:2344–2353. doi: 10.1152/jn.1996.75.6.2344. [DOI] [PubMed] [Google Scholar]
- 36.La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K(+) channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71–80. doi: 10.1016/j.pain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 Function and Modulation Are Target Tissue Dependent. The Journal of Neuroscience. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 43.Morissette M, Le Saux M, Di Paolo T. Effect of oestrogen receptor alpha and beta agonists on brain NMDA receptors. J Neuroendocrinol. 2008;20:1006–1014. doi: 10.1111/j.1365-2826.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 44.Murphy AZ, Suckow SK, Johns M, Traub RJ. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol Behav. 2009;97:205–212. doi: 10.1016/j.physbeh.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain. 2000;87:83–88. doi: 10.1016/S0304-3959(00)00272-4. [DOI] [PubMed] [Google Scholar]
- 46.Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distention in the T13-L2 spinal cord of the rat. J Neurophysiol. 1988;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- 47.Ness TJ, Gebhart GF. Characterization of superficial T13-L2 dorsal horn neurons encoding for colorectal distention in the rat: comparison with neurons in deep laminae. Brain Res. 1989;486:301–309. doi: 10.1016/0006-8993(89)90516-7. [DOI] [PubMed] [Google Scholar]
- 48.Ness TJ, Randich A, Gebhart GF. Further evidence that colorectal distention is a noxious visceral stimulus in rats. Neurosci Lett. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]
- 49.Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- 50.Olivar T, Cervero F, Laird JM. Responses of rat spinal neurones to natural and electrical stimulation of colonic afferents: effect of inflammation. Brain Res. 2000;866:168–177. doi: 10.1016/s0006-8993(00)02274-5. [DOI] [PubMed] [Google Scholar]
- 51.Olivar T, Laird JM. Differential effects of N-methyl-D-aspartate receptor blockade on nociceptive somatic and visceral reflexes. Pain. 1999;79:67–73. doi: 10.1016/S0304-3959(98)00152-3. [DOI] [PubMed] [Google Scholar]
- 52.Peng HY, Chen GD, Lai CY, Hsieh MC, Hsu HH, Wu HC, Lin TB. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ reflex sensitization in ovariectomized female rats. J Neurochem. 2010;113:54–66. doi: 10.1111/j.1471-4159.2010.06577.x. [DOI] [PubMed] [Google Scholar]
- 53.Peng HY, Chen GD, Tung KC, Chien YW, Lai CY, Hsieh MC, Chiu CH, Lai CH, Lee SD, Lin TB. Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. Am J Physiol Endocrinol Metab. 2009;297:E416–E426. doi: 10.1152/ajpendo.00129.2009. [DOI] [PubMed] [Google Scholar]
- 54.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex Differences in Hippocampal Estradiol-Induced N-Methyl-D-Aspartic Acid Binding and Ultrastructural Localization of Estrogen Receptor-Alpha. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- 56.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 57.Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34 (Suppl 1):S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su X, Sengupta JN, Gebhart GF. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997;78:1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- 59.Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. NeuroReport. 2000;11:2113–2116. doi: 10.1097/00001756-200007140-00011. [DOI] [PubMed] [Google Scholar]
- 61.Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides. 1999;20:267–273. doi: 10.1016/s0196-9781(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 62.Traub RJ, Murphy AZ. Colonic inflammation induces Fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93–102. doi: 10.1016/s0304-3959(01)00381-5. [DOI] [PubMed] [Google Scholar]
- 63.Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- 64.Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A Rat Model of Chronic Post-Inflammatory Visceral Pain Induced by Deoxycholic Acid. Gastroenterology. 2008;135:2075–2083. doi: 10.1053/j.gastro.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traub RJ, Zhai QZ, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression and the visceromotor reflex. Neurosci. 2002;113:205–211. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 66.Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J Neurophysiol. 2005;94:3788–3794. doi: 10.1152/jn.00230.2005. [DOI] [PubMed] [Google Scholar]
- 67.Wang G, Tang B, Traub RJ. Pelvic nerve input mediates descending modulation of homovisceral processing in the thoracolumbar spinal cord of the rat. Gastroenterology. 2007;133:1544–1553. doi: 10.1053/j.gastro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang R, Liu Y, Gu X, Zhang J, Zheng Y, Ma Z. Intrathecal administration of roscovitine attenuates cancer pain and inhibits the expression of NMDA receptor 2B subunit mRNA. Pharmacol Biochem Behav. 2012 doi: 10.1016/j.pbb.2012.03.025. [DOI] [PubMed] [Google Scholar]