Abstract
A length-dependent neuropathy with pain in the feet is a common complication of diabetes (painful diabetic neuropathy, PDN). It was hypothesized that pain may arise from sensitized-hyperactive cutaneous nociceptors, and that this abnormal signaling may be reduced by topical administration of the α2-adrenergic agonist, clonidine, to the painful area. This was a randomized, double-blind, placebo-controlled, parallel-group, multi-center trial. Nociceptor function was measured by determining the painfulness of 0.1% topical capsaicin applied to the pre-tibial area of each subject for 30 minutes during screening. Subjects were then randomized to receive 0.1% topical clonidine gel (n=89) or placebo gel (n=90) applied t.i.d. to their feet for 12 weeks. The difference in foot pain at week 12 in relation to baseline, rated on a 0-10 numerical pain rating scale (NPRS), was compared between groups. Baseline NPRS was imputed for missing data for subjects who terminated the study early. The subjects treated with clonidine showed a trend toward decreased foot pain compared to the placebo-treated group (the primary endpoint; p=0.07). In subjects who felt any level of pain to capsaicin, clonidine was superior to placebo (p<0.05). In subjects with a capsaicin pain rating ≥2 (0-10, NPRS), the mean decrease in foot pain was 2.6 for active compared to 1.4 for placebo (p=0.01). Topical clonidine gel significantly reduces the level of foot pain in PDN subjects with functional (and possibly sensitized) nociceptors in the affected skin as revealed by testing with topical capsaicin. Screening for cutaneous nociceptor function may help distinguish candidates for topical therapy for neuropathic pain.
Keywords: Topical Clonidine, Capsaicin, Diabetic Neuropathy, PDN, Pain, Clinical Trial
Introduction
A length-dependent neuropathy is one of the most common complications of diabetes. Pain, typically felt in the feet, is a common feature. The analgesic efficacy of oral medications such as pregabalin and duloxetine in PDN is highly variable and many patients have difficulty with side effects [25]. It is therefore desirable to find alternative therapies. Prior reports from studies in animals and man have suggested that clonidine may be effective in relieving neuropathic pain when applied topically to the painful area [6;17]. Clonidine is an α2-adrenergic receptor agonist, that was originally approved as an oral product to treat hypertension. Intrathecally applied clonidine was later shown to produce analgesia for both acute and chronic pain [10]. Alpha-2 receptors are also present on nociceptors in the epidermis [26]. Activation of these G-protein coupled receptors leads to release of an inhibitory G-protein which in turn down regulates adenylate cyclase and other second messengers thought to play a role in initiating and maintaining the abnormal excitability of nociceptors [16]. The origin of neuronal signals leading to pain in PDN is unknown, though nociceptors expressed in the skin are a potential target [3]. Given the robust expression of alpha-2 receptors in cutaneous nociceptors, evidence that nociceptors in the skin may be sensitized in neuropathic pain models, and prior behavioral and clinical data indicating analgesic effects of clonidine, a double blind randomized study was performed to determine efficacy, tolerability, and safety of topical clonidine to treat PDN. In some patients the skin is profoundly denervated, and in these cases topical therapy is likely futile. In other patients, the nociceptor innervation of the skin is preserved [11;23;32] and to the extent that abnormal signaling in these nociceptors leads to pain, topical clonidine may have a therapeutic role. Here we provide evidence that the analgesic effect of topical clonidine varies with the painfulness of topical capsaicin applied near the foot. This supports the hypothesis that the efficacy of this topical therapy depends on the presence of nociceptors in the skin, and possibly on the level of sensitization.
Materials and Methods
Design
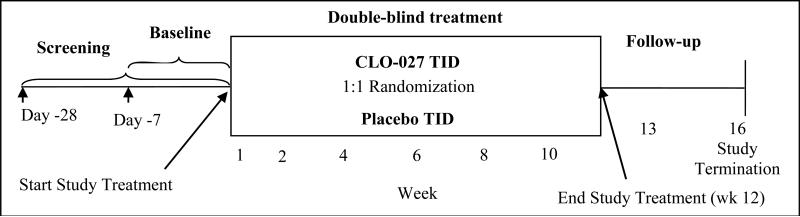
The study was a randomized, double-blind, placebo-controlled, parallel-group, conducted at multiple centers throughout the United States that consisted of a screening phase (28 +/- 7 days prior to the baseline visit), a baseline phase (7 days prior to treatment plus the baseline visit assessments [day 1]), a 12-week double-blind treatment phase, and a follow up period (Figure 1). The protocol and informed consent documents were approved by the appropriate Institutional Review Boards and the trial was registered (ClinicalTrials.gov identifier: NCT00695565). Written informed consent was obtained from each patient before initiation of study procedures.
Figure 1.
Timeline
Screening of subjects
All subjects were diagnosed to have a length dependent painful sensory neuropathy affecting the feet attributable to type 1 or type 2 diabetes mellitus based on history, physical examination, and laboratory data. The symptoms followed a stocking type distribution. Subjects were excluded if they had clinical evidence of other causes of foot pain such as Morton's neuroma, tarsal tunnel syndrome, plantar fasciitis, lumbar radiculopathy, and arthritis. Subjects were also excluded if they had another condition with greater pain intensity, an unstable medical/psychological condition, or an open lesion/skin condition in the area of gel application. The complete list of inclusion and exclusion criteria is provided in Table 1. Physical examination, vital signs, application site assessment, complete medical history, clinical lab specimen examination, ECG, urine drug screen and pregnancy test were conducted at screening. Participants also completed questionnaires and underwent sensory testing. Participants discontinued use of “as needed” (PRN) pain medications other than acetaminophen (paracetamol). If they were on other daily pain medications, they were allowed to continue these as long as they agreed to continue stable daily dosing throughout the study.
Table 1.
Inclusion/Exclusion criteria
| Inclusion Criteria | |
|---|---|
| 1 | Between 18 and 80 years of age |
| 2 | Established diagnosis of diabetes (type I or II) with pain attributable to a symmetrical stocking distribution neuropathy in the lower extremities |
| 3 | Average daily pain score ≥4 on an 11-point 0-10 numerical pain rating scale (NPRS) in the area of PDN during the baseline phase |
| 4 | At least a six month history of PDN pain, but ≤five years (prior to screening) |
| 5 | Stable glycemic control regimen for at least 3 months |
| 6 | Stable analgesic regimen for at least 21 days prior to randomization |
| 7 | Willing to maintain current medications at their same dose throughout the study |
| Exclusion Criteria | |
|---|---|
| 1 | Other chronic pain with greater intensity than their PDN pain |
| 2 | Other chronic pain within the region of PDN |
| 3 | Any serious or unstable medical or psychological condition |
| 4 | Hypotension |
| 5 | History of illicit drug or alcohol abuse within a year |
| 6 | Cognitive or language difficulties that would impair understanding/completion of the assessment instruments |
| 7 | Pregnant or lactating females, planning to become pregnant, or using unreliable means of birth control |
| 8 | Received other experimental drugs within two months of randomization |
| 9 | Prior use of topical clonidine gel |
| 10 | Open lesions or skin conditions in the area of gel application |
| 11 | Known sensitivity or intolerance to clonidine |
Baseline assessment
Subjects recorded the average pain intensity in their feet over the past 24 hour period (average daily pain) using the numerical pain rating scale (NPRS). Eligible subjects (baseline pain rating ≥ 4 [average of 7 days prior to treatment]; diaries greater than 80% complete) underwent baseline measurements which included reassessment of screening procedures and questionnaires, and laboratory assessments (including sampling of plasma levels of clonidine). A subset of participants (97) underwent a 3 mm skin punch biopsy 6 cm above the ankle of either leg in order to quantify intraepidermal nerve fiber density (IENFD). Epidermal C-fibers co-localize with markers of nociceptors and are thought to relate to pain functioning in the skin [26]. This procedure was elective and sites as well as participants could opt out from participation based on their preference. Labeling was performed with the pan axonal marker PGP 9.5 and density assessment followed validated techniques [35]. (Therapath: www.therapath.com)
Questionnaires
The Brief Pain Inventory (BPI) [4] was used to assess pain and functioning. Subjects also underwent tests of sleep (Chronic Pain Sleep Inventory; CPSI) [13], and depression along with anxiety (Hospital Anxiety and Depression Scale; HADS) [40]. Clinician and Patient Global Impressions of Change (CGIC, PGIC, respectively) were assessed by having the investigator and patient independently rate the overall change in pain status at the final treatment visit [8].
Psychophysical assessment
During screening, 0.1% capsaicin was applied over a 1 cm diameter area on the pre-tibial area midway between the calf and ankle of both legs. The pretibial area was selected for placement of the capsaicin stimulus rather than the foot, because the pretibial area is typically also affected by the length dependent neuropathy, and it was believed that the pain from capsaicin stimulus might otherwise be confused with the ongoing pain in the foot. Notably the area selected was near the area chosen for skin biopsy used to anatomically quantitate the severity of the small fiber neuropathy (data presented in Results). An occlusive dressing (e.g., Tegaderm™) was applied over the capsaicin application site, and left in place for 30 minutes. Subjects then rated the capsaicin induced pain on a 0-10 scale (NPRS) for each leg independently. The responses from the right and left sides were highly correlated (r2 = 0.93, p = 0.001). Therefore the mean pain rating of the two sides was used in further analyses. Vibratory testing was performed on the dorsum of both large toes through use of a 128 Hz tuning fork [20]. Normal, reduced, or absent mechanical sensation was assessed through use of a 10g (5.07) von Frey monofilament (Center for Specialized Diabetes Foot Services; Mid-Delta Health Systems, Inc.) applied to the dorsum of the great toe [20]. An assessment of thermal discrimination was conducted to test the subject's ability to differentiate warmth from cold on the dorsum of each foot using a heated or cooled metal rod.
Randomization and blinding
Participants were randomized in blocks to receive either 0.1% topical clonidine gel or placebo gel in a 1:1 ratio, double-blinded fashion. Subjects were stratified with regard to baseline pain severity, such that similar numbers of patients with moderate and severe pain were included in the active and placebo groups. The placebo formulation was identical in appearance, consistency, packaging, and labeling. Placebo and active drug were supplied by Arcion Therapeutics.
Treatment period
Participants recorded their average pain over the last 24 hours in a diary each evening before going to bed throughout the study using the numerical pain rating scale (NPRS). Subjects returned to the clinic for visits at weeks 1, 2, 4, 6, 8, 10, and 12 for assessments. Pharmacokinetics and other laboratory tests, physical examination, application site assessments, vital signs, ECG, urine drug screen, urine pregnancy test, HADS, BPI, and CPSI were obtained at clinic visits. Subjects also returned to clinic approximately one week after discontinuation of study medication for a follow up safety evaluation.
Drug application
Study medication was self-administered to both feet in the morning, afternoon, and evening. A “dose” was defined as the amount of gel delivered with one complete pump (a metered dose) of the mechanical dispensing bottle per foot. Participants were instructed to apply the gel evenly to the toes, between the toes, and top and bottom of the feet extending up to the ankle. The clonidine bottles contained 0.1% clonidine and dispensed 0.65 g of gel (0.65 mg clonidine) per dose. The total daily clonidine dose was 3.9 mg (two feet, three times/day; 0.65 mg × 6). Prior unpublished studies suggested that .05% is not an effective dose of clonidine and 0.2% had no greater efficacy than 0.1% clonidine. Safety was assessed by tracking the frequency and severity of adverse events, and comparing pre- and post-treatment urinalysis, blood chemistry and hematology. Safety was additionally evaluated by assessing local dermal changes and changes in heart rate and blood pressure at study visits.
Statistical Analysis
Analyses of covariance (ANCOVA) were performed on NPRS change scores (from baseline to 12 week average daily diary ratings; landmark analysis - primary outcome) and (continuous) secondary outcome measures controlling for baseline scores and treatment-by-study center interaction. Secondary outcomes of interest included pain (BPI), sleep (CPSI), depression and anxiety (HADS), clinician and patient global impressions of change (CGIC and PGIC), as well as physiological factors. CGIC and PGIC categories were compared between active and placebo groups using a generalized linear model based on maximum likelihood with specification of the distribution as multinomial. Analyses were also conducted by capsaicin score (a pre-specified variable of interest), by separate analyses using the intra-patient capsaicin scores as a dichotomous, independent factor in the ANCOVA. Missing data for subjects who terminated early were imputed using the baseline scores (BOCF).
Results
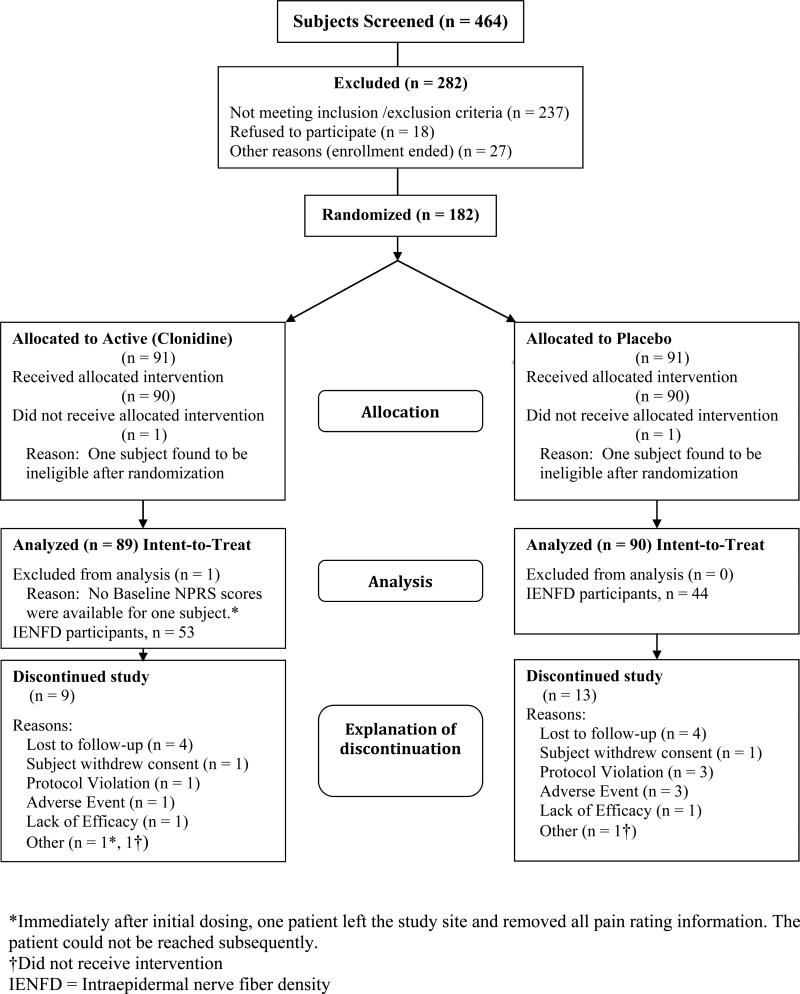
Of 464 screened subjects, 182 were randomized (Figure 2). Of the randomized subjects, one participant from each group was found to be ineligible after randomization (but before dosing) had occurred. In another subject, the baseline NPRS scores were lost after treatment on day 1 and the subject was subsequently lost to follow up. Of the 179 remaining subjects, 90 received placebo gel and 89 received active gel. Patient demographics, clinical characteristics at baseline, summary questionnaire data and other variables of interest are presented in Table 2.
Figure 2.
CONSORT (Consolidated Standards of Reporting Trials) diagram
Table 2.
Demographics, clinical characteristics, and outcomes at baseline, and 12 weeks minus baseline
| Variable | Placebo (n=90) | 0.1% Clonidine (n=89) | p-value |
|---|---|---|---|
| Baseline (BL) | Baseline (BL) | ||
| Age (in years), mean (SD) | 57.6 (9.5) | 59.4 (9.9) | 0.22 |
| Sex, n (%) | |||
| Female | 48 (53) | 45 (51) | 0.77 |
| Male | 42 (47) | 44 (49) | |
| Race, n (%) | |||
| Non-Hispanic white | 46 (51) | 56 (63) | 0.19 |
| Hispanic | 17 (19) | 7 (8) | |
| African descent | 24 (27) | 24 (27) | |
| Other | 3 (3) | 2 (2) | |
| Type of diabetes mellitus, n (%) | |||
| Type 1 | 4 (4) | 5 (6) | 0.56 |
| Type 1.5 | 0 (0) | 1 (1) | |
| Type 2 | 86 (96) | 83 (93) | |
| Duration of diabetes (in years), mean (SD) | 9.6 (7.8) | 10.7 (8.0) | 0.35 |
| Duration of foot pain (in years), mean (SD) | 2.9 (1.3) | 3.0 (1.3) | 0.55 |
| Nerve fiber density (in fibers/mm), mean (SD); (n) | 3.2 (3.5); (44) | 2.3 (2.6); (53) | 0.14 |
| Concomitant medications (%) | 48 | 39 | 0.25 |
| Capsaicin pain rating, mean (SD) (at Screening; 0-10 NPRS, average of right and left foot) | 1.8 (2.7) | 1.8 (2.4) | 0.97 |
| Baseline (BL) | Change (12 week minus BL) | Baseline (BL) | Change (12 week minus BL) | ||
|---|---|---|---|---|---|
| Average pain severity from diary, daily mean (SD, 0-10 NPRS) | 6.4 (1.4) | -1.7 (1.9) | 6.5 (1.5) | -2.3 (2.2) | 0.07§ |
| Experienced >30% reduction in pain, n (%) | 36 (40.0) | 43 (48.3) | 0.34 | ||
| Experienced >50% reduction in pain, n (%) | 26 (28.9) | 31 (34.8) | 0.49 | ||
| HbA1c, mean (SD) | 7.5 (1.6) | 0.1 (0.7) | 7.4 (1.6) | 0.03 (0.6) | 0.47§ |
| BPI - Severity Scale, sum (SD) | 25.3 (5.8) | -6.0 (8.2) | 25.4 (6.4) | -7.7 (8.9) | 0.23§ |
| BPI – Average pain, mean (SD) | 6.3 (1.4) | -1.6 (1.9) | 6.5 (1.5) | -2.2 (2.2) | 0.06§ |
| BPI - Functional Interference Scale, sum (SD) | 38.8 (15.1) | -11.8 (14.9) | 36.4 (16.9) | -13.5 (17.2) | 0.53§ |
| CPSI - Overall sleep quality, mean (SD); (increase = improvement) | 37.9 (23.4) | 15.9 (32.1) | 39.7 (24.7) | 18.1 (30.7) | 0.40§ |
| HADS – Anxiety Scale, sum (SD) | 7.0 (4.1) | -0.5 (2.5) | 6.2 (4.1) | -0.7 (3.0) | 0.30§ |
| HADS – Depression Scale, sum (SD) | 5.9 (4.1) | -0.4 (2.4) | 5.0 (3.7) | -0.8 (2.1) | 0.11§ |
Concomitant medications included anticonvulsants, antidepressants, and opioids; Brief Pain Inventory (BPI; Severity range 0-40, Average pain range 0 – 10, Functional Interference range 0-70); Chronic Pain Sleep Inventory (CPSI; range 0-100); Hospital Anxiety and Depression Scale (HADS Anxiety range 0-21, Depression range 0-21; <8 is normal, threshold for moderate is 11).
p-values represent analyses of differences from week 12 to baseline in the active compared to placebo group; no changes were observed at baseline between groups.
The two randomized groups were well balanced with regard to demographic characteristics (Table 2). The mean pain level at baseline was 6.5 (n=179). Nine subjects were diagnosed with type 1 diabetes; one had Type 1.5, and all remaining subjects had type 2 diabetes. The mean duration of diabetes was 10 years with a mean pain duration of three years. Use of concomitant neuropathic pain medications was overall 44% (39% in the active group and 48% in the placebo group).
Efficacy
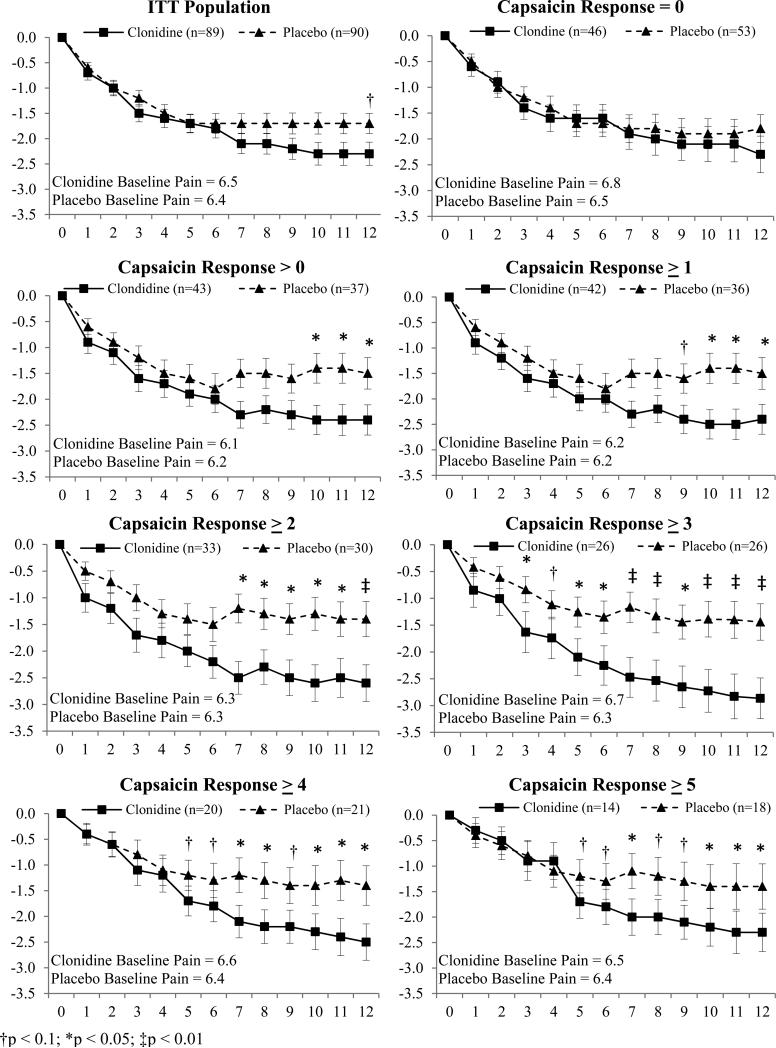
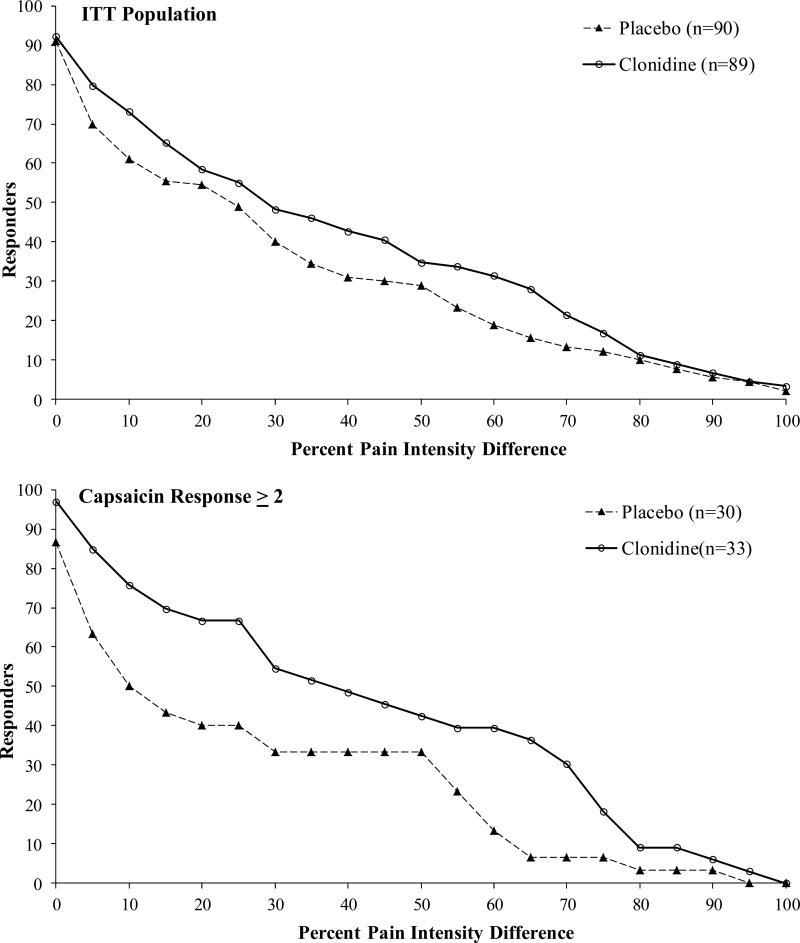
Pain in the intent-to-treat (ITT) population decreased with time as shown in Figure 3. At 12 weeks the decrease in pain in the clonidine group (-2.3; 0-10, NPRS) was greater than the drop in pain in the placebo group (-1.7 (Δ=0.6, p=0.07). The responder analysis shown in Figures 4A and 4B demonstrates the cumulative proportion of patients who had specified percent decreases in the NPRS at 12 weeks. For example, 43% of the clonidine subjects had a 30% drop in pain compared to 36% of the placebo subjects. Changes in the BPI, CPSI, and HADS did not reach statistical significance (Table 2).
Figure 3.
Change in pain by capsaicin response level [(Mean ± SEM) Y-axis = ΔNPRS; X-axis = Week]. Effects of Clonidine over Placebo varied with capsaicin response determined during screening. Weekly means of “average pain over the last 24 hours” rated on 0-10 numerical pain rating scale (NPRS).
Figure 4.
Cumulative proportion of responder's analysis (CPRA) graph displaying proportion of patients who had a given percentage decrease in pain compared to baseline at week 12 with Clonidine or Placebo. Effects of Clonidine over Placebo varied with capsaicin response determined during screening. Ratings of pain were obtained through 0-10 NPRS.
To assess nociceptor function, topical 0.1% capsaicin cream was applied under occlusion for 30 minutes in the pretibial area. The capsaicin stimulus evoked pain in 45% of subjects (48% active group; 41% placebo group). The change in pain from baseline in the active versus placebo groups varied with the capsaicin rating as shown in Figure 3. In the subjects that did not detect capsaicin (rating of 0; n = 99), clonidine had no statistically significant differential effect over placebo at any time point. However, in the subjects who detected any level of pain to capsaicin (capsaicin > 0), clonidine was significantly more effective than placebo (difference in mean = 0.9, 95% CI 0.01 to 1.76; p < 0.05; Figure 3). The magnitude of separation between the clonidine and placebo treated patients became more pronounced with increasing capsaicin ratings. In subjects with a capsaicin pain rating ≥ 2 (0-10, NPRS), the mean decrease in pain was 2.6 (0-10, NPRS) for active compared to 1.4 for the placebo (difference in mean = 1.2, 95% CI 0.21 to 2.22; p = 0.01). The responder analysis in Figure 4b is shown for subjects with capsaicin pain ratings ≥ 2.
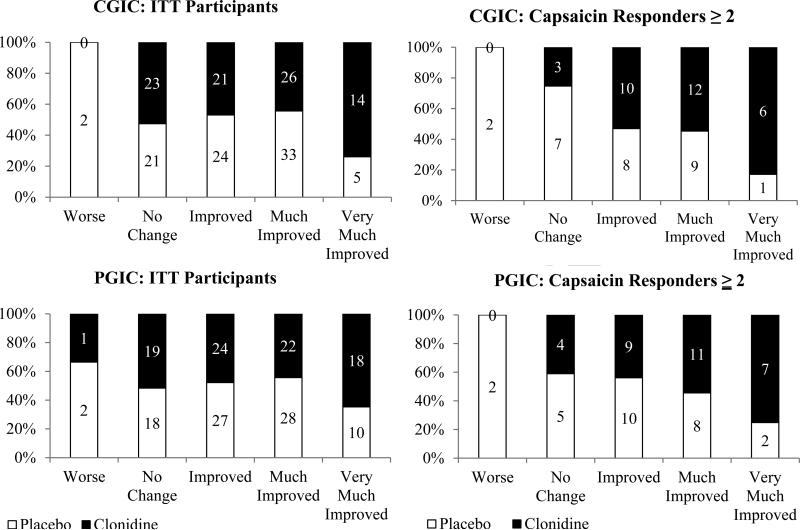
Male and female subjects did not differ with regard to the response to capsaicin, nor were there any identified ethnic or racial differences. Capsaicin responders also did not significantly differ from non-responders with respect to duration of diabetes, duration of neuropathic pain, baseline HbA1c, and baseline pain (NPRS – data not shown). The analysis of secondary endpoints demonstrated a similar pattern of efficacy in the capsaicin responders (Table 3). Among capsaicin responders, PGIC and CGIC were statistically significant in favor of clonidine (p = 0.034 and p = 0.018, respectively; Figure 5), as was change in sleep quality (p = 0.034). A similar analysis was performed based on the results of non-nociceptor sensory function (vibration, assessment of tactile sensibility, and thermal discrimination). No significant correlations emerged between any of these measures and clonidine efficacy or results of capsaicin testing.
Table 3.
Demographics and outcomes (change from baseline (12 weeks minus baseline)) for capsaicin responders (capsaicin pain rated ≥2; 0-10 NPRS)
| Variable | Placebo (n=30) | 0.1% Clonidine (n=33) | p-value | ||
|---|---|---|---|---|---|
| Baseline (BL) | Δ from BL to 12 week visit | Baseline (BL) | Δ from BL to 12 week visit | ||
| Duration of diabetes (in years), mean (SD), | 8.8 (5.9) | 12.2 (9.3) | 0.10 | ||
| Duration of foot pain (in years), mean (SD) | 2.7 (1.3) | 2.8 (1.2) | 0.57 | ||
| Nerve fiber density (in fiber/mm), mean (SD); (n) | 4.3 (4.2); (18) | 2.9 (3.0); (24) | 0.20 | ||
| Capsaicin pain rating, mean (SD) NPRS | 5.2 (2.0) | 4.5 (2.0) | 0.20 | ||
| Average pain severity from diary, mean (SD) NPRS | 6.3 (1.4) | -1.4 (1.8) | 6.3 (1.5) | -2.6 (2.0) | 0.01§ |
| HbA1c, mean (SD) | 7.3 (1.5) | 0.26 (0.6) | 7.4 (1.8) | 0.0 (0.6) | 0.17§ |
| BPI – Severity scale, sum (SD) | 25.4 (5.8) | -5.3 (7.8) | 25.1 (7.3) | -7.8 (7.2) | 0.18§ |
| BPI – Average pain, mean (SD) | 6.3 (1.5) | -1.3 (1.7) | 6.5 (1.6) | -2.2 (1.9) | 0.06§ |
| BPI-Functional Interference Scale, sum (SD) | 37.2 (17.1) | -8.7 (13.2) | 37.1 (17.5) | -13.0 (15.2) | 0.43§ |
| CPSI -Overall sleep quality, mean (SD); (increase = improvement) | 37.7 (26.9) | 4.4 (32.7) | 44.5 (21.5) | 13.4 (30.4) | 0.03§ |
| HADS – Anxiety Scale, sum (SD) | 7.8 (4.5) | -0.2 (2.2) | 6.4 (4.4) | -0.6 (2.7) | 0.30§ |
| HADS – Depression Scale, sum (SD) | 6.1 (4.5) | -0.8 (2.1) | 4.8 (3.5) | -0.4 (1.8) | 0.60§ |
Brief Pain Inventory (BPI; Severity range 0-40, Average pain range 0-10, Functional Interference range 0-70); Chronic Pain Sleep Inventory (CPSI, range 0-100); Hospital Anxiety and Depression Scale (HADS Anxiety range 0-21, Depression range 0-21; <8 is normal, threshold for moderate is 11).
p-values represent analyses of differences from week 12 to baseline in the active compared to placebo group; no changes were observed at baseline between groups.
Figure 5.
Clinician and patient global impression of change (CGIC; PGIC) at point of study termination (12 weeks) for capsaicin responders [(≥2); Y-axis = percent of patients]. CGIC and PGIC were assessed by having the investigator and patient independently rate overall global impression of change in the subject's pain status at the final treatment visit using a 7 point verbal rating scale. The investigator and subject were asked: “Relative to Baseline, please rate from among the following choices the subject's total improvement whether or not, in your judgment, it is due entirely to study drug treatment: ‘very much improved,’ ‘much improved,’ ‘minimally improved,’ ‘no change,’ ‘minimally worse,’ ‘much worse,’ or ‘very much worse’.” Percentages are displayed by treatment group on the y-axis, the n for each group is included within each bar.
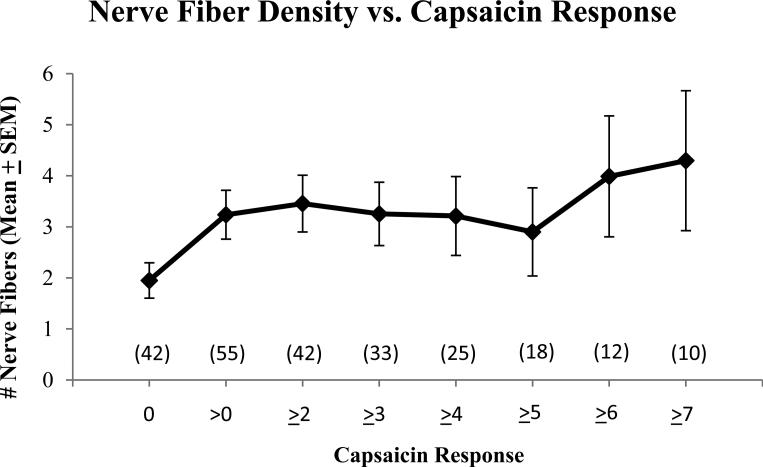
Ninety-seven of the 179 subjects underwent a 3 mm skin punch biopsy. Nerve fiber count varied with capsaicin response (Figure 6). The mean IENFD was 2.7 fibers/mm, SD=3.1; lower limit of normal is 5.4 fibers/mm[15]). Those who reported any level of pain from the capsaicin (responses > 0) had significantly greater intra-epidermal nerve fiber densities (p = 0.042) when compared with subjects rating “0” to capsaicin.
Figure 6.
Nerve fiber count by capsaicin response. Participating subjects (n=97) received a 3 mm punch skin biopsy on the lower extremity six cm above the ankle. Intra-epidermal nerve fiber density (IENFD) was determined using the pan-axonal marker PGP 9.5 by a central laboratory (Therapath: www.therapath.com). Sites were selected for participation based on willingness to do skin biopsy and a prespecified intent to do the biopsy in about one half of the subjects.
Pharmacokinetics
The typical plasma level of clonidine for treating hypertension is over 1000 pg/ml [29]. In the current examination, the clonidine levels at 2 weeks were similar to those at 12 weeks and generally below the level of detection (10 pg/ml). There were two outliers with levels > 200 pg/ml (796 pg/ml and 315 pg/ml). The reason for these outliers was not apparent and neither subject had side effects or blood pressure changes consistent with or attributable to excessive clonidine exposure.
Safety
As indicated in Table 4 no severe adverse events were attributed to treatment with clonidine. Skin site reactions were mild and only observed in the placebo group. No significant differences were observed in cardiovascular parameters (blood pressure, heart rate, ECG, serum chemistry, hematology, urine, PT, PTT or HbA1c, and physical examination.
Table 4.
Adverse events associated with treatment by group
| System/Organ Class | Placebo Gel (N=90) | Clonidine Gel (N=89) |
|---|---|---|
| Number of patients with ≥1 related adverse event, n (%) | 11 (12.2) | 3 (3.4) |
| General disorders and administration site conditions | 2 (2.2) | 1 (1.1) |
| Musculoskeletal and connective tissue disorders | 2 (2.2) | 0 (0) |
| Nervous system disorders | 2 (2.2) | 2 (2.2) |
| Psychiatric disorders | 1 (1.1) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | 0 (0) | 1 (1.1) |
| Skin and subcutaneous tissue disorders | 5 (5.6) | 0 (0) |
General disorders = Application site irritation/reaction; Musculoskeletal and connective tissue disorders = muscle tightness/pain in extremity; Nervous system disorders = Burning sensation/Dizziness/Headache; Psychiatric disorders = disorientation; Respiratory, thoracic and mediastinal disorders = dyspnoea; Skin and subcutaneous tissue disorders = dry skin/eczema/pruritus/rash.
Discussion
The results of this study suggest that treatment with topical clonidine reduces pain from diabetic neuropathy and indicates that efficacy depends on the relative level of functionality of nociceptors in the skin. The primary endpoint related to the effects of clonidine in the overall population. Though there was a trend favoring efficacy (p = 0.07), statistical significance was not achieved. The statistical analysis plan stipulated a further analysis relative to the innervation status of nociceptors in the skin as revealed by testing with topical capsaicin. Studies have shown that the loss of small fiber function and pain sensibility in the skin varies widely among diabetic patients [3;18;33]. The abnormal signals in the nociceptive pathways that lead to pain may in principal extend along the neuroaxis (from the skin to the brain). If the skin is severely denervated with regard to nociceptor function, then the target for clonidine, presumed to be the α2-adrenergic receptor on the epidermal nociceptors, would be lost and clonidine would not have efficacy [2;3;14]. To test for functioning of nociceptors in the skin, topical capsaicin cream 0.1% was applied to the skin above the ankle for 30 minutes at the screening visit. Analgesic efficacy of clonidine over placebo increased with the subjects’ response to the capsaicin stimulus. Thus, these data suggest that the analgesic effect of clonidine depends on the presence of functional capsaicin-responsive nociceptors in the skin, and raises the broader issue that neuropathic pain treatments may be guided by results of sensory testing.
The separation between clonidine and placebo was 1.2 on the NPRS scale in subjects with a capsaicin response of ≥ 2 (0-10 scale). In the overall population the separation was 0.6. This separation is within the range seen in other approved therapies. Pregabalin and duloxetine are widely used as oral therapies to treat PDN. Tolle et al [36] reported benefit of pregabalin of 1.1 over placebo (difference between change in pain in placebo versus active group) with 600 mg dosing at 12 weeks. With 300 mg dosing the difference from placebo was only 0.2. In a recent study in Japan [28] (n = 317) the difference over placebo at 300 mg/day was 0.6; at 600 mg/day the difference was 0.7. Duloxetine at the dose of 60mg/day the difference over placebo was about 1.3 at 12 weeks [38], but only 1.0 when the imputation for missing data was BOCF [9].
Other sensory and skin biopsy data
Other tests of sensory functions (mechanical, vibration, thermal) did not correlate with the responses to clonidine. These tests were done as simple screens and lacked quantitative rigor. More sophisticated tests of heat and mechanically induced pain have been done in specialized centers [14], but would be difficult to perform in the context of a large multi-center clinical trial. Nevertheless, future studies aimed at assessments of other nociceptor functions and their relation to efficacy of topical treatment will be of value. The capsaicin test reported here was technically simple to apply, took little time, and did not depend on the use of specialized equipment or personnel [34].
To test for anatomical evidence of cutaneous C-fiber pathology, 97 of the 179 subjects underwent a 3-mm skin biopsy. Intra-epidermal nerve fibers were labeled with the pan-axonal marker PGP 9.5, and density of fibers was quantified. The C-fiber density in the epidermis, presumed to correspond mainly to the innervation density of nociceptors [1;24;26], was significantly lower in subjects who lacked a response to capsaicin. This anatomical analysis simply assessed the density of epidermal fibers, not the expression of α2-adrenergic receptors or the presence of sensitization. The capsaicin response, in contrast, is in a sense a functional assay and could suggest sensitization in TRPV1-positive fibers as well as the anatomical presence of nociceptors.
Mechanism of action
The blood levels of clonidine in this study were typically below the level of detection (10 pg/ml). Thus the effects were almost certainly peripherally-mediated. Li et al [17] studied clonidine treatment in a rodent neuropathic pain model and determined that clonidine reversed allodynia and hyperalgesia when applied to the affected paw but not when it was applied to the contralateral side. Application of the clonidine patch relieved hyperalgesia in patients with complex regional pain syndrome but only in the area of application [5]. Evidence indicates that clonidine is also efficacious in the treatment of PDN when delivered through a transdermal patch applied remotely to the painful area (e.g., shoulder or anterior chest region) [39]. In this case the analgesic mechanism of clonidine may be central as well as peripheral given that intrathecally administered clonidine has been shown to have analgesic properties [7;21;30].
The discovery that the targeted alpha-2a receptor is expressed directly on nociceptors in the epidermis [27] further supports the hypothesis that clonidine effects are mediated by direct effects in the skin. Clonidine is an agonist for an inhibitory G-protein coupled receptor [22;31]. Activation of these receptors likely decreases levels of adenylate cyclase and cAMP. Increased levels of these second messengers have been identified as a source of increased excitability of nociceptors and as a mechanism of neuropathic pain. Adenylate cyclase upregulation may also lead to phosphorylation of the TRPV1 channel and therefore sensitize nociceptors to capsaicin stimulation. This observation provides further impetus to use capsaicin testing as a tool to identify candidates for clonidine treatment [12;19;37].
Conclusions
Topical clonidine gel significantly reduced the level of pain in subjects with diabetic neuropathy in whom there are functional (and possibly sensitized) nociceptors in the affected skin. This study provides support for the view that quantitative sensory testing may aid in identification of the appropriate treatment for a given patient. The treatment with clonidine was safe and without the problematic side effects typically associated with systemic therapies. Further research is warranted to corroborate the efficacy and safety of topical clonidine as a treatment of PDN and possibly other neuropathic pain states. Drugs with effects in preclinical trials targeting specific mechanisms have often failed in phase 2 and 3 efficacy trials. This could be due in part to the heterogeneity of mechanisms in the patients. In future drug trials it may prove useful to screen for nociceptor function in the skin as a way to optimize identification of effective topical therapies.
Summary.
Topical clonidine significantly reduces pain associated with diabetic neuropathy in subjects with functional nociceptors in the affected skin as revealed by testing with topical capsaicin.
Acknowledgments
This work was supported by Arcion Therapeutics and CMC was supported by K23 NS070933.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of interest
CMC was awarded a travel grant from Arcion to present and attend the Neuropathic Pain Conference in 2008. BS, MK, and WKS consult for Arcion. KB and JNC are employed by Arcion. The other authors have no conflicts of interest.
Reference List
- 1.Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–466. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JN. Nerve lesions and the generation of pain. Muscle Nerve. 2001;24:1261–1273. doi: 10.1002/mus.1143. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 5.Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 6.Dogrul A, Uzbay IT. Topical clonidine antinociception. Pain. 2004;111:385–391. doi: 10.1016/j.pain.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Eisenach JC, De KM, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The Clinical Importance of Changes in the 0 to 10 Numeric Rating Scale for Worst, Least, and Average Pain Intensity: Analyses of Data from Clinical Trials of Duloxetine in Pain Disorders. The Journal of Pain. 2010;11:109–118. doi: 10.1016/j.jpain.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.FDA Center for drug evaluation and research - Duloxetine. 2004 [Google Scholar]
- 10.Hassenbusch SJ, Gunes S, Wachsman S, Willis KD. Intrathecal clonidine in the treatment of intractable pain: a phase I/II study. Pain Med. 2002;3:85–91. doi: 10.1046/j.1526-4637.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 11.Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 13.Kosinski M, Janagap CC, Gajria K, Schein J. Psychometric Testing and Validation of the Chronic Pain Sleep Inventory. Clinical Therapeutics. 2007;29:2562–2577. doi: 10.1016/j.clinthera.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kramer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27:2386–2391. doi: 10.2337/diacare.27.10.2386. [DOI] [PubMed] [Google Scholar]
- 15.Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavand'homme PM, Ma W, De KM, Eisenach JC. Perineural alpha(2A)-adrenoceptor activation inhibits spinal cord neuroplasticity and tactile allodynia after nerve injury. Anesthesiology. 2002;97:972–980. doi: 10.1097/00000542-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Sekiyama H, Hayashida M, Takeda K, Sumida T, Sawamura S, Yamada Y, Arita H, Hanaoka K. Effects of topical application of clonidine cream on pain behaviors and spinal Fos protein expression in rat models of neuropathic pain, postoperative pain, and inflammatory pain. Anesthesiology. 2007;107:486–494. doi: 10.1097/01.anes.0000278874.78715.1d. [DOI] [PubMed] [Google Scholar]
- 18.Lin HJ, Chiu HC, Tchen PH, Fu CC. Cutaneous thermal thresholds in normal subjects and diabetic patients without symptoms of peripheral neuropathy. J Formos Med Assoc. 1990;89:857–862. [PubMed] [Google Scholar]
- 19.Malbon CC, Tao J, Wang HY. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem J. 2004;379:1–9. doi: 10.1042/BJ20031648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clinical Neurology and Neurosurgery. 2006;108:477–481. doi: 10.1016/j.clineuro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol. 1988;146:223–228. doi: 10.1016/0014-2999(88)90296-8. [DOI] [PubMed] [Google Scholar]
- 22.Ongioco RR, Richardson CD, Rudner XL, Stafford-Smith M, Schwinn DA. Alpha2-adrenergic receptors in human dorsal root ganglia: predominance of alpha2b and alpha2c subtype mRNAs. Anesthesiology. 2000;92:968–976. doi: 10.1097/00000542-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Petersen KL, Fields HL, Brennum J, Sandroni P, Rowbotham MC. Capsaicin evoked pain and allodynia in post-herpetic neuralgia. Pain. 2000;88:125–133. doi: 10.1016/S0304-3959(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 24.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 25.Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK, Garcia-Cebrian A, Monz B. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. doi: 10.1186/1471-2377-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-Da-Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T, Shoji S. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28:109–116. doi: 10.1111/j.1464-5491.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 29.Schaller MD, Nussberger J, Waeber B, Porchet M, Brunner HR. Transdermal clonidine therapy in hypertensive patients. Effects on office and ambulatory recorded blood pressure values. JAMA. 1985;253:233–235. [PubMed] [Google Scholar]
- 30.Schechtmann G, Wallin J, Meyerson BA, Linderoth B. Intrathecal clonidine potentiates suppression of tactile hypersensitivity by spinal cord stimulation in a model of neuropathy. Anesth Analg. 2004;99:135–139. doi: 10.1213/01.ANE.0000115150.83395.48. [DOI] [PubMed] [Google Scholar]
- 31.Shi TS, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution and regulation of alpha(2)-adrenoceptors in rat dorsal root ganglia. Pain. 2000;84:319–330. doi: 10.1016/s0304-3959(99)00224-9. [DOI] [PubMed] [Google Scholar]
- 32.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29:883–887. doi: 10.2337/diacare.29.04.06.dc05-2180. [DOI] [PubMed] [Google Scholar]
- 34.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 35.Tavee J, Zhou L. Small fiber neuropathy: A burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]
- 36.Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12:203–213. doi: 10.1016/j.ejpain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, Raskin J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 39.Zeigler D, Lynch SA, Muir J, Benjamin J, Max MB. Transdermal clonidine versus placebo in painful diabetic neuropathy. Pain. 1992;48:403–408. doi: 10.1016/0304-3959(92)90092-P. [DOI] [PubMed] [Google Scholar]
- 40.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]