Abstract
Diphtheria toxin (DT)-based anti-CD3 immunotoxins have clinical relevance in numerous applications including autoimmune disease therapies and organ transplantation tolerance protocols. Pre-existing anti-DT antibodies acquired either by vaccination against diphtheria toxin or infections with C. diphtheriae may interfere or inhibit the function of these anti-CD3 immunotoxins. Previously, a full-length anti-rhesus monkey CD3 immunotoxin, FN18-CRM9, was shown to be less effective at depleting circulating T cells in animals with pre-existing anti-DT antibody titers than in animals without antibodies, and subsequent doses were ineffective. In this study, the T cell depletion function of a truncated DT based recombinant anti-monkey CD3 immunotoxin, A-dmDT390-scfbDb (C207), as part of a reduced intensity conditioning regimen prior to hematopoietic cell transplantation, was compared between two groups of monkeys: those with and without pre-existing anti-diphtheria titers. T cell depletion was comparable in both groups of monkeys, and therefore appeared to be unaffected by the presence of moderate levels of pre-existing anti-diphtheria antibodies.
Keywords: immunotoxin, T cell depletion, antibody, diphtheria toxin
1. Introduction
Anti-CD3 immunotoxins (CD3-IT) are valuable in their ability to specifically deplete T-cells without disturbing other immune cell types. CD3-ITs are often constructed using diphtheria toxin (DT), a 535 amino acid exotoxin, capable of crossing eukaryotic cell membranes and initiating cell death via inhibition of protein synthesis. Full-length DT is made up of three functionally different domains: a catalytic domain responsible for the inhibition of protein synthesis, a transmembrane domain whose conformational change allows for entry into the endosomal membrane permitting the catalytic domain to enter the cytoplasm, and a receptor binding domain which binds the cell surface receptor [1,2]. Recently, construction of immunotoxins using a truncated diphtheria toxin lacking the receptor binding domain has been most optimal. The single-chain construct offers several advantages over its full-length counterpart including a lower molecular weight allowing for easier access into tissue, as well as reduced toxicity [3].
In preclinical and clinical studies, anti-CD3 immunotoxins have been used for the treatment of T-cell lymphomas in humans [4] and as an agent for T-cell depletion in experimental transplantation studies in animal models [5-7]. Pre-existing antibodies to DT as a result of vaccination or infections with C. Diphtheria or cross reactive antibodies, may potentially neutralize anti-CD3 immunotoxins presenting a major an obstacle in the use of these agents. The effect of pre-existing human anti-DT antibodies on the use of the full-length immunotoxin directed against human CD3ε, UCHT1-CRM9, and a truncated DT390 single-chain IT was previously examined[3]. Compared to the full-length immunotoxin which was completely neutralized, the truncated single-chain IT constructed with a C-terminal deletion of the receptor binding domain was only moderately inhibited. Patient serum was considered positive if a positive ELISA reaction (2-fold above background) was observed at a 1:100 dilution. T cell depletion using a full-length anti-rhesus monkey CD3 immunotoxin , FN18-CRM9 was also negatively affected in monkeys who possessed pre-existing anti-DT titers by ELISA at 1:100 serum dilution [8].
Recently, the truncated DT mutant DT390, devoid of the receptor binding domain, was used in the construction of the recombinant anti-monkey CD3 immunotoxin [9]. In this study, we assessed whether pre-existing anti-DT antibodies at the moderate levels found in naive rhesus macaques would impair T-cell depletion with this recombinant anti-monkey CD3 immunotoxin. Based on data with the human single chain IT, we hypothesized that the recombinant anti-monkey CD3 recombinant immunotoxin would only be moderately inhibited or avoid inhibition altogether by pre-existing antibody, and T cell depletion would be unaffected. Furthermore, we examined antibody responses in monkeys with and without pre-existing anti-DT antibody following anti-CD3 immunotoxin treatment.
2. Materials and Methods
2.1. Conditioning Regimen and HCT
Prior to hematopoietic cell transplantation on day 0, recipients were treated with 8 doses (25 ug/kg/dose) of an anti-monkey CD3 recombinant immunotoxin [10] administered twice daily over 4 days prior to transplantation (day -4 to -1), low dose (100 cGy) whole body irradiation (day -2), and a 45 day course of Cyclosporine A (day 0 to 45) (Pathiraja et al. manuscript in preparation).
2.2. Anti-DT ELISA
We measured pre-existing and induced anti-CD3 immunotoxin IgG by ELISA adapted from Woo et al [11]. ELISA Maxisorp plates (Nunc, Rochester, NY, USA) were coated with 100 μL/well of the anti-monkey CD3-immunotoxin at 5 ug/ml in PBS. Plates were washed with PBS (Cellgro, Manassas, VA) + 0.1% Tween 20 (polyoxyethylenesorbitan monolaurate Sigma- Aldrich, St. Louis, MO, USA) and blocked 3% Gelatin (Type B: From Bovine Skin, Sigma) in PBS. Serial dilutions (1:10; 1:100; 1:1000; 1:10,000) of rhesus macaque serum in triplicates (100 μl/well) were loaded in the blocked plates and incubated at 37 °C for 1 hr. Goat anti-DT (Serotec) was used to generate a standard curve for measuring levels of anti-DT in sera. The plates were probed with rabbit anti-human IgG HRP or rabbit anti-goat HRP for 1 hr at 37 °C, developed with ABTS (Southern Biotech, Birmingham, Al), and read at 405 nm. Results are presented as OD values and antibody titers. Serum samples measured following immunotoxin administration were measured at a single time point between 19-125 days following the final dose.
2.3. Immunofluorescence staining and flow cytometric analysis
Flow cytometry (FACS) analysis was performed using a Becton Dickinson FACScan (San Jose, California, USA). Heparinized peripheral blood was distributed into staining tubes (Falcon 2054) at 100 μL/tube and washed once using 2 mL flow cytometry buffer (HBSS containing Ca2+ and Mg2+/0.1% BSA/0.1% NaN3). Cells were then stained with 10 uL of direct fluorescein isothiocyanate (FITC)-labeled CD3 (SP34) or phycoerythrin-streptavidin (PE)-conjugated CD3 (SP34) mAb for 30 minutes at room temperature. RBCs were lysed and the cells were fixed using FACS lysing solution (Becton Dickinson) before acquisition. Data were analyzed using Winlist mode analysis software (Verity Software House, Topsham, Maine, USA).
3. Results
3.1. T cell depletion was comparable in monkeys with and without detectable levels of pre-existing anti-DT IgG titers
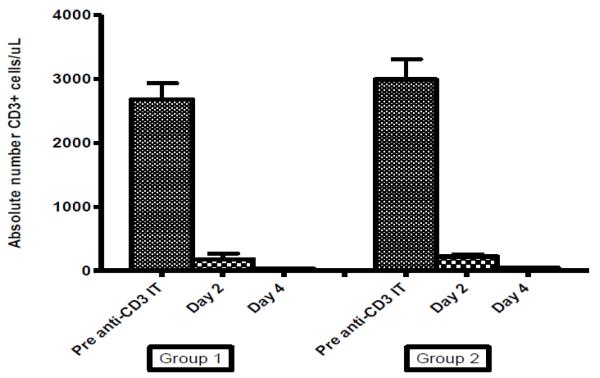
Of the eight monkeys analyzed in this study (Table 1), three (Group 1) had anti-DT serum antibody levels not significantly different than background levels at 1:100 dilution. Five monkeys (Group 2) had significant (2-fold above background) levels of anti-DT antibody prior to treatment with the recombinant anti-monkey CD3 immunotoxin administration without prior immunization record against DT. CD3+ T cell levels were measured by flow cytometry immediately prior to the first dose of the recombinant anti-monkey CD3 immunotoxin, and after four doses and eight doses (Figure 1). The average numbers of CD3+ T-cells prior to conditioning were similar between the two groups of monkeys (group 1- 2677 cells/uL vs. group 2- 2988 cells/uL). Following four doses of the recombinant anti-monkey CD3 immunotoxin, the CD3+ T cell levels declined in both groups to an average of 178 cells/uL in group 1 and 222 cells/uL in group 2. CD3+ T cell levels in both groups following eight doses of immunotoxin and 100 cGy TBI were below 100 cells/uL (28 and 44).
Table 1.
| Pre | Post | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monkey | OD | Titer | Concentration (ng/mL) | OD | Titer | Concentration (ng/mL) |
Pre Anti- CD3 IT (cells/uL) |
Day 2 (cells/uL) |
Day 4 (cells/uL) |
| Group 1 | |||||||||
| S0209 | 0.269+ 0.047 |
<1:10 | <100 | 1.758 + 0.220 |
1:1000 | 233,720 | 2,430 | 269 | 26 |
| S0409 | 0.193+ 0.019 |
<1:10 | <100 | 0.502 + 0.040 |
1:100 | 2,277 | 3,181 | 88 | 18 |
| S0609 | 0.262 + 0.079 |
1:10 | <100 | 0.414 + 0.066 |
1:100 | 1,467 | 2,371 | N/A | 41 |
| Group 2 | |||||||||
| S0610 | 0.348 + 0.224 |
1:100 | 1,842 | 0.363 + .001 |
1:100 | 1.178 | 2,146 | 187 | 39 |
| S1010 | 0.571 + 0.093 |
1:100 | 2,354 | 0.713 + 0.024 |
1:100 | 2,391 | 3,406 | 363 | 28 |
| S8710 | 1.646 + 0.116 |
1:1000 | 6,423 | 1.378 + 0.061 |
1:1000 | 6,588 | 2,455 | 180 | 70 |
| S9110 | 0.641 + 0.143 |
1:100 | 1,300 | 0,389 + 0.020 |
1:100 | 1,195 | 3,026 | 255 | 69 |
| S8610 | 0.710 + 0.003 |
1:100 | 697 | 0.645 + 0.005 |
1:100 | 2,106 | 3,908 | 125 | 16 |
Figure 1.
T cell depletion levels in monkeys without pre-existing antibody titers (Group 1; n=3) and monkeys with pre-existing antibody titers (Group 2; n=5). The absolute number of CD3+ cells/uL were measured in the peripheral blood of animals prior to CD3-IT administration, on Day 2 following 4 doses of anti-CD3 IT, and on Day 4 following 8 doses of anti-CD3 IT + 100 cGy TBI. T cell levels were comparable between the groups at all time points.
3.2. Anti-DT antibody develops following recombinant anti-monkey CD3 immunotoxin administration in monkeys without pre-existing anti-DT antibody
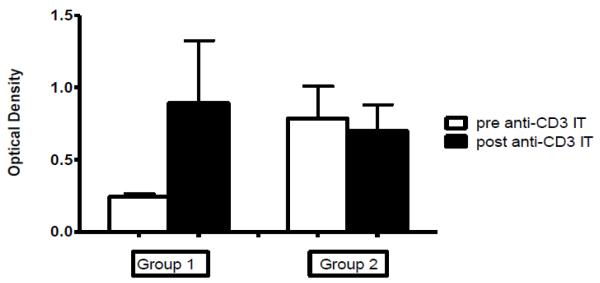
Prior to hematopoietic cell transplantation, the recombinant anti-monkey CD3 immunotoxin was administered as part of the conditioning regimen. Subsequently, serum was analyzed by ELISA for increases in anti-DT Ab titers (Figure 2). All three of the animals without significant levels of anti-DT antibody prior to immunotoxin administration displayed an increase in the OD values read at 1:100 serum dilution and in the highest serum dilution that showed a positive reaction. Serum from monkeys with pre-existing anti-DT antibody titers did not show an overall increase in OD values nor the highest serum dilution that showed a positive reaction.
Figure 2.
Average OD values of anti-CD3 IT IgG antibody in monkeys without pre-existing antibody titers (Group 1; n=3) and monkeys with pre-existing antibody titers (Group 2; n=5) prior to and following anti-CD3 IT administration. We analyzed serum from 8 rhesus macaques for anti-CD3 IT antibody titers prior to and between 19-125 days following anti CD3-IT exposure. Group 1 animals (n=3) without pre-existing titers demonstrated an increase in titers and antibody concentration following exposure. Group 2 animals (n=5) with pre-existing titers did not show an overall increase titers or concentration.
4. Discussion
Anti-CD3 immunotoxins have clinical relevance for numerous applications including autoimmune disease therapies, T-cell leukemias, and organ transplantation. We have developed a novel reduced intensity conditioning regimen which involves the use of the recombinant anti-monkey CD3 immunotoxin for T cell depletion for the induction of transplantation tolerance (Pathiraja et al manuscript in preparation). Whereas many humans develop anti-DT titers as a result of direct immunizations against DT, some rhesus macaques may acquire anti-DT titers as a result of infections with toxigenic strains of C. diphtheriae [3] or other cross-reactive antibodies. Pre-existing circulating anti-DT antibodies could potentially have a neutralizing effect on DT-based immunotoxins. In this brief report, we provide evidence for exceptional T cell depletion using a recombinant anti-monkey CD3 immunotoxin despite the presence of moderate levels of pre-existing anti-DT antibodies found in the naïve rhesus macaques available to study.
Previously, the use of a full-length DT based immunotoxin in rhesus macaques with pre-existing Ab was shown to be less effective in depleting T-cells in the blood and delaying T cell repopulation [8]. In a study by Neville et al., the full-length anti-rhesus monkey CD3 immunotoxin, FN18-CRM9, constructed by chemically conjugating a binding site mutant of diphtheria toxin, CRM9 and an anti-rhesus monkey CD3 mAb, FN18, depleted T cells to a “much lesser extent” in monkeys with detectable anti-CRM9 titers at a 1:100 dilution of sera [8]. Furthermore, subsequent doses were without effect and T-cell repopulation was observed earlier in those monkeys.
As part of this study, we assessed the effect of pre-existing anti-DT antibodies on the T cell depletion function of the recombinant anti-monkey CD3 immunotoxin, A-dmDT390 scfbDb (C207), which consists of “the catalytic and translocation domains of DT (DT390)” fused to the affinity matured “single-chain fold-back diabody of FN18” [9]. Studies in humans have shown that compared to a full-length anti-human CD3-immunotoxin, UCHT1-CRM9, which was completely neutralized by anti-DT antibodies in human sera detectable at 1:100 dilutions, a recombinant anti-human CD3 immunotoxin A-dmDT390biscFv (UCHT1) was only moderately inhibited under the same conditions [3]. In a recent clinical trial assessing the therapeutic effects of anti-CD3 recombinant diphtheria immunotoxin treatment on cutaneous T cell lymphoma in five patients, there was no association of pre-treatment antibody titer with Cmax, the peak immunotoxin serum level, after infusion [12]. All patients in the study had received immunizations against DT during childhood, resulting in a positive signal by enzyme immunoassay. Concentrations of circulating anti-DT antibodies prior to receiving any immunotoxin were calculated between 1 and 5 ug/mL, similar to those observed in this study. Concentrations of anti-DT antibodies increased following immuntoxin treatment in all patients.
Our data demonstrate that T cell depletion function of the recombinant anti-monkey CD3 immunotoxin is unaffected by the presence of moderate levels of pre-existing anti-DT antibodies in naïve rhesus macaques. Peripheral blood CD3+ levels in both groups of monkeys following 4 and 8 doses (100 ug/kg total) of anti-monkey CD3 immunotoxin were depleted equally. It is possible that the presence of higher levels of anti-DT antibody may interfere with depletion function; however, our study could not address this due to lack of availability of animals with higher titers. These data may help facilitate the use of the recombinant anti-monkey CD3 immunotoxin in rhesus macaque models of transplantation.
Highlights.
T cell depletion in rhesus macaques using recombinant anti-CD3 immunotoxin
Effect of pre-existing anti-DT antibodies on T cell depletion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Bennett MJ, Choe S, Eisenberg D. Refined structure of dimeric diphtheria toxin at 2.0 A resolution. Protein Sci. 1994;3:1444–1463. doi: 10.1002/pro.5560030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bell CE, Eisenberg D. Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide. Biochemistry. 1996;35:1137–1149. doi: 10.1021/bi9520848. [DOI] [PubMed] [Google Scholar]
- [3].Thompson J, Hu H, Scharff J, Neville DMJ. An anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin avoids inhibition by pre-existing antibodies in human blood. J Biol Chem. 1995;270:28037–28041. doi: 10.1074/jbc.270.47.28037. [DOI] [PubMed] [Google Scholar]
- [4].Woo JH, Lee YJ, Neville DM, Frankel AE. Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials. Methods Mol Biol. 2010;651:157–175. doi: 10.1007/978-1-60761-786-0_10. [DOI] [PubMed] [Google Scholar]
- [5].Cina RA, Wikiel KJ, Lee PW, Cameron AM, Hettiarachy S, Rowland H, Goodrich J, Colby C, Spitzer TR, Neville DM, Jr., Huang CA. Stable Multilineage Chimerism without Graft versus Host Disease Following Nonmyeloablative Haploidentical Hematopoietic Cell Transplantation. Transplantation. 2006;81:1677–1685. doi: 10.1097/01.tp.0000226061.59196.84. [DOI] [PubMed] [Google Scholar]
- [6].Knechtle SJ, Fechner JH, Jr., Dong YC, Stavrou S, Neville DM, Jr., Oberley T, Buckley P, Armstrong N, Rusterholz K, Hong XN, Tsuchida M, M M. Hamawy, Primate renal transplants using immunotoxin. Surgery. 1998;124:438–446. [PubMed] [Google Scholar]
- [7].Knechtle SJ, Vargo D, Fechner J, Zhai Y, Wang J, Hanaway MJ, Scharff J, Hu HZ, Knapp L, Watkins D, Neville DM., Jr. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation. 1997;63:1–6. doi: 10.1097/00007890-199701150-00002. [DOI] [PubMed] [Google Scholar]
- [8].Neville DMJ, Scharff J, Hu HZ, Rigaut K, Shiloach J, Slingerland W, Jonker M. A new reagent for the induction of T-cell depletion, anti-CD3-CRM9, J Immunother. Emphasis Tumor Immunol. 1996;19:85–92. doi: 10.1097/00002371-199603000-00001. [DOI] [PubMed] [Google Scholar]
- [9].Ma S, Hu H, Thompson J, Stavrou S, Scharff J, Neville DMJ. Genetic construction and characterization of an anti-monkey CD3 single-chain immunotoxin with a truncated diphtheria toxin. Bioconjug. Chem. 1997;8:695–701. doi: 10.1021/bc9701398. [DOI] [PubMed] [Google Scholar]
- [10].Kim GB, Wang Z, Liu YY, Stavrou S, Mathias A, Goodwin KJ, Thomas JM, Neville DM. A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel. 2007;20:425–432. doi: 10.1093/protein/gzm040. [DOI] [PubMed] [Google Scholar]
- [11].Woo JH, Bour SH, Dang T, Lee YJ, Park SK, Andreas E, Kang SH, Liu JS, Neville DM, Jr., Frankel AE. Preclinical studies in rats and squirrel monkeys for safety evaluation of the bivalent anti-human T cell immunotoxin, A-dmDT390-bisFv(UCHT1) Cancer Immunol Immunother. 2008;57:1225–1239. doi: 10.1007/s00262-008-0457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Frankel AE, Zuckero SL, Mankin AA, Grable M, Mitchell K, Lee YJ, Neville DM, Woo JH. Anti-CD3 recombinant diphtheria immunotoxin therapy of cutaneous T cell lymphoma. Curr Drug Targets. 2009;10:104–109. doi: 10.2174/138945009787354539. [DOI] [PubMed] [Google Scholar]