Abstract
Objective
Maternal anti-Ro autoantibodies associate with cardiac manifestations of neonatal lupus (cardiac NL), yet only 2% of women with this reactivity have an affected child. Identification of a more specific marker would channel intense monitoring to fetuses at greater risk. This study aims to determine whether autoantibodies against Ro52 amino acids 200–239 (p200) confer added risk over autoantibodies to full length Ro52, Ro60 or La.
Methods/Results
Anti-Ro-exposed pregnancies resulting in cardiac NL or no cardiac manifestations were identified from the Research Registry for Neonatal Lupus and PR Interval and Dexamethasone Evaluation. Umbilical cord (n=123) and maternal (n=115) samples were evaluated by ELISA. The frequencies of p200, Ro52, Ro60 and La autoantibodies were not significantly different between affected and unaffected children. However, neonatal anti-Ro52 and Ro60 titers were highest in cardiac NL and their unaffected siblings compared to unaffected neonates without a cardiac NL sibling. Although both maternal anti-Ro52 and p200 autoantibodies were less than 50% specific for cardiac NL, anti-p200 was the least likely of the Ro autoantibodies to be false positive in mothers who have never had an affected child. Titers of anti-Ro52 and p200 did not differ during a cardiac NL or unaffected pregnancy from the same mother.
Conclusion
Maternal reactivity to p200 does not confer an added risk to fetal conduction defects over full length Ro52 or Ro60 autoantibodies. Mothers who may never be at risk for having an affected child have lower anti-Ro60 titers and may require less stringent echocardiographic monitoring compared to women with high titer autoantibodies.
One of the strongest clinical associations with autoantibodies (Abs) directed to components of the SSA/Ro-SSB/La ribonucleoprotein complex is the development of neonatal lupus (NL) in an offspring. Cardiac and cutaneous diseases are the seminal manifestations of NL. In contrast to the reversible short lived cutaneous disease, the cardiac disease of NL (cardiac NL) is fatal in nearly one fifth of cases and most surviving children require a pacemaker for life (1). Cardiac NL develops during 18 to 24 weeks of gestation and is typically characterized by fibrosis of the atrioventricular node which can extend to the working myocardium and endocardium (2). The rapidity of clinically detectable injury is supported by the reports of normal sinus rhythm progressing to irreversible third degree block within 1–2 weeks (3, 4). At present serial echocardiographic evaluation of all fetuses exposed to anti-SSA/Ro Abs is recommended to detect potentially reversible incomplete blocks (5). Identification of a specific biomarker of cardiac NL would channel intense monitoring to those fetuses at greatest risk of disease.
Autoantibodies to the 52kD-SSA/Ro (Ro52), 60kD-SSA/Ro (Ro60), and 48kD-La proteins were first associated with cardiac NL over two decades ago (6). Two non-mutually exclusive hypotheses have been proposed to explain the pathogenic mechanism by which these Abs to normally sequestered intracellular antigens initiate injury in the fetal heart. The first posits that the intracellular Ro/La antigens translocate to the surface of cardiomyocytes undergoing apoptosis during physiological remodeling and are bound by Abs. The formation of pathogenic Ab-apoptotic cell immune complexes promotes pro-inflammatory and pro-fibrotic responses (7–9). The second hypothesis is based on molecular mimicry wherein Abs cross-react with L-type calcium channels and cause dysregulation of calcium homeostasis (10–12). While several studies have attempted to identify specific epitopes within the Ro and La antigens that associate with cardiac NL, most of these studies report epitopes common to the anti-Ro/La response regardless of fetal outcome. Moreover, different Ab subsets are identified depending on the immunoassay employed. For example, the sensitivity of peptide or recombinant protein ELISAs for anti-Ro60 Abs is low and may result in false negatives (13–16).
There has been recent excitement in the Ab response against the p200 epitope, spanning Ro52 amino acids (aa) 200–239, as a candidate biomarker conferring an increased maternal risk for the development of cardiac NL in an offspring (17, 18). While several groups have confirmed the high prevalence of the p200 response in women giving birth to a child with cardiac NL, there have been inconsistencies regarding its utility in high risk assessment relative to the pregnancy exposure (19). Consensus has not been reached as to whether this Ab response is also similarly observed in anti-Ro exposed healthy children when all other maternal Ab reactivities to components of the Ro/La complex are equivalent. Moreover, it has not been determined whether Abs to the p200 region of Ro52 confer any added risk over that observed to full length Ro52. A limitation of most previous studies is that prevalence and titer of maternal Abs have not been measured during the time of fetal exposure. To address the clinical utility of the p200 response as a diagnostic indicator of cardiac NL, this study evaluated umbilical cord blood and maternal serum during affected and unaffected pregnancies for reactivity to p200, full length Ro52, Ro60 and La.
MATERIALS AND METHODS
Patients
The eligibility criteria for this study was enrollment in the Research Registry for Neonatal Lupus (20), which contains neonates with documented cardiac or cutaneous NL and their unaffected siblings, or enrollment in the PR Interval and Dexamethasone Evaluation (3) which required mothers to be pregnant and positive for anti-Ro Abs as determined by the Clinical Laboratory Improvement Amendments (CLIA) approved commercial immunology laboratory of New York University/Langone Medical Center. Umbilical cord plasma and the matched maternal serum (when available) were obtained, with informed consent. Three groups of pregnancies were defined in this study: Group 1 comprised pregnancies in which the offspring had documented cardiac NL (samples were available from 48 mothers (50 pregnancies), and 59 neonates); Group 2 comprised unaffected pregnancies subsequent to a previous affected pregnancy (30 mothers (32 pregnancies) and 35 neonates); and Group 3 comprised unaffected pregnancies where all previous pregnancies (at least one) were unaffected (31 mothers (33 pregnancies) and 29 neonates). Maternal samples were taken between gestational week 20 and birth. Maternal diagnoses pertained to the last follow-up visit. Serum was also obtained from 28 (anti-Ro/La-negative) healthy controls. Studies were approved by the Institutional Review Board.
Recombinant full length Ro52 and p200 synthetic peptide
Recombinant human Ro52 was generated using the expression plasmid pET28 system in Escherichia coli BL21 (DE3; Novagen, Madison, WI) and affinity-purified by nickel column chromatography (21). A synthetic peptide representing aa 200–239 of Ro52 was purchased with biotin conjugated at the NH2-terminus (Mimotopes, Victoria, Australia).
Ro52 and p200 ELISA
Full length Ro52 (3 μg/ml) in phosphate buffered saline (PBS) was coated onto 96-well microtiter plates overnight, 4°C. After washing (PBS/0.1% Tween20) non-specific sites were blocked with 0.1% gelatin/PBS. Human sera was applied (1/500 in blocking buffer), 2 hours at room temperature. Alkaline-phosphatase conjugated rabbit anti-human IgG (γ-chain specific) (Sigma, St Louis, MO) was used (1:3000) with phosphatase substrate tablets and optical density (OD) at 405nm was determined after 15 minutes. The p200 ELISA was done as described (18) with minor modifications. Biotinylated-p200 peptide (3 μg/ml in 0.03M Na2CO3, 0.07M NaHCO3, 0.1%NaN3) was applied to pre-blocked streptavidin microplates (R&D Systems, MN) overnight. Plates were washed (0.15M NaCl, 0.006M NaH2PO4.H2O, 20%NaN3/0.05% Tween20/2% BSA), human sera applied (1/500) and binding of human IgG detected as described above. Reactivity was expressed as binding units based on a ratio with one high-titer patient selected as a standard. Binding units = [(OD of sample)/OD standard)] x100. If the OD of the sample was greater than the standard, the sample would be further diluted and binding units multiplied by the dilution factor. Sera were considered positive if the binding units were 3 SD above the mean binding units of 28 healthy controls.
Ro60 and La ELISA
Due to the low sensitivity of recombinant Ro60 ELISA for detecting anti-Ro60 (15, 16), a commercial ELISA using Ro60 purified from bovine spleen and thymus was employed. Anti-La was also detected by a commercial ELISA using La purified from calf and rabbit thymus according to manufacturer’s recommendations (Diamedix Corporation, Miami, FL). Ro60 and La ELISAs were done by the NYU/Langone Medical Center CLIA approved immunology laboratory.
Statistical Analysis
Calculations were performed using GraphPad Prism software version 5.04 (GraphPad Software, San Diego, CA). The prevalence of Abs was compared by Fisher’s Exact test. Anti-Ro52, p200, Ro60, and La Ab titers were compared in samples that were positive for the respective Ab by Mann Whitney or paired T-test where appropriate. Linear regression was used to evaluate correlations. P values less than 0.05 were considered significant.
RESULTS
p200 is an immunodominant epitope in the Ro52 Ab response regardless of fetal clinical outcome
Umbilical cord blood from 123 Ab-exposed infants was studied, 59 with cardiac NL (Group 1), 35 without cardiac NL but who had a sibling with cardiac NL (Group 2), and 29 without cardiac NL and had only healthy siblings (Group 3). The sex and race were not significantly different among the neonates studied. As expected, children with cardiac NL were of lower birth weight than their unaffected siblings (p=0.03) and frequently born prematurely compared to unaffected siblings (p=0.004) and unaffected non-siblings (p=0.005). All neonates were positive for anti-Ro60 and 120 (98%) were positive for anti-Ro52. Autoantibodies against p200 were found in 87% of the neonates with anti-Ro52 reactivity. Anti p200 Abs were less frequent in unaffected non-siblings (Group 3) compared to affected neonates and siblings (Groups 1 and 2) however this trend did not reach significance (Table 1).
Table 1.
Clinical and demographic characteristics of neonates.
| Group 1 (N=59) | Group 2 (N=35) | Group 3 (N=29) | P-value 1 vs. 2 | P-value 1 vs. 3 | |
|---|---|---|---|---|---|
| Sex of Child | |||||
| Male | 24 (40%) | 22 (63%) | 7 (24%) | 0.06 | 1.0 |
| Female | 34 (58%) | 13 (37%) | 10 (35%) | ||
| NA | 1 (2%) | 0 (0%) | 12 (41%) | ||
| Race/ethnicity | |||||
| White | 47 (80%) | 28 (80%) | 14 (48%) | 1.0 | 1.0 |
| Black | 3 (5%) | 2 (6%) | 0 (0%) | ||
| Hispanic | 2 (3%) | 0 (0%) | 4 (14%) | ||
| Asian | 5 (9%) | 3 (8%) | 0 (0%) | ||
| Other/NA | 2 (3%) | 2 (6%) | 11 (38%) | ||
| Antibody Status | |||||
| Anti-Ro52 | 58 (98%) | 35 (100%) | 27 (93%) | 1.0 | 0.3 |
| Anti-p200 | 50 (85%) | 33 (94%) | 21 (72%) | 0.2 | 0.2 |
| Anti-Ro60 | 59 (100%) | 35 (100%) | 29 (100%) | 1.0 | 1.0 |
| Anti-La | 30 (51%) | 23 (66%) | 17 (57%) | 0.2 | 0.5 |
| Gestational Age (wks) | 36.9 (±0.3)* | 37.9 (±0.3)† | 38.3 (±0.4)‡ | 0.004 | 0.005 |
| Birth weight (g) | 2746 (±104)§ | 3123 (±147)|| | 3078 (±181)¶ | 0.03 | 0.1 |
Group 1: cardiac NL, 3rd degree block (n=47), 2nd degree (n=2),1st degree (n=3), cardiomyopathy (n=5), sinus bradycardia (n=3)
Group 2: unaffectedwith at least one sibling with cardiac NL, cutaneous NL (n=4)
Group 3: unaffectedwith unaffected siblings, cutaneous NL (n=3), hepatic/hematological complications (n=1).
n=55,
n=35,
n=15,
n=34,
n=18,
n=9
NA=not available
Maternal serum from 115 pregnancies was assessed, 50 cardiac NL pregnancies (Group 1), 32 unaffected pregnancies subsequent to a cardiac NL pregnancy (Group 2), and 33 unaffected pregnancies where all previous pregnancies were unaffected (Group 3). The clinical diagnosis and race of the mothers were not significantly different across the groups. All mothers were positive for anti-Ro60 and 111 (97%) were positive for anti-Ro52 Abs. Reactivity against the p200 epitope was more frequent in Group 1 (88%) and Group 2 (97%) compared to Group 3 (67%). The presence of anti-p200 had 88% sensitivity and 33% specificity for cardiac NL (p=0.026, Group 1 vs. 3) while full length Ro52 Ab testing showed 100% sensitivity and 12% specificity for cardiac NL (p=0.022, Group 1 vs. 3). There was no significant difference between Groups 1 and 2 for p200 or Ro52 reactivity. Anti-La was equivalent across all groups (Table 2).
Table 2.
Clinical and demographic characteristics of pregnantmothers.
| Group 1 (N=50) | Group 2 (N=32) | Group 3 (N=33) | P-value 1 vs. 2 | P-value 1 vs. 3 | |
|---|---|---|---|---|---|
| Clinical Diagnosis | 0.5 | 0.2 | |||
| Asymptomatic | 15 (30%) | 7 (22%) | 5 (15%) | ||
| UAS | 11 (22%) | 8 (25%) | 6 (18%) | ||
| SS | 13 (26%) | 12 (38%) | 8 (24%) | ||
| SLE/SS | 5 (10%) | 3 (9%) | 5 (15%) | ||
| SLE | 4 (8%) | 2 (6%) | 7 (22%) | ||
| Other/NA | 2 (4%) | 0 (0%) | 2 (6%) | ||
| Race/ethnicity | 1.0 | 0.5 | |||
| White | 41 (82%) | 25 (79%) | 11 (33%) | ||
| Black | 2 (4%) | 2 (6%) | 0 (0%) | ||
| Hispanic | 2 (4%) | 0 (0%) | 3 (9%) | ||
| Asian | 4 (8%) | 3 (9%) | 1 (3%) | ||
| Other/NA | 1 (2%) | 2 (6%) | 18 (55%) | ||
| Antibody Status | |||||
| Anti-Ro52 | 50 (100%) | 32 (100%) | 29 (88%) | 1.0 | 0.022 |
| Anti-p200 | 44 (88%) | 31 (97%) | 22 (67%) | 0.2 | 0.026 |
| Anti-Ro60 | 50 (100%) | 32 (100%) | 33 (100%) | 1.0 | 1.0 |
| Anti-La | 26 (52%) | 19 (59%) | 17 (52%) | 0.6 | 1.0 |
Group 1: cardiac NL pregnancy, 3rd degree block (n=42), 2nd degree (n=1), 1st degree (n=3), cardiomyopathy (n=5), sinus bradycardia (n=2). Twins discordant for cardiac NL (n=4).
Group 2: unaffectedpregnancy, mother had aprevious child with cardiac NL. Healthy (n=29), cutaneous NL (n=4).
Group 3: unaffectedpregnancy, mother has only unaffected children, cutaneous NL (n=3), hepatic/hematological complications (n=1).
UAS= undifferentiated autoimmune syndrome, SS= Sjögren’s syndrome, SLE= systemic lupus erythematosus, NA= not available
High titer anti-Ro52 and Ro60 Abs are associated with cardiac NL
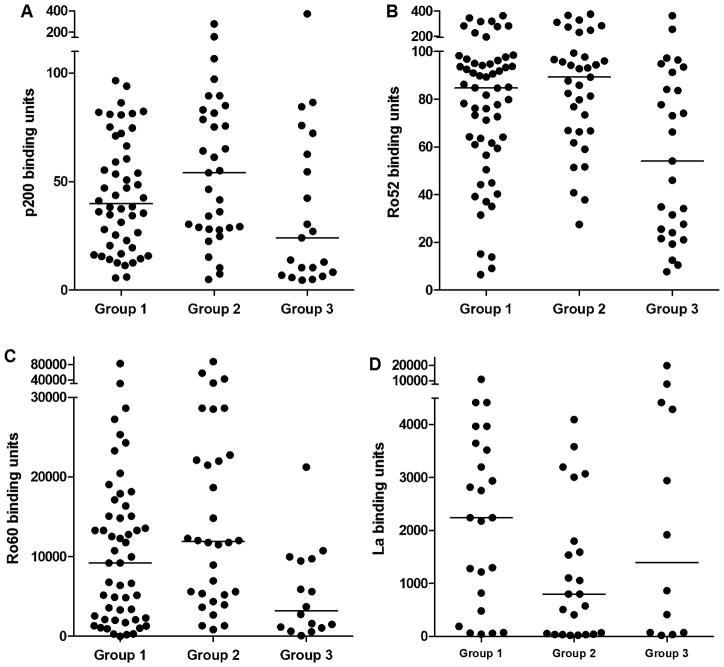
The titer of anti-p200, anti-Ro52, anti-Ro60 and anti-La was next evaluated in umbilical cord samples that were positive for the respective Abs. Titers of anti-p200 were higher in neonates with cardiac NL (Group 1) (40±7) and unaffected siblings (Group 2) (54±9) compared to unaffected non-siblings (Group 3) (24±17) however, this trend did not reach significance. Anti-Ro52 titers were also higher in Group 1 (85±11) and Group 2 (89±17) compared to Group 3 (54±15), p=0.02 (Group 1 vs. Group 3) and 0.002 (Group 2 vs. Group 3). Higher titer was not restricted to Ro52 Abs as anti-Ro60 titer was also increased in Group 1 (9,216±1,796) and Group 2 (11,904±3,254) compared to Group 3 (3,232±1,425), p=0.03 (Group 1 vs. Group 3) and p=0.002 (Group 2 vs. Group 3) (Figure 1). In contrast, titers of anti-La were not significantly different (2,240±482 for Group I, 1,357 ±312 for Group 2, and 1,392±1,648 for Group 3). Linear regression showed that anti-p200, Ro52, Ro60, and La Ab titer was independent of gestational age (for anti-p200 p=0.09, R2=0.07; for anti-Ro52 p=0.57, R2=0.008; for anti-Ro60 p=0.10, R2=0.07; for anti-La p=0.70, R2=0.004). Titers of anti-Ro52 positively correlated with p200 Abs (p<0.01, R2=0.2) in all neonates.
Figure 1. Umbilical cord blood titer of anti-p200, anti-Ro52, anti-Ro60 and anti-La.
Reactivity of umbilical cord plasma from neonates with cardiac NL (Group 1), unaffected siblings of neonates with cardiac NL (Group 2), or unaffected neonates with only unaffected siblings (Group 3) against p200 (Ro52 aa 200–239) peptide (A), full length recombinant Ro52 (B), native Ro60 (C), or native La (D). The mean ELISA binding units of duplicate determinations is plotted for each neonate that was considered positive (>3 SD above the median binding units for 28 healthy controls). Cut-offs for positivity are >6.4 for Ro52, >4.5 for p200 and >19 for Ro60 and La. Solid lines represent the median ELISA binding units for each group.
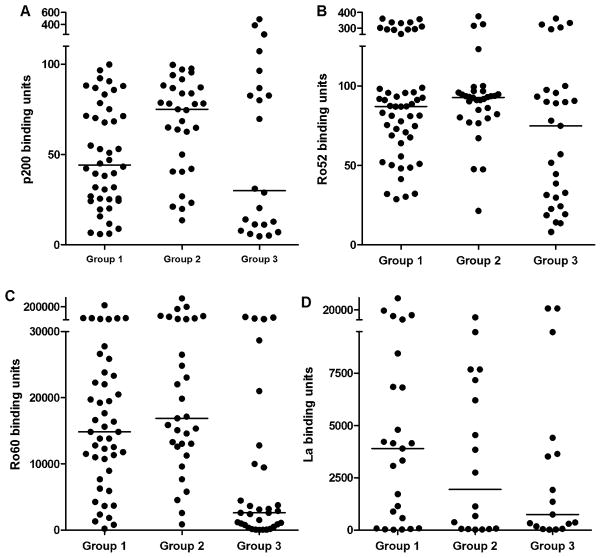
In serum from pregnant mothers, titers of anti-p200 were higher in mothers who were pregnant with a cardiac NL child, Group 1 (44±4) or an unaffected sibling of a cardiac NL child, Group 2 (75±5) compared to pregnant mothers who only had unaffected children, Group 3 (30±27), however, this trend did not reach significance. The maternal titers of anti-Ro52 paralleled p200 Ab titers (87±15 for Group 1, 93±14 for Group 2, and 75±20 for Group 3, p=NS for all comparisons). Levels of anti-Ro60 were significantly higher in Group 1 (14,336±4,896) and Group 2 (16,896 ±12,125) compared to Group 3 (2,624±3,245), p=0.0002 (Group 1 vs. Group 3) and p<0.0001 (Group 2 vs. Group 3). Titers of La Abs were not significantly different for Group 1 (3,904±1472), Group 2 (1,944±963), or Group 3 (752±1677).
There was a positive correlation between maternal and neonatal Ab titer for p200 (p<0.0001, R2=0.7), anti-Ro52 (p=0.0008, R2=0.4), and anti-Ro60 (p<0.0001, R2=0.8). The ratio of maternal to fetal Ab titers were as follows: anti-p200 Group 1: 1.7±0.4, Group 2: 1.4±0.1, Group 3: 1.4±0.3; anti-Ro52 Group 1: 1.5±0.2, Group 2: 1.4±0.2, Group 3: 1.5±0.3; anti-Ro60 Group 1: 3.1±1.2, Group 2: 2.0±0.1, Group 3: 1.9±0.4; and anti-La Group 1: 2.6±0.4, Group 2: 2.0±0.2, Group 3: 1.6±0.2.
Anti-p200 and Ro52 Ab titer is equivalent in cardiac NL and unaffected pregnancies from the same mother and in twins discordant for cardiac NL
Serum samples from 11 mothers obtained during two pregnancies, cardiac NL and unaffected, were available for study. For the groups as a whole, there were no significant differences in anti-p200 titers during a pregnancy complicated by cardiac NL (61±31) or resulting in a healthy child (66±29), p=0.2. Titers of Ro52 Ab were also equivalent (cardiac NL vs. healthy, 73±23 vs. 80±27, p=0.2) (Table 3a).
Table 3a.
Anti-Ro52 and p200 antibody titers in serum from mothers during pregnancy.
| Mother | Pregnancy 1–Cardiac NL | Pregnancy 2–Unaffected | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Ro52 | p200 | DOB | sample date | Ro52 | p200 | DOB | sample date | |
|
| ||||||||
| 1 | 88.6 | 92.2 | 09/02 | 03/02 | 90.3 | 83.9 | 05/03 | 05/03 |
| 2 | 83.0 | 53.0 | 11/03 | 11/03 | 86.1 | 64.9 | 03/09 | 12/08 |
| 3 | 87.1 | 53.1 | 08/04 | 08/04 | 67.1 | 62.6 | 09/05 | 05/05 |
| 4 | 28.7 | 3.9 | 05/92 | 05/92 | 47.5 | 13.6 | 01/94 | 01/94 |
| 5 | 81.2 | 83.2 | 05/05 | 05/05 | 76.9 | 68.6 | 01/07 | 01/07 |
| 6 | 72.9 | 96.7 | 08/05 | 06/05 | 80.1 | 99.6 | 09/08 | 09/08 |
| 7 | 91.1 | 86.9 | 11/06 | 11/06 | 123.1 | 95.6 | 04/09 | 04/09 |
| 8 | 70.8 | 47.0 | 04/07 | 04/07 | 93.1 | 40.5 | 11/09 | 11/09 |
| 9 | 74.7 | 30.7 | 05/07 | 05/07 | 92.6 | 78.2 | 06/10 | 06/10 |
| 10 | 32.1 | 31.8 | 09/03 | 06/03 | 21.3 | 23.3 | 11/07 | 11/07 |
| 11 | 95.7 | 88.0 | 05/04 | 05/04 | 100.0 | 92.9 | 05/07 | 05/07 |
DOB= date of birth
Umbilical cord blood was available from 9 sibling pairs, including 4 twin sets, of whom one twin had cardiac NL and the other was unaffected. Titers of anti-p200 were equivalent among cardiac NL (47±28) and unaffected siblings (50±27), p=0.4. Anti-Ro52 titers were also equivalent in cardiac NL (84±49) and unaffected siblings (97±73), p=0.2 (Table 3b).
DISCUSSION
The search for specific biomarkers that predict mothers at high risk of delivering a child with cardiac NL is challenging given the rarity of disease. Definitive conclusions regarding whether the anti-p200 response significantly increases the risk of cardiac NL over that associated with Abs to full length Ro52 or other components of the Ro/La complex are limited by several factors. None of the previous studies a) restricted the evaluation of maternal sera to that obtained during pregnancy, b) assessed umbilical cord blood from both affected and unaffected siblings, or c) simultaneously evaluated maternal and neonatal sera for both anti-p200 and full length Ro52 responses. Accordingly, each of these limitations was addressed in the current study. Anti-Ro52 and Ro60 Abs were prevalent in the mothers during their pregnancies complicated by cardiac NL and their subsequent pregnancies of healthy children, findings which were paralleled by equivalent frequencies in the neonates. The presence of both anti-Ro52 and p200 Abs was more common in mothers pregnant with a child with cardiac NL or who had previously had a child with cardiac NL compared to mothers who never had a child with cardiac NL. Although both anti-Ro52 and p200 Abs were of low specificity for cardiac NL, anti-p200 was the least likely of the Ro autoantibodies to be false positive in mothers who have never had an affected child. However, because the sensitivity of anti-p200 was lower than Ro52 Abs, testing solely for reactivity to p200 is insufficient for pregnant mothers of unknown Ab status. Based on a 2% prevalence of cardiac NL in anti-Ro60 positive mothers (22), the presence of anti-Ro52 and anti-p200 minimally increases the risk of cardiac NL to 2.2% and 2.6% respectively in 1000 pregnancies. Overall, the presence of p200 Abs was common in each group suggesting that p200 is a dominant epitope in the anti-Ro52 response regardless of fetal outcome.
One of the difficulties in comparing the frequency and titer of Ab specificities between mothers of affected and unaffected children is that the recurrence rate of cardiac NL, while ten-fold higher than the overall risk of disease is not 100%. Absent a change in Ab profile from one pregnancy to another as demonstrated for the majority of families studied herein, other factors must contribute to defining risk for a fetus. However, at least from the perspective of defining a high risk Ab profile potentially independent of the fetal component, comparison to mothers who have never had a child with cardiac NL and have had at least two healthy pregnancies is likely to be informative. While it is acknowledged that cardiac NL can occur after even 5 healthy children (23), the Group 3 neonates and their mothers did reveal lower titers of Abs against Ro52, and Ro60 compared to the cardiac NL neonates and their unaffected siblings. Most studies attempting to identify high risk reactivities have compared affected pregnancies to those in which the fetus remains healthy and the mother has never had a child with cardiac NL. The findings reported herein are consistent with the literature. Specifically, in a smaller initial study by Salomonsson and colleagues, an increased titer of anti-p200, anti-Ro60 and anti-La was reported in cases of heart block compared to mothers who gave birth to unaffected children (17). More recently Jaeggi and colleagues reported that cardiac complications in fetuses were associated with higher titers of maternal anti-Ro Abs (did not distinguish between anti-Ro52 or anti-Ro60) compared to mothers carrying an unaffected child, with the exception that mothers of unaffected children who had previously had a child with cardiac NL also had high titer anti-Ro comparable to affected pregnancies (24). These observations indicate that measuring p200, Ro52, or Ro60 Ab titers in a mother who has previously had a child with cardiac NL will not add value in predicting the outcome of any subsequent pregnancies. However, mothers who may never be at risk for having an affected child have lower Ab frequencies and titers.
It has been previously reported that titers of Abs against epitopes of Ro52 were increased in mothers between 18 and 30 weeks gestation (13) and that Ro52 Abs gradually decline from early to late pregnancy (25). Moreover, hemodilution during pregnancy secondary to the expected physiologic increase of plasma volume may result in changes in Ab concentrations. These findings were not observed in the current study as consecutive pregnancies were exposed to a similar frequency and titer of maternal Abs regardless of the time of maternal serum sampling. Moreover, equivalent levels of anti-p200 and Ro52 Abs were observed in each twin pair. This finding was not consistent with an earlier study by Harley and colleagues in which the fraternal twin with cardiac NL had only marginally detectable anti-Ro Abs compared to high titer anti-Ro Abs in the unaffected twin (26). However, our findings were in agreement with a subsequent study of twins discordant for cardiac NL in which equivalent levels of anti-Ro and La Abs were reported (27).
Given that p200 Abs are absent in 15% of the cardiac NL neonates reported in this study and 40% of anti-Ro52-positive mothers who had a child with heart block in a recent population-based study (28), it is unlikely that anti-p200 Ab are the sole cause of autoimmune heart block in fetuses. In a murine study, passive transfer of anti-Ro and anti-La antibodies from mothers of children with heart block results in bradycardia and PR prolongation in 70% and 90% of pups respectively (29). In contrast, active immunization with the p200 peptide results in 20% of rat pups with first degree block and none with complete block (30). These data suggest that Abs to p200 are neither necessary nor sufficient to account for all cases of cardiac NL. A more recent study in which pregnant rats were passively immunized with monospecific Abs against human p200, identified a 100% penetrance of 1st degree block and 81% sinus bradycardia in pups (31). These observations raise the possibility that p200 Abs may be an important initiator of injury but that Abs against Ro60 (and perhaps La) RNA binding antigens are required for full expression of atrioventricular nodal disease and advanced block. The clinical significance of 1st degree block remains controversial since it has not been rigorously established whether 1st degree block progresses to 2nd or 3rd degree. In a recent prospective interinstitutional study, of 150 fetuses with persistently normal PR interval throughout the observation period of gestational weeks 19 to 24, a diagnosis of complete block was subsequently made in 1. Of 15 untreated fetuses either with PR prolongation or with type 1 second-degree block, progressive heart block developed in none of them. Three of these 15 fetuses (20%) had a neonatal diagnosis of 1st degree block that spontaneously resolved or had not progressed on follow-up examinations (32).
Autoantibodies against both full length Ro52 and native Ro60 have a higher sensitivity for cardiac NL compared to p200. However, the specificity of these responses is low suggesting that other fetal or environmental factors are required for full disease expression. These factors are likely to be fetal genes, which might amplify inflammatory and fibrosing responses (33–35) or protective factors that inhibit Ab mediated tissue injury (36, 37).
Figure 2. Maternal serum titer of anti-p200, anti-Ro52, anti-Ro60 and anti-La.
Reactivity of serum from mothers pregnant with a cardiac NL child (Group 1), an unaffected child born subsequent to a cardiac NL child (Group 2), or an unaffected child and had only unaffected children (Group 3) against p200 peptide (A) full length recombinant Ro52 (B), native Ro60 (C), or native La (D). The mean ELISA binding units of duplicate determinations is plotted for each neonate considered positive (>3 SD above the median binding units for 28 healthy controls). Cut-offs for positivity are >6.4 for Ro52, >4.5 for p200 and >19 for Ro60 and La. Solid lines represent the median ELISA binding units for each group.
Table 3b.
Anti-Ro52 and p200 antibody titers in umbilical cord blood from sibling (1–5) or twin pairs (6–9) discordant for cardiac NL.
| Sibling pair | Sibling 1–Cardiac NL | Sibling 2 - Healthy | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Ro52 | p200 | DOB | Sex | Ro52 | p200 | DOB | Sex | |
|
| ||||||||
| 1 | 89.3 | 75.2 | 05/04 | M | 94.2 | 78.6 | 05/07 | M |
| 2 | 84.8 | 55.4 | 08/04 | M | 79.8 | 46.5 | 09/05 | M |
| 3 | 94.8 | 15.6 | 04/07 | F | 99.3 | 15.3 | 10/09 | F |
| 4 | 194.4 | 38.5 | 05/07 | M | 282.4 | 61.2 | 06/10 | F |
| 5 | 15.2 | 11.4 | 09/03 | F | 27.5 | 24.7 | 11/07 | F |
| 6 | 91.0 | 66.4 | 08/07 | M | 87.7 | 75.7 | 08/07 | F |
| 7* | 81.7 | 41.2 | 03/08 | M | 81.3 | 30.4 | 03/08 | M |
| 8* | 44.9 | 22.9 | 10/09 | M | 66.9 | 29.0 | 10/09 | M |
| 9 | 59.4 | 94.0 | 04/10 | F | 51.4 | 89.5 | 04/10 | F |
monozygotic twins
DOB= date of birth
Significance and Innovation.
This study is the first assessment of maternal anti-Ro52 and p200 autoantibodies in umbilical cord blood from cardiac NL and unaffected siblings, thereby eliminating any doubt as to exposure to autoantibody.
For a mother with a cardiac NL child, the frequencies and titers of anti-Ro52 and p200 autoantibodies are not informative with regard to the risk of recurrence.
Although both anti-Ro52 and p200 autoantibodies were of low specificity for cardiac NL, anti-p200 was the least likely of the Ro autoantibodies to be false positive in mothers who have never had an affected child. However, the sensitivity of anti-p200 was lower than Ro52 Abs therefore testing solely for reactivity to p200 is insufficient.
Mothers with low titer anti-Ro60 may require less stringent echocardiographic monitoring compared to women with high titer autoantibodies.
Acknowledgments
Financial Support: This work was supported by Australian National Health and Medical Research Council Postgraduate Training Fellowship Grant (595989) to Joanne Reed and National Institutes of Health Grants R01 AR42455-16 and N01-AR-4-2271 and Mary Kirkland Center for Lupus Research Grant.
References
- 1.Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–35. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buyon JP, Friedman DM. Neonatal Lupus. In: Lahita RG, Tsokos G, Buyon JP, Koike T, editors. Systemic Lupus Erythematosus. 5. San Diego: Academic Press; 2011. pp. 541–67. [Google Scholar]
- 3.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62:1138–46. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buyon JP, Clancy RM. Neonatal Lupus: Basic Research and Clinical Perspectives. Rheum Dis Clin N Am. 2005;31:299–313. doi: 10.1016/j.rdc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Buyon JP, Ben-Chetrit E, Karp S, Roubey RA, Pompeo L, Reeves WH, et al. Acquired congenital heart block. Pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J Clin Invest. 1989;84:627–34. doi: 10.1172/JCI114208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buyon JP, Clancy RM. Dying right to live longer: positing apoptosis as a link between maternal autoantibodies and congenital heart block. Lupus. 2008;17:86–90. doi: 10.1177/0961203307085115. [DOI] [PubMed] [Google Scholar]
- 9.Clancy RM, Alvarez D, Komissarova E, Barrat FJ, Swartz J, Buyon JP. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol. 2010;184:2148–55. doi: 10.4049/jimmunol.0902248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutjdir M, Chen L, Zhang ZH, Tseng CE, DiDonato F, Rashbaum W, et al. Arrhythmogenicity of IgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Circ Res. 1997;80:354–62. doi: 10.1161/01.res.80.3.354. [DOI] [PubMed] [Google Scholar]
- 11.Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res. 1998;44:11–9. doi: 10.1203/00006450-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Karnabi E, Qu Y, Wadgaonkar R, Mancarella S, Yue Y, Chahine M, et al. Congenital heart block: identification of autoantibody binding site on the extracellular loop (domain I, S5-S6) of alpha(1D) L-type Ca channel. J Autoimmun. 2010;34:80–6. doi: 10.1016/j.jaut.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsch C, Hoebeke J, Dali H, Ricchiuti V, Isenberg DA, Meyer O, et al. 52-kDa Ro/SSA epitopes preferentially recognized by antibodies from mothers of children with neonatal lupus and congenital heart block. Arthritis Res Ther. 2006;8:R4. doi: 10.1186/ar1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricchiuti V, Briand JP, Meyer O. Epitope mapping with synthetic peptides of 52-kD SSA/Ro protein reveals heterogeneous antibody profiles in human autoimmune sera. Clin Exp Immunol. 1994;95:397–407. doi: 10.1111/j.1365-2249.1994.tb07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed JH, Jackson MW, Gordon TP. A B cell apotope of Ro60 in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1125–9. doi: 10.1002/art.23377. [DOI] [PubMed] [Google Scholar]
- 16.Gordon P, Khamashta MA, Rosenthal E, Simpson JM, Sharland G, Brucato A, et al. Anti-52 kDa Ro, Anti-60 kDa Ro, and Anti-La Antibody Profiles in Neonatal Lupus. J Rheum. 2004;31:2480–7. [PubMed] [Google Scholar]
- 17.Salomonsson S, Dorner T, Theander E, Bremme K, Larsson P, Wahren-Herlenius M. A Serological Marker for Fetal Risk of Congenital Heart Block. Arthritis Rheum. 2002;46:1233–41. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 18.Strandberg L, Winqvist O, Sonesson S, Mohseni S, Salomonsson S, Bremme K, et al. Antibodies to amino acid 200–239 (p200) of Ro52 as serological markers for the risk of developing congenital heart block. Clin Exp Immunol. 2008;154:30–7. doi: 10.1111/j.1365-2249.2008.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, et al. Maternal antibody responses to the 52-kd SSA/Ro p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 20.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 21.Miranda-Carus ME, Boutjdir M, Tseng C-E, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161:5886–92. [PubMed] [Google Scholar]
- 22.Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40:27–41. doi: 10.1007/s12016-009-8190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55:2778–84. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Strandberg L, Salomonsson S, Bremme K, Sonesson S, Wahren-Herlenius M. Ro52, Ro60 and La IgG autoantibody levels and Ro52 IgG subclass profiles longitudinally throughout pregnancy in congenital heart block risk pregnancies. Lupus. 2006;15:346–53. doi: 10.1191/0961203306lu2309oa. [DOI] [PubMed] [Google Scholar]
- 26.Harley JB, Kaine JL, Fox OF, Reichlin M, Gruber B. Ro (SS-A) antibody and antigen in a patient with congenital complete heart block. Arthritis Rheum. 1985;28:1321–5. doi: 10.1002/art.1780281202. [DOI] [PubMed] [Google Scholar]
- 27.Watson RM, Scheel JN, Petri M, Kan JS, Provost TT, Ratrie H, 3rd, et al. Neonatal lupus erythematosus. Report of serological and immunogenetic studies in twins discordant for congenital heart block. Br J Dermatol. 1994;130:342–8. doi: 10.1111/j.1365-2133.1994.tb02931.x. [DOI] [PubMed] [Google Scholar]
- 28.Salomonsson S, Dzikaite V, Zeffer E, Eliasson H, Ambrosi A, Bergman G, et al. A population-based investigation of the autoantibody profile in mothers of children with atrioventricular block. Scand J Immunol. 2011;74:511–7. doi: 10.1111/j.1365-3083.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 29.Mazel JA, El-Sherif N, Buyon J, Boutjdir M. Electrocardiographic abnormalities in a murine model injected with IgG from mothers of children with congenital heart block. Circulation. 1999;99:1914–8. doi: 10.1161/01.cir.99.14.1914. [DOI] [PubMed] [Google Scholar]
- 30.Salomonsson S, Sonesson SE, Ottosson L, Muhallab S, Olsson T, Sunnerhagen M, et al. Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201:11–7. doi: 10.1084/jem.20041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrosi A, Dzikaite V, Park J, Strandberg L, Kuchroo VK, Herlenius E, et al. Anti-Ro52 monoclonal antibodies specific for amino acid 200–239, but not other Ro52 epitopes, induce congenital heart block in a rat model. Ann Rheum Dis. 2012;71:448–54. doi: 10.1136/annrheumdis-2011-200414. [DOI] [PubMed] [Google Scholar]
- 32.Jaeggi ET, Silverman ED, Laskin C, Kingdom J, Golding F, Weber R. Prolongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block. A prospective observational study on the effects of maternal antibodies on 165 fetuses. J Am Coll Cardiol. 2011;57:1487–92. doi: 10.1016/j.jacc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Clancy RM, Backer CB, Yin X, Kapur RP, Molad Y, Buyon JP. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]
- 34.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 35.Clancy RM, Marion MC, Kaufman KM, Ramos PS, Adler A, Harley JB, et al. Identification of candidate loci at 6p21 and 21q22 in a genome-wide association study of cardiac manifestations of neonatal lupus. Arthritis Rheum. 2010;62:3415–24. doi: 10.1002/art.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed JH, Clancy RM, Purcell AW, Kim MY, Gordon TP, Buyon JP. {beta}2-Glycoprotein I and Protection from Anti-SSA/Ro60-Associated Cardiac Manifestations of Neonatal Lupus. J Immunol. 2011;187:520–6. doi: 10.4049/jimmunol.1100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clancy RM. When the levee doesn’t break: A novel role of beta2-glycoprotein I to protect against congenital heart block. Arthritis Rheum. 2009;60:636–8. doi: 10.1002/art.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]