Abstract
Objective
To assess differences in gynecologic history and lightheadedness during menstrual cycle phases among patients with POTS and healthy control women.
Methods
In a prospective, questionnaire-based study carried out at Paden Autonomic Dysfunction Center, Vanderbilt University, between April 2005 and January 2009, a custom-designed questionnaire was administered to patients with POTS (n=65) and healthy individuals (n=95). The results were analyzed via Fisher exact test and Mann–Whitney U test.
Results
Patients with POTS reported increased lightheadedness through all phases of the menstrual cycle phases as compared with healthy controls. Both groups experienced the greatest lightheadedness during menses, and a decrease in lightheadedness during the follicular phase. Patients with POTS reported a higher incidence of gynecologic diseases as compared with healthy controls.
Conclusion
The severity of lightheadedness was found to vary during the menstrual cycle, which may relate to changes in estrogen levels. Patients with POTS also reported an increase in estrogen-related gynecologic disease.
Keywords: Aldosterone, Estrogen, Lightheadedness, Menstrual cycle, Orthostatic tachycardia
1. Introduction
Postural tachycardia syndrome (POTS) is a disorder of chronic orthostatic intolerance that disproportionately affects women of childbearing age [1]. More than 500 000 women are affected by POTS in the USA [2], with symptom onset beginning between 15 and 50 years and a 5:1 female predominance [3]. The characteristic symptoms of POTS (palpitations, dyspnea, lightheadedness, and blurred vision) occur during standing, but resolve with recumbence [4]. The most marked physiologic feature of POTS is an excessive increase in heart rate that occurs on standing in the absence of hypotension [3], and POTS is associated with a very poor quality of life and considerable functional disability [5,6].
The pathophysiology of POTS is complex and not completely understood. Associated features include increased sympathetic tone (reflected by elevated levels of norepinephrine) [7–9], partial autonomic neuropathy [10], and low blood volume [11]. The rennin–angiotensin–aldosterone system (RAAS) plays a vital role in regulating of blood volume. We and others previously reported that many patients with POTS have inappropriately low levels of aldosterone despite their low blood volume [12,13]. Estrogen and progesterone have been shown to affect blood volume [14], and estrogen has an influence on RAAS regulation [15,16]. The effect of progesterone on aldosterone has been investigated with varying results [17]. Because the overwhelming majority of patients with POTS are women of reproductive age [8], we considered that there might be a higher incidence of estrogen-related gynecologic disorders in women affected with POTS, and that there may be cyclic variability in POTS-related lightheadedness.
The goals of the present study were, first, to identify whether patients with POTS experience variations in severity of lightheadedness during different phases of the menstrual cycle; second, to determine whether patients with POTS experience a higher prevalence of gynecologic disorders compared with age-matched healthy individuals; and third, to identify differences in pregnancy complications and outcome between women with POTS and age-matched healthy individuals.
2. Materials and methods
In a prospective questionnaire-based study carried out at the Vanderbilt Autonomic Dysfunction Center, Vanderbilt University, Nashville, USA, women aged between 18 and 65 years were recruited after being diagnosed with POTS during clinical assessment between April 1, 2005, and January 31, 2009. The Vanderbilt University Investigational Review Board approved the study, and written informed consent was obtained from each individual before they completed the questionnaire. The questionnaire was administered to patients with POTS as a part of the Autonomic Dysfunction Center Screening Protocol (IRB#030751). The study was approved on October 14, 2003, and the reproductive questionnaire used was approved in an amendment on March 18, 2004. The web-based version of the questionnaire was approved on July 13, 2006. The healthy women completed the same questionnaire within a separate study entitled “Symptoms of Postural Tachycardia Syndrome (POTS) throughout the Menstrual Cycle” (IRB#080116), which was approved on March 31, 2008.
The study participants met the diagnostic criteria for POTS, developing symptoms of orthostatic intolerance accompanied by a heart rate rise of 30 beats per minute or more within the first 10 minutes of standing in the absence of hypotension (a fall in blood pressure of 20/10 mm Hg or more) [4]. Symptoms worsened on standing and improved with recumbency. All patients had at least a 6-month history of symptoms in the absence of any additional chronic debilitating disorder or prolonged bed rest, were aged at least 18 years, and were not on medication that might impair autonomic tone for 5 half-lives prior to assessment.
Healthy women aged between 18 and 60 years were recruited via email advertisement. They were free of syncope and were otherwise self-described as healthy; no formal physical exam was administered. Healthy volunteers were offered small monetary compensation for completing the questionnaire.
A self-administered questionnaire was developed to assess gynecologic symptoms and reproductive history among female patients with POTS and control women (Supplementary Material S1). The questions contained in the questionnaire were divided into 5 sections: (1) current reproductive status; (2) complete menstrual cycle history, including information regarding length and heaviness of flow, irregular patterns of bleeding, faintness during different phases of the menstrual cycle, and intensity of premenstrual symptoms; (3) established diagnosis of gynecologic disorders; (4) medication history, focusing specifically on hormonal contraceptives and hormone replacement therapy, and any accompanying changes in faintness during use of these drugs; and (5) obstetric history, including abnormalities and/or complications during pregnancy and an assessment of faintness during the 3 pregnancy trimesters. The questionnaire defined all terms (menstrual cycle, menstrual period, and cycle day) before any questions were asked.
Non-dichotomous questions used Likert scales with anchors. Questions asking for a subjective rating of faintness used a 5-point scale, where 1 and 2 corresponded to feeling less faint, 3 corresponded to no change, and 4 and 5 corresponding to feeling more faint. Questions regarding the intensity of premenstrual symptoms were answered via a 6-point scale, from 1 (no change) to 6 (extreme change).
Questionnaires were initially distributed on paper, but switched to an electronic web-based version approximately halfway through the study. Adjustments were necessary between the paper and electronic versions with regard to faintness during different phases of the menstrual cycle. Participants completing the paper version answered this question on the 5-point Likert scale described above, whereas the electronic version used a 1–100 scale. As a result, answers from paper questionnaires were converted from the 1–5 scale to the 100-point scale, where 1=1, 2=25, 3=50, 4=75, and 5=100. To ascertain whether faintness got better or worse during different phases of the menstrual cycle, every individual score at each phase was normalized to the early luteal phase, giving an early luteal score of 1 for each participant.
Electronic study data were collected and managed via Research Electronic Data Capture (REDCap)—a secure, web-based application designed to support data capture for research studies [18]. Paper and electronic data were manually entered into Excel spreadsheets (Microsoft, Redmond, WA, USA), which were merged and imported into SPSS version 16.0 (IBM, Armonk, NY, USA) for statistical analysis. Data were stored in a password-protected database.
Categoric data were analyzed via Fisher exact test. Continuous data (e.g. age) were presented as mean ± standard error (SEM), and differences between POTS patients and control individuals were analyzed with Student t test. Ordinal data (e.g. symptoms ratings on a Likert scale) were presented as median values (25th, 75th percentile), and differences were analyzed via the non-parametric Mann–Whitney U test. A repeated-measure analysis of variance model was used to compare changes over time within a group. P values were calculated for the changeover time (PTIME) and for the interaction or differences between the 2 lines representing the 2 groups (PINT). A P value of less than 0.05 was considered statistically significant.
3. Results
The mean age was 33 ± 1 years for both POTS patients (range 18–57 years) and control women (range 19–56 years). Answers of “not applicable” or “not sure” were discarded before the data were assessed and analyzed. As a result, the number of valid answers varied from question to question. The age at menarche was similar for patients with POTS and healthy controls (12.7 ± 1.5 years versus 12.7 ± 1.3 years; P=0.575). There was no significant difference in length of menstrual cycle, duration of bleeding, or duration of heaviest blood flow (Table 1). A detailed history of abnormal menses patterns was acquired for both groups (Table 2). Patients with POTS reported a higher incidence of secondary amenorrhea (absence of menstruation for >2 months without pregnancy [19]) compared with control women (37% versus 16%; P=0.005; Table 2). Women with POTS also reported a higher incidence of metrorrhagia, menorrhagia, and prolonged spotting as compared with control women; however, this difference was not significant (Table 2).
Table 1.
Menstrual history of patients with POTS and healthy controls
| POTS (n=65) | Controls (n=92) | P value | |
|---|---|---|---|
| Age at menarche, y a | 12.7 ± 0.9 | 12.7 ± 1.3 | 0.727 |
| How far apart are/were periods b | 3 (2,3) | 3 (2,3) | 0.671 |
| Duration of bleeding b | 2 (2,3) | 2 (2,3) | 0.451 |
| Severity of heaviest blood flow b | 2 (2,3) | 2 (2,3) | 0.153 |
| Duration of heaviest blood flow b | 1 (1,2) | 1 (1,1) | 0.051 |
| Length of irregular pattern of bleeding b | 2 (2,4) | 3 (2,4) | 0.101 |
Abbreviation: POTS, postural tachycardia syndrome.
Values are given as mean ± SEM and were analyzed via Student t test.
Values are given as median (25th, 75th percentile) and were analyzed via Mann–Whitney U test. Values were on a Likert scale where 1 represented the minimal severity or duration, and 5 represented the worst. One patient with POTS did not provide an answer.
Table 2.
Abnormal menstrual bleeding patterns among patients with POTS and healthy controls a
| Question | POTS (n=65) | Controls (n=92) | P value (Fisher exact test) |
|---|---|---|---|
| Are/were periods regular | 42 (68) | 75 (82) | 0.056 |
| More than 1 period per month | 28 (43) | 39 (42) | >0.999 |
| Continuous spotting for >10 d | 19 (29) | 23 (25) | 0.586 |
| Continuous moderate-to-heavy flow for >10 d | 12 (19) | 10 (11) | 0.243 |
| Missed period (1 month and not pregnant) | 33 (51) | 37 (40) | 0.197 |
| Amenorrhea b | 24 (37) | 15 (16) | 0.005 |
Abbreviation: POTS, postural tachycardia syndrome.
Values are given as number (percentage) unless indicated otherwise.
Defined as 2 consecutive months without a period in the absence of pregnancy.
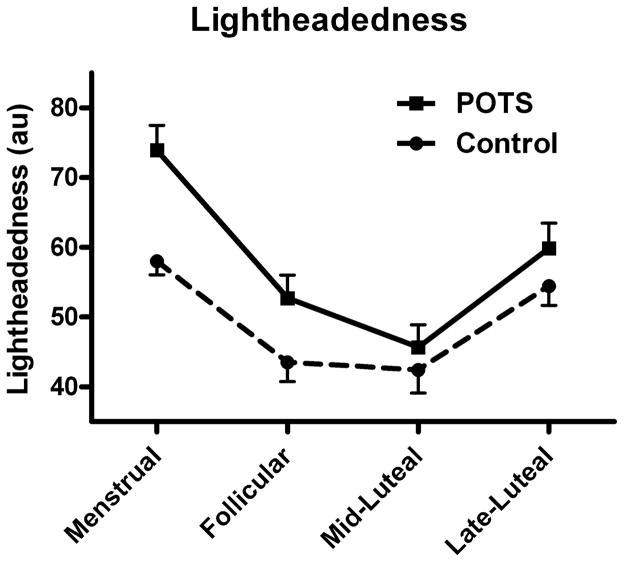
Both women with POTS and healthy controls experienced cyclic variability in subjective faintness (lightheadedness) at various phases of the menstrual cycle (Figure 1). The pattern of variability in lightheadedness was similar between the 2 groups (PINT=0.609). Both groups experienced greatest lightheadedness during menses, with a decrease during the follicular phase and a nadir in the mid-luteal phase, before an increase again in the late-luteal phase. Patients with POTS experienced significantly more lightheadedness compared with control women in each phase of the menstrual cycle: menstruation (74 ± 4 arbitrary units [au] versus 58 ± 2 au; P<0.001), and follicular (53 ± 3 au versus 44 ± 3 au; P=0.001), mid-luteal (46 ± 3 au versus 42 ± 3 au; P=0.010), and late-luteal (60 ± 4 au versus 54 ± 3 au; P=0.026) phases. Among women with POTS, the level of lightheadedness was significantly greater during the follicular phase than during the mid-luteal phase (47 ± 3 versus 42 ± 2 au; P=0.049).
Figure 1.
Perception of lightheadedness during different phases of the menstrual cycle. Shown is self-reported lightheadedness during different phases of the menstrual cycle for patients with postural tachycardia syndrome (POTS) (unbroken line) and healthy controls (broken line). Severity of lightheadedness varied depending on the phase of the menstrual cycle, peaking in the menstrual phase. At each phase, patients with POTS perceived greater lightheadedness compared with control individuals (menstrual P<0.001; follicular P=0.001; mid-luteal P=0.01; late-luteal P=0.026).
Lifetime use of hormonal contraceptives were similar between the POTS (89%) and the control (84%) groups (P=0.361). Significantly fewer patients with POTS than healthy controls were current users of oral contraceptives (29% versus 47%; P=0.032). There was no significant difference in the use of hormone replacement therapy between women with POTS and control women, in terms of either lifetime use (13% versus 5%; P=0.145) or current use (6% versus 2%; P=0.225).
There was a notable difference in the prevalence of gynecologic abnormalities between the 2 groups (Table 3). Women with POTS had a significantly higher incidence of dysfunctional uterine bleeding (14% versus 4%; P=0.042), endometriosis (20% versus 5%; P=0.009), uterine fibroids (25% versus 10%; P=0.015), galactorrhea (9% versus 0%; P=0.004), and ovarian cysts (43% versus 13%; P<0.001). When a subset of control women and POTS patients with a history of using oral contraceptive pills (OCPs) were evaluated (Supplementary Material S2) for gynecologic abnormalities, the difference in endometriosis (21% versus 5%; P=0.007), uterine fibroids (26% versus 10%; P=0.02), galactorrhea (9% versus 0%; P=0.01), and ovarian cysts (43% versus 13% P<0.001) remained significant. There was no significance difference in gynecologic abnormalities between the POTS patients and the controls who reported never using OCPs; however, this subgroup was small.
Table 3.
Self-reported gynecologic abnormalities among patients with POTS and healthy controls a
| Gynecologic abnormality | POTS (n=65) | Controls (n=92) | P value (Mann–Whitney U test) |
|---|---|---|---|
| Anovulation b | 3 (5) | 2 (2) | 0.401 |
| Dysfunctional bleeding | 9 (14) | 4 (4) | 0.042 |
| Endometriosis | 13 (20) | 5 (5) | 0.009 |
| Uterine fibroids | 16 (25) | 9 (10) | 0.015 |
| Galactorrhea | 6 (9) | 0 (0.0) | 0.004 |
| Hirsutism | 3 (5) | 3 (3) | 0.690 |
| Hyperprolactinemia b | 1 (2) | 1 (1) | >0.999 |
| Hypopituitarism | 0 (0.0) | 1 (1) | >0.999 |
| Infertility c | 2 (3) | 3 (3) | >0.999 |
| Ovarian cysts | 28 (43) | 12 (13) | <0.001 |
| Polycystic ovarian syndrome | 3 (5) | 3 (3) | 0.485 |
| Premature menopause | 3 (5) | 1 (1) | 0.307 |
| Regular menopause | 2 (3) | 6 (7) | 0.471 |
Abbreviation: POTS, postural tachycardia syndrome.
Values are given as number (percentage) unless indicated otherwise.
Answers were missing from 1 patient with POTS.
Answers were missing from 2 patients with POTS.
Late-luteal (defined as the 5 days before menses) premenstrual symptoms were rated on a Likert scale from 1 (mildest) to 5 (worst) by both POTS patients and controls. Although most of the symptoms assessed were reported to be similar in both groups (Table 4), there were differences in feelings of depression, sadness, and stress. Notably, a significantly greater number of healthy women reported feelings of stress (P=0.001), irritability (P=0.006), and sadness (P=0.022) before their periods than did women with POTS (Table 4).
Table 4.
Prevalence of premenstrual symptoms among patients with POTS and healthy controls a
| Premenstrual symptom | POTS (n=65) | Controls (n=92) | P value (Mann–Whitney U test) |
|---|---|---|---|
| Breast pain | 3.0 (2.0, 4.50) | 3.0 (2.0, 4.0) | 0.665 |
| Unable to cope with ordinary demands b | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.065 |
| Feel under stress | 2.0 (1.0, 3.0) | 2.5 (2.0, 4. 0) | 0.001 |
| Irritable/bad temper | 3.0 (1.0, 4.0) | 3.0 (2.0, 4.0) | 0.006 |
| Feel sad or blue | 2.0 (1.0, 4.0) | 3.0 (2.0, 4.0) | 0.022 |
| Backache, joint and muscle pain; stiffness | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | >0.999 |
| Weight gain | 3.0 (1.0, 4.0) | 3.0 (2.0, 3.8) | 0.145 |
| Abdominal heaviness, discomfort, or pain | 3.0 (2.0, 5.0) | 3.0 (2.0, 4.0) | 0.502 |
| Edema, puffiness, or swelling | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | 0.295 |
| How long have symptoms been experienced | 2.0 (2.0, 3.0) | 2.0 (1.0, 2.0) | 0.878 |
Abbreviation: POTS, postural tachycardia syndrome.
Values are given as median (25th, 75th percentile) unless indicated otherwise. Values were on a Likert scale where 1 represented the least severity and 5 represented the worst.
One patient with POTS provided no answer.
There was no difference between patients with POTS and control women in pregnancy rates (48% versus 32%; P=0.253) or numbers of live births (1.8 ± 1.1 versus 1.4 ± 0.9; P=0.141). Among the participants who had been pregnant in the past, there were no differences in the incidence of difficulties during getting pregnant or complications during pregnancy such as gestational diabetes, high blood pressure, spontaneous abortion, pre-eclampsia, preterm delivery, or vaginal bleeding (Table 5). Neither women with POTS nor healthy women felt a significant difference in faintness during pregnancy as compared with their non-pregnant state, nor was there a difference in faintness among the 3 trimesters for both groups (data not shown).
Table 5.
Complications during pregnancy among patients with POTS and healthy controls a
| POTS (n=29) | Controls (n=23) | P value (Fisher exact test) | |
|---|---|---|---|
| Any complications | 20 (69) | 12 (48) | 0.253 |
| Gestational diabetes | 1 (3) | 0 (0.0) | >0.99 |
| High blood pressure | 7 (23) | 3 (13) | 0.489 |
| Spontaneous abortion | 13 (42) | 6 (26) | 0.263 |
| Pre-eclampsia | 2 (7) | 3 (13) | 0.640 |
| Preterm delivery | 4 (13) | 1 (4) | 0.380 |
| Vaginal bleeding | 7 (23) | 4 (17) | 0.741 |
Abbreviation: POTS, postural tachycardia syndrome.
Values are given as number (percentage) unless indicated otherwise.
4. Discussion
The present results demonstrated 3 key findings. First, both women with POTS and healthy individuals reported that perceived lightheadedness varied with the menstrual cycle, but women with POTS had greater lightheadedness at all time-points tested. Second, women with POTS reported a higher incidence of gynecologic abnormalities. Third, there were no reported differences in POTS symptoms during the 3 trimesters of pregnancy.
Both patients with POTS and healthy women perceived increased lightheadedness in the late-luteal phase with a peak during menstruation, and perceived lightheadedness to be the least during the follicular phase. Physiologically, estrogen is highest on day 14 of the menstrual cycle and lowest during menstruation [20] (when estrogen would have the least effect on the RAAS, blood volume, and systemic vascular resistance). Fu et al. [21] recently reported that women with POTS experienced an increase in lightheadedness at the start of menstruation as compared with the mid-luteal phase. In addition, stroke volume and cardiac output were lower and vascular resistance was higher during menstruation [21]. These data support our hypothesis that changes in estrogen and its effects on the RAAS might contribute to the lightheadedness reported in POTS.
The present data suggest that there is a higher prevalence of certain gynecologic abnormalities among women affected by POTS (Table 3), although the study sample size was relatively small. Women with POTS had a significantly higher incidence of uterine fibroids, dysfunctional uterine bleeding, ovarian cysts, endometriosis, and galactorrhea. With the exception of dysfunctional uterine bleeding, this higher incidence remained even when the analysis was restricted to women reporting OCP use. All of the aforementioned gynecologic disorders are estrogen-dependent or can be associated with increased levels of estradiol [20]. These results suggest that there might be a link between POTS and a higher incidence of several hormone-dependent gynecologic disorders; however, we cannot exclude the confounding possibility that the higher rates of diagnosis are due to greater medical attention among women with POTS.
The rates of pregnancy and live births did not different between patients with POTS and healthy controls. The patients with POTS did not report any differences in lightheadedness during their pregnancy or after their pregnancy. These findings are consistent with observations by Kimpinski et al. [22], who found that parous women with POTS did not report a difference in lightheadedness among the pregnancy trimesters, and that there was even a non-significant trend toward an improvement in symptoms. This lack of a significant difference in lightheadedness during pregnancy might be due to the increase in blood volume [23], a hypothesis shared by Kimpinski et al. [22].
We and others have found that many patients with POTS have low blood volume [12,24]. Despite their low blood volume, patients with POTS were found to have paradoxically low aldosterone levels and inappropriately low plasma renin activity, while having elevated concentrations of circulating angiotensin II [12,25]. These studies strongly implicate a perturbed RAAS axis leading to low blood volume as a potential mechanism that contributes to POTS pathophysiology.
Stachenfeld [26] has shown that changes in extracellular fluid occur in young healthy women who are chemically “oophorectomized” via a gonadotropin-releasing hormone antagonist [26]. In this series of experiments, each participant’s endogenous estrogen and progesterone production was blocked, and the patients were given estrogen alone, progesterone alone, or a combination of both. Stachenfeld [26] found that estrogen increased sodium and water retention, and also affected extracellular fluid distribution. In the present study, both patients with POTS and healthy women were least symptomatic from lightheadedness during the follicular phase when estrogen has a positive effect on extracellular fluid volume. It seems that estrogen might have a “protective effect” against lightheadedness. Future prospective evaluation should include the effects of estrogen and progesterone on blood volume regulation and the RAAS among both patients with POTS and healthy individuals.
The main limitation of the study is that it was questionnaire-based and used historic recall data from both women with POTS and control women, and thus has a possible recall bias. Although both groups completed the same questionnaire, it is possible that the unwell patients with POTS might recall more details as compared with the control women. The sample size of the study—although fairly large for typical studies of POTS—was limited. The small sample size was further accentuated for some analyses, such as pregnancy, that applied to only a subset of the individuals.
In summary, in the present questionnaire-based study, lightheadedness varied with the menstrual cycle among both patients with POTS and healthy women. In both groups, lightheadedness increased in the late-luteal phase and peaked during menstruation. In addition, patients with POTS reported a higher prevalence of some estrogen-related gynecologic diseases in comparison to healthy women. Pregnancy did not seem to affect lightheadedness in either group of women.
Supplementary Material
Synopsis.
Patients with postural tachycardia syndrome reported an increase in estrogen-related gynecologic disease and varying degrees of lightheadedness during different phases of the menstrual cycle.
Acknowledgments
The study was supported in part by National Institutes of Health grants R01 HL071784, R01 NS055670, R01 HD 046228, P01 HL56693, and 1 UL1 RR024975 (Clinical and Translational Science Award); and the Paden Dysautonomia Center.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ali YS, Daamen N, Jacob G, Jordan J, Shannon JR, Biaggioni I, et al. Orthostatic intolerance: a disorder of young women. Obstet Gynecol Surv. 2000;55(4):251–9. doi: 10.1097/00006254-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317(2):75–7. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45(4 Suppl 5):S19–25. [PubMed] [Google Scholar]
- 4.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 5.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77(6):531–7. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 6.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7(2):204–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120(9):725–34. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69(8):790–8. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 9.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111(21):2734–40. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 10.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343(14):1008–14. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 11.Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci. 2007;334(1):57–60. doi: 10.1097/MAJ.0b013e318063c6c0. [DOI] [PubMed] [Google Scholar]
- 12.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–82. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 13.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58(2):167–75. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–9. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Macova M, Armando I, Zhou J, Baiardi G, Tyurmin D, Larrayoz-Roldan IM, et al. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology. 2008;88(4):276–86. doi: 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol. 2005;98(6):1991–7. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 17.Szmuilowicz ED, Adler GK, Williams JS, Green DE, Yao TM, Hopkins PN, et al. Relationship between aldosterone and progesterone in the human menstrual cycle. J Clin Endocrinol Metab. 2006;91(10):3981–7. doi: 10.1210/jc.2006-1154. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists. Amenorrhea (ACOG technical bulletin 128) Washington, DC: ACOG; 1989. [Google Scholar]
- 20.Couchman GM, Hammond CB. Physiology of Reproduction. In: Scott JR, DiSaia PD, Hammond CB, Spellacy WN, editors. Danforth’s Obstetrics and Gynecology. 7. Philadelphia: Lippincott; 1994. pp. 29–49. [Google Scholar]
- 21.Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010;56(1):82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimpinski K, Iodice V, Sandroni P, Low PA. Effect of pregnancy on postural tachycardia syndrome. Mayo Clin Proc. 2010;85(7):639–44. doi: 10.4065/mcp.2009.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol. 1983;245(5 Pt 1):R720–9. doi: 10.1152/ajpregu.1983.245.5.R720. [DOI] [PubMed] [Google Scholar]
- 24.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104(3):298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 2006;110(2):255–63. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev. 2008;36(3):152–9. doi: 10.1097/JES.0b013e31817be928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.