Abstract
The concept of bacterial translocation and gut-origin sepsis as a cause of systemic infectious complications and the multiple organ dysfunction syndrome (MODS) in surgical and ICU patients has emerged over the last several decades, although the exact clinical relevance of these phenomenon continue to be debated. Thus, the goal of this review will be to trace the evolution of gut-origin sepsis and gut-induced MODS and put these disorders and observations into clinical perspective. Additionally, the mechanisms leading to gut-derived complications will be explored as well as therapeutic options to limit or prevent these complications. From this work, several major conclusions emerge. First, that bacterial translocation occurs clinically and is responsible for increased infectious complications in patients undergoing major abdominal surgery. However, the phenomenon of bacterial translocation is not sufficient to explain the development of MODS in ICU patients. Instead, the development of MODS in these high risk patients is likely due to gut injury and the systemic spread of non-microbial, tissue injurious factors that reach the systemic circulation via the intestinal lymphatics. These observations have resulted in the gut lymph hypothesis of MODS.
Keywords: Bacterial translocation, organ failure, sepsis
Introduction
The idea of the gut being the reservoir for systemic infections during stress states dates back to the 1940's, when live bacteria of enteric origin were found in the peritoneal washings of dogs after hemorrhagic shock (1). However, this notion of gut origin sepsis fell out of favor until the early 1980's when the concept of bacterial translocation was proposed as a potential mechanism to explain systemic infection in patients with organ failure (2). Although it is now generally, but not universally, accepted that gut barrier failure can lead to systemic infection and/or a systemic septic state in various preclinical models, some controversy remains about its clinical relevance. The controversy over the clinical relevance of bacterial translocation and gut-origin sepsis is confounded by these terms meaning different things to different people. Therefore, the first step in discussing this topic is to establish a common vocabulary (Table 1) and secondly to stress the important point that bacterial translocation and gut-derived sepsis may occur independently of each other. That is, bacterial translocation may occur in the absence of gut-derived sepsis, or the patient may have gut-derived sepsis in the absence of documented bacterial translocation. Consequently, attempts to understand the clinical relevance and potential therapeutic approaches to bacterial translocation and gut-origin sepsis should not necessarily link the two together but should examine each process separately. As will be discussed in more detail later, the phenomenon and clinical relevance of bacterial translocation has been studied largely in patients undergoing abdominal surgery where it is possible to sample intestinal lymph nodes. These patient populations where bacterial translocation has been directly measured are generally not critically ill, and although they have an increased risk of developing post-operative infections, they have a low-likelihood of developing multiple organ dysfunction syndrome (MODS). In contrast, the incidence and clinical relevance of gut-origin sepsis and its consequences, such as organ failure, have been studied mainly in critically ill or injured intensive care unit (ICU) patients, in whom the diagnosis of gut-origin sepsis is based primarily on measurements of increased gut permeability and not bacterial translocation.
Table 1.
Bacterial translocation and gut-derived sepsis: a common vocabulary
| Bacterial Translocation is best defined as the process by which intestinal bacteria or Candida cross the intestinal mucosal barrier to reach the mesenteric lymph nodes from which they may or may not spread systemically and cause infection. |
| The diagnosis of bacterial translocation requires the identification of intestinal bacteria in the intestinal lymph nodes. |
| Gut-derived sepsis is best defined as the process whereby gut-derived pro-inflammatory, tissue-injurious microbial and non-microbial factors induce or contribute to the development of SIRS, ARDS or MODS. This process may or may not occur in presence or absence of gut-origin systemic infection or bacterial translocation. |
| The diagnosis of gut-derived sepsis is based on measurements of gut barrier function (permeability) in conjunction with the clinical response of the patient. |
The clinical controversy over bacterial translocation and gut-origin sepsis
During the past three decades, the concept that the gut and its contents can induce, contribute to, or perpetuate the systemic inflammation response (SIRS), acute lung injury (ALI), acute respiratory distress syndrome (ARDS) and MODS, as well as serve as a reservoir for bacterial infection has gained attention. During this time period, multiple studies have examined the role and relevance of intestinal barrier failure, bacterial translocation and gut-derived sepsis in multiple patient populations. As will be discussed later, based on both clinical and experimental studies, the answer to the questions, >do bacterial translocation and gut-derived sepsis exist?= appears to be yes. However, the clinical relevance of bacterial translocation in the pathogenesis of sepsis and organ failure is more controversial. The controversy revolves around the failure to consistently find gut-derived bacteria or bacterial products, such as endotoxin, in the blood of critically ill or injured septic-appearing patients with MODS. Thus, the first step in clarifying the controversy over the clinical relevance of the stressed gut in the pathogenesis of sepsis, ARDS and MODS is to expand the focus beyond bacterial translocation to include gut barrier failure and the systemic spread of gut-derived nonmicrobial, proinflammatory and tissue-injurious factors. This proposal is based on observations that loss of gut barrier function, even in the absence of systemic bacteremia or endotoxemia, can cause a septic state and contribute to distant organ dysfunction (3). This septic response appears to occur through the release of nonmicrobial factors into the intestinal lymphatics which, upon reaching the systemic circulation, are sufficient to cause both distant organ injury and a septic state (3). Simply stated, it is now time to dissociate the process of bacterial translocation from the pathophysiology of gut-derived sepsis and MODS, since, although these two conditions may occur together, gut-origin sepsis and MODS do not require bacterial translocation and bacterial translocation by itself may not lead to MODS.
Bacterial Translocation and gut-origin sepsis; The evidence
It is well recognized that most infections are caused by organisms colonizing the host that generally originate at sites of mucosal injury, ciliary dysfunction or integumentary damage. This means that infections principally begin where bacteria breach the patient=s local mechanical defenses. Since the intestine is the reservoir for enormous numbers of bacteria (>1010 organisms per gram of tissue) and their toxic products, such as endotoxin and peptidoglycan, failure of the gut barrier could be easily seen to lead to systemic bacteremia and a septic state. In this context, the concept of bacterial translocation gained clinical attention in the 1980's because it clarified the clinical observation of how critically ill or injured patients could develop endotoxemia or bacteremia with enteric organisms without an identifiable focus of infection being found, even at autopsy (4). However, studies to establish whether bacterial translocation occurs in patients were more difficult to perform than the preclinical animal studies carried out to establish the concept of bacterial translocation and gut-origin sepsis (5). This is because a laparotomy to harvest and culture mesenteric lymph nodes is necessary to definitively establish that bacterial translocation has occurred. Although any or all of the following clinical observations might suggest that bacterial translocation is occurring, these findings are indirect and not definitive: 1) association between increased gut permeability and MODS in high risk patient groups, 2) association between gut mucosal acidosis (ischemia) and distant organ failure, and 3) clinical trials indicating that enteral feeding or selective gut decontamination improves clinical outcome. Consequently, the initial human proof-of-principle studies establishing that intestinal bacteria do translocate to intestinal lymph nodes were carried out in patients undergoing abdominal surgery either for inflammatory bowel disease (6) or simple small bowel obstruction (7).
Subsequently, similar results were observed in a study measuring bacterial translocation in organ donors (8). In this study, bacterial translocation was documented in 67% of the organ donors and the bacteria recovered from the lymph nodes and other tissues were identical to those isolated from the bowel contents. Since then, six additional clinical series totaling 2125 patients undergoing abdominal surgery have shown that the incidence of bacterial translocation to the mesenteric lymph nodes ranged from 5% to 21% and that in each of these studies, bacterial translocation was associated with a significant twofold to threefold increase in the rate of infectious complications (9–14). Furthermore, in about half of these patients, the same organisms were identified in the mesenteric lymph nodes as in the postoperative infectious focus (9–14). The notion that the gut was the reservoir for these translocating bacteria was further validated by genomic studies showing that the bacteria in the mesenteric lymph nodes originated from the patients= gut flora (15). Thus, studies of surgical patients undergoing laparotomy have validated the concept that bacterial translocation is a clinical event and that its occurrence is associated with a significantly higher incidence of systemic infectious complications.
Bacterial translocation has been implicated as the mechanism by which the ischemicnecrotic pancreas becomes infected in patients with severe pancreatitis (16,17). This conclusion is based on work showing that intestinal permeability is increased in patients with severe pancreatitis and that this increased gut permeability was associated with endotoxemia, organ failure and morbidity (17). Bacterial translocation also occurs in patients with cirrhosis and liver failure and the incidence of bacterial translocation increased from 3% to 31% as the magnitude of the liver dysfunction (Child score) increased (18). Further, bacterial translocation appears to be a mechanism by which bacterial peritonitis occurs in cirrhotics with ascites (19).
However, although bacterial translocation has been documented to occur in patients undergoing major operations, in organ donors and in patients with severe pancreatitis or cirrhosis, the studies linking bacterial and/or endotoxin translocation to MODS in critically ill or injured patients in the ICU was indirect and was primarily based on increased gut permeability to orally administered probes. These studies showed that gut permeability was increased in thermally injured, trauma and ICU patients, but in less than half of these studies was a clear association between the magnitude of the increase in gut permeability and infectious complications or endotoxemia found, although the evidence linking increased gut permeability to the development of MODS was stronger (20–26). Thus, based on clinical studies using gut permeability as a surrogate marker for bacterial translocation or gut-origin sepsis, there is suggestive, but not conclusive, evidence that loss of gut barrier function contributes to the development of systemic infection and MODS. In an attempt to directly correlate bacterial and endotoxin translocation with the subsequent development of ARDS and MODS, Moore et al (27) carried out a prospective study where portal vein catheters were placed in severely injured trauma patients shortly after their arrival to the hospital. Serial portal blood samples were then tested for translocating bacteria or the presence of endotoxin. While 30% of the enrolled patients subsequently developed MODS, only 2% of all the portal vein cultures collected were positive for bacterial growth and none of the portal blood samples contained endotoxin. Given the compelling results of this study, doubt was cast on the clinical relevance of bacterial translocation in the development of MODS. Although bacterial translocation did appear to predispose to infectious complications in post-operative surgical patients, this clinical study (27) and other inconsistent results in preclinical and clinical studies, caused us and others to re-evaluate the role of bacterial translocation in the pathogenesis of MODS.
This notion that loss of gut barrier function might not be involved in the development of SIRS and MODS was based on the supposition that it is bacteria and their products exiting the gut through the portal circulation that contributes to the development of trauma-induced sepsis and organ failure. One potential explanation for the failure of Moore et al (27) to find bacteria or endotoxin in the portal system of patients with MODS is that gut-derived, MODS-inducing factors were exiting the gut via the intestinal lymphatics rather than the portal blood (Figure 1). This observation is consistent with preclinical studies showing that the primary route of translocating bacteria as well as other gut-derived molecules reaching the systemic circulation is via the intestinal lymphatics (28). It would also help explain why increased gut permeability and gut ischemia are better predictors of the development of MODS than the presence of gut-derived bacteremia or endotoxemia. Based on an extensive series of studies in rodents, pigs and non-human primates, it now appears that the early onset of SIRS and organ failure after trauma, shock or other systemic stress states are due to nonbacterial, tissue-injurious, pro-inflammatory factors liberated from the stressed gut that reach the systemic circulation through the mesenteric lymphatics rather than the portal venous system (reviewed in ref 3). This work has resulted in the gut-lymph hypothesis of SIRS, ARDS and MODS.
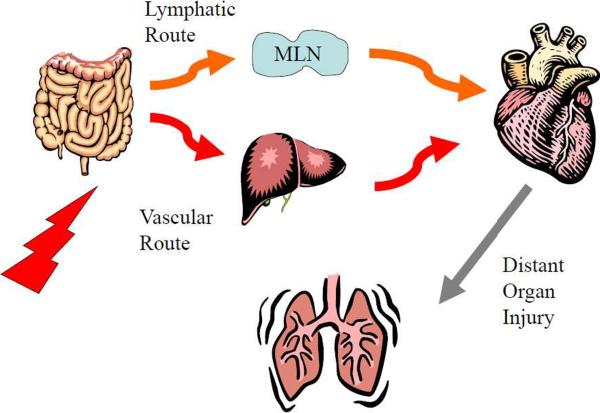
Figure 1.
Schematic illustration of lymphatic and portal pathways by which gut-derived factors and/or bacteria can reach the systemic circulation. In the portal route, the liver would be the first major organ encountered by these gut-derived factors, while in the lymphatic route, the factors in lymph would enter the systemic circulation at the level of the subclavian vein and after passing through the heart would go into the pulmonary circulation. Thus, in the intestinal lymph route, the lung is the first major vascular bed that drains the gut
This gut lymph hypothesis is based on several major experimental observations. First, ligation of the major intestinal lymph duct, which prevents intestinal lymph from reaching the systemic circulation, prevents the development of early ARDS and MODS. Secondly, in vitro studies of mesenteric lymph from shocked, but not sham-shocked, animals leads to neutrophil activation, cardiomyocyte and endothelial cell injury as well as red blood cell dysfunction (3). Lastly, injection of shocked but not sham-shocked lymph into healthy mice or rats recreates a systemic septic state and causes ARDS and MODS (3). Since these pro-inflammatory and tissue-injurious lymph specimens were sterile and did not contain detectable levels of endotoxin or bacterial DNA (29), the biologically active factors in the lymph samples appeared to be non-microbial. Likewise, the biologic activity of lymph does not appear to be cytokine-mediated (3). While investigations into the exact nature of the biologically-active factors in lymph continues, recent studies suggest that these non-microbial factors act as danger signals and exert their adverse systemic effects by stimulating TLR4 and perhaps other pattern recognition receptors in a fashion similar to bacteria (29). These observations are consistent with the recently proposed >danger model = (30) where non-microbial, host-derived products of tissue injury lead to a sterile systemic inflammatory state that can progress to ARDS and MODS if the systemic inflammatory response is sufficiently great. Thus, although the gut-lymph hypothesis remains to be fully tested clinically, it does resolve the paradox of how gut-derived sepsis and MODS can occur and yet neither bacteria nor endotoxin be consistently found in the portal or systemic circulations. This hypothesis also explains the relationship between increased gut permeability and MODS (Figure 2).
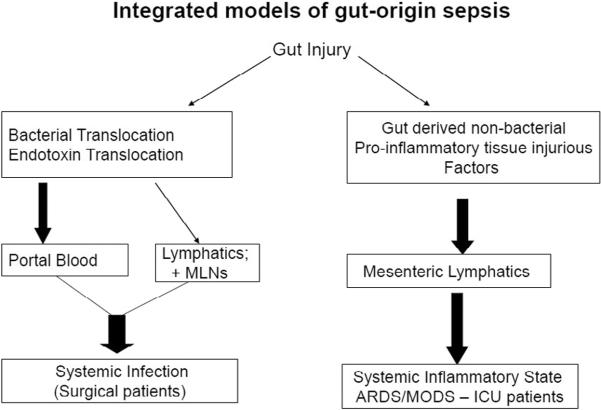
Figure 2.
Schematic overview illustrating the proposed integrated models of gut-origin sepsis. In this model system, gut injury or dysfunction can lead to bacterial translocation and systemic infection and/or the release of non-microbial gut-derived factors that potentiate the development of a septic state, ARDS and MODS. The bacterial translocation pathway leading to an increased incidence of systemic infections has been documented in several groups of surgical patients undergoing major procedures. The gut lymph pathway however appears to be the major mechanism of gut-origin sepsis in ICU patients.
Pathogenesis and Therapy
As a general principle, therapies directed at preventing or limiting bacterial translocation and/or gut injury are based on an understanding of the host=s physiologic defenses that maintain normal intestinal barrier function, limit stress-induced gut injury and help maintain a stable gut flora as well as the pathophysiologic changes associated with bacterial translocation and/or gut barrier failure. Conceptually, these therapeutic approaches can be divided into two major groups. First, therapies directed at maintaining a stable gut flora and thereby limiting the risk of bacterial translocation and the development of systemic infections and secondly, therapies focusing on limiting the development of gut injury and gut barrier failure and hence reducing the incidence of gut-origin sepsis and MODS. Although some therapies have over lapping effects, this conceptual approach of categorizing various therapies by their goals has the advantage of providing a rationale therapeutic framework and thereby facilitating rationale therapeutic decision making.
The two major clinical therapeutic approaches directed at limiting intestinal bacterial overgrowth with potential pathogenic bacteria are selective digestive tract decontamination (SDD) and the use of prebiotics and probiotics. The strategy of SDD is based on the concept that life-threatening infections in critically ill or injured patients originate from the gut and the use of oral nonabsorbable antibiotics plus a brief course of systemic antibiotics prevents intestinal bacterial overgrowth and thereby limits gut-origin infections and improves clinical outcome. Although most of the early studies documented that SDD reduces the incidence of infection by about 50%, they were not able to consistently document a survival advantage. However, some recent single center prospective trials as well as meta-analyses indicate that SDD reduces mortality in multiple ICU patient populations (31–33). While the reduction in mortality with SDD has varied between studies, most have shown an absolute reduction of mortality of greater than 3–4%, which translates into an over all reduction of mortality of approximately 11%. Because of the risk of antibiotic resistance, several studies have used a selective oral decontamination (SOD) approach rather than SDD. The efficacy of SOD versus SDD in preventing ventilator associated pneumonia and improving mortality was recently tested in a multi-center clinial trial enrolling 5939 patients (31). The results of this study showed that the two therapeutic approaches were comparable with SDD reducing 28 day mortality by 3.5% and SOD reducing mortality by 2.9%. Thus, the use of SDD or SOD to control oropharyngeal and intestinal colonization with potentially pathogenic bacteria appears a viable therapeutic option.
A new approach to controlling the gut flora is the use of enterally administered prebiotics, probiotics and synbiotic combinations as adjuncts to more traditional therapies (34). Prebiotics are specific plant fibers and probiotics are mainly specific strains of lactobacillius, whereas synbiotics are a combination of the two. The largest number of clinical trials using these agents have been performed in patients undergoing elective major abdominal procedures and a recent meta-analyses of these studies was published in 2009 (35). This meta-analysis of 9 clinical studies, documented that the perioperative administration of probiotics and/or synbiotics reduced the overall postoperative infection rate by more than 50% and significantly decreased length of stay, although there was no mortality advantage. This failure to show a mortality benefit was not surprising, since the mortality rate of these studies was low and averaged about 3%. The second largest group of prospective controlled randomized trials was carried out in mechanically ventilated ICU patients testing whether the use of probiotics/synbiotics would decrease the incidence of ventilator-associated pneumonia (VAP). A meta-analysis of these trials found that probiotic-treated patients had 40% less VAP than the control group as well as decreased length of stay, although again there was no survival advantage (36). The one major exception to these clinical studies showing probiotics/synbiotics are clinically beneficial, was a study in patients with severe acute pancreatitis where the probiotic-treated patients had an increased incidence of infectious complications and a higher mortality rate (37). While the explanation for these conflicting results remains to be resolved, in a subsequent subgroup analysis of the patients in this pancreatitis study, the authors found that the adverse effects of the probiotic therapy was largely confined to the group of patients who already had early organ failure (38). From this observation, the authors proposed that probiotics may result in adverse consequences when administered into patients with compromised gastrointestinal tracts where gut permeability is likely to be increased and intestinal oxygen delivery reduced. Thus, in thinking about the use of probiotics, prebiotics and synbiotics, the timing of administration may be critical. For example, the prophylactic administration of these agents electively prior to major operations seems to be a uniformaly beneficial therapy. This may be because gut barrier function is intact and the patient=s gut flora has not been perturbed. In contrast, administering these agents at high dosages to patients with established gut injury and increased gut permeability may result in these low virulence bacteria translocating across the injured mucosa thereby contributing to increased systemic inflammation and altered immune defenses. As more information becomes available, these types of questions can be answered.
Because of the potentially important relationship between nutrition, infection and gut barrier function, this area has received significant clinical and experimental attention over the last several decades. One of the most important concepts that has evolved from this work is that the gut has specific nutritional needs distinct from the rest of the body and that lack of enteral feeding itself can result in impaired gut barrier function (39). This concept is based on both clinical and preclinical studies documenting that lack of enteral feeding as well as standard total parenteral nutrition rapidly leads to gut atrophy as well as changes in gut function. Thus, a major clinical strategy directed towards supporting gut structure and function has been nutritional and various nutritional strategies have been developed focusing, at least in part, on supporting gut barrier function and hence reducing the incidence of gut-origin sepsis and MODS. These nutritional therapeutic approaches can be divided into 1) perioperative strategies used for high-risk elective surgical patients and 2) those utilized in ICU patients. One example of the effectiveness of perioperative nutrition is in patients undergoing major abdominal surgery where early enteral nutrition was found to significantly reduce mortality (40), a finding that was later confirmed in a 2009 meta-analysis (41). Early enteral nutrition has also shown clinical benefit in patients with severe pancreatitis who are at increased risk of gut-origin sepsis and bacterial translocation (42).
In ICU patients, nutritional therapies, including the use of key gut-protective immunonutrients, such as glutamine, omega-3 fatty acids and trace elements such as selenium, have been shown to reduce the incidence of infectious complications, improve gut barrier function, reduce length of stay and in some series improved survival (43). This complex area has been recently reviewed in detail by Martindale et al (43). Thus, although these specific nutritional components and approaches may have multiple non-gut effects, including roles as anti-oxidants and immuno-inflammatory modulators, it appears that at least part of their effectiveness is at the level of the gut. Consequently, it appears clear that early enteral feeding and enteral and/or parenteral administration of glutamine and omega-3-fatty acids is gut protective and clinically beneficial in high-risk surgical as well as ICU patients.
In addition to the clinically established therapies discussed above, preclinical and early Phase I and Phase II clinical trials are being carried out testing various other gut-protective approaches. These include various anti-oxidant volume resuscitative therapies directed at limiting intestinal ischemia-reperfusion injury, various agents directed at neutralizing potential gut-derived proinflammatory mediators as well as agents directed at limiting the host response to microbial products as well as danger signal molecules. As more basic information is gained on the exact mechanisms of stress, shock and ischemia-induced gut injury as well as the pathophysiology of gut-derived sepsis, new therapeutic options should emerge.
Conclusions
In summary, gut barrier failure has evolved from a theory in which bacteria translocating to distant organs cause injury into one in which bacteria and gut ischemia invoke an intestinal inflammatory response and gut-derived inflammatory products lead to distant organ injury. In this paradigm, gut ischemia appears to be the dominant link by which splanchnic hypoperfusion is transduced from a hemodynamic into an immuno-inflammatory event and it appears to do so via the release of biologically-active factors into the mesenteric lymphatics. Importantly, the clinical conditions found to be associated with bacterial translocation, loss of gut barrier function, gut-derived sepsis and organ dysfunction are largely consistant with that predicted from preclinical studies. Having established that gut-derived infection, sepsis and MODS occurs in patients, we believe that further research will focus on the mechanisms of gut barrier failure as well as the exact nature and function of gut-derived, pro-inflammatory nonmicrobial factors.. Hopefully, the results of these mechanistic studies will lead to new and effective therapies that improve clinical outcomes.
Acknowledgments
Work supported by NIH grant RO1 GM059841
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schweinburg FB, Frank HA, Frank ED, et al. Transmural migration of intestinal bacteria during peritoneal irrigation in uremic dogs. Proc Soc Exp Biol Med. 1949;71:150–153. doi: 10.3181/00379727-71-17114. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA, Maejima K, Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985;25:385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA, Xu DZ, Lu Q. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction syndrome: A new look at gut origin sepsis. J Organ Dys. 2006;2:70–79. [Google Scholar]
- 4.Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrere JS. Multipleorgan failure: Generalized autodestructive inflammation? Arch Surg. 1985;120:1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. Bacterial translocation of the gut flora. J Trauma. 1990;30:S184–S189. doi: 10.1097/00005373-199012001-00037. [DOI] [PubMed] [Google Scholar]
- 6.Ambrose NS, Johnson M, Burdon DW, Keighley MR. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br J Surg. 984(71):623–625. doi: 10.1002/bjs.1800710821. [DOI] [PubMed] [Google Scholar]
- 7.Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124:699–701. doi: 10.1001/archsurg.1989.01410060065013. [DOI] [PubMed] [Google Scholar]
- 8.van Goor H, Rosman C, Grond J, et al. Translocation of bacteria and endotoxin in organ donors. Arch Surg. 1994;129:1063–1066. doi: 10.1001/archsurg.1994.01420340077014. [DOI] [PubMed] [Google Scholar]
- 9.O'Boyle CJ, MacFie J, Mitchell CJ, et al. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedman PC, Macfie J, Sagar P, et al. The prevalence of gut transloation in humans. Gastroenterology. 1994;107:643–649. doi: 10.1016/0016-5085(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 11.Woodcock NP, Sudheer V, El-Barghouti N, et al. Bacterial translocation in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2000;87:439–442. doi: 10.1046/j.1365-2168.2000.01417.x. [DOI] [PubMed] [Google Scholar]
- 12.MacFie J, O'Boyle C, Mitchell CJ, et al. Gut origin of sepsis: A prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin KF, Kallam R, O'Boyle C, MacFie J. Bacterial translocation may influence long-term survival in colorectal cancer patients. Dis Colon Rectum. 2006;50:323–330. doi: 10.1007/s10350-006-0827-4. [DOI] [PubMed] [Google Scholar]
- 14.MacFie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87–93. doi: 10.1002/bjs.5184. [DOI] [PubMed] [Google Scholar]
- 15.Reddy BS, MacFie J, Gatt M, et al. Commensal bacteria do translocate across the intestinal barrier in surgical patients. Clin Nutr. 2007;26:208–215. doi: 10.1016/j.clnu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kazantsev GB, Hecht DW, Rao R, et al. Plasmid labeling confirms bacterial translocation in pancreatitis. Am J Surg. 1994;167:201–206. doi: 10.1016/0002-9610(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 17.Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 18.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 19.Gines P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 20.LeVoyer T, Cioffi WG, Jr, Pratt L, et al. Alterations in intestinal permeability after thermal injury. Arch Surg. 1992;127:26–29. doi: 10.1001/archsurg.1992.01420010032005. [DOI] [PubMed] [Google Scholar]
- 21.Faries PL, Simon RJ, Martella AT, et al. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma. 1998;44:1031–1035. doi: 10.1097/00005373-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Roumen RM, Hendriks T, Wevers RA, Goris JA. Intestinal permeability after severe trauma and hemorrhagic shock is increased without relation to septic complications. Arch Surg. 993(128):453–457. doi: 10.1001/archsurg.1993.01420160095016. [DOI] [PubMed] [Google Scholar]
- 23.Langkamp-Henken B, Donovan TB, Pate LM, et al. Increased intestinal permeability following blunt and penetrating trauma. Crit Care Med. 1995;23:660–664. doi: 10.1097/00003246-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Walsh DS, Thavichaigarn P, Dheeradhada C, et al. Prolonged alteration in gut permeability following nonthermal injury. Injury. 1996;27:491–494. doi: 10.1016/0020-1383(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 25.Harris CE, Griffiths RD, Freestone N, et al. Intestinal permeability in the critically ill. Intensive Care Med. 1992;18:38–41. doi: 10.1007/BF01706424. [DOI] [PubMed] [Google Scholar]
- 26.Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 27.Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: A clinical perspective with major torso trauma. J Trauma. 1991;31:629–636. doi: 10.1097/00005373-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Mainous MR, Tso P, Berg RD, Deitch EA. Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch Surg. 1991;126:33–37. doi: 10.1001/archsurg.1991.01410250037005. [DOI] [PubMed] [Google Scholar]
- 29.Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB, Ramanathan M, Xu DZ, Deitch EA, Feinman R. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6:e14829. doi: 10.1371/journal.pone.0014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. 2002. [DOI] [PubMed] [Google Scholar]
- 31.van Nieuwenhoven CA, Buskens E, van Tiel FH, Bonten MJ. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA. 2001;286:335–40. doi: 10.1001/jama.286.3.335. [DOI] [PubMed] [Google Scholar]
- 32.Stoutenbeek CP, Saene HKF, Little RA, et al. The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med. 2006;33:261–70. doi: 10.1007/s00134-006-0455-4. [DOI] [PubMed] [Google Scholar]
- 33.de Smet AMGA, Kluytmans JAJW, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 34.Bengmark S, Martindale R. Prebiotics and synbiotics in clinical medicine. Nutr Clin Pract. 2005;20:244–261. doi: 10.1177/0115426505020002244. [DOI] [PubMed] [Google Scholar]
- 35.Pitsouni E, Alexiou V, Saridakis V, et al. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2009;65:561–70. doi: 10.1007/s00228-009-0642-7. [DOI] [PubMed] [Google Scholar]
- 36.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:954–62. doi: 10.1097/CCM.0b013e3181c8fe4b. [DOI] [PubMed] [Google Scholar]
- 37.Besselink MGH, van Santvoort HC, Buskens E. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 38.Besselink MG, van Santvoort HC, Renooij W, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250:712–9. doi: 10.1097/SLA.0b013e3181bce5bd. [DOI] [PubMed] [Google Scholar]
- 39.Gatt M, MacFie J. Randomized clinical trial of gut-specific nutrients in critically ill surgical patients. Br J Surg. 2010;97:1629–36. doi: 10.1002/bjs.7155. [DOI] [PubMed] [Google Scholar]
- 40.Andersen HK, Lewis SJ, Thomas S. Cochrane Database of Syst Rev. 2006;4:CD004080. doi: 10.1002/14651858.CD004080.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Lewis SJ, Andersen HK, Thomas S. Early Enteral Nutrition Within 24h of Intestinal Surgery Versus Later Commencement of Feeding: A Systematic review and Meta-analysis. J Gastrointest Surg. 2008;13:569–75. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 42.Petrov MS, van Santvoort HC, Besselink MGH, et al. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008;143:1111–7. doi: 10.1001/archsurg.143.11.1111. [DOI] [PubMed] [Google Scholar]
- 43.Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–61. doi: 10.1097/CCM.0b013e3181a40116. [DOI] [PubMed] [Google Scholar]