Abstract
Purpose
Study the impact of daily rotations and translations of the prostate on dosimetric coverage during RT.
Methods and Materials
Real–time tracking data for 26 patients were obtained during RT. IMRT plans meeting RTOG0126 dosimetric criteria were created with 0, 2, 3, and 5 mm PTV margins. Daily translations and rotations were used to reconstruct prostate delivered dose from the planned dose. D95 and V79 are computed from the delivered dose to evaluate target coverage and the adequacy of PTV margins. Prostate equivalent rotation is a new metric introduced in this study to quantify prostate rotations by accounting for prostate shape and length of rotational lever-arm.
Results
Large variations in prostate delivered dose were seen among patients. Adequate target coverage was met in 39%, 65%, and 84% of the patients for plans with 2, 3, and 5 mm PTV margins, respectively. While no correlations between prostate delivered dose and daily rotations are seen, the data shown clear correlation with prostate equivalent rotation.
Conclusions
Prostate rotations during RT could cause significant underdosing even if daily translations were managed. These rotations should be managed with rotational tolerances based on prostate equivalent rotations.
Keywords: intra-fraction rotation, equivalent rotation, margins, setup correction, organ motion
Introduction
As in-room guidance technologies become more advanced, and the uncertainty in target location is reduced, margin expansions made to treatment beams can also be reduced. Current investigations have estimated that PTV expansions of 10 mm or less are sufficient to account for prostate motion. Much of this literature focuses on prostate inter-fraction translational motion.1–4 Recently, the introduction of real-time electromagnetic tracking has allowed intra-fraction translational motion of the prostate to be studied. It was shown that 2 mm PTV are sufficient to provide adequate coverage in the presence of actively managed translational motion.5,6
Li Jin et al.7 performed a comprehensive estimation of the population margins for prostate RT and found that after inter-fraction motion was largely removed by electromagnetic guidance, prostate rotation had a much larger impact than intra-fraction motion7. They demonstrated that managing rotation would allow significant reduction in margins to about 4–6 mm.7 Despite several studies investigating prostate rotations and its impact on dosimetric coverage,6–9 the relationship between prostate rotation, dosimetric coverage, and rotation management has yet to be elucidated. The magnitude and frequency of intra-fraction prostate rotation has recently been reported based on electromagnetic tracking data.10–12 The data showed that rotations are predominantly about the Right-Left axis with a standard deviation of roughly ±7°.
In this study, prostate delivered dose was constructed for 26 patients. The dosimetric impact of average daily rotations and translations (six degrees of freedom) was investigated and the correlations between dosimetric coverage and the prostate shape, rotation magnitude, and the location of the centroid of rotations were evaluated.
Materials and Methods
I. Patient Data
Real-time tracking data were recorded at 10 Hz for twenty-six prostate cancer patients during IMRT utilizing the Calypso® System (Calypso Medical Technologies, Seattle, WA) on an IRB-approved study. On average, total tracking time per fraction was about 8 minutes. Three transponders were placed into the left mid-base, right mid-base, and apex of the prostate transrectally with ultrasound guidance. Planning CT scans were obtained 6–11 days after transponders placement to allow for resolution of prostate edema. Consequent CT scans were obtained during weeks three, six, and post treatment to check for transponder migrations.
II. Treatment planning
CTV volumes were created by contouring the prostate (without seminal vesicles) for low-risk patients and extending to include the proximal 1 cm of seminal vesicles for intermediate risk patients on the planning CT. Although patients were treated with a 5 mm uniform PTV margin, this study retrospectively evaluated uniform PTV expansions of 0, 2, 3, and 5 mm. Seven-field IMRT plans meeting RTOG012613 dosimetric criteria were created and optimized for each PTV margin.
III. Prostate localization and tracking
The prostate was localized daily, based on pre-determined transponder positions relative to isocenter on the treatment planning CT. Target translations from isocenter were kept to within 3 mm by manually interrupting the beam and applying a couch shift. No intervention was applied for rotations.
IV. Tracking data
Daily three-dimensional coordinates of each transponder relative to isocenter, were exported from Calypso System. First, the average of the tracked transponder location was centered on its original position from the planning CT. Then, the tracking data were used to determine the rigid rotation about three axes from the planned location to that measured during each tracking session. In this way we determine prostate daily average rotation including both inter- and average intra-fractional rotations.
This was done using single value decomposition (SVD) to obtain the rigid rotation. All transponder locations were used in the SVD analysis to determine the rotations that minimized the mean squared distance relative to the planned transponders locations.
V. Prostate delivered dose
Dose calculation tools were implemented in our in-house treatment planning system (UMPlan) to reconstruct the prostate delivered dose from the planned dose utilizing the prostate measured translations and rotations. The prostate was rigidly translated and rotated, (average six degree of freedom transformation was used) within the planned dose cloud. The delivered dose was reconstructed for each fraction and summed to determine the total dose delivered throughout the treatment course.
VI. Delivered dose and prostate motion
DVHs of the prostate were computed from the delivered dose to evaluate the adequacy of PTV margins under target motion. For purposes of evaluation, an adequate clinical coverage is achieved when the dose delivered to 100% of the prostate CTV was ≥ 95% of the planned dose. Changes of D95 and V79 with prostate rotations were examined for possible correlations. Only rotations about the right-left axis were evaluated since rotations about other axes are usually small.
VII. Daily average Equivalent rotations
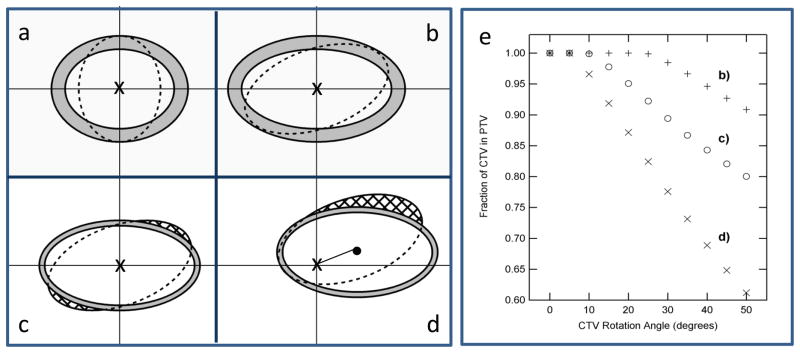
The consequence of rotation on target coverage will depend on the magnitude of rotation, the lever-arm of rotation (distance from rotation center to prostate center of mass, CM), and prostate elongation along the superior-inferior axis. Those effects were simulated and illustrated in Figure 1. Sagittal views of two targets rotating about their CM are shown in panels (a) and (b) with the dashed lines outlining the CTV and the shaded region showing the PTV expansion. A slightly elongated target (a) can rotate up to 90º and remain within the PTV, while a more elongated target (b) could rotate up to only 20º to remain within the PTV. Panels (c) and (d) illustrate the impact of rotation lever-arm on target coverage. A 20º rotation about the target CM forced 6% of the target outside the 2 mm PTV margin while 21% of the volume was forced outside the PTV by moving the rotational centroid 1cm away from the target CM. Simulation of the percentage CTV remaining within the PTV for cases shown in panels b, c, and d are shown in panel e. Note how a small rotation of an elongated target could be equivalent to a larger rotation of a less elongated target. Similarly, a large rotation about the target CM could be equivalent to a smaller rotation with a lever-arm. Evidently, prostate measured rotation, θ, is an insufficient indicator of dose decrement. We introduce a new metric, equivalent rotation, to quantify target rotations by accounting for prostate elongation and the length of the lever-arm of rotation. Equivalent rotation, θ' is defined as
Figure 1.
Simulations illustrating the impact of target elongation, offset of rotational centeroid, and magnitude of rotations on target coverage. Panel (a) a target with a 5 mm PTV expansion (grey region) can be rotated by 90° about its center while remaining within the PTV while a more elongated target (panel b) can rotate 20° only to stay within the PTV. A 20° rotation about target center (panel c) causes 6% of the target to be outside the PTV while a 20° off-center rotation (panel d) results in a 13% of target outside the PTV. Panel (e) illustrates the percentage of the CTV that remains within the static PTV for these cases.
| Eqn. 1 |
The geometry factor, G, is a dimensionless factor defined as
| Eqn. 2 |
R is the ratio of the prostate average dimension in the anterior-posterior direction to its length along the superior-inferior axis. Δ the distance between the prostate CM and transponders centroid (i.e. centroid of rotations) normalized to the prostate length along the superior-inferior axis. It is important to note that both R and Δ are additive, i.e. they will impact rotation independently. The target coverage will be evaluated for changes in θ' instead of θ which allows for meaningful comparisons of target coverage among patients.
Results
I. Planned dose and PTV margins
The adequacy of prostate coverage from the planned dose was assessed using the D95 = 79 Gy and the V79 = 95% criteria. These criteria were achieved in all patients for 0, 2, 3, and 5 mm PTV margins. While decreasing the PTV margin did not affect the CTV coverage, it did affect the rectum and bladder coverage. On average, the bladder V60 increased from 5% – 10% and V65 increased from 2–5% when the PTV margin increased from 2–5 mm. The rectumV70 increased from 3–7% when the margin increased from 2–5 mm.
II. Delivered dose and PTV margins
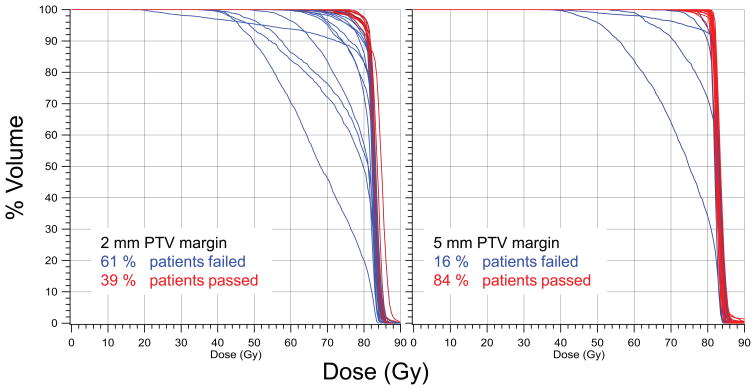
Adequate CTV coverage was achieved in 84%, 65%, and 39% of the patients using 5, 3, and 2 mm PTV margins, respectively. The DVHs, calculated from the prostate delivered dose, shown in Figure 2 demonstrate how the adequacy of the PTV margins varies among patients. .
Figure 2.
DVH’s computed from the prostate delivered dose.
III. Dosimetric coverage and prostate motion
a) Daily Average Rotations
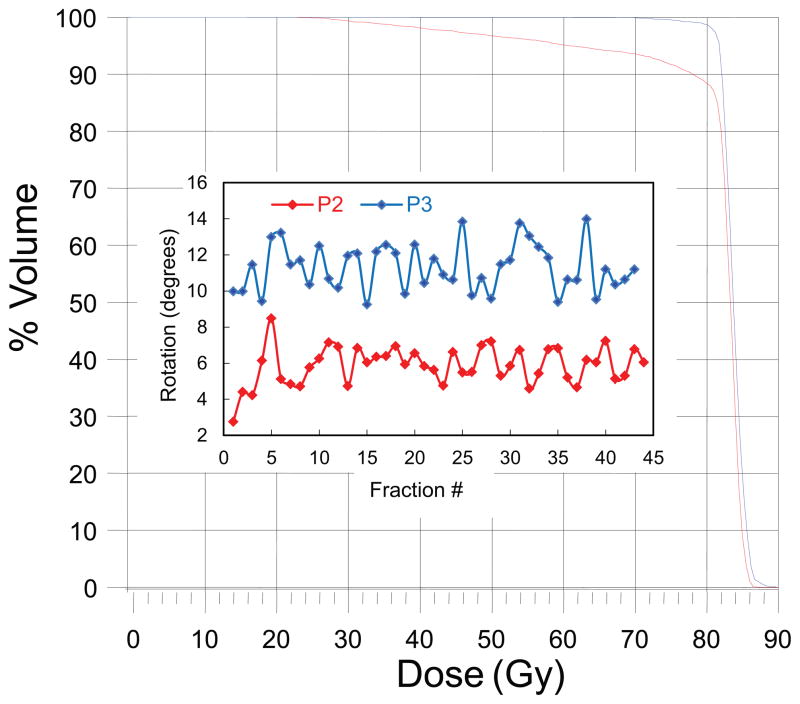
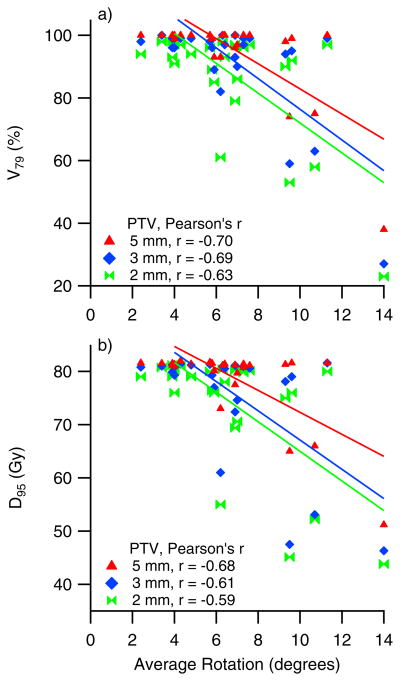
The prostate average rotations about the right-left axis versus fraction number for two patients are shown in Figure 3. The larger average rotation seen in patient P3 (θave = 11º) compared to P2 (θave = 6º) may intuitively suggest a larger dose decrement to the target in patient P3. However, the DVHs displayed in Figure 3 show a larger dose decrement in patient P2. Similar results have been seen in other patients as is illustrated in Figure 4 where target coverage is plotted against prostate average rotations for the whole patient cohort. This data indicate that some patients are more sensitive to rotation than others. In addition, the lack of a clear relationship between D95 or V79 with angle indicate that there are other factors that influence target coverage besides rotation angle. The Pearson’s r values for a linear regression fit of the data above 4 degrees yields are −0.62 to −0.70 for V79 and −0.59 to −0.68 for D95.
Figure 3.
Prostate average rotations about the RL axis along with the DVH’s calculated from the delivered dose are shown in this Figure.
Figure 4.
This figure illustrates the lack of correlations between target coverage (D95 and V79) and measured rotations about the RL axis. Data shown represent all patients for all PTV margins used in the analysis.
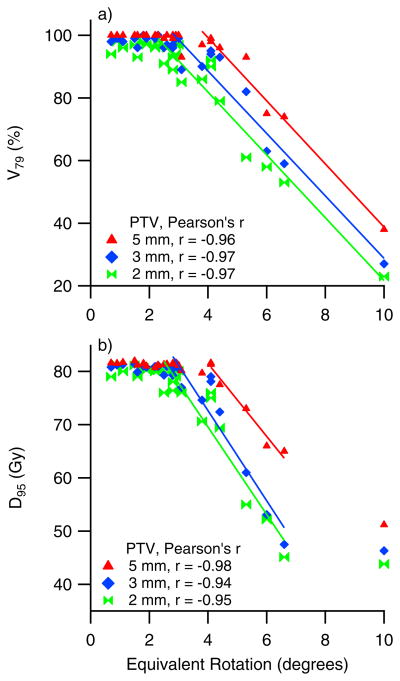
b) Equivalent rotations
Patient-specific prostate elongation, R, and lever arm, Δ, parameters were used to determine prostate equivalent rotation, θ'. D95 and V79 are plotted in Figure 5 against θ', rotations about the right-left axis only were considered. With a 5 mm PTV margin, dosimetric coverage remains adequate when θ' values are kept below 4, after which it begins to fall with increasing θ'. Similar trends are seen for 2 mm and 3 mm PTV margins. Decreasing the PTV margin causes a steeper fall off of D95 which starts at smaller θ' values, e.g. 2–3 for 2mm and 3 mm PTV margins, respectively.
Figure 5.
The correlations between target coverage and prostate equivalent rotations are demonstrated in these plots of D95 and V79 plotted vs. prostate equivalent rotations
Considering prostate equivalent rotations, instead of rotations, explains the inconsistency in the dosimetric coverage seen in patients P3 and P2 (discussed above). The larger equivalent rotation in patient P2 is consistent with larger decrement to the delivered dose seen in this patient as compared to patient P3. The Pearson’s r values for a linear regression fit of the linear portion of the data are about 0.97 for V79 and −0.94 to −0.98 for D95. These values indicate that once the shape of the prostate and the lever-arm of rotation are considered through use of Eqn. 1 and 2, in addition to rotation angle, a clear trend emerges in the data.
Discussion
Studies quantifying the dosimetric impact of prostate rotations on target coverage during radiotherapy are essential to guide clinicians in the management of prostate rotations. This study provides results on the quantification of the dosimetric impact of prostate daily average rotations (inter- and average intra-fractional rotations) and translations utilizing real-time tracking data from an extended cohort of patients. We show that even after virtually eliminating prostate translation during treatment, rotation can still have a substantial impact on prostate delivered dose.
Cranmer-Sargison8, reported that a 5° degree rotation about the RL axis can be detrimental to target coverage in some patients. Hertin et al.9, on the other hand, concluded that prostate rotations around RL axis have minimal impact on prostate coverage given that translations were managed. This conclusion was based on evaluating dosimetric impact of prostate rotations using simulated treatment plans with different rotation errors.9 This disagreement could be attributed to using simulated rotational errors on 5 patient CT data vs. real rotations measured in 26 patients or the nature of simulated error.
Unlike rotations, the management of translations during prostate radiotherapy is well addressed in the literature and could be easily achieved using a 3–5 mm action threshold for intervention as typically used with electromagnetic or radiographic guidance. However, prostate rotations and translations are inter-related and if managed separately could produce misleading results.5, 6 Li et al5, for example, concluded that using 2 mm PTV margin expansion cause no reduction in prostate dose. That was based on the analysis of intra-fraction translations on prostate dose using data from 35 patients with various PTV margins. It is not surprising that adequate coverage could be achieved with 2 mm PTV margin if the effects of prostate rotations are not accounted for.
Large variations in prostate rotations were seen among patients in this study confirming incidence of prostate rotations previously reported in the literature.9,10 It was anticipated based on these variations that prostate delivered dose would also vary among patients. The data shown in figure 2 demonstrate these variations for each PTV margin used in the analysis. It is surprising, though, to see underdosing in 16% of the patients when using a 5 mm PTV margin. This expansion is been used in some clinical practices and considered large enough to achieve adequate coverage, especially with radiographic image or electromagnetic guidance. Interestingly, adequate coverage was achieved in 40% of the patients using 2 mm margin despite prostate rotations. A tight margin could be used in such patients to avoid potential toxicity to neighboring normal tissues.
We investigate if these dosimetric variations could be sufficiently explained by variations in prostate rotations seen among patients. Our data showed that the variations in prostate rotations seen among patients are inconsistent with the dosimetric coverage achieved in these patients, see figures 4. Consequently, it is not possible to determine a rotational tolerance and PTV margin that would apply to all patients. Some patients appear to be more sensitive to rotations indicating that the impact of rotation on target coverage is patient-specific and, therefore, rotational tolerances and PTV margins should be individualized. Cranmer-Sargison8 recommended that prostate rotations should be kept below 5° degrees. However, using a 5° tolerance for all patients would be unnecessarily tight for some patients leading to unwarranted corrective action and a disruption to clinical flow. For other patients, such tolerance could lead to target underdosing. The challenge is how to, prospectively, identify whether a patient belongs to one group or the other.
To address this issue, we investigated variations seen among patients that may have contributed to the absence of correlations seen in Figure 4 such as prostate shape and the location of the fiducial markers within the prostate. The simulations shown in Figure 1 demonstrate how prostate shape and the location of the rotational centroid relative to prostate center of mass impact the consequence of rotations. These variations must be accounted for before a rotational tolerance and a PTV margin are used equally for all patients. Therefore, a new metric, θ', was defined to quantify prostate rotations accounting for prostate shape and length of lever arm of rotation as is defined in Eq 1. Figure 5 shows how well θ' correlates with prostate dosimetric coverage. Because the DVHs were computed with rotations about all three axes and the data separates into clear trends when only rotations about the left-right axis are plotted, this indicates that the dosimetric consequences of rotations about the other axes are relatively small, though extension of this work about all three rotational axes may further improve the results. The data also suggests that for patients with large prostate elongation and/or with a rotational center close to the periphery of the prostate, a smaller rotational tolerance and/or a larger PTV margin should be considered. Clearly, the data showed that rotational tolerances and PTV margins could be individualizing given how a 5 mm PTV margin is unnecessarily large for 39% of patients and could have been safely reduced to 2 mm while still achieving adequate coverage. On the other hand, tighter rotational tolerances should be used in 16% of the patients to achieve adequate coverage even when using 5 mm margin. The remaining 45% of patients would benefit from reduced PTV margins, though requiring some degree of intervention to manage rotations.
In our institute, we are able to prospectively determine individualized rotational tolerances and PTV margins utilizing the dose calculation tool developed in this study and CT data. It’s not likely that many clinics will be able to perform similar analysis, the data in Figure 5 could provide some guidance to clinicians on setting rotational tolerances during prostate RT. keeping in mind that Figure 5 may change when using different treatment planning techniques and/or optimization criteria
The reliability of the analysis employed in this study depends on whether or not the prostate shape and the lever-arm length remain stable throughout the patient’s course of treatment. Studies in the literature support the rigidity of the prostate during RT and showed negligible intra-fractional volumetric variations14 and small inter-fraction deformation of the prostate which is independent of rectal filling.15 Other studies showed that the implanted FM demonstrates a long-term stability.16
For future analysis, real-time intra-fractional variations in prostate rotations will be used instead of average rotations. Real-time beam and multi-leaf collimator information acquired during treatment will be used17 to enable the reconstruction of the prostate delivered dose using instantaneous tracking data. Currently, the use of prostate average rotations could be justified by the very small variations in intra-fractional rotations seen in most patients. It has previously been reported that the standard deviation of rotations about the left-right axis ranges from 2º to 5º, over all fractions for a single patient, with larger variations for patients with larger systematic rotations. For any given fraction the standard deviation would typically be on this order or smaller. Variations in the rotation about the anterior-posterior and inferior-superior axes typically are significantly smaller, ranging from 0.5º to 2º. Consequently, dosimetric variations from the results presented here, due to intra-fraction motions, exist are expected to be small.
CONCLUSION
The dosimetric impact of prostate inter-fractional and average intra-fractional rotations has been quantitatively evaluated utilizing tracking data from 26 patients. Our study showed significant underdosing in 16% of the patient populations even when using 5 mm PTV margin if rotations are left unmanaged during RT.
This study revealed that the magnitude of prostate rotations is insufficient predictor of dose decrement to target during RT. And that the consequence of rotations depends on prostate shape and the location of the rotational centroid within the prostate. Prostate equivalent rotation, a newly defined metric is introduced to quantify prostate rotations accounting for prostate measured rotations, prostate shape, and rotational centroid location. Prostate equivalent rotations did correlate with the prostate coverage and can be used as a predictor of dose decrement. This study showed that rotational tolerances should be individualized and could be prospectively derived with proper dose calculation tools.
Acknowledgments
This work was supported by NIH grant R21 CA110485-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodford C, Yartsev S, Dar AR, et al. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen megavoltage computed tomography images. Int J Radiat Oncol Biol Phys. 2007;69:1316–1322. doi: 10.1016/j.ijrobp.2007.07.2369. [DOI] [PubMed] [Google Scholar]
- 2.Wu QJ, Thongphiew D, Wang Z, et al. On-line re-optimization of prostate IMRT plans for adaptive radiation therapy. Phys Med Biol. 2008;53:673–691. doi: 10.1088/0031-9155/53/3/011. [DOI] [PubMed] [Google Scholar]
- 3.Lee L, Le QT, Xing L. Retrospective IMRT dose reconstruction based on cone-beam CT and MLC log-file. Int J Radiat Oncol Biol Phys. 2008;70:634–644. doi: 10.1016/j.ijrobp.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Lee RJ, Handrahan D, et al. Intensity-modulated radiotherapy using implanted fiducial markers with daily portal imaging: assessment of prostate organ motion. Int J Radiat Oncol Biol Phys. 2007;68:912–919. doi: 10.1016/j.ijrobp.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Li HS, Chetty IJ, Enke CA, et al. Dosimetric consequences of intrafraction prostate motion. Int J Radiat Oncol Biol Phys. 2008;71:801–812. doi: 10.1016/j.ijrobp.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Litzenberg DW, Balter JM, Hadley SW, et al. Influence of intrafraction motion on margins for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:548–553. doi: 10.1016/j.ijrobp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Sheng J, Lihu J, Pollack A, et al. Gains from real-time tracking of prostate motion during external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2009;75:1613–1620. doi: 10.1016/j.ijrobp.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Cranmer-Sargison G. A treatment planning investigation into the dosimetric effects of systematic prostate patient rotational set-up errors. Med Dosim. 2008;33:199–205. doi: 10.1016/j.meddos.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.van Herten YR, van Kamer JB, van Wieringen N, et al. Dosimetric evaluation of prostate rotations and their correction by couch rotations. Radiother Oncol. 2008;88:156–162. doi: 10.1016/j.radonc.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Willoughby TR, Kupelian PA, Pouliot J, et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Noel CE, Santanam L, Olsen JR, et al. An automated method for adaptive radiation therapy for prostate cancer patients using continuous fiducial-based tracking. Phys Med Biol. 2010;55:65–82. doi: 10.1088/0031-9155/55/1/005. [DOI] [PubMed] [Google Scholar]
- 13.RTOG0126. RTOG 0126: A phase III Randomized Study of High Dose 3D-CRT/IMRT Versus Standard Dose 3D-CRT/IMRT in Patients Treated for Localized Prostate Cancer 2004.
- 14.Deurloo KE, Steenbakkers RJ, Zijp LJ, et al. Quantification of shape variation of prostate and seminal vesicles during external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:228–238. doi: 10.1016/j.ijrobp.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Nichol AM, Brock KK, Lockwood GA, et al. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007;67:48–56. doi: 10.1016/j.ijrobp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Litzenberg DW, Willoughby TR, Balter JM, et al. Positional stability of electromagnetic transponders used for prostate localization and continuous, real-time tracking. Int J Radiat Oncol Biol Phys. 2007;68:1199–1206. doi: 10.1016/j.ijrobp.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Litzenberg DW, Hadley SW, Tyagi N, et al. Synchronized dynamic dose reconstruction. Med Phys. 2007;34:91–102. doi: 10.1118/1.2388157. [DOI] [PubMed] [Google Scholar]