Abstract
Background
The mucin 1 (MUC1) heterodimeric oncoprotein is overexpressed in human prostate cancers with aggressive pathologic and clinical features. However, few insights are available regarding the functional role of MUC1 in prostate cancer.
Methods
Effects of MUC1-C on AR expression were determined by RT-PCR, immunoblotting and AR promoter activation. Coimmunoprecipitations, direct binding assays and chromatin immunoprecipitation (ChIP) studies were performed to assess the interaction between MUC1-C and AR. Cells were analyzed for invasion, growth in androgen-depleted medium and sensitivity to MUC1-C inhibitors.
Results
The present studies in androgen-dependent LNCaP and LAPC4 prostate cancer cells demonstrate that the oncogenic MUC1-C subunit suppresses AR expression. The results show that MUC1-C activates a posttranscriptional mechanism involving miR-135b-mediated downregulation of AR mRNA levels. The results further demonstrate that MUC1-C forms a complex with AR through a direct interaction between the MUC1-C cytoplasmic domain and the AR DNA-binding domain. In addition, MUC1-C associates with AR in a complex that occupies the PSA promoter. The interaction between MUC1-C and AR is associated with induction of the epithelial-mesenchymal transition (EMT) and increased invasion. MUC1-C also conferred growth in androgen-depleted medium and resistance to bicalutamide treatment. Moreover, expression of MUC1-C resulted in sensitivity to the MUC1-C inhibitor GO-203 with inhibition of growth in vitro. GO-203 treatment also inhibited growth of established tumor xenografts in nude mice.
Conclusions
These findings indicate that MUC1-C suppresses AR expression in prostate cancer cells and confers a more aggressive androgen-independent phenotype that is sensitive to MUC1-C inhibition.
Keywords: MUC1-C, androgen receptor, prostate cancer, androgen-independent growth, targeted therapy
Introduction
Mucin 1 (MUC1) is a heterodimeric complex that is aberrantly overexpressed in human prostate and other carcinomas [1]. As such, MUC1 has been considered an attractive target for the development anti-cancer agents. However, early attempts at targeting MUC1, particularly with antibodies, were largely unsuccessful [2]. In this regard, MUC1 consists of two subunits that result from autocleavage of a single polypeptide product from the MUC1 gene [1]. The MUC1 N-terminal cleavage fragment (MUC1-N) contains the characteristic glycosylated tandem repeat structure of mucin family members [1]. MUC1-N forms a cell surface complex with the MUC1 C-terminal fragment that spans the cell membrane and includes a transforming cytoplasmic domain [1,3,4]. The demonstration that MUC1-C is oncogenic provided the basis for the design of inhibitors that block its function [5]. In this capacity for inducing transformation, MUC1-C interacts with the epidermal growth factor receptor (EGFR) and other receptor tyrosine kinases (RTKs) at the cell membrane [1,6]. In addition, with overexpression as found in carcinoma cells, MUC1-C accumulates in the cytoplasm and localizes to the nucleus, where it contributes to the regulation of gene expression [1,7]. In the nucleus, MUC1-C interacts with certain transcription factors, such as NF-κB p65 and STAT3, and promotes activation of their target genes [8,9]. The MUC1 gene itself is activated by NF-κB p65 and STAT3, thus forming an autoinductive loop in which MUC1-C contributes to the overexpression of MUC1 in carcinoma cells [8,9]. As an integral part of this loop, the MUC1-C cytoplasmic domain contains a CQC motif that is necessary for the formation of MUC1-C dimers and the import of MUC1-C to the nucleus [10]. Blocking the MUC1-C CQC motif with cell-penetrating peptides thus inhibits nuclear MUC1-C localization and its transforming function [11].
MUC1 is overexpressed in prostate cancers that are associated with more aggressive disease [12–17]. In this context, MUC1 expression was detected in ~90% of primary prostate cancers that were Gleason grade ≥7 or were metastatic to lymph nodes [12,13]. Moreover, gene expression profiling of prostate cancers has shown that MUC1 is highly expressed in those with aggressive clinicopathologic features and an elevated risk of recurrence [18]. In human prostate cancer cell lines, MUC1 is expressed at high levels in the androgen-independent DU145 and PC3 models, which have low to undetectable androgen receptor (AR) abundance [8,19]. By contrast, the androgen-dependent LNCaP, androgen-responsive CWR22Rv1, and androgen-sensitive MDA PCa 2b prostate cancer cells express AR and little if any MUC1, supporting a potential inverse relationship between these two proteins [8,19]. Indeed, stable introduction of AR in PC3 cells was associated with downregulation of MUC1 expression [19]. The basis for this effect was attributed in part to AR occupancy of the MUC1 promoter and suppression of MUC1 gene transcription [19]. In addition, AR-mediated upregulation of miR-125b [20] was shown to contribute to suppression of MUC1 translation [19,21]. Thus, AR signaling suppresses MUC1 expression by transcriptional and posttranscriptional mechanisms. In concert with these observations, treatment of LNCaP, CWR22Rv1 and MDA PCa 2b cells with a MUC1-C inhibitor had no apparent effect on growth or survival [22]. However, the MUC1-C-positive DU145 and PC3 cells responded to MUC1-C inhibition with induction of cell death in vitro [22]. Established DU145 and PC3 tumor xenografts in nude mice were also sensitive to MUC1-C inhibitor treatment as evidenced by prolonged regressions [22]. These findings indicate that AR signaling downregulates MUC1 abundance and that MUC1 is overexpressed in certain prostate cancer cells, which in turn are sensitive to MUC1-C inhibitors.
The present studies demonstrate that MUC1-C suppresses AR expression in prostate cancer cells by a posttranscriptional miR-135b-mediated mechanism. The results also show that MUC1-C interacts directly with AR and forms complexes with AR on the promoter of the PSA gene. The interaction between MUC1-C and AR is associated with induction of (i) EMT, (ii) invasion, (iii) androgen-independent growth, and (iv) sensitivity to MUC1-C inhibition.
Materials and Methods
Cell culture
Human LNCaP prostate cancer cells (ATCC) were cultured in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum (HI-FBS), 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine. LAPC4 cells were grown in Iscove’s Modified Dulbecco’s medium (IMDM) with 5% HI-FBS, antibiotics and L-glutamine. LNCaP and LAPC4 cells were infected with lentiviruses expressing GFP or MUC1-C and selected in hygromycin. In certain experiments, the cells were grown in phenol red-free medium containing charcoal-stripped serum (CSS) (Cellgro). Cells were treated with bicalutamide (Sigma). Cells were also treated with the MUC1-C inhibitor GO-203 or the control peptide CP-2 as described [23].
Immunoprecipitation and immunoblotting
Lysates from subconfluent cells were prepared as described [8]. Soluble proteins were precipitated with anti-AR (H-280; Santa Cruz Biotechnology) or anti-MUC1-C [24]. Immune precipitates and lysates not subjected to precipitation were immunoblotted with anti-AR, anti-MUC1-C, anti-PSA (Santa Cruz Biotechnology), anti-β-actin (Sigma), anti-E-cadherin (Cell Signaling Technology) and anti-vimentin (Santa Cruz Biotechnology). Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare Biosciences).
RT-PCR analysis of mRNA expression
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen). RNAs were analyzed using the One-Step RT-PCR Kit with Platinum Taq (Invitrogen) and AR-specific primers or GAPDH-specific primers (Supplemental Table 1). Amplified fragments were analyzed by electrophoresis in 2% agarose gels.
AR promoter-luciferase assays
Cells were transfected with pGL3 or pGL3-pAR-Luc and, as an internal control, SV-40-Renilla-Luc (Promega) in the presence of Lipofectamine 2000. After 48 h, the cells were lysed in passive lysis buffer. The lysates were analyzed for firely and Renilla luciferase activities using the dual luciferase assay kit (Promega).
Analysis of miRNA expression
Total RNA was assessed for miR-135b and miR-147 expression using a small RNA specific RT-PCR kit (Systems Biosciences) with a universal reverse primer and specific forward primers (Supplemental Table 2). Human U6 small RNA was used as a control.
In vitro binding assays
GST, GST-MUC1-CD, GST-MUC1-CD(1–46), and GST-MUC1-CD(46–72) were prepared as described [8]. GST-AR, GST-AR(3–557), GST-AR(528–627) and GST-AR(625–910) were generated as described [25]. Purified GST-MUC1-CD and GST-AR(528–627) were cleaved with thrombin to remove the GST moiety. Adsorbates to glutathione-conjugated beads were analyzed by immunoblotting.
Chromatin immunoprecipitation (ChIP) assays
Soluble chromatin was prepared as described [26] and precipitated with anti-AR, anti-MUC1-C or a control nonimmune IgG. For re-ChIP assays, AR complexes from the initial ChIP were eluted and reimmunoprecipitated with anti-MUC1-C as described [19]. For PCR, 2 µl for a 50 µl DNA extraction were used with 25 to 35 cycles of amplification. The primers used for PCR and qPCR are listed in Supplemental Tables 3 and 4, respectively.
Cell invasion assays
Cell invasion assays were performed using 8 mm pores size transwell chambers (Costar) coated with 1:1000 Matrigel (BDBioscience) as described [27].
Tumor xenograft studies
Four- to 6-week-old castrated BALB/c nu/nu male mice were injected subcutaneously with 1 × 107 LNCaP/MUC1-C cells in the flank. When tumors were detectable, the mice were pair-matched into control and treatment groups of 10 mice each. Mice were excluded if the tumors were not within 15% of the mean tumor volume. PBS (control vehicle) or 7.5 mg/kg body weight GO-203 (dissolved in PBS) were administered daily by intravenous injection for 21 days. Tumor volume was calculated as described [23].
Results
MUC1-C downregulates AR expression
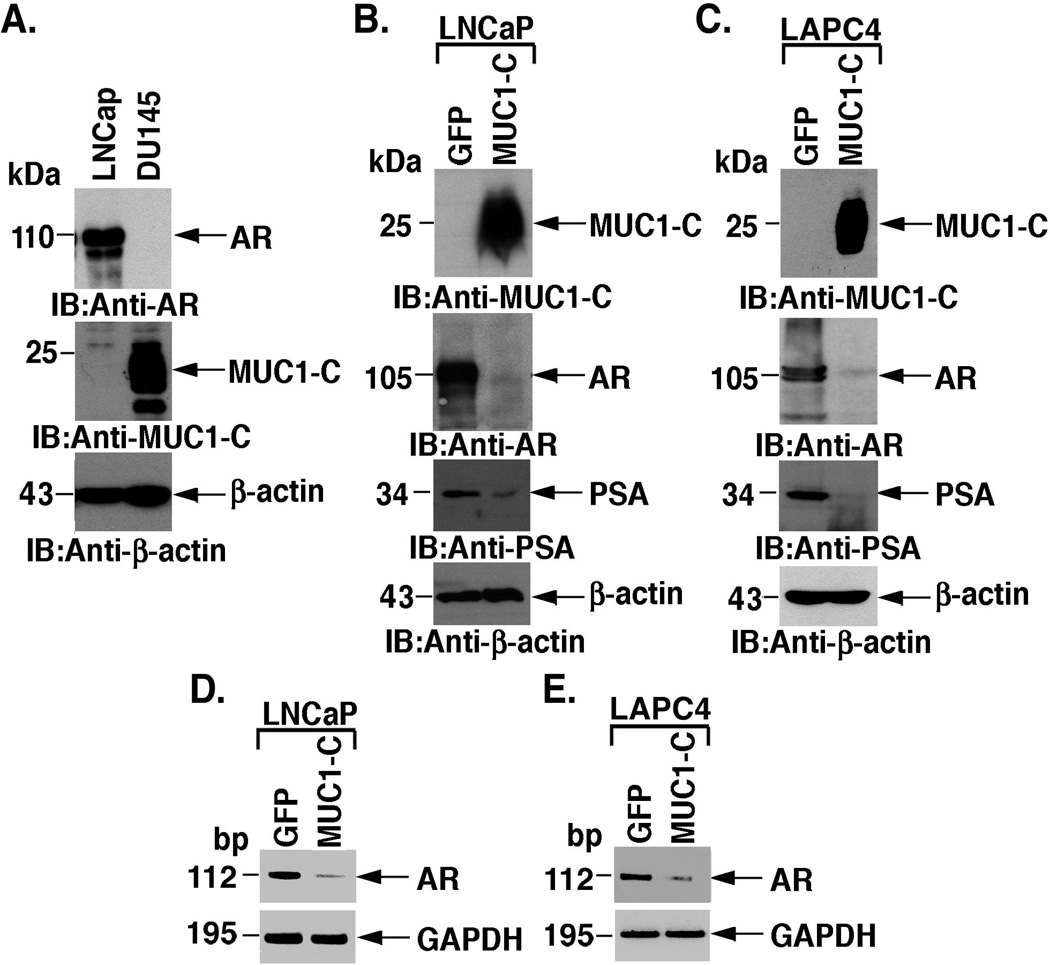
Previous studies showed that AR regulates MUC1-C expression in prostate cancer cells [19]. The androgen-dependent LNCaP prostate cancer cells express AR and low to undetectable levels of MUC1-C (Fig. 1A). By contrast, the androgen-independent DU-145 prostate cancer cells have the opposite pattern, that is, undetectable levels of AR and upregulation of MUC1-C (Fig. 1A). To assess the effects of MUC1-C on AR levels, we stably expressed MUC1-C or, as a control, GFP in LNCaP cells (Fig. 1B). Notably, MUC1-C expression was associated with marked downregulation of AR protein (Fig. 1B). MUC1-C expression was also associated with decreases in PSA levels, consistent with AR-dependent regulation of the PSA gene (Fig. 1B). To extend these observations, androgen-dependent LAPC4 prostate cancer cells were stably transfected to express GFP or MUC1-C. Like LNCaP cells, LAPC4 cells express AR, but not MUC1-C (Fig. 1C). Moreover, expression of MUC1-C in LAPC4 cells resulted in downregulation of AR and PSA protein (Fig. 1C). RT-PCR further demonstrated that MUC1-C decreases AR expression at the mRNA level in LNCaP (Fig. 1D) and LAPC4 (Fig. 1E) cells. These findings indicate that MUC1-C suppresses AR expression.
Figure 1. MUC1-C suppresses AR expression.
A. Lysates from LNCaP and DU145 cells were immunoblotted with the indicated antibodies. B and C. LNCaP (B) and LAPC4 (C) cells were stably transfected to express GFP or MUC1-C. Lysates were immunoblotted with the indicated antibodies. D and E. Total RNA from the indicated LNCaP (D) and LAPC4 (E) cells was analyzed by RT-PCR for AR and, as a control, GAPDH mRNA levels.
MUC1-C decreases AR mRNA levels by a miRNA-dependent mechanism
To assess the effects of MUC1-C on AR gene transcription, we transfected the LNCaP/GFP and LNCaP/MUC1-C cells with a vector containing the AR promoter upstream to the luciferase gene (pAR-Luc). Analysis of luciferase activity demonstrated that MUC1-C has little if any effect on AR promoter activation (Fig. 2A). Similar results were obtained in LAPC4 cells (data not shown), indicating that MUC1-C downregulates AR expression at the post-transcriptional level. Previous work has identified certain miRNAs downregulate AR expression in prostate cancer cells [28,29]. Among these, we found that expression of MUC1-C in LNCaP cells is associated with upregulation of miR-135b and miR-147 (Fig. 2B). To confirm that miR-135b downregulates AR mRNA levels, we overexpressed miR-135b in LNCaP cells. Analysis of AR mRNA levels at 48 and 96 h after transfection demonstrated decreases in AR transcripts (Fig. 2C). Overexpression of miR-135b was also associated with decreases in AR protein (Fig. 2D). These findings indicate that MUC1-C decreases AR expression at least in part by upregulation of miR-135b.
Figure 2. MUC1-C suppresses AR expression by a miR-135b-mediated mechanism.
A. LNCaP/GFP and LNCaP/MUC1-C cells were transfected with the empty pGL3 vector or 0.1 µg (open bars), 0.2 µg (shaded bars) and 0.5 µg (solid bars) pAR-Luc. As an internal control, the cells were also transfected with the SV-40-Renilla-Luc plasmid. Luciferase activity was measured at 48 h after transfection. The results are expressed as relative luciferase activity (mean±SD from three separate experiments) compared to that obtained from cells transfected with the empty pGL3 vector (assigned a value of 1). B. Total RNA from the indicated LNCaP cells was analyzed for miR-135b and miR-147 levels. U6 expression was included as a control. C and D. LNCaP cells were transfected with a control scrambled siRNA for 48 h or miR-135b for the indicated times. Total RNA was analyzed for AR and GAPDH mRNA levels by RT-PCR (C). Lysates were immunoblotted with the indicated antibodies (D).
MUC1-C cytoplasmic domain binds directly to AR
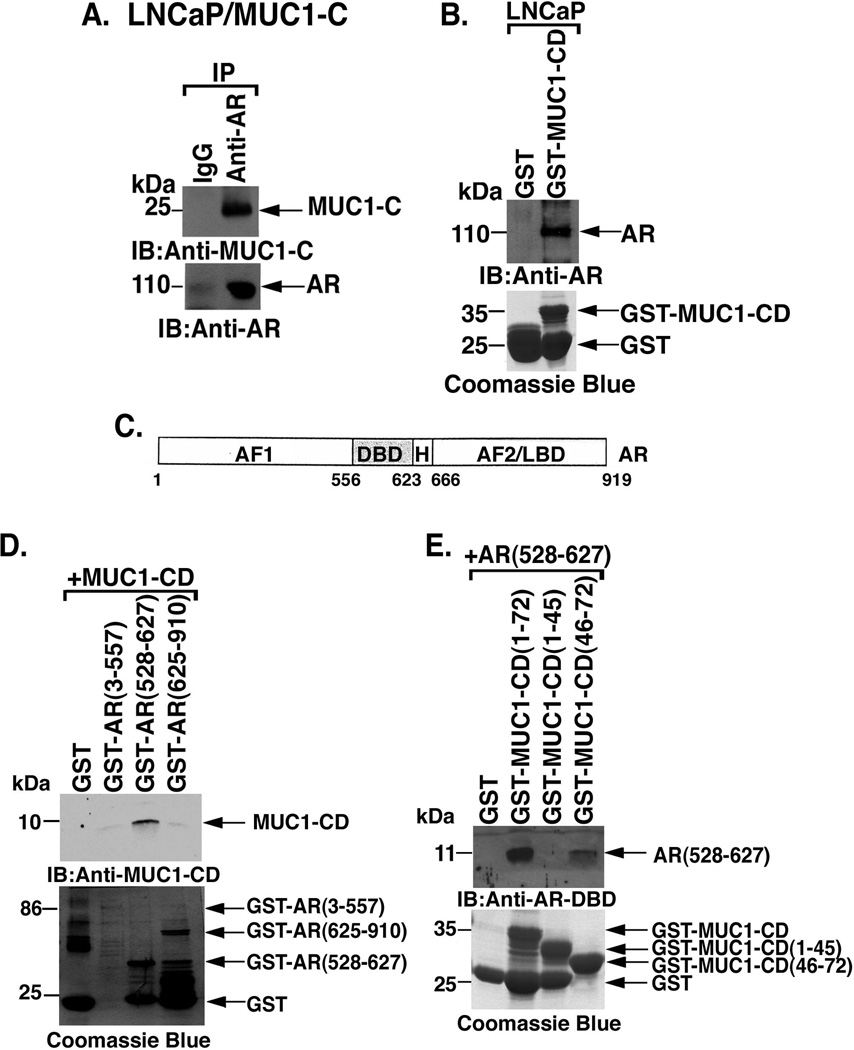
Previous work demonstrated that MUC1-C associates with ERα and that the MUC1-C cytoplasmic domain binds directly to the ERα DNA binding domain (DBD) [26]. To determine whether MUC1-C associates with AR, coimmunoprecipitation studies were performed on lysates from LNCaP/MUC1-C cells. The detection of AR-MUC1-C complexes provided support for the contention that MUC1-C interacts with AR (Fig. 3A). To define in part the basis for the AR-MUC1-C interaction, LNCaP cell lysates were incubated with GST or GST-MUC1-CD. The results show that AR binds to GST-MUC1-CD and not GST, indicating that AR associates with the MUC1-C cytoplasmic domain (Fig. 3B). AR consists of an AF1 domain, DNA-binding domain (DBD), hinge region (H) and the AF2/ligand binding domain (LBD) (Fig. 3C). Incubation of MUC1-CD with GST-AR(3–557), GST-AR(528–627) or GST-AR(625–910) demonstrated that MUC1-CD binds to the AR DBD region (Fig. 3D). To further assess the interaction between MUC1-CD and the AR DNA binding domain, we incubated AR(528–627) with GST or GST-MUC1-CD. Analysis of the adsorbates with an anti-AR-DBD antibody confirmed direct binding of MUC1-CD to the AR-DBD (Fig. 3D). The MUC1-C cytoplasmic domain consists of 72 amino acids. The results also demonstrate that MUC1-CD(46–72), and not MUC1-CD(1–46), confers the interaction (Fig. 3E). These findings indicate that the MUC1-C cytoplasmic domain binds directly to AR at the DBD.
Figure 3. MUC1-C cytoplasmic domain binds directly to the AR DNA-binding domain.
A. Lysates from LNCaP/MUC1-C cells were precipitated with anti-MUC1-C or a control IgG. The precipitates were immunoblotted with the indicated antibodies. B. Lysates from LNCaP cells were incubated with GST or GST-MUC1-CD. The adsorbates were immunoblotted with anti-AR. Input of the GST proteins was assessed by Coomassie blue staining. C. Schematic representation of the AR protein with the AF1 domain, the DNA-binding domain (DBD), hinge region (H) and the AF2/ligand binding domain (LBD). D. MUC1-CD was incubated with GST, GST-AR(3–557), GST-AR(528–627) or GST-AR(625–910). The adsorbates were immunoblotted with anti-MUC1-CD. Input of the GST proteins was assessed by Coomassie blue staining. E. AR(528–627) was incubated with GST, GST-MUC1-CD, GST-MUC1-CD(1–45) or GST-MUC1-CD(46–72). The adsorbates were immunoblotted with anti-AR-DBD. Input of the GST proteins was assessed by Coomassie blue staining.
MUC1-C associates with AR on the PSA promoter
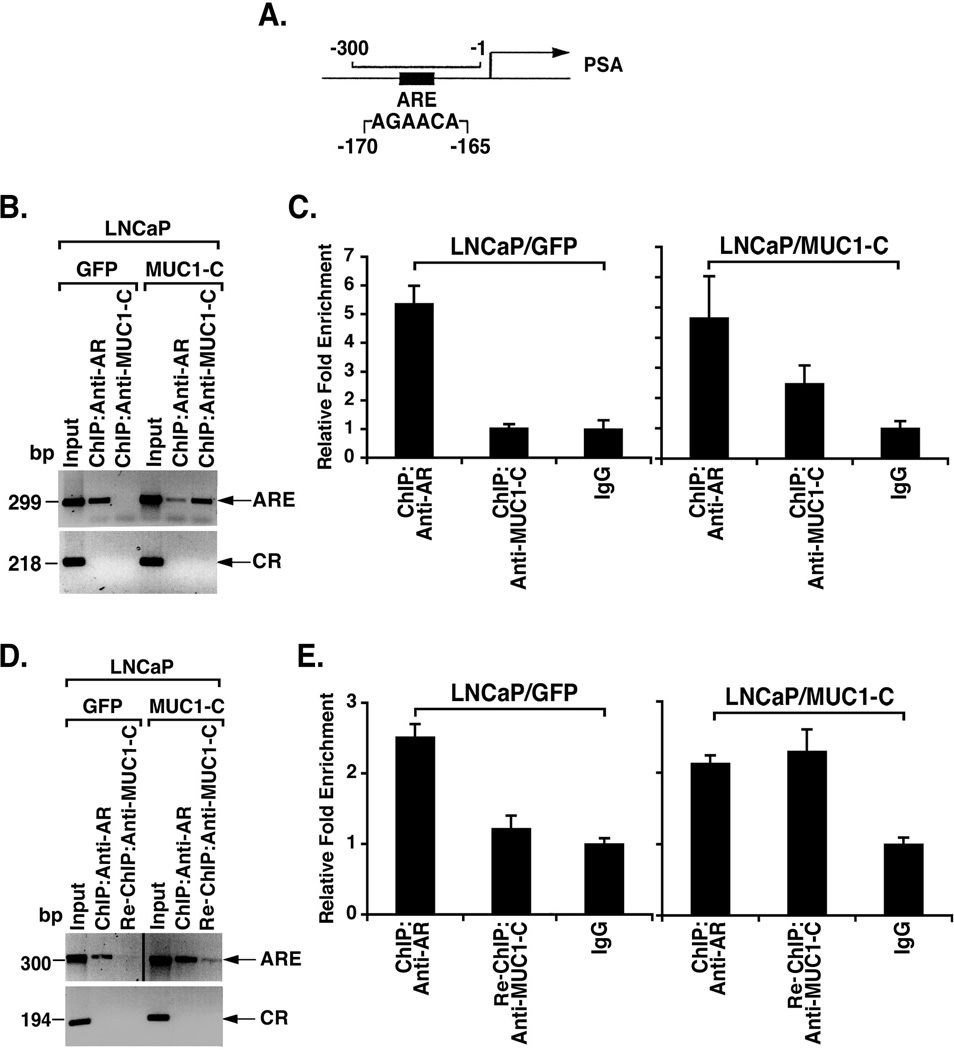
To determine whether AR associates with MUC1-C in the nucleus, we performed chromatin immunoprecipitation (ChIP) studies on the PSA gene promoter, which contains an androgen response element at positions −170 to −165 (AGAACA) (Fig. 4A). AR occupancy was detectable on the PSA promoter in LNCaP/GFP and LNCaP/MUC1-C cells (Fig. 4B). ChIP analysis further demonstrated that MUC1-C also occupies the PSA promoter in LNCaP/MUC1-C, but not LNCaP/GFP cells (Fig. 4B). ChIP qPCR analysis confirmed that both AR and MUC1-C occupy the PSA promoter in the LNCaP/MUC1-C cells (Fig. 4C). Re-ChIP studies were then performed to determine whether AR and MUC1-C are present as a complex. In contrast to the control LNCaP/GFP cells, re-ChIP analysis of the LNCaP cells demonstrated that AR associates with MUC1-C on the PSA promoter (Fig. 4D). Re-ChIP qPCR analysis confirmed that AR and MUC1-C form a complex on the PSA promoter in the LNCaP/MUC1-C cells (Fig. 4E). These findings indicate that MUC1-C interacts with AR on the PSA promoter.
Figure 4. MUC1-C associates with AR on the PSA promoter.
A. Schematic representation of the PSA gene promoter with positioning of the androgen response element (ARE). B and C. Soluble chromatin from LNCaP/GFP and LNCaP/MUC1-C cells was precipitated with anti-AR or anti-MUC1-C and then analyzed for PSA promoter sequences. The final DNA samples were amplified by PCR (B) and qPCR (C) with pairs of primers for the AR binding region (−300 to −1) or a control region (CR; −4893 to −4675). The results (mean±SD of three determinations) are expressed as the relative fold enrichment compared to that obtained with the IgG control (C). D and E. Soluble chromatin from LNCaP/GFP and LNCaP/MUC1-C cells was precipitated with anti-AR. The anti-AR precipitates were released, reimmunoprecipitated with anti-MUC1-C, and then analyzed for PSA promoter sequences. The final DNA samples were amplified by PCR (D) and qPCR (E). The results (mean±SD of three determinations) are expressed as the relative fold enrichment compared to that obtained with the IgG control (right).
MUC1-C induces EMT and invasion
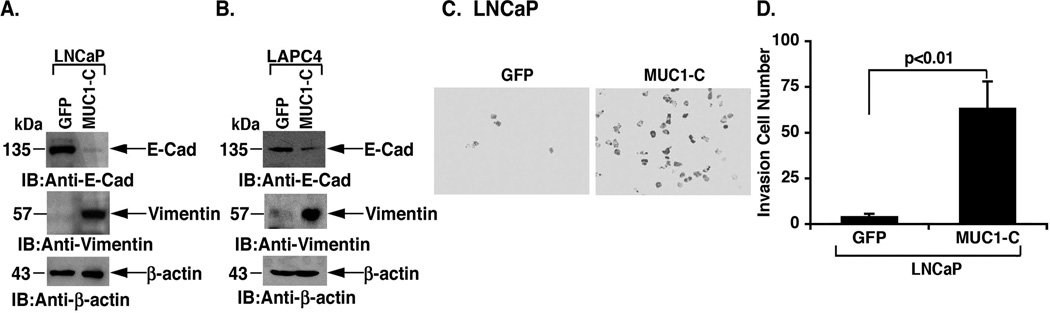
Overexpression of MUC1 in breast and pancreatic cancer cells has been associated with induction of EMT [30,31]. Moreover, downregulation of AR abundance has been linked to EMT and invasion of prostate cancer cells [32]. Accordingly, we asked if MUC1-C-induced suppression of AR expression is associated with markers of EMT. Indeed, the LNCaP/MUC1-C cells exhibited a decrease in E-cadherin levels as compared to that in LNCaP/GFP cells (Fig. 5A). In addition, MUC1-C expression was associated with upregulation of vimentin, consistent with the induction of EMT (Fig. 5A). Consistent with these results, MUC1-C decreased E-cadherin expression and upregulated vimentin levels in LAPC4 cells (Fig. 5B). To assess the effects of MUC1-C on invasion, studies were performed by seeding LNCaP cells in transwell plates. Invasion of the LNCaP/MUC1-C cells was increased visually compared to that found for the LNCaP/GFP cells (Fig. 5C). Quantification of the results confirmed that MUC1-C significantly increases LNCaP cell invasion (Fig. 5D).
Figure 5. Effects of MUC1-C on EMT and invasion.
A and B. Lysates from the stably transfected LNCaP (A) and LAPC4 (B) cells were immunoblotted with the indicated antibodies. C and D. LNCaP/GFP and LNCaP/MUC1-C cells (1 × 105) were seeded in 8 µm pore transwell chambers coated with 1:1000 matrigel and incubated for 24 h (C). The results are expressed as the invasion cell number (mean±SD of 5 fields) at 24 h (D).
MUC1-C confers androgen-independent growth
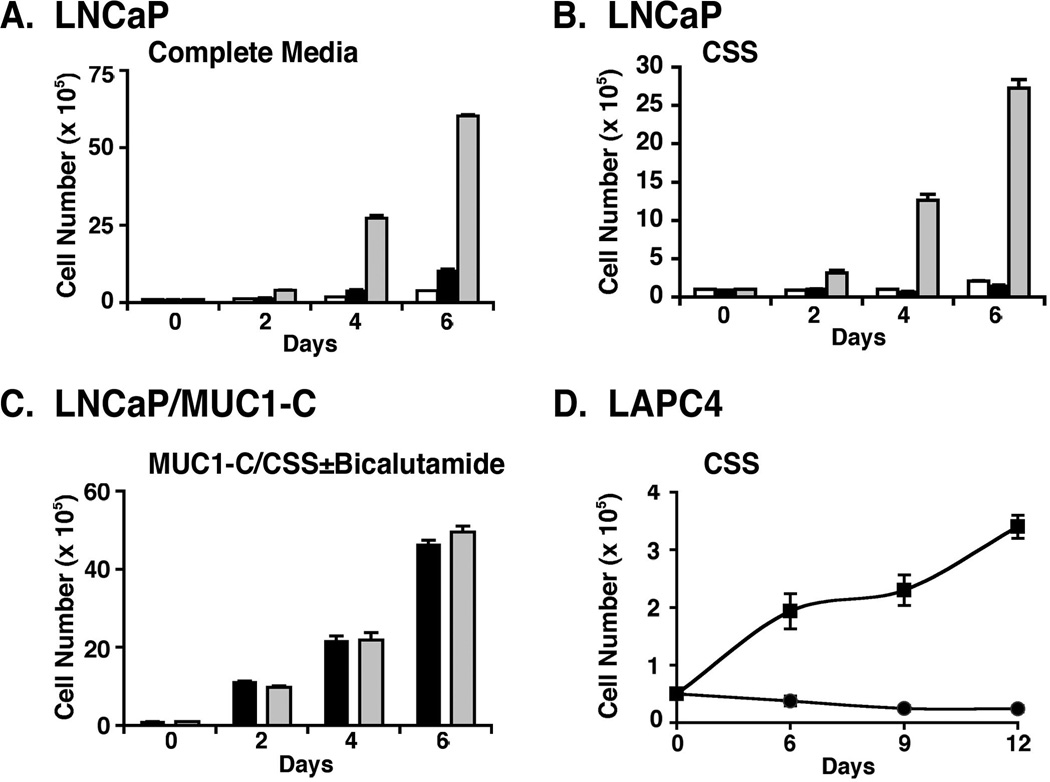
In association with the induction of EMT and invasion, LNCaP cell proliferation in complete medium was increased by MUC1-C expression (Fig. 6A). Moreover, as expected, proliferation of LNCaP and LNCaP/GFP cells was suppressed in charcoal-stripped medium (Fig. 6B). Importantly and in contrast, LNCaP/MUC1-C cells readily proliferated in this setting of androgen depletion (Fig. 6B). We also found that the LNCaP/MUC1-C cells grow in the presence of the anti-androgen, bicalutamide (Fig. 6C). Consistent with these results, LAPC4/MUC1-C, but not LAPC4/GFP, cells proliferated in androgen-depleted medium (Fig. 6D), indicating that MUC1-C confers androgen-independent growth.
Figure 6. MUC1-C confers androgen independent growth of LNCaP and LAPC4 cells.
A–B. LNCaP (open bars), LNCaP/GFP (solid bars) and LNCaP/MUC1-C (shaded bars) cells were seeded at 1 × 105 cells/ml in complete medium (A) or phenol red-free medium containing charcoal-stripped serum (CSS) (B). C. LNCaP/MUC1 cells were seeded at 1 × 105 cells/ml in CSS (solid bars) or CSS containing 100 µg/ml bicalutamide (shaded bars). D. LAPC4/GFP (circles) and LAPC4/MUC1-C (squares) cells were seeded at 0.5 × 105 cells/ml in CSS. Viable cell number was determined by trypan blue exclusion. The results are expressed as the cell number (mean±SD of three determinations).
Inhibition of MUC1-C blocks binding to AR and induces death of LNCaP/MUC1-C cells
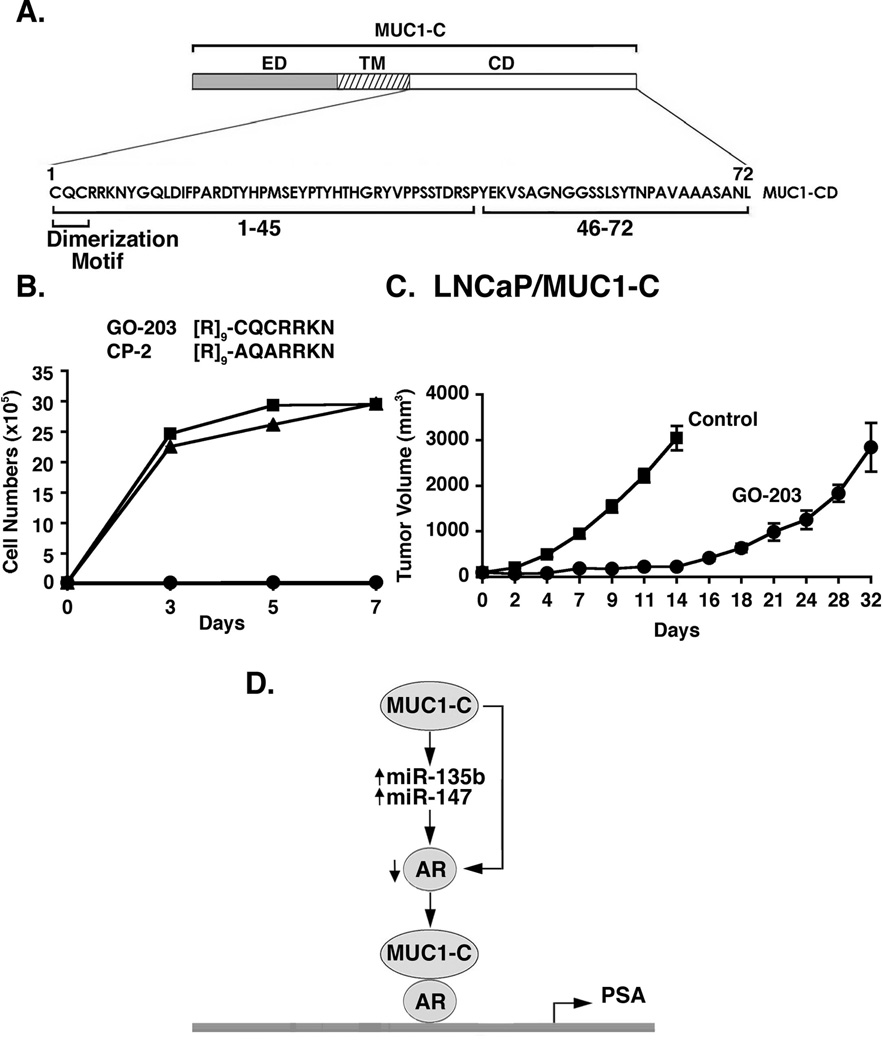
The MUC1-C cytoplasmic domain contains a CQC motif that is necessary for MUC1-C dimerization and interaction with other proteins (Fig. 7A) [10]. To inhibit MUC1-C dimerization, the cell-penetrating peptide GO-203 was synthesized that binds directly to the MUC1-C CQC motif in the cytoplasmic domain and blocks reactivity of this site (Fig. 7B) [11,23]. As a control, the CP-2 peptide, in which the Cys residues have been altered to Ala, is inactive in binding to the MUC1-C CQC motif (Fig. 7B). Previous studies have shown that inhibition of MUC1-C has no effect on growth of MUC1-negative LNCaP cells [22]. Notably, however, GO-203 was highly effective in inhibiting growth of LNCaP/MUC1-C cells (Fig. 7B). By contrast, the control CP-2 had no apparent effect on LNCaP/MUC1-C cell growth (Fig. 7B), indicating that expression of MUC1-C in LNCaP cells confers sensitivity to MUC1-C inhibition. To extend this analysis, we injected LNCaP/MUC1-C cells into the flanks of castrated nude mice. In contrast to LNCaP cells which do not grow in castrated mice [33], LNCaP/MUC1-C cells formed aggressive tumors that reached 3000 mm3 by day 14 when they were sacrificed (Fig. 7C). In addition, treatment with 7.5 mg/kg/d GO-203 intravenously for 21 d was associated with a significant delay in LNCaP/MUC1-C tumor growth (Fig. 7C).
Figure 7. Targeting of MUC1-C inhibits growth of LNCaP/MUC1-C cells.
A. Schematic of the MUC1-C subunit with the amino acid sequences of the MUC1-C cytoplasmic domain highlighting the CQC dimerization motif. B. D-amino acid sequences of the GO-203 and control CP-2 peptides. LNCaP/MUC1-C cells were left untreated (Control; squares) and treated with 5 µM GO-203 (circles) or CP-2 (triangles) each day for the indicated days. Cell numbers are expressed as the mean±SD of three determinations. C. Castrated male BALB/c nu/nu male mice were injected subcutaneously in the flank with 1 × 107 LNCaP/MUC1-C cells. The mice were pair-matched when the tumors were ~100±10 (mean±SE) mm3. Treatment groups consisted of 8 mice injected intravenously with PBS (vehicle control; squares) or 7.5 mg/kg GO-203 (circles) each day for 21 days. Tumor measurements were performed on the indicated days. Mice were weighed twice weekly. There was no weight loss in either group. D. Proposed model for the interaction between MUC1-C and AR.
Discussion and Conclusions
MUC1-C oncoprotein suppresses AR expression
Previous work in human prostate cancer cell lines demonstrated a reciprocal relationship between MUC1 and AR expression [19,22]. AR signaling was thus shown to suppress MUC1 abundance by direct downregulation of the MUC1 promoter and by a posttranscriptional miR-125b-mediated mechanism [19]. These findings, however, were not sufficient to explain why overexpression of MUC1 is associated with low AR levels in androgen-independent prostate cancer cell lines. In the present studies, androgen-dependent LNCaP and LAPC4 cells, which express AR, but not MUC1, were used as a model to assess the effects of the oncogenic MUC1-C subunit. Interestingly, we found that MUC1-C downregulates AR levels and that this response is conferred by a posttranscriptional mechanism (Fig. 7D). Other work had shown that AR expression is suppressed by certain miRNAs [28,29]; consequently, we searched for the induction of those miRNAs by MUC1-C and identified miR-135b. We also found that MUC1-C increases miR-147 expression, which has similarly been shown to downregulate AR expression [29]. Thus, MUC1-C increases levels of at least two miRNAs that decrease AR abundance (Fig. 7D), and the present results do not exclude the possibility that other miRNAs may be involved in MUC1-C-mediated AR suppression. Decreases in AR expression have been associated with induction of EMT and the invasion potential of prostate cancer cells [32]. Moreover, MUC1 has been shown to confer the induction of EMT in other types of carcinoma cells [30,31]. In concert with these findings, MUC1-C-mediated AR suppression was associated with induction of EMT as detected by loss of the epithelial marker E-cadherin and gain of the mesenchymal marker vimentin. EMT has been linked to invasion and metastases [34]. Accordingly, we also found that MUC1-C expression in LNCaP cells is associated with significant increases in invasion. Overexpression of MUC1 in primary prostate cancer cells has been linked to more aggressive disease, recurrence and metastases [12,13,18]. The present findings that MUC1-C induces EMT may thus explain, at least in part, how MUC1-C contributes to the aggressiveness of prostate cancers.
MUC1-C interacts directly with AR
MUC1-C localizes to the nucleus of carcinoma cells and contributes to the regulation of gene expression [1]. In breast cancer cells, MUC1-C binds directly to ERα at its DBD and stimulates ERα-mediated transcription [26]. The present studies demonstrate that the MUC1-C cytoplasmic domain binds to the AR DBD (Fig. 7D). Assessment of the binding with fragments of the 72 aa MUC1-C cytoplasmic domain showed that the region from aa 46 to 72 is responsible for the interaction. This region of the MUC1-C cytoplasmic domain is phosphorylated by EGFR and SRC [35,36], and contains a SAGNGGSSLS motif that confers direct binding to the Wnt effector β-catenin [4,37]. Protein inhibitor of activated STAT y (PIASy) binds to the AR DBD and suppresses AR activity without affecting its binding to DNA [38]. The MST1 serine-threonine kinase also interacts with the AR DBD and inhibits AR activity, but by a mechanism that involves decreases in DNA binding [39]. In addition, the FOS [40] and FKHR [25] transcription factors have been shown to interact with the AR DBD and affect its activity. To assess in part the functional signficance of the MUC1-C-AR interaction, ChIP studies showed that MUC1-C forms a complex with AR on the ARE of the PSA promoter (Fig. 7D). How MUC1-C affects AR-mediated transcription will require further study. However, the Wnt/β-catenin and PI3K/AKT pathways, which are both activated by MUC1-C [4,41,42], have been shown to stimulate AR function in positive feed-forward loops that lead to androgen-independent AR activation [43–45]. By extension, MUC1-C binds directly to β-catenin [4,37] and could contribute to the formation of AR-β-catenin complexes, which enhance AR-mediated transcriptional activity [46].
Does MUC1-C contribute to the survival of prostate cancer cells?
The development of cell-penetrating peptide [11] and small molecule [47] inhibitors of MUC1-C has permitted the assessment of malignant cell dependence on this oncoprotein for growth and survival. Studies in androgen-independent DU145 and PC3 prostate cancer cells demonstrated that MUC1-C inhibition is associated with slowing of growth and induction of death in a setting of MUC1-C overexpression and low AR abundance [22]. By contrast, inhibition of MUC1-C in androgen-dependent LNCaP cells had no effect on proliferation or viability [22], indicating that dependence on MUC1-C may be associated with androgen-independence. The present studies in LNCaP and LAPC4 cells expressing MUC1-C lend support to that contention. MUC1-C conferred cell growth in the presence of androgen depletion and bicalutamide, promoting the conversion from androgen-dependence to androgen-independence. Importantly, the LNCaP/MUC1-C cells also developed sensitivity to MUC1-C inhibition as indicated by loss of survival in vitro. MUC1-C inhibitor treatment of mice bearing established LNCaP/MUC1-C tumors further demonstrated growth delay. These results and those obtained with DU145 and PC3 tumors indicate that certain prostate cancer cells [22], particularly those with MUC1-C overexpression and androgen-independence, are sensitive to MUC1-C inhibitors. The development of castrate-resistant prostate cancer occurs in the presence of continued AR activation by multiple mechanisms that include AR gene amplification and mutations, intracellular production of AR ligands and interactions with other signaling pathways and effectors, such as β-catenin [48–51]. Thus, the present results and the demonstration that MUC1 is overexpressed in certain subsets of primary prostate cancers invokes the possibility that MUC1-C inhibitors may be effective in the treatment of this disease. In this regard, the first-in-man MUC1-C inhibitor, GO-203-2C, has entered Phase I evaluation in patients with refractory solid tumors. Based on the present results, it is anticipated that this agent will be studied for effectiveness against MUC1-C expressing castrate-resistant prostate cancers that are metastatic and unresponsive to available therapies.
Supplementary Material
Acknowledgements
This work was supported by Department of Defense Prostate Cancer Idea Award W81XWH-08-1-0093 and National Cancer Institute Grants CA97098 and CA42802.
D. Kufe holds equity in Genus Oncology and is a consultant to the company.
The authors thank Dr. Wenlong Bai for providing the GST-AR constructs.
Abbreviations
- MUC1
mucin 1
- MUC1-C
MUC1 C-terminal subunit
- MUC1-CD
MUC1 cytoplasmic domain
- AR
androgen receptor
- EMT
epithelial-mesenchymal transition
- PSA
prostate specific antigen
References
- 1.Kufe D. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer. 2009;9(12):874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kufe D. Targeting the MUC1 oncoprotein: a tale of two proteins. Cancer Biol Ther. 2008;7:81–84. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005;65(22):10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 5.Kufe D. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009;8(13):1201–1207. doi: 10.4161/cbt.8.13.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27(6):992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufe D. Oncogenic function of the MUC1 receptor subunit in gene regulation. Oncogene. 2010;29(42):5663–5666. doi: 10.1038/onc.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad R, Raina D, Joshi MD, Kawano T, Kharbanda S, Kufe D. MUC1-C oncoprotein functions as a direct activator of the NF-κB p65 transcription factor. Cancer Res. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad R, Rajabi H, Kosugi M, Joshi M, Alam M, Vasir B, Kawano T, Kharbanda S, Kufe D. MUC1-C oncoprotein promotes STAT3 activation in an auto-inductive regulatory loop. Science Signaling. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, Kufe D. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282(27):19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 11.Raina D, Ahmad R, Joshi M, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69(12):5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschenbaum A, Itzkowitz SH, Wang JP, Yao S, Eliashvili M, Levine AC. MUC1 expression in prostate carcinoma: correlation with grade and stage. Mol Urol. 1999;3(3):163–168. [PubMed] [Google Scholar]
- 13.Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ, Li Y. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis. 2005;22(7):565–573. doi: 10.1007/s10585-005-5376-z. [DOI] [PubMed] [Google Scholar]
- 14.Arai T, Fujita K, Fujime M, Irimura T. Expression of sialylated MUC1 in prostate cancer: relationship to clinical stage and prognosis. Int J Urol. 2005;12(7):654–661. doi: 10.1111/j.1442-2042.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrén O, Fall K, Andersson SO, Rubin MA, Bismar TA, Karlsson M, Johansson JE, Mucci LA. MUC-1 gene is associated with prostate cancer death: a 20-year follow-up of a population-based study in Sweden. Br J Cancer. 2007;97(6):730–734. doi: 10.1038/sj.bjc.6603944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbar C, Mascaux C, Wespes E. Expression of MUC1 and sialyl-Tn in benign prostatic glands, high-grade prostate intraepithelial neoplasia and malignant prostatic glands: a preliminary study. Anal Quant Cytol Histol. 2008;30(2):71–77. [PubMed] [Google Scholar]
- 17.Rabiau N, Dechelotte P, Guy L, Satih S, Bosviel R, Fontana L, Kemeny JL, Boiteux JP, Bignon YJ, Bernard-Gallon D. Immunohistochemical staining of mucin 1 in prostate tissues. In Vivo. 2009;23(2):203–207. [PubMed] [Google Scholar]
- 18.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101(3):811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajabi H, Joshi M, Jin C, Ahmad R, Kufe D. Androgen receptor regulates expression of the MUC1-C oncoprotein in human prostate cancer cells. The Prostate. 2011;71(12):1299–1308. doi: 10.1002/pros.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007;104(50):19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajabi H, Jin C, Ahmad R, McClary C, Kufe D. Mucin 1 oncoprotein expression is suppressed by the miR-125b oncomir. Genes & Cancer. 2010;1(1):62–65. doi: 10.1177/1947601909357933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi MD, Ahmad R, Raina D, Rajabi H, Bubley G, Kharbanda S, Kufe D. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther. 2009;8(11):3056–3065. doi: 10.1158/1535-7163.MCT-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, Shimamura T, Shapiro G, Supko J, Kharbanda S, Kufe D. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Therapeutics. 2011;10(5):806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panchamoorthy G, Rehan H, Kharbanda A, Ahmad R, Kufe D. A monoclonal antibody against the oncogenic mucin 1 cytoplasmic domain. Hybridoma. 2011 doi: 10.1089/hyb.2011.0070. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23(1):104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor α. Mol Cell. 2006;21(2):295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17(1):53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T, Wang Q, Balk S, Brown M, Lee GS, Kantoff P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69(8):3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71(5):1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 30.Horn G, Gaziel A, Wreschner DH, Smorodinsky NI, Ehrlich M. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Exp Cell Res. 2009;315(8):1490–1504. doi: 10.1016/j.yexcr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, Bajaj E, Shanmugam K, Lee YY, Hwang SI, Gendler SJ, Mukherjee P. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30(12):1449–1459. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24(3):769–777. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, He D, Xue Y, Wang R, Wu K, Xie H, Zeng J, Wang X, Zhau HE, Chung LW, Chang LS, Li L. PrLZ protects prostate cancer cells from apoptosis induced by androgen deprivation via the activation of Stat3/Bcl-2 pathway. Cancer Res. 2011;71(6):2193–2202. doi: 10.1158/0008-5472.CAN-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Ren J, Yu W, Li G, Kuwahara H, Yin L, Carraway KL, Kufe D. The EGF receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J Biol Chem. 2001;276:35239–35242. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J Biol Chem. 2001;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 38.Gross M, Liu B, Tan J, French FS, Carey M, Shuai K. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene. 2001;20(29):3880–3887. doi: 10.1038/sj.onc.1204489. [DOI] [PubMed] [Google Scholar]
- 39.Cinar B, Collak FK, Lopez D, Akgul S, Mukhopadhyay NK, Kilicarslan M, Gioeli DG, Freeman MR. MST1 is a multifunctional caspase-independent inhibitor of androgenic signaling. Cancer Res. 2011;71(12):4303–4313. doi: 10.1158/0008-5472.CAN-10-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z, Pan J, Balk SP. Androgen receptor-associated protein complex binds upstream of the androgen-responsive elements in the promoters of human prostate-specific antigen and kallikrein 2 genes. Nucleic Acids Res. 1997;25(16):3318–3325. doi: 10.1093/nar/25.16.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic PI3K/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–20612. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 43.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26(7):898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 44.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99(2):402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 45.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 46.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277(13):11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J, Rajabi H, Kufe D. MUC1-C oncoprotein is a target for small molecule inhibitors. Mol Pharm. 2011;79(5):886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 49.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 50.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17(12):3876–3883. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 51.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res. 2011;17(7):1649–1657. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.