Abstract
Background
New, effective chemotherapeutic agents are needed for intraocular retinoblastoma.
Methods
Our institutional clinical trial sought to estimate the rate of response to 2 courses of vincristine and topotecan (VT) window therapy in patients with bilateral retinoblastoma and advanced disease (Reese-Ellsworth Group IV or V) in at least one eye. The topotecan dose started at 3 mg/m2/day for 5 days, and was adjusted to target a systemic exposure of 140 ± 20 ng/ml*hr. The vincristine dose was 0.05 mg/kg for patients < 12 months of age and 1.5 mg/m2 for those > 12 months of age at diagnosis.
Results
From February 2005 to June 2010, 27 patients received VT window therapy. Median age at enrollment was 8.1 months (range, 0.7–22.1 months). Twenty-four patients (88.9%) responded to window therapy (95% CI, 71.3%–96.9%). Hematologic toxicity comprised grade 4 neutropenia (n=27), grade 3 anemia (n=19), and grade 3/4 thrombocytopenia (n=16). Thirteen patients had grade 3 non-hematologic toxicity. G-CSF support was added after 10 patients had been treated and significantly reduced the duration of grade 4 neutropenia (median, 7 vs. 24 days; P <0.001). Pharmacokinetic studies showed rapid changes in topotecan clearance rates during the first year of life.
Conclusions
The combination of topotecan and vincristine is effective for the treatment of advanced intraocular retinoblastoma. G-CSF treatment alleviates the duration of grade 4 neutropenia. Appropriate topotecan starting doses for patients 0–3, 3–6, 6–9, 9–12, and >12 months of age are specified.
Keywords: Retinoblastoma, topotecan, pharmakokinetic, first year of life, toxicity
INTRODUCTION
Primary systemic chemotherapy moved to the forefront of the management of intraocular retinoblastoma in the 1990s.1–3 Its aims were to reduce tumor volume and allow focal consolidation therapy while avoiding external-beam irradiation of young patients with a germline RB1 mutation that increases the risk of second tumors throughout life.4 Initial primary chemotherapy comprised carboplatin, vincristine, and etoposide with or without cyclosporine.1–3, 5
To reduce the risk of chemotherapy-induced acute myeloid leukemia,6 the previous St. Jude Children’s Research Hospital prospective study RET37 excluded etoposide and called for 8 cycles of carboplatin and vincristine at 3-week intervals. Focal therapy was withheld until disease progression was noted. Although the results were similar to those obtained with the three-drug regimens, further improvement was needed; we reasoned that better outcomes would require the earlier introduction of focal therapy or the use of new effective chemotherapeutic agents.
Topotecan was subsequently selected for investigation in a phase II St. Jude study. Topotecan had been shown to provide good cerebrospinal fluid (CSF) penetration in children with central nervous system (CNS) tumors8 and a 25%–30% response rate in patients with high-risk medulloblastoma.9 Other pediatric malignancies, including neuroblastoma10 and rhabdomyosarcoma,11 were also responsive to topotecan. Further, topotecan had shown efficacy against retinoblastoma in both preclinical12 and clinical13 studies. The blood-ocular barrier is similar to the blood-brain barrier, and preclinical studies at St. Jude had shown that topotecan readily penetrates the vitreous humor.12 Finally, therapeutic response in children with embryonal brain tumors was related to topotecan CSF penetration achievable only after high topotecan plasma exposure (e.g., AUC 140 ng/ml*hr), which is only tolerable on a daily for 5 days/week schedule.9
Metabolism and elimination of drugs is unique during the first few months of life,14 and bilateral retinoblastoma occurs at a much younger age than other childhood malignancies. Therefore, it was important to perform a prospective phase II study to evaluate response and toxicity in this age group. Here we describe the responses and toxicity profile associated with 2 courses of vincristine and topotecan window therapy in children with advanced intraocular retinoblastoma.
PATIENTS AND METHODS
Treatment Plan
The RET5 protocol (NCT0018688) was opened to accrual on February 4, 2005, after approval by the St. Jude Institutional Review Board. Written informed consent was obtained from the parent or guardian of each patient.
Patients with non-metastatic intraocular retinoblastoma were stratified according to disease laterality and stage. Eyes were grouped by the Reese-Ellsworth group as the study design progressed between 2002 and 2004 and was approved in 2005. During this period the newly designed classification (International Classification of Retinoblastoma)15 that provided better evaluation of response to intravenous chemotherapy was still being evaluated. Stratum A was for early-group bilateral or unilateral (unifocal or multifocal) retinoblastoma (Reese-Ellsworth Groups I–III). Stratum B was for bilateral retinoblastoma for which conservative management was considered and in which at least one eye had Reese-Ellsworth Group IV or V disease. Stratum C was for advanced (Reese-Ellsworth Group IV or V) unilateral retinoblastoma that required enucleation.
The primary objective of RET5 was to estimate the response rate of stratum B patients to two courses of window therapy with vincristine and topotecan (VT). Topotecan (a 0.5 h daily infusion for 5 days) was first administered at 3 mg/m2/day, and the dose was then adjusted to attain a targeted systemic exposure of 140 ± 20 ng/ml*hr. The vincristine dose was 0.05 mg/kg for patients age < 12 months and 1.5 mg/m2 for patients age ≥ 12 months at diagnosis.
To accurately evaluate response to the window VT therapy, focal consolidation was used only after the second course of chemotherapy. Patients who had a complete response or partial response (PR) to window therapy were to receive 3 additional courses of VT at 21-day intervals (courses 5, 8, and 11), alternating with vincristine-carboplatin (courses 3, 4, 6, 7, 9, and 10). Subsequent courses of chemotherapy were initiated only if absolute neutrophil count (ANC) was > 750/µL and platelets count was > 100,000/µL. Patients with less than a PR after window therapy were to receive 6 courses of vincristine, carboplatin and etoposide, given at 21-day intervals. After hematologic toxicity was observed in the first 10 patients, the protocol was amended to add granulocyte colony-stimulating factor (G-CSF) (5 mcg/kg/day), starting 24–36 hours after completion of each course of chemotherapy and given for 7–10 days, until ANC was >2,000/µL.
Definition of Response
Patients underwent serial, detailed funduscopic examinations under anesthesia. All tumors were carefully documented during each exam by using the RET-CAM II retinal camera (Clarity Medical, Pleasanton, CA), which provided digital storage and allowed immediate comparison to previous findings. Patients on stratum B also underwent serial magnetic resonance imaging of the orbits and Doppler ultrasonography of the eye as an adjunct means of measuring tumors and evaluating responses. A complete response was defined as complete calcification or regression of all documented tumors for at least 4 weeks and a PR as >50% but <100% reduction or calcification of the tumors, without the appearance of new lesions, for at least 4 weeks. Development of any new lesion (including vitreous seeds), irrespective of other responses, constituted progressive disease. Response was documented per eye and per patient. For analysis of the primary objective (the response rate of stratum B patients to two courses of window therapy), response was assessed per patient, using the lesser response of the 2 eyes.
Statistical Design and Analysis
The RET5 protocol was a single-arm, two-stage study designed to evaluate response to window therapy by using the sequential conditional probability ratio test16 to determine whether the response rate was ≤70% vs. ≥85% with a 10% significance level and 90% power. The planned sample size was 53 patients, and an interim analysis was planned after 27 stratum B patients had been evaluated for response. If 18 or fewer of the 27 patients had responses, closure of the study would be considered. Although RET5 was predicted to reach its full recruitment goal within 5 years, only 27 stratum B patients had been enrolled after 5 years. The protocol was closed in June 2010. A Blyth-Still-Casella 95% confidence interval for the rate of response to window therapy was calculated. The two-sided exact Wilcoxon rank-sum test was used to compare the duration of grade 4 neutropenia in patients who did vs. did not receive G-CSF with window therapy. An alpha value of P = 0.05 was prospectively chosen.
Pharmacokinetic Sampling and Analysis
Plasma samples were obtained according to a limited sampling model,17 before topotecan infusion and at hours 0.083, 1.5, and 2.5 after the end of infusion. At each time point, 2 mL of whole blood was collected from a site contralateral to the infusion and placed in a heparinized tube. Plasma was separated for assay of topotecan lactone concentration.18
To support pharmacokinetically guided topotecan dosing, the area under the concentration-time curve (AUC; topotecan systemic exposure) from time zero to infinity (AUC0→∞) was estimated by fitting a two-compartment model to topotecan lactone plasma concentration-time data by using a maximum a posteriori (MAP) Bayesian algorithm in ADAPT 5 software.18 To calculate age-based optimal topotecan starting dosages for future studies, all pharmacokinetic data were combined to develop a nonlinear mixed-effects model using NONMEM 7 software. Estimated fixed-effect parameters included volumes of the central (Vc) and peripheral (Vd) compartments, clearance rate (CL), and intercompartmental clearance rate (Q). Inter-individual variability of all fixed-effects parameters and inter-occasion variability of CL were estimated. The PRIOR subroutine in NONMEM was used to include previously estimated data from older patients. Age was tested as a covariate of CL by using a sigmoidal function, where age was adjusted to the estimated post-conceptual age. Monte Carlo simulations were performed with the final model to assess the optimal starting dosages for different age groups.
Pharmacokinetically Guided Topotecan Dosing
We used a previously described pharmacokinetically guided dosing approach18 to individualize topotecan dosage to attain a targeted plasma AUC of 120–160 ng/mL*hr. After the first topotecan dose of course 1, plasma samples were obtained, processed immediately, and analyzed. If the topotecan plasma AUC was within the target range, no adjustment was required. If not, the dosage was adjusted linearly on the basis of the patient’s topotecan clearance rate to attain the targeted AUC on day 2, and repeat pharmacokinetic studies were performed. Pharmacokinetic data from samples obtained on day 5 of course 1 were used to determine the starting dosage for the second course of therapy. During the second course, plasma samples were collected on day 1 and the dosage was adjusted as described for course 1. If dose adjustments were required during course 2, pharmacokinetic studies were repeated during course 5 (the third course of VT), beginning with dose 1. Pharmacokinetic targeting studies were similarly performed with dose 1 of course 8 (the fourth course of VT).
RESULTS
Patients
Table 1 shows the characteristics of the 27 stratum B patients. All had bilateral disease and at least one eye in Reese-Ellsworth Group IV or V. Median age at study enrollment was 8.1 months. Seven patients (26%) were <6 months of age and 23 (85%) were <12 months of age at diagnosis. Most patients were Caucasian (78%). Fifteen patients (56%) had advanced-stage (group IV or V) disease in both eyes. Two patients (7%), neither of whom received G-CSF, had 13q deletions.
Table 1.
Characteristics of patients enrolled on RET5 Stratum B (n=27)
| Age at enrollment (months) | |
| Median (range) | 8.1 (0.7–22.1) |
| Age at diagnosis (months) | |
| Median (range) | 7.9 (0.7–22.0) |
| Sex | |
| Male | 14 (51.9%) |
| Female | 13 (48.1%) |
| Race | |
| White | 21 (77.8%) |
| Black | 2 (7.4%) |
| Other | 4 (14.8%) |
| Reese-Ellsworth group (n=54 eyes)* | |
| II | 4 (7.4%) |
| III | 8 (14.8%) |
| IV | 18 (33.3%) |
| V | 24 (44.4%) |
| International Classification for Retinoblastoma | |
| A | 2 (3.7%) |
| B | 15 (27.8%) |
| C | 8 (14.8%) |
| D | 25 (46.3%) |
| E | 4 (7.4%) |
Includes one patient who underwent enucleation of one eye (RE group VB) before enrollment.
Response
Of the 27 patients, 23 had confirmed, sustained PR in both eyes and were considered to have an overall PR; 1 patient had undergone enucleation of the left eye at diagnosis and had a PR in the right eye that was sustained for 8 weeks. Thus, 24 of 27 patients (88.9%) responded to VT (95% CI, 71.3%–96.9%). One patient who experienced unacceptable toxicity discontinued window therapy and was categorized as a non-responder. Each of two patients had a PR in one eye but developed new lesions in the other eye within 4 weeks; they were therefore categorized as having progressive disease. Of the 53 eyes available for response evaluation, 49 (92.4%) had a PR to window therapy. The Reese-Ellsworth distribution of the 49 eyes was Group II (n=3), Group III (n=8), Group IV (n=17) and Group V (n=21). It is worth mentioning that all tumors showed partial calcification.
Courses and Dose Modifications
Twenty-six of the 27 stratum B patients received both courses of window therapy. The patient who did not complete both courses had a 13q deletion and did not receive G-CSF. After experiencing 21 days of grade 4 neutropenia during course 1, grade 4 thrombocytopenia and leukopenia, and grade 2 colitis and allergic reaction (drug fever), this patient was moved to a non-protocol vincristine-carboplatin regimen. The 26 patients who received both courses required a median of 47 days (range, 41–55 days) to complete window therapy (calculated from the start of window course 1 to the day before course 3 began). The median time required to complete window therapy was 49 days (range, 42–55 days) in the 9 patients treated without G-CSF and 44 days (range, 41–51 days) in the 17 patients who received G-CSF (P=0.095). Five patients required a delay of VT during course 2; 3 of the 5 did not receive G-CSF (delays of 5, 7 and 6 days); the 2 who received G-CSF had delays of 1 and 5 days.
Toxicity
Version 3.0 of the Common Terminology Criteria for Adverse Events was used to evaluate toxicity in our study. Myelosuppression was significant. All patients experienced grade 4 hematologic toxicity (neutropenia) during window therapy (Table 2). Nineteen of the 27 patients (70%) had grade 3 anemia and 16 (59%) had grade 3 or 4 thrombocytopenia. Thirteen patients (48%) experienced grade 3 or 4 non-hematologic toxicity (Table 2), including 6 of 10 patients (60%) treated without G-CSF and 7 of 17 patients (41%) who received G-CSF (P=0.44).
Table 2.
Grade 3 and 4 toxicities observed during RET5 window therapy
| Group | ||||
|---|---|---|---|---|
| G-CSF (n=17) |
No G-CSF (n=10) |
|||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| n | n | n | n | |
| Hematologic toxicities | ||||
| Hemoglobin | 11 | 8 | ||
| Leukocytes (total WBC) | 3 | 1 | 3 | |
| Neutrophils/granulocytes (ANC/AGC) | 17 | 10 | ||
| Platelets | 8 | 3 | 1 | 4 |
| Non-hematologic toxicities | ||||
| Allergic reaction | 1 | |||
| Anorexia | 2 | 1 | ||
| Colitis, infectious (e.g., Clostridium difficile) | 1* | 1† | ||
| Dehydration | 2 | |||
| Diarrhea | 2 | 1 | 2 | |
| Febrile neutropenia | 2 | 3 | ||
| Fever without neutropenia | 1 | 1 | ||
| Glucose, serum-low (hypoglycemia) | 1 | |||
| Infection with Grade 3 or 4 neutropenia | 2‡ | |||
| Mucositis/stomatitis (clinical exam), Oral cavity | 2 | |||
| Pulmonary | 1 | |||
| Rash/desquamation | 2 | 1 | ||
| Vomiting | 1 | 1 | ||
Abbreviations: AGC, absolute granulocyte count; WBC, white blood cell count
This patient had clinical findings consistent with colitis and adenovirus was identified in the stool.
This patient had Clostridium difficile colitis and required intravenous antibiotics.
One patient had balanitis and the other had respiratory syncytial virus infection.
The median duration of grade 4 neutropenia was 24 days (range, 10–33 days) for patients treated without G-CSF and 7 days (range, 4–11 days) for those treated with G-CSF (P<0.0001). Five patients had grade 3 febrile neutropenia during window therapy, including 3 of 10 patients (30%) treated without G-CSF and 2 of 17 patients (12%) treated with G-CSF (P=0.33). The febrile neutropenia resolved within 1 day in all patients but one; that patient was treated without G-CSF and had grade 3 febrile neutropenia for 11 days.
Twenty of the 27 patients (74%) required packed red blood cell transfusions during window therapy; 13 patients (48%) required 1 transfusion and 7 (26%) required 2. Eight of 10 patients (80%) treated without G-CSF and 12 of 17 patients (71%) who received G-CSF required PRBC transfusions. Seven of the 27 patients (26%) required platelet transfusion during window therapy, including 3 of 10 patients (30%) treated without G-CSF and 4 of 17 patients (24%) treated with G-CSF. Six patients received 1 platelet transfusion and 1 received 2 platelet transfusions.
Pharmacokinetic Analysis
A total of 111 pharmacokinetic studies were performed in 26 patients. After the first (fixed) dose of cycle 1, 8 of 26 patients (31%) were within the targeted exposure range. The median topotecan lactone clearance rate after this dose was 18.8 L/hr/m2 (range, 9.8–36.8 L/hr/m2). Of the remaining 85 pharmacokinetic studies, 54 (64%) revealed systemic exposure within the target range. The topotecan lactone clearance rate was observed to increase rapidly with age, particularly in very young children. Only 3 patients required no topotecan dose modification.
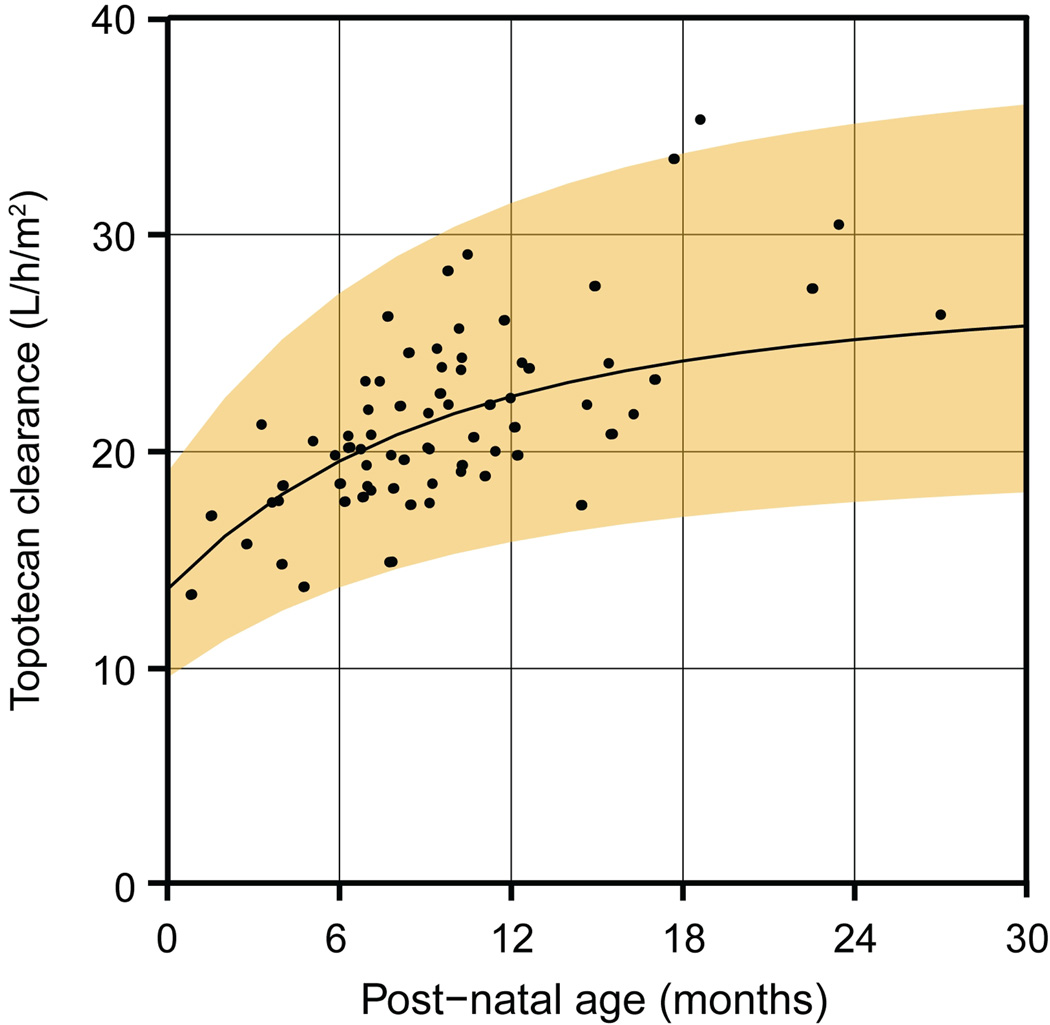
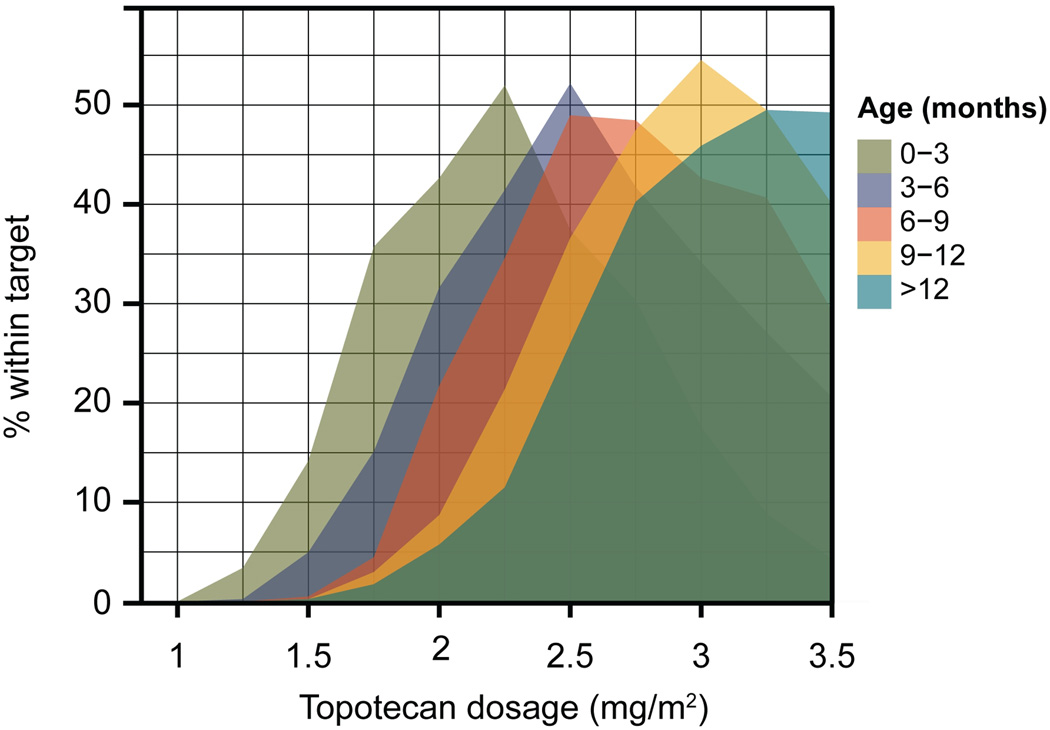
A nonlinear mixed-effects modeling and simulation analysis was performed to relate topotecan clearance to age and determine age-appropriate starting dosages. Systemic clearance of topotecan lactone changed rapidly during the first year of life (Figure 1). The effect of age on clearance was appropriately described by a sigmoidal relationship. Monte Carlo simulations based on the final model, including the effect of age on clearance, were used to assess the probability of attaining the targeted exposure over a range of starting dosages in different age groups (Figure 2). This analysis established the optimal starting dosages of topotecan for young children of different ages (Table 3).
Figure 1.
The topotecan lactone clearance rate increases with age in a sigmoidal manner. Points represent individual clearance rates on day 1 of each cycle in which a pharmacokinetic study was performed. The curve and shaded region represent the median and 90% prediction interval determined by the nonlinear mixed-effects model.
Figure 2.
Determination of optimal age-based starting dosages of topotecan. The final nonlinear mixed-effects model was used to perform Monte Carlo simulations of topotecan lactone plasma concentration in patients of different ages over a range of dosages. The topotecan lactone AUC calculated for each patient in the simulation was used to determine the probability of achieving the targeted 120–160 ng/mL*hr systemic exposure within each age group.
Table 3.
Suggested starting dosages of topotecan in young children
| Age (months) | Topotecan dosage |
|---|---|
| 0 to < 3 | 2.25 mg/m2 |
| 3 to < 6 | 2.5 mg/m2 |
| 6 to < 9 | 2.75 mg/m2 |
| 9 to < 12 | 3.0 mg/m2 |
| 12 and older | 3.5 mg/m2 |
DISCUSSION
Although our study demonstrated that the combination of topotecan and vincristine is an effective therapy of previously untreated advanced intraocular retinoblastoma, we believe that most of the observed response is due to topotecan. Our group demonstrated previously that vincristine had the lowest effect on retinoblastoma cell cultures and no effect on retinoblastoma in preclinical mouse models.12 Carboplatin and etoposide have been the mainstay of chemotherapy for intraocular retinoblastoma for 2 decades.1–3, 5, 7 However, these agents pose a long-term risk of second malignancy1, 6 and ototoxicity,19, 20 and patients with advanced intraocular retinoblastoma remain at high risk of requiring external-beam irradiation or enucleation. Chantada et al used topotecan in 9 patients but with metastatic (n=6) or relapsed/refractory (n=3) previously treated intraocular retinoblastoma.13 Our response rate of 89% is similar to those reported in prospective studies of other therapeutic agents in untreated patients.1, 5
We also describe the toxicity profile of topotecan in the youngest group reported to date. Only a few studies11, 21, 22 have used the daily × 5 days regimen, which we chose because a higher topotecan systemic exposure (AUC 140 ng/ml*hr) was tolerated in patients with CNS tumors.9 Comparison of toxicity data across studies is confounded not only by different intravenous topotecan regimens23 but also by differences in age, previous treatment, dosing, utilization of G-CSF, and methods of reporting toxicity (per course vs. per patient) (Table 4). Additionally, there are intra-study differences in topotecan doses and/or utilization of G-CSF. Overall, our toxicity profile was comparable to those reported with the use of similar regimens, and VT therapy was well tolerated after G-CSF was introduced. Patients treated with G-CSF had a significantly shorter duration of grade 4 neutropenia, a lower frequency of grade 3–4 non-hematologic toxicity, and less delay in completing window therapy.
Table 4.
Studies that have used topotecan daily for 5 days of every 21 days
| Reference (Year) |
Study type |
No. Patients |
Tumor (No. patients) |
Previous therapy |
Median months of age (range) |
Topotecan dose (mg/m2/day) |
TSE (ng/ml*hr) |
GCSF use | Toxicity- related mortality |
|---|---|---|---|---|---|---|---|---|---|
| Tubergen et al (1996)20 | Phase I | 40 | ST 30 BT 10 |
Yes | 144 (36–240) |
1.4 1.7 |
NA | No Yes |
None |
| Nitschke et al (1998)21 | Phase II | 141 | ST 140 RB 1 |
Yes | 144 (12–180) |
2 | NA | For grade 4 N, F&N, Delay > 1 week |
1 |
| Pappo et al (2001)11 | Window | 48 | Met RMS | No | 120 (0–228) |
2 2.4 |
NA | For grade 4 N* Yes |
2 |
| Stewart et al (2004)9 | Window | 44 | MB | No | 88 (38–203) |
2 5.5† |
140 ± 20 | Yes Yes |
None |
| Chantada et al (2004)13 | Response | 9‡ | Relapsed & refractory RB |
Yes§ | 36 (7–90) |
2 | NA | No | None |
| Current Study |
Window | 27 | Advanced IO non- Met RB |
No | 8.1 (0.7–22) |
3 | 140 ± 20 | No (n=10) Yes (n=17) |
None |
Abbreviations: No., number; ST, solid tumor; BT, brain tumor; RB, retinoblastoma; Met, metastatic; RMS, rhabdomyosarcoma; MB, medulloblastoma; IO, intraocular; TSE, targeted systemic exposure; NA, not available; N, neutropenia; F, fever.
7 patients received 2 mg/m2 of topotecan without GCSF.
The first 10 patients received a 30-min infusion at 2 mg/m2; the regimen was modified to a 4-h infusion starting at 5.5 mg/m2.
6 extraocular, 3 intraocular
1 patient with metastatic RB at diagnosis had not received previous therapy.
Our use of pharmacokinetically guided topotecan dosage was initially intended to minimize interpatient variability in topotecan systemic exposure and to target a range of plasma drug exposure putatively associated with cytotoxic effects in the vitreous body and retina. The rapid change in topotecan lactone clearance rates observed during the first year of life partially explained the limited success of pharmacokinetically guided dose adjustment. However, pharmacokinetically guided dosing prevented the recurrence of systemic overexposure to topotecan observed after the first dose, especially in very young children. This finding presumably reflects a lower topotecan systemic clearance in young children with developing renal function (the primary mechanism of topotecan elimination). Age <0.5 vs. >0.5 years was previously shown to be a significant covariate in a topotecan population pharmacokinetic study.24 However, only a few infants <0.5 years of age were included. The present study greatly enhances our ability to accurately select appropriate doses for these children. We acknowledge that pharmacokinetically guided dosing requires extensive infrastructure and may be available at only a few centers.25 However, in its absence the information in Table 4 may be used to select both the initial dosage and appropriate increases in dosage as an infant ages. These starting doses will be used in our future protocols and our standard of care for topotecan therapy in infants less than 1 year of age.
Another potential limitation to wider acceptance of our regimen is the higher toxicity observed compared to other standard therapies for intraocular retinoblastoma.1–3, 5 We believe that if our approach demonstrates a higher success rate in avoiding enucleation and/or radiation therapy, such extra toxicity could be justified. In addition, different strategies such as topotecan without vincristine or fewer cycles of topotecan could be investigated in the future. Such approaches could decrease toxicity (especially non-hematologic) without compromising efficacy.
Childhood cancers during the first year of life are unique and are increasingly diagnosed.26 The physiology of infants is unique as well, compounding the complexity of treatment. Our comprehensive toxicity and pharmacokinetic data may be useful in treating many cancers in this age group.
Acknowledgements
We thank Sharon Naron for editing the manuscript and Klo Spelshouse for assistance with artwork.
This work was supported by grants CA21765 and CA23099 from the National Institutes of Health, by the American Lebanese Syrian Associated Charities (ALSAC), and by Research to Prevent Blindness, Inc., and St. Giles Foundation.
Footnotes
This work was presented in part at the annual meeting of the American Academy of Ophthalmology, San Francisco, CA, in October 2009, the American Society of Clinical Oncology, Chicago, USA in June 2011, and at the International Society of Ocular Oncology, Buenos Aires, Argentina, in November 2011.
Financial disclosures: The authors declare no financial conflicts of interest.
REFERENCES
- 1.Gallie BL, Budning A, DeBoer G, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114:1321–1328. doi: 10.1001/archopht.1996.01100140521001. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1330–1338. doi: 10.1001/archopht.1996.01100140530002. [DOI] [PubMed] [Google Scholar]
- 3.Murphree AL, Villablanca JG, Deegan WF, 3rd, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1348–1356. doi: 10.1001/archopht.1996.01100140548005. [DOI] [PubMed] [Google Scholar]
- 4.Woo KI, Harbour JW. Review of 676 second primary tumors in patients with retinoblastoma: association between age at onset and tumor type. Arch Ophthalmol. 2010;128:865–870. doi: 10.1001/archophthalmol.2010.126. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DL, Himelstein B, Shields CL, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;8:12–17. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- 6.Gombos DS, Hungerford J, Abramson DH, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007;114:1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21:2019–2025. doi: 10.1200/JCO.2003.09.103. [DOI] [PubMed] [Google Scholar]
- 8.Baker SD, Heideman RL, Crom WR, Kuttesch JF, Gajjar A, Stewart CF. Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemother Pharmacol. 1996;37:195–202. doi: 10.1007/BF00688317. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CF, Iacono LC, Chintagumpala M, et al. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol. 2004;22:3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 10.Kretschmar CS, Kletzel M, Murray K, et al. Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: a Pediatric Oncology Group study. J Clin Oncol. 2004;22:4119–4126. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 11.Pappo AS, Lyden E, Breneman J, et al. Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: an Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 2001;19:213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 12.Laurie NA, Gray JK, Zhang J, et al. Topotecan combination chemotherapy in two new rodent models of retinoblastoma. Clin Cancer Res. 2005;11:7569–7578. doi: 10.1158/1078-0432.CCR-05-0849. [DOI] [PubMed] [Google Scholar]
- 13.Chantada GL, Fandiño AC, Casak SJ, Mato G, Manzitti J, Schvartzman E. Activity of topotecan in retinoblastoma. Ophthalmic Genet. 2004;25:37–43. doi: 10.1076/opge.25.1.37.28996. [DOI] [PubMed] [Google Scholar]
- 14.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 15.Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;13:2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Xiong X, Tan M, Boyett J. Sequential conditional probability ratio tests for normalized test statistic on information time. Biometrics. 2003;59:624–631. doi: 10.1111/1541-0420.00072. [DOI] [PubMed] [Google Scholar]
- 17.Turner PK, Iacono LC, Panetta JC, et al. Development and validation of limited sampling models for topotecan lactone pharmacokinetic studies in children. Cancer Chemother Pharmacol. 2006;57:475–482. doi: 10.1007/s00280-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 18.Santana VM, Zamboni WC, Kirstein MN, et al. A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clin Cancer Res. 2003;9:633–640. [PubMed] [Google Scholar]
- 19.Jehanne M, Lumbroso-Le Rouic L, Savignoni A, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52:637–643. doi: 10.1002/pbc.21898. [DOI] [PubMed] [Google Scholar]
- 20.Qaddoumi I, Bass KJ, Wu J, et al. Carboplatin associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tubergen DG, Stewart CF, Pratt CB, et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: a Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1996;18:352–361. doi: 10.1097/00043426-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Nitschke R, Parkhurst J, Sullivan J, Harris MB, Bernstein M, Pratt C. Topotecan in pediatric patients with recurrent and progressive solid tumors: a Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Bomgaars L, Berg SL, Blaney S. The development of camptothecin analogs in childhood cancers. Oncologist. 2001;6:506–516. doi: 10.1634/theoncologist.6-6-506. [DOI] [PubMed] [Google Scholar]
- 24.Schaiquevich P, Panetta JC, Iacono LC, et al. Population pharmacokinetic analysis of topotecan in pediatric cancer patients. Clin Cancer Res. 2007;13:6703–6711. doi: 10.1158/1078-0432.CCR-07-1376. [DOI] [PubMed] [Google Scholar]
- 25.Park JR, Scott JR, Stewart CF, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:4351–4357. doi: 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman HR, Bleyer WA. Infants and adolescents with cancer: special considerations. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 452–463. [Google Scholar]