Abstract
Study Objectives:
An ideal biomarker for sleep should change rapidly with sleep onset, remain at a detectably differential level throughout the sleep period, and exhibit a rapid change with waking. Currently, no molecular marker has been identified that exhibits all three properties. This study examined three substances (lactate, glucose, and glutamate) for suitability as a sleep biomarker.
Design:
Using amperometric biosensor technology in conjunction with electroencephalograph (EEG) and electromyograph (EMG) monitoring, extracellular concentrations of lactate and glucose (Cohort 1) as well as lactate and glutamate (Cohort 2) were recorded over multiple sleep/wake cycles.
Patients or Participants:
There were 12 C57Bl/6J male mice (3-5 mo old).
Interventions:
Sleep and waking transitions were identified using EEG recordings. Extracellular concentrations of lactate, glucose, and glutamate were evaluated before and during transition events as well as during extended sleep and during a 6-h sleep deprivation period.
Measurements and Results:
Rapid and sustained increases in cortical lactate concentration (approximately 15 μM/min) were immediately observed upon waking and during rapid eye movement sleep. Elevated lactate concentration was also maintained throughout a 6-h period of continuous waking. A persistent and sustained decline in lactate concentration was measured during nonrapid eye movement sleep. Glutamate exhibited similar patterns, but with a much slower rise and decline (approximately 0.03 μM/min). Glucose concentration changes did not demonstrate a clear correlation with either sleep or wake.
Conclusions:
These findings indicate that extracellular lactate concentration is a reliable sleep/wake biomarker and can be used independently of the EEG signal.
Citation:
Naylor E; Aillon DV; Barrett BS; Wilson GS; Johnson DA; Johnson DA; Harmon HP; Gabbert S; Petillo PA. Lactate as a biomarker for sleep. SLEEP 2012;35(9):1209-1222.
Keywords: Biosensor, continuous in vivo monitoring, electroencephalography, electromyography, glucose, glutamate, lactate, mouse, sleep
INTRODUCTION
Biosensors are a proven technology that can routinely and rapidly measure changes in analyte concentration on a second-by-second timescale in the extracellular fluid of freely moving animals. This tool is particularly well suited to quantify analytes that change in response to physiologic transitions that occur very quickly, such as during wake and sleep. Recent biosensor studies on glutamate changes as a function of sleep/wake have been crucial in defining the role of glutamate in the sleep process.1,2–3 Other studies using biosensors for either glucose4 or lactate5 have examined these substances individually across the sleep/wake cycle in rats. To date, no study has simultaneously examined the changes in lactate with either glutamate or glucose in the same animal. The reliable, robust, and reproducible measurements provided by biosensors are suitable for the development of predictive models– especially those relating to metabolic biomarkers. In this study, we simultaneously recorded from two biosensors (lactate + glucose or lactate + glutamate) from within the frontal cortex of mice over multiple sleep/wake cycles. The biosensor recordings demonstrate that changes to brain lactate concentration are highly correlative with sleep/wake state and firmly establish lactate as a suitable metabolic biomarker.
The National Institutes of Health Definitions Working Group classifies a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes.”6 An ideal biomarker for sleep would demonstrate rapid changes at sleep/wake transitions and remain at a constant level throughout the duration of the sleep or wake episode. Currently, no known biomarker is accurate enough to be a substitute for electroencephalographic measurement of sleep/wake activity.
A major problem in modern sleep research is the ability to quickly and accurately evaluate sleep and wake states independent of the electroencephalograph (EEG) signal.7 Sleep and wake states are typically classified using continuous recording of surgically implanted electrodes for recording EEG and electromyography (EMG) signals. This approach yields a large amount of data that must be independently and expertly scored and categorized before an accurate picture of sleep emerges. Although effective in smaller studies, scaling the analysis of EEG and EMG signals to cohort sizes required for large-scale screening paradigms is difficult. Identification of a metabolic biomarker that accurately reflects sleep/wake state would convey a significant benefit in conducting large-scale sleep studies in rodent populations.
For this study we measured EEG and EMG biopotential signals in conjunction with high-resolution electrochemical biosensors to record lactate, glucose, and glutamate concentration changes within the mouse cortex throughout multiple, uninterrupted sleep/wake cycles. In addition to being the first study to examine this phenomenon in a mouse model, this is the first time multiple, simultaneous biosensor measurements have been recorded as a function of sleep in any species. This study also marks the first high-resolution detailed examination of three neurometabolic substances across multiple sleep and wake periods.
METHODS
Animals
Twelve C57Bl/6J male mice between 3-5 mo of age (3.9 ± 0.2 mo) with an average weight of 29.8 ± 0.9 g were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were maintained on a 12:12 h light-dark cycle with food and water available ad libitum throughout the study. All animal procedures were approved by the University of Kansas Institutional Animal Care and Use Committee (IACUC).
Biosensor Construction
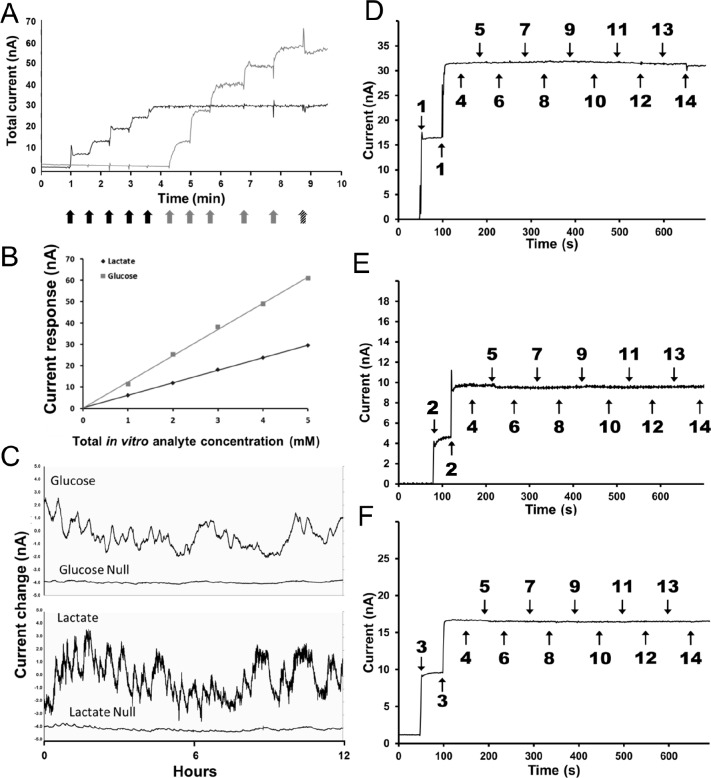
All biosensors were constructed according to previously published methods.1,8 Briefly, the working electrode is formed by a Teflon-coated Pt-Ir wire with a 180 μM diameter (Medwire, Mount Vernon, NY). Silver wire was concentrically wrapped around the insulated portion of the working electrode then chloridized (0.3 M FeCl3 in 0.1 M aqueous HCl) to serve as the reference electrode. A 1-mm section of the working electrode was then exposed to form the sensing cavity, which was then sequentially coated with a selectivity membrane followed by a sensing membrane. For construction of the glutamate biosensor sensing membrane, glutamate oxidase (E.C. 1.4.311, Yamasa Corp., Choshi-Chiba Japan) was used as part of the sensing layer; for construction of the glucose biosensor sensing membrane, glucose oxidase (E.C. 1.1.3.4, BBI Enzymes, Blaenavon, Gwent, UK) was used as part of the sensing layer; and for construction of the lactate biosensor sensing membrane, lactate oxidase (E.C. 1.1.3.15, Sekisui Diagnostics, Tokyo, Japan) was used as part of the sensing layer. Electroactive interferents were excluded from detection by the presence of a semipermeable membrane. This allows for the diffusion of hydrogen peroxide while retarding most relevant electroactive interferents in the brain, including neutral, negatively and positively charged species. The characterization of the membrane's ability to exclude electroactive interferents has been comprehensive, such that the signal correctly reflected the concentration of each analyte of interest.1,8,9 All biosensors are calibrated in vitro before implantation using standard protocols.1 After explantation, all biosensors were immediately (< 10 min) post-calibrated in a 37°C bath containing 100 mM phosphate buffered saline (pH 7.4) buffer solution. Titration of the biosensor response was accomplished by stepwise addition of l-glutamate (10 μM), l-glucose (1 mM), or l-lactate (100 μM) at regular intervals (Figure 1A and 1B). Amperometric changes in individual biosensor readings were used for measurement of concentration changes in vivo. The specificity of the biosensor's analyte detection was further validated by simultaneous implantation of a working sensor and a null sensor (constructed in an identical fashion but lacking the sensing enzyme) contralaterally within the same animal and recording over a 12-h period (Figure 1C).
Figure 1.
(A) Post-calibration curve for both a lactate (black trace) and a glucose (gray trace) sensor implanted in a mouse. Black arrows indicate addition of 0.1 mM lactate, gray arrows indicate addition of 1mM glucose, and the hatched arrow indicates 250 mM ascorbic acid addition. (B) Linearity of the sensor response to both lactate and glucose additions. (C) Simultaneous recording of active sensors for glucose and lactate along with the null sensors for each analyte implanted contralaterally within the same animal. Typical in vitroInterference test plots for biosensors: lactate (D), glutamate (E), and glucose (F). Biosensors were calibrated in 100 mM phosphate buffered saline (pH 7.4) at 37°C. The addition of analytes and interferents shown on the plots are as follows: (1) L-lactate 0.1 mM, (2) L-glutamate 10 μM, (3) D-glucose 1.0 mM, (4) norepinephrine 1.0 μM, (5) serotonin 1.0 μM, (6) dopamine 1.0 μM, (7) gamma-amino-butyric acid 1.0 μM, (8) tryptophan 2.0 μM, (9) L-cysteine 2.0 μM, (10) L-tyrosine 2.0 μM, (11) L-glutathione 2.0 μM, (12) L-glutamine 2.0 μM, (13) L-aspartic acid 2.0 μM, and (14) L-ascorbic acid 250 μM.
The selectivity of the biosensors used in this study has been previously established in the literature.1,8,10,11 Figure 1C illustrates that in the absence of the biorecognition element (the oxidase enzyme), there is no significant change in current levels. This clearly demonstrates that the sensors are not influenced by any endogenous compounds present in the brain. Furthermore, it demonstrates that only in the presence of the biorecognition element (the oxidase enzyme) do the biosensors respond to the analyte of interest. Figures 1D, 1E, and 1F further demonstrate the selectivity of the biosensors in vitro. Each selectivity plot clearly shows the response of the biosensor to its analyte of interest (Figure 1D: lactate, Figure 1E: glutamate, Figure 1F: glucose) at physiologically relevant concentrations (lactate: 0.1 mM, glutamate: 10 μM, glucose: 1.0 mM). Common endogenous electroactive interferents (norepinephrine, serotonin, dopamine, and ascorbic acid) as well as non-electroactive compounds (L-aspartic acid, L-glutamine, etc.) that represent possible candidates for enzyme substrates were included in the selectivity test. In all three cases (lactate, glutamate, and glucose) the biosensor signal was not influenced by any of the interfering compounds. These in vitro selectivity plots, in combination with the null sensor data, clearly demonstrate the selectivity of the biosensors.
Surgery
Under ketamine/xylazine anesthesia, cannulas for biosensor recording (Part #MD-2255, BASi, West Lafayette, IN) were placed bilaterally within the frontal cerebral cortex (A/P +1.7 to +1.9, M/L ± 1.5, D/V −;0.5). EEG recording screws with attached wires (Part #8403, Pinnacle Technology, Inc., Lawrence, KS) were placed contralaterally across the cortex and EMG probes (#10IR5T, MedWire, Mount Vernon, NY) were inserted into the nuchal muscles. All electrode leads were connected to a head mount (Part #8402, Pinnacle Technology, Inc.) and sealed with dental acrylic resin. After recovery from surgery (7 days), animals were placed in the recording chamber and connected to a flexible tether for 24 h before biopotential and biosensor recording. In one cohort of animals (n = 6), lactate (Part #7004-Lactate, Pinnacle Technology, Inc.) and glucose (Part #7004-Glucose, Pinnacle Technology, Inc.) biosensors were inserted into the guide cannulas; in the second cohort of mice (n = 6), glutamate (Part #7004-Glutamate, Pinnacle Technology, Inc.) biosensors were paired with lactate biosensors. Biosensor insertion was done immediately after the 24-h tether acclimation period and recording of the biosensor signal commenced immediately. Accuracy of the sensor placement was confirmed through examination of brain tissue during postmortem analysis (Figure 2).
Figure 2.
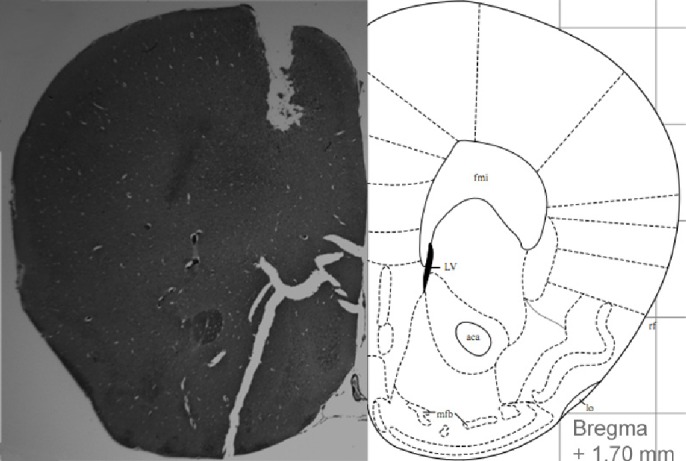
Histologic slice showing sensor placement (left) and corresponding stereotaxic atlas page (right).
EEG/EMG Data Collection
A preamplifier unit (Part #8406-SL, Pinnacle Technology, Inc.) rigidly attached to the mouse headmount provided 1st-stage amplification (100×) and initial high-pass filtering (1st-order 0.5 Hz for EEG and 10 Hz for EMG). All signals were then routed to the conditioning/acquisition system (Part #8401, Pinnacle Technology, Inc.) via a tether and low-torque commutator (Part #8408, Pinnacle Technology, Inc.). The 8401 amplifier/conditioning unit provided an additional fifty-fold signal amplification, additional high-pass filtering, and an 8th order elliptic low-pass filter (50 Hz EEG and 200 Hz EMG). The signals were then sampled at 250 Hz, digitized using a 14-bit analog to digital converter and routed to a PC-based acquisition and analysis software package via USB.
Sleep Deprivation
After baseline recordings, 6-h of sleep deprivation were administered using an automated sleep deprivation unit (Part #8229, Pinnacle Technology, Inc.). Sleep deprivation was achieved using a continuously slowly rotating bar (10 rotations/min) suspended 1 inch above the floor and bedding within the mouse cage. This device has been previously demonstrated to provide effective short-term sleep deprivation in mice12 and has been proven compatible with sleep restriction paradigms. In all cases, sleep deprivation was administered during the first 6-h of the lights-on period. During sleep deprivation periods, food and water were available ad libitum. Video monitoring of animal activity and EEG data were analyzed during sleep deprivation application to confirm that at least a 90% reduction in nonrapid eye movement (NREM) sleep and a 100% reduction in rapid eye movement (REM) sleep occurred during this period.
Sleep/Wake and Biosensor Analysis
EEG and EMG data were classified by an expert scorer as 10-sec epochs of wake, NREM, or REM sleep based on standard criteria13 using the Sirenia® analysis package (Pinnacle Technology, Inc.). Biosensor measurements were determined by averaging 1-sec biosensor readings over the course of each 10-sec epoch. Converted biosensor measures were matched to scored sleep epochs for comparison of analyte concentrations between states. Sensor drift in baseline measurements was corrected by subtraction to a fitted 1st -order exponential curve.
Analysis of individual lactate concentration change events (peak–trough) measured from both the beginning and end of the sleep deprivation time period demonstrated no significant changes (P = 0.77) after application of the 1st-order exponential curve subtraction. Thus, use of the 1st-order exponential curve to help provide a more tractable baseline over time and from animal to animal does not significantly alter the original experimental data. The 1st-order exponential curve does, however, provide an acceptable method for quantifying long-term biosensor data.
Statistical Comparisons
All statistical analyses were performed using the Statistica 7 package (StatSoft Inc, Tulsa, OK). Time series comparisons were conducted using a repeated measures analysis of variance (RM-ANOVA) and Tukey post hoc comparisons. Statistical significance is reported for P < 0.05. Unless otherwise noted, all error bars represent standard error of the mean.
RESULTS
This study used high temporal resolution amperometric biosensors to simultaneously measure lactate, glucose, and glutamate concentration changes in the extracellular space during natural waking, NREM, and REM sleep periods and during a short period of sleep deprivation. Sleep/wake times and bout architecture are shown in Table 1 and agree favorably with previously published reports using this species.14,15 An example of EEG and EMG waveforms plotted simultaneously with raw, uncorrected biosensor traces for lactate and glucose from a single animal is shown in Figure 3. Biosensor recordings are well suited for quantifying sec-by-sec changes in neurotransmitter release during sleep in a manner that does not perturb the equilibrium of the local environment9 and, as revealed in Figures 1B through 1F, this biosensor design is extremely selective for the analyte of interest. By calibrating each biosensor after explantation, the recorded in vivo current can be transformed into meaningful changes in analyte concentration allowing multiple biosensors implanted in multiple animals to be directly compared.
Table 1.
Breakdown of overall WAKE, NREM, and REM sleep times as well as sleep fragmentation computed using a 30-sec bout length.

Figure 3.
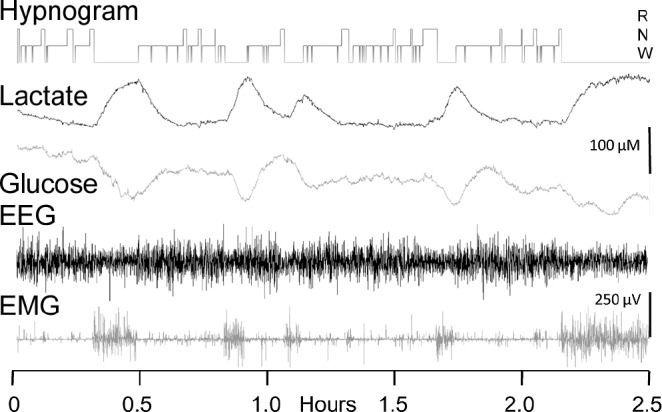
Data traces showing simultaneous, synchronized lactate and glucose biosensor traces along with corresponding electroencephalographic and electromyographic activity over a 2.5-h period. A hypnogram indicating scored sleep/wake states is displayed at the top of the traces. The biosensor traces are plotted uncorrected for sensor drift.
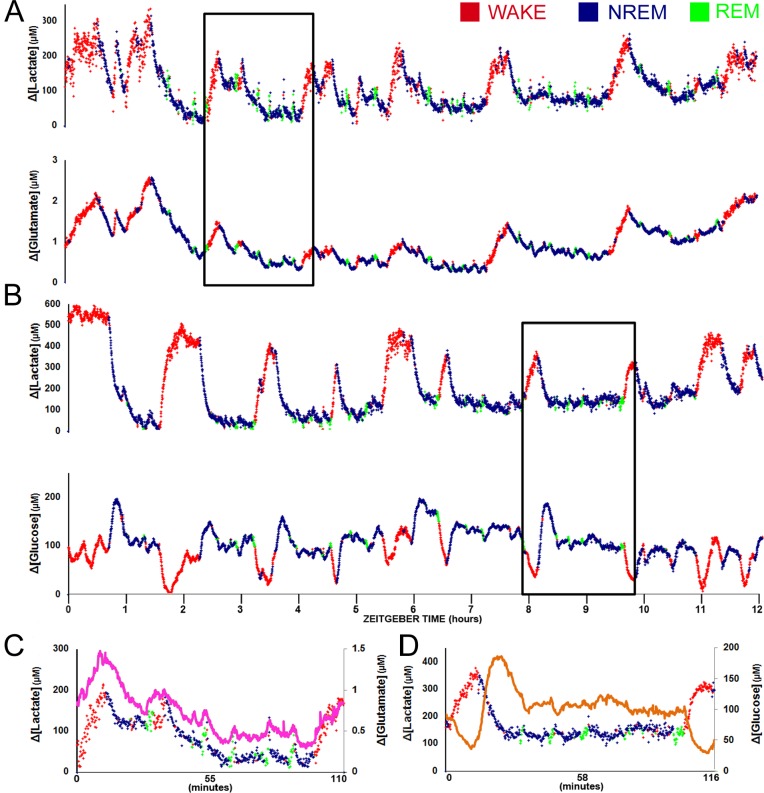
In the first cohort (n = 6) changes in lactate and glutamate concentrations were measured bilaterally within the prefrontal cortex in conjunction with cortical EEG and intramuscular EMG activity. Upon waking, changes in extracellular lactate concentration immediately increased, remained elevated throughout the duration of the wake episode, and rapidly declined at NREM sleep onset. Extracellular glutamate (fuchsia line in Figure 4C) demonstrated a similar pattern, but the time course of the increase and decrease were significantly slower than that observed for lactate (Figures 4A and 4C). The second cohort (n = 6) consisted of an identical cannula location placement with a glucose sensor substituted for the glutamate sensor. As expected, lactate showed an identical response as that seen in the first cohort, but in contrast to glutamate, changes in glucose concentration (orange line in Figure 4D) showed a transient decline with waking and transient increase at sleep onset (Figures 4B and 4D).
Figure 4.
Multiple sleep/wake cycles recorded using simultaneous electroencephalographic and (A) lactate/glutamate biosensors or (B) lactate/glucose biosensors plotted during the lights-on period. Epochs scored as wake are noted in red, non-rapid eye movement (NREM) sleep epochs are colored blue, and rapid eye movement (REM) sleep epochs are indicated in green. Concentration change for each analyte is indicated on the y-axis. The lower graphs (C, D) correspond to time periods on the upper graphs indicted by the solid box. In all expanded graphs, lactate concentration change is plotted in a manner similar to that of the large-scale graphs with colors indicating sleep/wake state and the secondary analyte (C) glutamate or (D) glucose plotted as on overlay in fuchsia or orange, respectively.
Analyte changes were quantified for all wake episodes longer than 15 min that terminated after 1 min of continuously recorded sleep. Specific concentration measurements using the biosensors relied on changes measured relative to a defined baseline value. For this purpose, the baseline comparison period for each wake episode was calculated using the average analyte concentration value from the 5 min of sleep immediately preceding wake onset. This method was used to directly average biosensor concentration readings across the recording period for each animal. Lactate biosensor readings for both experimental cohorts were statistically equivalent during wake (2-way RM-ANOVA DF = 1, F = 2.07, P = 0.18). Thus, the wake periods of both cohorts could be combined for analysis. By analogy, the changes in lactate levels during sleep periods of both cohorts could also be combined (2-way RM-ANOVA DF = 1, F = 3.18, P = 0.10).
The decision to use a 1-min time period was based on our data and experience with biosensor analysis. Individual lactate levels can be observed to increase almost immediately upon waking and exhibit a clearly significant elevation within 1 min after a transition. If the biosensor data are reanalyzed using a classic bout length of 30 sec, the same trends are observed. Statistically, there is no group-effect difference when the same dataset is analyzed in 30-sec versus 60-sec bouts (2-way RM-ANOVA, lactate wake: DF = 1, F = 0.04,P = 0.861; glutamate wake: DF = 1, F = 0.10, P = 0.776, lactate sleep: DF = 1, F = 1.15, P = 0.304; glutamate sleep: DF = 1, F = 0.74, P = 0.405). The advantage of the 60-sec bout length is that the number of bouts and the bout length increases relative to the 30-sec bout length, which further increases the statistical power of the biosensor analysis. Thus, NREM epochs < 60 sec occurring within the 15- to 35-min periods were not considered to be sufficient to be taken into account as sleep episodes for the purpose of biosensor analysis, even though such periods are frequent during the dark period.
For all mice used in this study, we observed an average of 9.7 ± 2.6 wake episodes per animal that were 15 min or longer in length and 3.2 ± 2.0 wake episodes per animal that were longer than 35 min. Likewise, we observed 12.2 ± 2.8 sleep episodes that were 15 min or longer and 6.6 ± 2.1 sleep episodes longer than 35 min. Spontaneous wake periods longer than 35 min are observed in this species (albeit with small periods of NREM epochs present) and we measured between 1 and 4 of these in each animal over the course of a 12- to 18-h recording period.
Immediately after waking from sleep, all analytes demonstrated a significant deviation from baseline sleep levels (lactate: DF = 41, F = 18.99, P < 0.001; glutamate: DF = 41, F = 12.96, P < 0.001; glucose: DF = 41, F = 5.51, P < 0.01). Extracellular lactate demonstrated the most dramatic change, increasing by 15.4 ± 1.7 μM/min for approximately the first 6 min of waking, before slowing accumulation to a maximum increase of approximately 150 μM above baseline within 25 min (Figure 5A). Mice are polyphasic and can exhibit spontaneous, long wake bouts at all times of the day. We measured lactate concentration in more than 100 spontaneous wake episodes, 35% of which were longer than 30 min and 10% of which were longer than 1-h. In each of these events, lactate remained at least five standard deviations above the baseline concentration for the duration of the wake episode and demonstrated no sign of declining. We do not believe that observation of longer waking episodes would enhance or alter the significance of these findings.
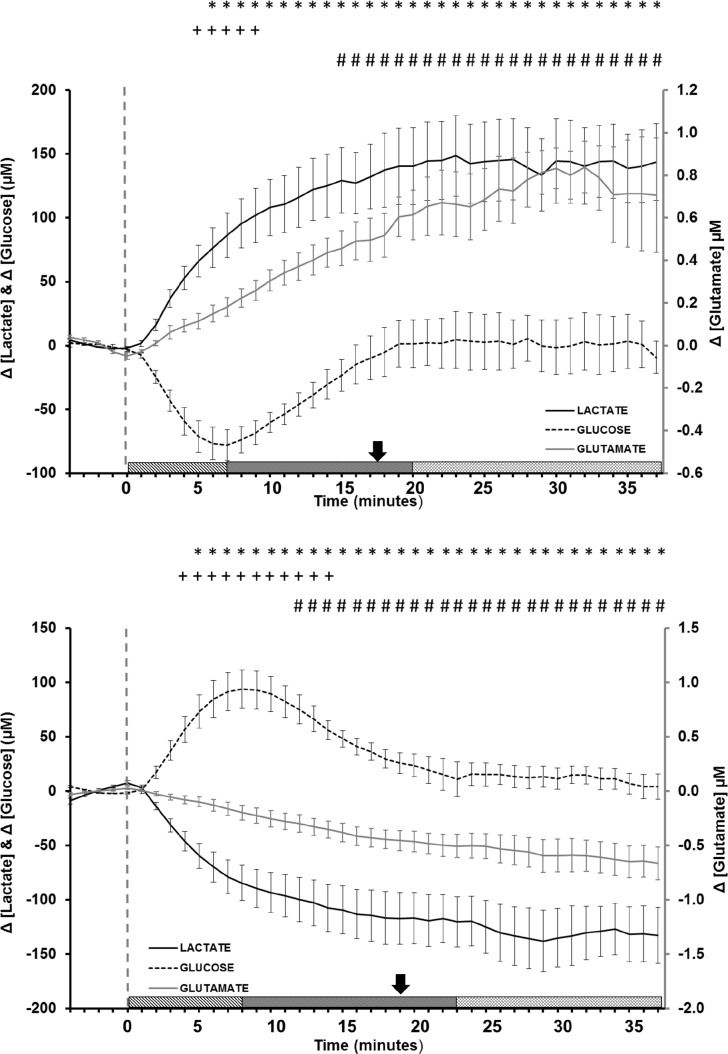
Figure 5.
Average concentration change as a function of time for lactate (solid black line), glucose (dashed black line), and glutamate (solid gray line) during (A) wake and (B) sleep episodes longer than 15 min. The baseline comparison period was calculated using the average value from the 5 min immediately preceding stage onset (vertical dashed gray line). Symbols indicate P < 0.05 (*lactate, +glucose, #glutamate) over each analyte's calculated baseline (Tukey post hoc). Bars represent standard error of the mean. The patterned bars at the bottom represent the three response phases for glucose: initiation phase (IP), transition phase (TP), and equilibrium phase (EP). The arrow directly above the bars represents the transition from the IP to the EP for lactate and glutamate.
Similar to that of lactate, glutamate concentration also steadily increased by 0.034 ± 0.005 μM/min before plateauing at a maximum increase of 0.8 μM above baseline within 40 min after waking. Extracellular glucose levels demonstrated a nonlinear response, declining at a rate of 13.6 ± 2.6 μM/min and reaching a nadir of approximately −90 μM in within the first 7 min of waking before returning to near-baseline levels within 20 min of waking. After 20 min of continuous waking, glucose concentration changes were found to be highly variable and were independently observed to continue increasing, to decline, or to remain steady in comparison with the sleep baseline. The variability of the glucose response at long time periods is attributed to the individual actions and activities of each animal.
Figure 5B illustrates the significant changes in analyte concentration noted during transition into NREM sleep (lactate: DF = 41, F = 22.61, P < 0.001; glutamate: DF = 41, F = 18.76, P < 0.001; glucose: DF = 41, F = 12.73, P < 0.001). Analogous to previously measured transitions, 5 min of waking activity immediately preceding the sleep episode onset was used to establish the baseline for subsequent changes. The analyte response noted during prolonged sleep (> 15 min) can be observed to be inverse of the waking pattern. Both lactate and glutamate concentrations declined during sleep (13.9 ± 1.2 μM/min and 0.028 ± 0.002 μM/min, respectively). These rates of decline were similar in magnitude to the rates of rise in analyte concentration noted during wake. Lactate reached a minimum concentration change of approximately 140 μM within 30 min of entering sleep, although almost 75% of this decline was noted to occur within the first 7 min of the NREM period. Glucose concentration exhibited an opposite trend to that seen upon waking and immediately increased by 16.7 ± 1.2 μM/min, reaching a maximum concentration increase of approximately 100 μM within 8 min of entering sleep before returning to baseline before the onset of the 1st REM period.
Similar to waking transitions, increases in lactate concentration were clearly noted during longer REM sleep periods. Measurement of analyte changes during REM sleep relied upon a technique similar to that used for previous transitions. Due to the shortened nature of REM episodes in the mouse (typically < 3 min in length) only 1 min of NREM sleep immediately before REM onset was used to establish the baseline. After 2.5 min of continuous REM sleep, extracellular lactate concentration was significantly elevated (14.1 ± 4.6 μM, P = 0.001, 1-sample t-test) and glucose concentration trended lower (-8.4 ± 5.4 μM, P = 0.09, 1-sample t-test). REM sleep periods of less than 1 min in length did not demonstrate any appreciable change in analyte concentration.
Our data reveal a fundamental relationship between lactate and sleep/wake transitions. The observed changes in lactate concentration are significant for sleep/wake transitions, and also show significance for REM sleep bouts that are longer than 2.5 min. REM bouts shorter than 1 min did not show an appreciable change in lactate concentration. REM bouts between 1 and 2.5 min were associated with a steady increase in lactate concentration, but this increase was not statistically significant in all cases. Thus, we have limited our analysis to REM bouts that are longer than 2.5 min. Shorter REM bouts would be difficult to distinguish by lactate concentration alone, and currently would still require an EEG for complete accuracy.
It should be noted that lactate consistently declines by approximately 100 μM during NREM sleep periods, whereas REM sleep periods may result in increases up to only 20 μM for brief periods of time. Thus, even when maximally elevated, REM sleep increases are insufficient to overcome the overall decline in lactate concentration during NREM sleep periods. For longer REM bouts, a change in lactate concentration is always present, and is statistically significant, but is typically approximately 20% the magnitude of those changes seen for sleep/wake transitions. The magnitude of change can also be used, even the absence of an EEG single, to help identify REM sleep bouts > 2.5 min.
For an analyte to be an accurate biomarker indicative of wake and sleep transitions, concentrations should change in a consistent and predictable manner corresponding to changes in sleep or wake state. Here, the lactate concentration reflects a wake state by a rapid rise upon waking that remains at a consistently elevated level throughout the entire waking period. Furthermore, the lactate concentration reflects sleep by rapidly declining at sleep onset and remaining at a consistently depressed level throughout the entire sleep period.
By defining a significant change in lactate elevation to be five standard deviations above baseline concentration, an analysis of 147 wake transitions found lactate concentrations to be significantly above baseline within 1 min of waking (51 ± 9 sec). Glutamate was much more variable, taking almost 5 min (284 ± 66 sec) to reach a significant, steady-state increase with waking. The longevity of this elevation was assessed by pooling all waking events longer than 5 min in length across all recording periods (Figure 6A). Lactate increase correlated nearly perfectly with time awake for every wake episode measured (R2 = 0.989, P < 0.001). Glutamate also demonstrated a significant correlation (R2 = 0.939, P < 0.001), but this correlation was less significant in comparison with lactate and requires a longer timescale to achieve sustained significance. Both of these analytes likewise declined with sleep onset and remained significantly below wake concentrations for the duration of all sleep episodes longer than 5 min (Figure 6B). The correlation between the duration of lactate decline during sleep was nearly as striking (R2 = 0.986, P < 0.001) as the lactate increase during wake. Again, consistent with the trends observed during waking, the change in extracellular glutamate concentrations were significant but less so in comparison with lactate, and required a longer timescale to achieve sustained significance.
Figure 6.
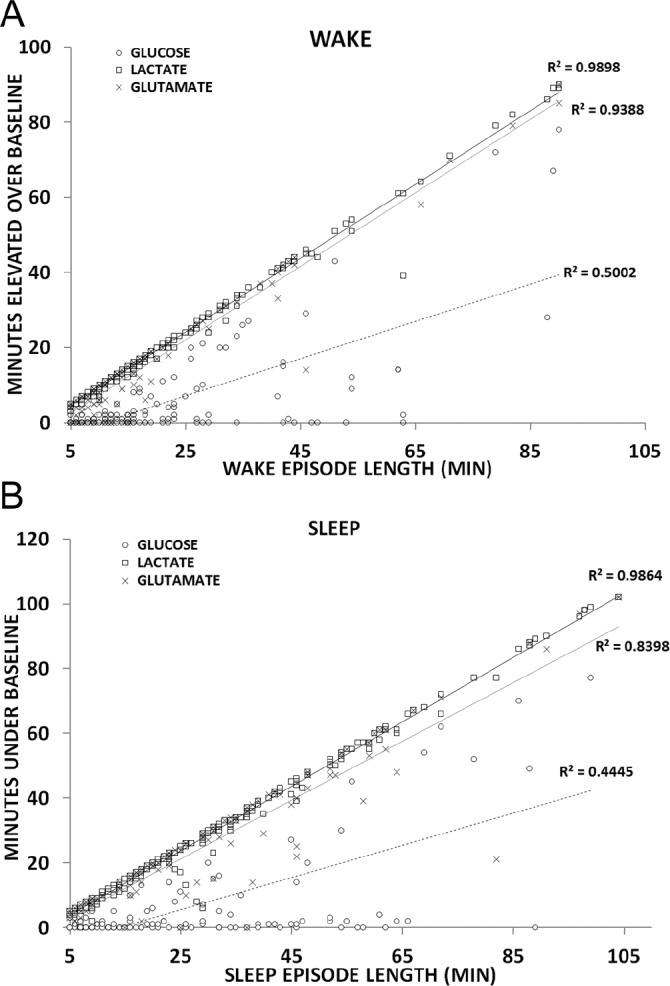
Correlational analysis between wake episode length and concentration changes for lactate (▫), glucose (○) and glutamate (×) for all (A) wake or (B) sleep periods longer than 5 min.
If changes in extracellular lactate concentration are a useful sleep/wake biomarker, this should also be reflected during periods of enforced sleep restriction. To examine if the lactate concentration remained high even during periods of long-term sleep restriction, five animals were subjected to enforced sleep deprivation using a slowly rotating bar at the beginning of the lights-on period immediately after baseline recording. This approach to sleep restriction emulates gentle handling in a user-defined manner without the need for constant human intervention.12
As shown in Figure 7A, enforced, long-term sleep deprivation resulted in a sustained increase in lactate concentration throughout the entire period of wakefulness followed by a rapid decline beginning immediately with the onset of recovery sleep. During enforced wakefulness, all mice demonstrated a rapid and sustained increase in lactate concentration at an initial rate similar to that observed during a typical wake transition (15.6 ± 4.4 μM/min). Although not significantly different (P = 0.22), analysis of the lactate concentration change after 30 min of continuous sleep deprivation (196 ± 60 μM) was found to be higher than the average measured change after 30 min of spontaneous waking (144 ± 33 μM).
Figure 7.
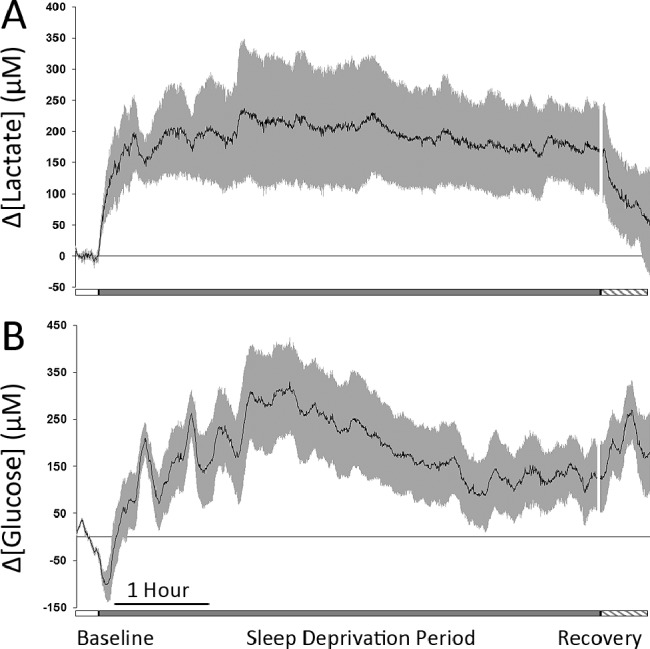
(A) Average lactate concentration change in five animals during 15 min baseline (open bar), 6-h of enforced sleep deprivation (gray bar), and 30 min of recovery sleep (hatched bar). (B) Average glucose concentration change in five animals during 15 min baseline (open bar) and 6-h of enforced sleep deprivation (gray bar). In both graphs, the black line indicates the mean change in concentration from baseline values and the gray bars indicate standard error of the mean.
During this same period, glucose concentration demonstrated the characteristic decrease immediately upon awakening followed by an increased concentration over initial baseline concentrations and extremely high variability throughout the course of the enforced wake period (Figure 7B). To better clarify the variability of glucose concentration during extended wake periods not involving forced sleep deprivation, we analyzed 61 spontaneous wake episodes lasting from 15-90 min. We found that for wake episodes lasting between 15 and 20 min, glucose concentrations were higher than pre-sleep concentrations 31.6% of the time; for episodes lasting from 21-30 min, glucose concentrations were higher than pre-sleep concentrations 45.0% of the time; and for episodes longer than 31 min glucose concentration was at or above pre-sleep concentrations 68.2% of the time. At the onset of NREM recovery sleep, the observed glucose surge was similar to that consistently observed during normal sleep. Although it was not directly measured in this study, extracellular glutamate concentration has been reported to initially increase, then gradually decline, over the course of a 6-h sleep deprivation period.2,16
DISCUSSION
This study presents the first high-resolution, simultaneous sampling of lactate, glucose, and glutamate over the course of multiple sleep/wake cycles in any animal. Data obtained from continuous sampling of in vivo biosensors in conjunction with EEG activity demonstrated that changes in extracellular lactate and glutamate concentrations within the cerebral cortex increased upon waking. Changes in extracellular central nervous system (CNS) glucose concentration first exhibited a notable and transient decline before returning to the levels measured during sleep; in many instances the levels continued to increase with extended waking. Lactate and glutamate concentrations remained high throughout the duration of waking, whereas glucose concentration tended to fluctuate. NREM sleep onset resulted in a reversal of these trends with the decline in lactate concentration exhibiting the most rapid time course. Lactate and glutamate both remained near their nadir over the entire course of the sleep episode, although during extended periods of REM sleep (longer than 1 min), lactate concentration increased in a manner similar to that during wake, but only reached statistical significance after 2.5 min.
Our finding that lactate rapidly increased upon waking while extracellular glucose simultaneously declined has not been previously demonstrated. Earlier reports suggested that extracellular lactate increased with waking,5,17,18–19 consistent with our data reported herein. However, these earlier studies were all of lower resolution and, with the exception of the study by Shram and et al.,5 the analysis was done on brain homogenates in contrast with the freely moving participants used in this current study. None of the previous studies analyzed the fine architecture of individual sleep/wake periods vis-à-vis changes in lactate concentration.
The primary energy analytes that are needed to help support neuronal action potentials are glucose (the primary energy source), lactate (the critical intermediate energy store), and glutamate. Earlier studies have independently examined changes in lactate,5 glucose,4,20 and glutamate1,2–3 concentration at various time points across the sleep/wake cycle, and the results are largely in agreement. This study is the first to simultaneously examine these changes in conjunction with biopotential recordings from multiple biosensors. Extracellular lactate concentrations in the brain have been reported to be lower during NREM sleep than during waking and REM,5,17,18–19 whereas glucose concentration is reportedly increased during the NREM period.4,20 In concordance with its role as the primary excitatory neurotransmitter, extracellular glutamate increases with wake and during REM sleep.1,2
Action potentials and postsynaptic processing are estimated to account for more than 70% of the total energy consumed by neurons with estimates of signal-related energy use to be as high as 30–50 μM adenosine triphosphate/g/min.21 This heightened demand requires the availability of a continuous energy supply for sustained neuronal firing. In an apparent paradox, these energy-hungry neurons store negligible amounts of glucose and glycogen, instead relying on astroglial cells to provide essential fuel for activity.22 Although a portion of circulating glucose is directly taken in by neurons, it is estimated that up to 80% is absorbed directly by astrocytes23,24 where it is converted to both glycogen and lactate. Lactate then moves into the intracellular space via monocarboxylic transporter proteins (MCT1 and MCT4) and delivered to neurons via uptake through the MCT2 transporter. This mechanism is commonly referred to as the astrocyte-neuron lactate shuttle hypothesis (ANLSH)25 and is supported by extensive experimental evidence.26,27–28 Not surprisingly, lactate has been shown to be efficiently oxidized by neurons under aerobic conditions,29 whereas in vitro studies have demonstrated a neuronal preference for the uptake of lactate over glucose.30,31 These studies suggest that both lactate and glucose provide complimentary roles as primary neuronal energy sources.
Studies by Magistretti et al.26,32,33 on neuroenergetic pathways provide a detailed description of why lactate, the preferred energy substrate for use by active neurons during wake, should be highly correlative with sleep/wake state. The local fuel reservoir behind the blood-brain-barrier is lactate, which is formed rapidly in astrocytes and whose concentration can be maintained at high levels under aerobic conditions to support acute and chronic neuronal energy needs. This picture further mitigates the conflict between immediate energy demands upon neuronal firing and the glucose transport limitations of the blood-brain barrier.
The main hypothesis of the ANLSH is that enhanced lactate consumption occurs upon neuronal activation and that the source of the lactate is from stores in the extracellular space via neighboring astrocytes. This hypothesis was investigated by Hu and Wilson, who examined the interplay of lactate and glucose under conditions of heightened neuronal activity (e.g., application of a stimulus to the brain of an anesthetized rat10). Their landmark biosensor study on brain energy dynamics clearly demonstrated that under conditions of acute aerobic energy demand the brain preferentially uses extracellular lactate stores to make adenosine triphosphate–a process that is highly efficient. Magistretti et al. used the data from Hu and Wilson to develop a mathematical model that provided a comprehensive description of brain lactate kinetics.34 The composite work of Magistretti et al. and others provides a complete depiction of neuronal energy dynamics and elucidate the critical role of lactate in supporting action potential firing.
Recent studies by Suzuki et al.35 have demonstrated that disruption of the neuronal lactate transporters MCT1, MCT2, and MCT4 in the hippocampus resulted in amnesia and have suggested that lactate plays an important role in memory consolidation. Suzuki et al.35 further reported that microinjections of l-lactate into the hippocampus restored long-term memory ability following MCT1 and MCT4 disruption but failed to restore memory in the case of MCT2 disruption. It is well established that even short-term sleep impairment results in memory-related difficulties36 and Suzuki et al. clearly state in their hypothesis that “lactate import into neurons is necessary for long-term memory.” Because this biosensor design only measures change within the extracellular space, an increase in lactate concentration with prolonged waking could indicate an increase in supply, a decrease in uptake or, more likely, a combination of these two factors. Our findings from the cortex are entirely consistent with those of Suzuki et al. Confirming our findings in the hippocampus, where Suzuki et al. performed their studies, would provide important further evidence that memory impairment resulting from sleep deprivation is likely due to alterations in the MCT2 transporter and deficient lactate uptake into neurons despite increased extracellular concentrations.
Although lactate and glutamate concentrations change in a consistent manner with sleep and waking, the measured changes in extracellular glucose demonstrated a very different pattern by declining at waking and rising during the initial NREM sleep onset. This specific pattern of short-term glucose perturbation with sleep/wake cycling has not been previously documented. A previous study by Netchiporouk et al.4 used a similar biosensor methodology to examine only glucose changes across the sleep/wake cycle. However, their analysis did not correlate glucose changes as a function of scored epochs, but instead grouped all NREM and all REM episodes together. They concluded that, in comparison with waking, glucose increased during all NREM sleep periods and decreased during REM sleep. This result is not inconsistent with our findings, and, if all of our NREM glucose results were averaged, the result would indicate higher overall levels during sleep. However, the increased sampling resolution provided by biosensor technology used in this study allows a clear elucidation of the transient change in extracellular glucose during sleep/wake transitions.
The initial glucose surge may represent continued glucose delivery necessary for increasing CNS glycogen stores, an event that occurs within 10 min after sleep onset.37 Likewise, the decline in glucose at wake transitions may be explained by a rapid absorption of available glucose by both resource-hungry neurons and astrocytes paired with an immediate lack of new glucose delivery from the bloodstream. A similar phenomenon documenting a decline in extracellular glucose in conjunction with increased neuronal activity has been reported during learning tasks. McNay et al.38 have reported clear declines in extracellular hippocampal glucose concentration lasting approximately 20 min when a rat is introduced into an elevated maze learning task. Similar to these learning tasks, waking from sleep also involves a heightened level of neuronal activation. This initial glucose decline likely represents a large uptake of available glucose by both astrocytes and neurons during increased neuronal activity.25,32,39
After the initial glucose decline upon waking, glucose concentration changes were found to be highly variable. Our data show that each animal had an independent response after approximately 20 min, where the change in glucose concentration could be observed to continue rising, decline, or remain steady. Furthermore, our analysis of glucose activity during spontaneous waking periods indicated that the longer an animal remains continuously awake, there is a greater probability, but not a certainty, that the glucose concentration will increase above the waking baseline values. Despite the fact that glucose is the primary energy source, it does not correlate in a 1-to-1 manner with sleep/wake, and as such is unsuitable for use as a biomarker. Individual animal actions are important with respect to glucose concentrations. This specific experiment was not designed to examine locomotor activity, feeding activity, or food restriction with regard to CNS glucose activity.
Lactate concentration changes were also found to be elevated during the enforced sleep deprivation period. Relative to the change observed during spontaneous waking, lactate change during sleep deprivation was increased (approximately 25%). This heightened level may reflect increased stress due to sleep deprivation and/or increased motor activity induced by the rotating bar. As noted by Shram et al.,5 “lactate is very sensitive to an animal's activity and its variation can be detected in any situation displaying a spontaneous motor activity.” Because both stress and activity have been explicitly noted to increase brain lactate concentration,5,40 we cannot rule out either mechanism as responsible for increased elevation of lactate levels relative to spontaneous waking measurements. A direct assessment of stress hormones might assist in dissecting the exact physiologic mechanisms of the increase in lactate concentration, especially under the conditions of sleep deprivation, but would not change our fundamental conclusion that lactate concentration increases during wake, decreases during sleep, and remains high during periods of enforced wakefulness. Regardless of the mechanism, lactate concentration remains low throughout the entirety of extended sleep periods, a finding that is consistent with our hypothesis that a change in lactate concentration is an excellent method for assessing sleep/wake state.
Other studies have been undertaken for all of these analytes that provide estimated absolute analyte ranges, although there is no study-to-study agreement as to the true absolute concentration. Estimates of lactate concentrations within the rat brain using microdialysis have been measured between 350 μM in the striatum41 to 410 μM in the cortex,42 whereas absolute extracellular concentrations of glucose in the brain range from 0.6–1.0 mM.43,44–45 These measurements usually fail to account for the physiologic state of the animal, which, as shown in this study, can cause a dramatic change in the value observed. Extracellular glutamate concentrations in the rat have been reported to be within an extended range of 1-5 μM,46–51 whereas reported absolute values in the mouse prefrontal cortex are similar (3.3 ± 1.3 μM).52 It should be noted that the microdialysis-determined absolute level analyte concentration is difficult to directly compare to the changes in analyte concentration determined by a biosensor. This is due, in part, to the temporal difference in the standard measurement (sec for biosensors versus min for microdialysis).
The magnitude of an analyte's absolute level may be used to assess the significance of the analyte change determined by the biosensor measurement. For example, if the changes in analyte concentration determined by the biosensor far exceed the reported absolute level value, it would suggest that one or both of the measurements may be compromised. For lactate, the changes in lactate concentration determined by the biosensors in this study show a range of concentration changes (± 150 μM) that correspond to 35% of the absolute value determined by microdialysis. For glucose, the changes in concentration determined by the biosensors show a range of ± 80 μM. This measured change corresponds to 10-15% of the absolute value determined by microdialysis and is consistent with other measured extracellular fluid declines in glucose concentration associated with neuronal activation.38 For glutamate, the changes in the glutamate concentration determined by the biosensors show a range of concentration changes of ± 0.6 μM that correspond to 20% of the absolute concentration determined by microdialysis in mice. This provides further validation of our biosensor measurements.
The biosensors used in this study are not designed to measure the absolute concentration of an analyte in an in vivo space. Rather, these biosensors are specifically designed to provide reliable, reproducible, and consistent measures of the sec-by-sec changes in analyte concentration over a period of time. The data derived from biosensors are sufficiently robust and the reproducibility of their measures is sufficiently high that predictive models can be derived. For example, our data reveal a heretofore unrecognized yet fundamental relationship between changes in lactate concentration and the sleep/wake cycle. The variations in analyte concentration presented in this study are in agreement with published values for these analytes. The concentration variations reported herein all fall well within the literature estimates of the absolute concentrations and at no time is the reported variation at odds with the absolute concentrations described in the literature.
Such clear correspondence with sleep/wake cycling suggests that both lactate and glutamate may be viable biomarkers. Our results on the changes in extracellular glutamate concentration compare favorably with previous studies that reported increases in glutamate in the cerebral cortex during wake in mice1,53,54 and rats.2 Both glutamate and lactate concentrations change markedly upon waking and remained significantly elevated in comparison with levels measured during NREM sleep. This is true for both natural waking (Figure 5A) and enforced wakefulness periods (Figure 7).2,53 However, the lactate concentration changed much more rapidly, showing clear increases measured within 1 min after waking or NREM sleep onset (i.e., a response of 5 standard deviations from baseline levels).
Measured glutamate concentrations were more variable and slower to respond to the physiologic state, typically taking 5 min or longer to reach the same level of change. Furthermore, changes in lactate concentration could clearly be seen during REM sleep episodes longer than 1 min, and these changes were significantly higher after 2.5 min. Finally, it is known that glutamate concentration initially peaks and then slowly declines during extended waking periods (> 3-h) in both mice16 and rats.2 By contrast, this study demonstrates that changes in extracellular lactate concentration remain high throughout extended periods of wakefulness, yet consistently and rapidly returned to baseline levels only when sleep restriction was terminated. Our findings clearly identify extracellular CNS lactate fluctuations to be a superior biomarker for indication of sleep/wake states in mice. We fully expect that lactate would also prove to be an excellent sleep/wake biomarker in other species.
A framework for the evaluation of an analyte response as a possible biomarker for sleep/wake and, more generally, for any physiologic state change can be generalized based on these results. Once a baseline is established, the change in physiologic state can result in up to three distinct response phases (time periods) based on the measured change in analyte concentration. The first response phase is the initiation phase (IP), which coincides with the physiologic state change. During the IP, the change in analyte concentration (either increasing or decreasing), relative to the defined baseline, is rapid and distinct. The second is the equilibrium phase (EP), which reflects the maintenance of the physiologic state. During the EP, analyte concentration stabilizes for the duration of the physiologic state and at a value that ideally is distinct from the baseline. The third is the transition phase (TP), which signals a change in analyte concentration opposite of that observed in the IP. A TP is not always present, and is not limited to a single instance during the physiologic state. These three time periods are shown for the glucose response for wake (Figure 5A) and sleep (Figure 5B). The transition from IP to EP for lactate and glutamate are indicated by a single arrow in Figures 5A and 5B. Neither analyte displays a TP.
To be a viable biomarker, we hypothesize that the response of an analyte over the time course of a physiologic state should not include a TP (e.g., changes in glucose concentration), and that the concentration observed during the EP must be distinct from the original baseline value (e.g., changes in lactate and glutamate concentrations). Further, our data also suggest the best biomarker will be the analyte that presents the most rapid change in concentration during the IP and the greatest absolute change from baseline during the EP (lactate versus glutamate concentration changes).
Our data show that the response of glucose during sleep/wake transitions is distinct from lactate and glutamate. Glucose shows all three phases, with a distinct TP during both wake and sleep episodes at 7-8 min. During the EP, the observed glucose concentration returns to baseline at 20-23 min during sleep and wake. By contrast, neither the lactate nor glutamate responses show a TP, and both achieve EP concentrations that are significantly elevated (wake) or depressed (sleep) relative to the appropriate baseline after approximately 18 min. Although lactate and glutamate are both potential biomarkers for sleep/wake, the lactate response is more robust and more rapid during IP and shows a larger change relative to the baselines during EP for both sleep and wake episodes. Thus, based on these criteria, and consistent with all the data in this study, the fundamental relationship between lactate and sleep/wake transitions indicates that lactate is a biomarker for this physiologic state change.
This study represents the first experimental support of the ANLSH on a sec-by-sec basis in a conscious, freely-moving animal. Our data are consistent with the prediction of the ANLSH in that extracellular lactate concentrations increase during periods of heightened neuronal activity and decrease during periods of neuronal quiescence. The clear demarcation of physiologic state change from sleep to wake and wake to sleep provides a unique model for examining in vivo neuronal activity in the absence of other confounds.
Time awake is the primary driving force and strongest predictor of the magnitude of the homeostatic response upon entering into sleep. The exceptionally high correlation between increased lactate during wake and decreased lactate during sleep represents a previously undescribed phenomenon with respect to CNS lactate levels. When considered together, it is not unreasonable to assume that elevated lactate or lactate byproducts may underlie some component of sleep homeostasis. This opens the possibility of new avenues for investigation of the sleep homeostatic mechanism.
Whereas increases in cortical lactate concentration during REM sleep have been reported in previous studies,5 this is the first report whereby sec-by-sec resolution has been used to explicitly measure these changes in detail. Our results show that lactate concentration changes are sufficient to identify significant sleep/wake changes. Our results also suggest that lactate concentration changes alone may be sufficient to identify REM periods longer than 1 min within extended sleep bouts. REM sleep periods of less than 1 min in length are not generally detectable by this method. Based on previous data,25 the increase in lactate with waking and REM sleep is almost certainly due to an increased lactate release from astrocytes in response to increased neuronal glutamate release and higher metabolic demands of this sleep stage.55 These findings are intriguing from a physiologic standpoint and suggest that increased lactate demand by neurons may be an important component of REM sleep. Given the measured decline in lactate with NREM onset, reduced lactate during the sleep period may be partially responsible for the limited duration of REM sleep in rodents.
These measured lactate changes are unlikely due to alterations in circulating lactate as plasma and liver lactate concentrations have been shown to have a circadian rhythm, increasing during the dark period and decreasing during the light period.56 Additionally, the increase in lactate during REM sleep, a period of neuronal activity similar to waking but without associated body movement, lends further support to the idea that these changes are brain-specific. Alterations in brain lactate concentration under pharmacologically induced sleep (e.g., melatonin or zolpidem) or wake (e.g., caffeine or modafinil) still remain to be investigated. However, these data point to a dynamic and intertwined association between glucose, lactate, and glutamate that can be directly related to changes in neuronal firing activity between wake, NREM and REM sleep states.
It is important to recognize that this study is not designed to assess genotypic or phenotypic changes in sleep architecture but rather offers a new approach to rapidly screen changes in sleep/wake architecture that may arise, for example, from genotypic or phenotypic alterations. Indeed, the ability to rapidly assess the classic analysis of sleep/wake architecture changes relies solely on the scoring of EEG and EMG data, which is recognized as time-intensive and laborious. It should be noted that although lactate concentration change alone is excellent for identification of wake periods lasting longer than 1 min, this method is less sensitive to shorter periods. Fine-grained analysis of very short wake or sleep bouts lasting 1 min or less may not be detectable using this method. The reliability of the changes in lactate concentration as a function of sleep/wake offers a new and exciting paradigm to rapidly assess whether such changes exist in the absence of an EEG/EMG based score.
This study reveals that monitoring changes in lactate levels is sufficient to accurately describe sleep/wake transitions and demonstrates the usefulness of lactate as a sleep/wake biomarker. When compared with traditional EEG monitoring, biosensor implantation and recording requires a similar level of technical expertise. However, one major advantage demonstrated by this study is that post-processing of the collected data can be accomplished faster and easier using changes in lactate concentration to rapidly define overall periods of sleep and wake. Simultaneous biosensor and biopotential recordings are complementary and together can be used to uncover previously unknown physiologic relationships. The incorporation of lactate changes into sleep analysis paradigms may help augment and improve automated scoring and may find use in developing new strategies for automated sleep analysis. Furthermore, unlike biopotential monitoring, biosensors also provide information regarding the change in concentration of the analyte as a function of time. These data may prove useful in unraveling neurologically relevant metabolic pathways, including neuroenergetic pathways.
DISCLOSURE STATEMENT
This research was supported by NIH grant# 5R44MH076318-03, The Defense Advanced Research Projects Agency (DARPA) and the Army Research Office (ARO) award number W911NF-10-1-0066 - Fred Turek, PI. All authors except Dr. Wilson are employed by Pinnacle Technology, Inc.
ACKNOWLEDGMENTS
The authors thank Mr. Chris Jubic and Ms. Bailey Knott for their assistance in assembling some of the graphics, Mrs. Michelle Canipe for editorial assistance, and Dr. Mike Thompson (Lawrence Memorial Hospital) for assistance with histology.
REFERENCES
- 1.Naylor E, Aillon DV, Gabbert S, et al. Simultaneous real-time measurement of EEG/EMG and L-glutamate in mice: a biosensor study of neuronal activity during sleep. J Electroanal Chem. 2011;656:106–13. doi: 10.1016/j.jelechem.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–9. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John J, Ramanathan L, Siegel JM. Rapid changes in glutamate levels in the posterior hypothalamus across sleep-wake states in freely behaving rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2041–9. doi: 10.1152/ajpregu.90541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur. J. Neurosci. 2001;13:1429–34. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 5.Shram N, Netchiporouk L, Cespuglio R. Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci. 2002;16:461–6. doi: 10.1046/j.1460-9568.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 6.Buysse DJ, Chair . International classification of sleep disorders: Diagnostic and coding manual, revised. Rochester: American Sleep Disorders Association; 2001. [Google Scholar]
- 7.Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–8. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–25. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- 9.Wilson GS, Johnson MA. In-vivo electrochemistry: what can we learn about living systems? Chem Rev. 2008;108:2462–81. doi: 10.1021/cr068082i. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–90. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Wilson GS. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J Neurochem. 1997;68:1745–52. doi: 10.1046/j.1471-4159.1997.68041745.x. [DOI] [PubMed] [Google Scholar]
- 12.Naylor E, Harmon H, Gabbert S, Johnson DA. Automated sleep deprivation: simulated gentle handling using a yoked control. Sleep. 2010;33(Supplement):A108. [Google Scholar]
- 13.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 14.Naylor E, Bergmann BM, Krauski K, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–43. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002;25:691–9. [PubMed] [Google Scholar]
- 16.Naylor E, Aillon D, Gabbert S, et al. Simultaneous measurement of EEG and glutamate during normal sleep and sleep deprivation in a mouse model. Poster session presented at: Neuroscience; 2010 Nov 13-17; San Diego, CA. [Google Scholar]
- 17.Reich P, Geyer SJ, Karnovsky ML. Metabolism of brain during sleep and wakefulness. J Neurochem. 1972;19:487–97. doi: 10.1111/j.1471-4159.1972.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu H, Tabushi K, Hishikawa Y, Kakimoto Y, Kaneko Z. Concentration of lactic acid in rat brain during natural sleep. Nature. 1966;212:936–7. [PubMed] [Google Scholar]
- 19.Cocks JA. Change in the concentration of lactic acid in the rat and hamster brain during natural sleep. Nature. 1967;215:1399–400. doi: 10.1038/2151399a0. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D, Harmon H, Naylor E, et al. An integrated EEG/EMG/glucose system for mice and rats. Sleep. 2007;30(Supplement):A24. [Google Scholar]
- 21.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89:537–52. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- 23.Vega C, Martiel JL, Drouhault D, Burckhart MF, Coles JA. Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol. 2003;546(Pt 2):551–64. doi: 10.1113/jphysiol.2002.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuquet J, Quilichini P, Nimchinsky EA, Buzsaki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci. 2010;30:15298–303. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 26.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 27.Magistretti PJ, Sorg O, Yu N, Martin JL, Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev Neurosci. 1993;15:306–12. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- 28.Pellerin L, Pellegri G, Bittar PG, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–9. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 29.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–6. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 30.Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Esaki T, Shimoji K, et al. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–84. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–7. doi: 10.1111/j.1749-6632.1996.tb34449.x. [DOI] [PubMed] [Google Scholar]
- 33.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–63. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A. 2005;102:16448–53. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Karnovsky ML, Reich P, Anchors JM, Burrows BL. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41:1498–501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 38.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokoloff L, Takahashi S, Gotoh J, Driscoll BF, Law MJ. Contribution of astroglia to functionally activated energy metabolism. Dev Neurosci. 1996;18:344–52. [PubMed] [Google Scholar]
- 40.De Bruin LA, Schasfoort EM, Steffens AB, Korf J. Effects of stress and exercise on rat hippocampus and striatum extracellular lactate. Am J Physiol. 1990;259(4 Pt 2):R773–9. doi: 10.1152/ajpregu.1990.259.4.R773. [DOI] [PubMed] [Google Scholar]
- 41.Demestre M, Boutelle M, Fillenz M. Stimulated release of lactate in freely moving rats is dependent on the uptake of glutamate. J Physiol. 1997;499(Pt 3):825–32. doi: 10.1113/jphysiol.1997.sp021971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shram NF, Netchiporouk LI, Martelet C, Jaffrezic-Renault N, Bonnet C, Cespuglio R. In vivo voltammetric detection of rat brain lactate with carbon fiber microelectrodes coated with lactate oxidase. Anal Chem. 1998;70:2618–22. doi: 10.1021/ac971299f. [DOI] [PubMed] [Google Scholar]
- 43.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem. 2001;75:325–37. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 44.Fellows LK, Boutelle MG. Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res. 1993;604:225–31. doi: 10.1016/0006-8993(93)90373-u. [DOI] [PubMed] [Google Scholar]
- 45.Forsyth R, Fray A, Boutelle M, Fillenz M, Middleditch C, Burchell A. A role for astrocytes in glucose delivery to neurons? Dev Neurosci. 1996;18:360–70. doi: 10.1159/000111429. [DOI] [PubMed] [Google Scholar]
- 46.Zhang FF, Wan Q, Li CX, et al. Simultaneous assay of glucose, lactate, L-glutamate and hypoxanthine levels in a rat striatum using enzyme electrodes based on neutral red-doped silica nanoparticles. AnalBioanal Chem. 2004;380:637–42. doi: 10.1007/s00216-004-2804-x. [DOI] [PubMed] [Google Scholar]
- 47.Shiraishi M, Kamiyama Y, Huttemeier PC, Benveniste H. Extracellular glutamate and dopamine measured by microdialysis in the rat striatum during blockade of synaptic transmission in anesthetized and awake rats. Brain Res. 1997;759:221–7. doi: 10.1016/s0006-8993(97)00258-8. [DOI] [PubMed] [Google Scholar]
- 48.Niwa O, Torimitsu K, Morita M, Osborne P, Yamamoto K. Concentration of extracellular L-glutamate released from cultured nerve cells measured with a small-volume online sensor. Anal Chem. 1996;68:1865–70. doi: 10.1021/ac951154d. [DOI] [PubMed] [Google Scholar]
- 49.Miele M, Boutelle MG, Fillenz M. The source of physiologically stimulated glutamate efflux from the striatum of conscious rats. J Physiol. 1996;497(Pt 3):745–51. doi: 10.1113/jphysiol.1996.sp021805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Rodriguez F, Medina-Ceja L, Wilson CL, Jhung D, Morales-Villagran A. Changes in extracellular glutamate levels in rat orbitofrontal cortex during sleep and wakefulness. Arch Med Res. 2007;38:52–5. doi: 10.1016/j.arcmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Watson CJ, Lydic R, Baghdoyan HA. Sleep duration varies as a function of glutamate and GABA in rat pontine reticular formation. J Neurochem. 2011;118:571–80. doi: 10.1111/j.1471-4159.2011.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther. 2008;324:725–31. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naylor E, Aillon D, Petillo PA, et al. Simultaneous measurement of EEG/EMG and glutamate during normal sleep and sleep deprivation in a mouse model. Paper presented at: Monitoring Molecules in Neuroscience. Proceedings of the 13th International Conference on In Vivo Methods; 2010 Sept 12-16; Brussels, Belgium. [Google Scholar]
- 54.Naylor E, Aillon D, Barrett BS, et al. Lactate acts as a primary neuronal energy source duirng waking and REM sleep. Sleep. 2011;34(supplement):A41. [Google Scholar]
- 55.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahlersova E, Ahlers I, Toropila M, Smajda B, Datelinka I. Circadian rhythm of the lactate and pyruvate concentration in rat liver and blood. Physiol Bohemoslov. 1981;30:213–20. [PubMed] [Google Scholar]