Abstract
Study Objectives:
To ascertain whether objectively measured snoring increases mortality, cardiovascular disease, or stroke risk over the effects of obstructive sleep apnea and other established risk factors.
Design:
Community-based cohort.
Participants:
400 residents of the Western Australian town of Busselton.
Interventions:
N/A.
Measurements:
Snoring and obstructive sleep apnea were quantified via the percentage of the night spent snoring and the respiratory disturbance index as measured by a single night recording in November-December 1990 by a home sleep apnea monitoring device (MESAM IV), along with a range of cardiovascular disease risk factors. Follow-up for deaths and cardiovascular hospitalizations was ascertained via record linkage until the end of 2007.
Results:
Our analytical sample of 380 people was made up of the 397 people for whom the authors had follow-up data, minus 17 people who reported a previous stroke or heart attack at baseline (n = 380/400 = 95% of cohort). Snoring was observed for a mean/median of 32.0/27.4% of the night (standard deviation = 23.9%; range = 0-97.2%). There were 46 deaths, 68 cardiovascular events, and 24 strokes during 17 yr of follow-up. Snoring as either a categoric or continuous variable was not significantly associated with death, incident cardiovascular disease, or stroke in both unadjusted Cox regression models and in models that adjusted for obstructive sleep apnea and other risk factors.
Conclusions:
No measure of snoring was associated with all-cause mortality, or incident cardiovascular disease or stroke over 17 yr in this community-based sample.
Citation:
Marshall NS; Wong KKH; Cullen SRJ; Knuiman MW; Grunstein RR. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the Busselton Health Study. SLEEP 2012;35(9):1235–1240.
Keywords: Survival, population, sleep disordered breathing
INTRODUCTION
Sleep apnea is an independent risk factor for premature mortality and cardiovascular disease (CVD), including stroke.1–7 Intermittent hypoxic insults are generally thought to be the most likely causal pathway.8 But it is also possible that repetitive arousal from sleep/sleep restriction and the large intrathoracic pressure swings characteristic of obstructive sleep apnea (OSA) might cause CVD.9
More recently, snoring-induced vibration injury to the carotid vascular bed has been implicated as a mechanism by which snoring may increase CVD, stroke in particular, independently of the effects of OSA.10,11 Snoring in association with carotid intima-media thickness and the presence of plaques in the carotids have also been reported by others.12
The hypothesis that snoring alone may prime or precipitate stroke is certainly biologically plausible. In people who have already developed arteriosclerosis, snoring might provide a mechanical stimulus to plaque rupture.13 In addition, snoring might begin the arteriosclerotic process. An anesthetized rabbit model indicated that snoring vibration through the carotids could lead to vascular injury and endothelial dysfunction.14,15 This finding may be concordant with the concept of vibration-induced white finger injuries (a secondary form of Reynaud’s syndrome).16
Nevertheless, the hypothesis that snoring alone causes cardiovascular or cerebrovascular disease remains controversial from clinical6,11,17 and epidemiologic8,11,18,19 viewpoints. Although sleep apnea has now been confirmed in 2 community-dwelling cohorts to be an independent predictor for stroke2,3 and in 3 cohorts is a risk factor for all-cause mortality,1,4,7 this does not equate to snoring alone being a risk factor. Indices for sleep apnea weighted toward intermittent hypoxia show stronger relationships to mortality or CVD.8 Another problem is that in studies in which OSA has been objectively quantified, snoring alone does not remain a prognostic factor. For example, in a multicenter clinical cohort primary snorers were at no higher risk of CVD or mortality than control patients investigated for suspected OSA.6 It was recently highlighted16 that no long-term epidemiologic cohort had reported associations with objective measurement of snoring.
Snoring and sleep apnea are of course correlated, but they are not completely overlapping phenomena.20 For the purpose of treatment allocation they have often placed on a severity continuum from no sleep disordered breathing to simple primary snoring through to mild, moderate, and then severe sleep apnea.6 However, in the Busselton cohort some people with sleep apnea snored very little, whereas many heavy snorers may have no apnea whatsoever.21 Most of the existing cohort studies have not separated or objectively measured the 2 correlated, independent conditions with continuous measures of severity. Therefore, the effects of snoring while controlling for sleep apnea do not appear to have been directly tested in a community-based cohort. Because data on snoring sounds as a percentage of the night, as well as objective sleep apnea severity, had been collected as part of the Busselton Sleep Study,21 we investigated whether snoring was associated with 17-yr mortality, CVD, or stroke while controlling for sleep apnea and other leading risk factors.
METHODS
In November-December 1990 we used the MESAM IV device (Madaus Medizen-Elektronik, Freiberg, Germany) to assess snoring and sleep apnea in 400 residents of the rural town of Busselton in the state of Western Australia who already belonged to the ongoing Busselton Health Study (BHS). The MESAM IV, is a 4-channel portable home-monitoring device used to quantify sleep disordered breathing via the measurement of snoring by audio recording, heart rate, oxygen saturation, and body position. The full methods for implementation and manual scoring of snoring and OSA are described in the original paper.21 The parent Busselton study is an ongoing representative and comprehensive survey of residents in the Shire of Busselton in the South-West region of Western Australia. The survey invites the participation of all individuals on the Commonwealth of Australia electoral roll, enrollment on which is compulsory for all Australian citizens age 18 yr and older.
Sample Construction
Men
The 2-stage sampling process has already been described elsewhere.21 Briefly, all men age 40-65 yr on the BHS Register were sent the initial sleep questionnaire (n = 758); 486 men responded. These 486 men were randomly telephoned until a sample of 311 had been recruited for an at-home overnight study (all available study appointments were filled). Five respondents who were invited to participate in the overnight study declined the invitation. Two hundred ninety-four of these overnight study recordings were of adequate quality to score, and 293 men had matched longitudinal data.
Women
All women age 40-65 yr on the register (n = 810) were sent the sleep questionnaire; 537 women responded. At-home overnight studies were undertaken in equal numbers from the 3 categories of snoring (never, sometimes, and almost always/always, each n = 38) so as to ensure some cases of OSA. Women were randomly telephoned until a sample of 114 had been recruited and all the available remaining study appointments had been filled. Six women who were telephoned declined participation. One hundred six of these recordings were of adequate quality to score, and 104 women had matched longitudinal data. Fewer women than men were sampled for financial and logistic reasons because in 1990 sleep apnea was thought to be a relatively rare condition in women before the publication of the Wisconsin cohort in 1993.22
Outcomes: Mortality and Vitality Ascertainment, Cardiovascular and Stroke Events
Mortality status, time to death, time to first cardiovascular event (fatal or nonfatal hospitalization), and time to first stroke event with follow-up to the end of 2007 were ascertained via the Western Australian Health Research Linked Database, which contains linked death and hospital admissions data for all people who resided in Western Australia between 1980 and the present.23 The system includes interstate deaths via the National Death Index.24 Survival in Western Australia was confirmed via listings in the telephone directory, direct contact with relatives, or listing on the electoral role. Incident CVD events were defined as a hospital admission with principal diagnosis of coronary heart disease (ICD-9 410-414; ICD-10 I20-25), stroke (ICD-9 430-437; ICD-10 I60-68, G45), congestive heart failure (ICD-9 428; ICD-10 I50) or peripheral arterial disease (ICD-9 440-448; ICD-10 I70-79), or death with an assigned underlying cause of CVD (ICD-9 390-459; ICD-10 I00-99, G45). Stroke events were similarly defined as hospital admission or death from stroke.
Exposure Variables
Exposure to snoring was quantified by the percentage of the night spent snoring measured via power spectral analysis of the sound signal. The microphone was placed inside a plastic casing to avoid movement sound artefact and taped next to the larynx of the participant. A snoring event was scored if the relative power in the frequencies between 100 and 800 kHz exceeded 50% of the total power. A snoring grade of 0-9 was given to each epoch according to the amount of snoring in that epoch (0 = no snoring; 9 = continuous repetitive snoring). Where an epoch was continuous snoring interrupted only by hypopneas/apneas, that epoch also was scored as a 9. The following formula was used to calculate the percentage of sleep spent snoring: [sum of grades for all epochs/(number of epochs*9)]*100, and has been described previously.21,25 Loud snoring was additionally scored when the sound exceeded 1.1mV at 100 Hz.
Exposure to sleep apnea was quantified by the Respiratory Disturbance Index (RDI), which is calculated by summing the total number of respiratory disturbances and dividing by the participant estimated hours of sleep to give an event rate per hr. Respiratory disturbances were defined as oxygen desaturations of ≥ 3% from the preceding baseline level that were accompanied by either an increased heart rate of at least 10 beats/min and/or a burst of snoring associated with commencement and termination of the desaturation event (i.e., an audible apnea). Hours of sleep were estimated using lights-off and lights-on time, a method that would have overestimated sleep time and therefore caused a systematically lower estimation of RDI than would have been estimated via traditional polysomnography (PSG).
Anthropometric variables were measured by study investigators on the evening before the sleep study and included height, weight, and abdominal height while supine (sagittal diameter), as well as neck, waist, and hip circumference. Blood pressure was then measured twice via an electronic sphygmomanometer (Spacelabs, Redmond, WA) with measurements at least 5 min apart in the supine position and after the participant had been lying quietly for at least 10 min. The mean of these measurements was then calculated.
History of stroke, heart attack, diabetes, and medically diagnosed angina were measured via self-report questionnaire. Smoking status was ascertained via questionnaire by asking whether the patient was a current or former smoker, or had never been a smoker. Former and current smokers were queried about smoking duration and use to calculate pack years. Alcohol consumption patterns were queried to calculate the approximate grams of alcohol consumption per week. Fasting blood samples were taken the morning after the overnight study for blood glucose, total cholesterol, and high-density lipoprotein cholesterol and analyzed using standard assay methods at the Western Australian State Health Laboratories.
Data Handling and Statistical Analyses
Analyses were undertaken (NSM) using SAS software (v 9.2 SAS Institute Cary, NC, USA). Snoring was considered continuously and also categorically using quartiles and we replicated all of these analyses also for loud snoring, but found no significant associations. For sleep apnea we used the same standard clinical cut points26 we reported in the OSA-mortality paper.7 No or subclinical sleep apnea served as the reference category (i.e., 0 to 4 respiratory disturbances per hr) and the 2 exposed categories were mild (5 to 14 RDI) and moderate-to-severe OSA (≥ 15 RDI). We were unable to examine the association of severe OSA with mortality because only 3 participants had an RDI of ≥ 30 at baseline.
Previously established risk factors for mortality were analyzed with the variables transformed or parameterized to maximize model fit (using improved Akaike’s information criteria) and to reduce the chance that any observed association between snoring and mortality was due to poor controlling of known risk factors. Mean arterial pressure (2/3*Systolic + 1/3*Diastolic) gave the best blood pressure fit to the mortality model. The best fit for body habitus was body mass index classified into normal/overweight/obese (< 25, 25 to < 30, or 30 kg/m2). Smoking defined categorically (Never, Former, Current) had the best fit, whereas pack yr was not a significant mortality risk factor.
Univariate associations between risk factors and time-to-event outcomes were investigated with Cox proportional hazards models using chi-square tests for significance (PROC PHREG). Categoric risk factors for mortality were tested (in PROC LIFETEST) to provide Kaplan-Meier curves. All risk factors were also investigated for their association with snoring using chi-square, Fisher exact, analysis of variance, or Kruskal-Wallis tests where appropriate.
Cox regression modeling was used to estimate the effect of snoring (as a continuous variable and as a categoric quartile variable) on the time-to-event outcomes before (unadjusted) and after adjustment for OSA and other risk factors. Regardless of univariate association, the following risk factors were forced into the fully adjusted model because of known associations with snoring, OSA, or mortality: age, sex, obesity, smoking status, blood pressure, total cholesterol, high-density lipoprotein cholesterol, angina, and diabetes. Other risk factors were examined for independent association with mortality when they exhibited some evidence of a univariate association with either mortality or with sleep apnea (P < 0.1). Fasting blood glucose was associated with snoring (Table 1). However, it was not associated with mortality (P > 0.7) when added to the fully adjusted model and did not change the size or significance of the association between snoring and mortality. Thus, it was not included in the final model.
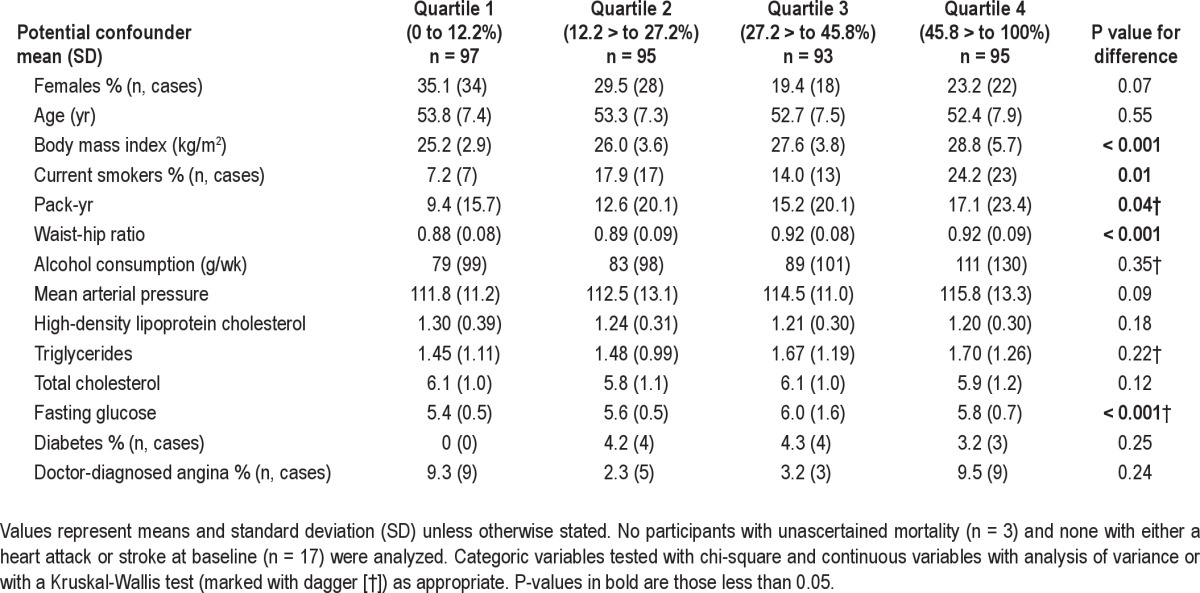
Table 1.
Association of snoring with other established risk factors
RESULTS
We had snoring and follow-up data in 397 participants (99.25% of original cohort). Those participants who indicated at baseline that they had already had a stroke (n = 4) or a heart attack (n = 13) were excluded from our primary analyses because these conditions, particularly stroke, are suspected to cause snoring and OSA and in addition their presence confuses the causal chain. As such, our analytical sample consisted of 380 people (n = 102 females; 95% of the original cohort). We observed an almost full range of percentage of the night spent snoring (range 0-97.2%; mean 32.0%; standard deviation (SD) 23.9%; median 27.4%; loud snoring range 0-80.7%; mean 9.0%, SD 15.7%; median 2.3%). Twenty-two percent of people snored more than 50% of the night and 4.7% were loud snorers for more than 50% of the night. The quartiles of snoring severity in the final analytic sample were Q1: 0-12.2% n = 97, Q2: 12.21-27.2% n = 95, Q3: 27.21-45.8% n = 93, Q4: 45.81-100%, n = 95. Snoring was significantly, but not strongly correlated with the RDI (Figure 1; Spearman rho = 0.5, P < 0.001). In the 380 participants a total of 46 (12.1%) died, 68 (17.9%) experienced a cardiovascular event, and 24 (6.3%) experienced a stroke event.
Figure 1.

Scatterplot showing the strength of the correlation between sleep time spent snoring and the respiratory disturbance index. Spearman correlation (rho 0.5, P < 0.001) for the association between the amount of the night spent snoring and the major index of sleep apnea severity, the respiratory disturbance index, shows that although these are correlated phenomena they do not completely overlap. The second worst snorer, for instance, also had no sleep apnea.
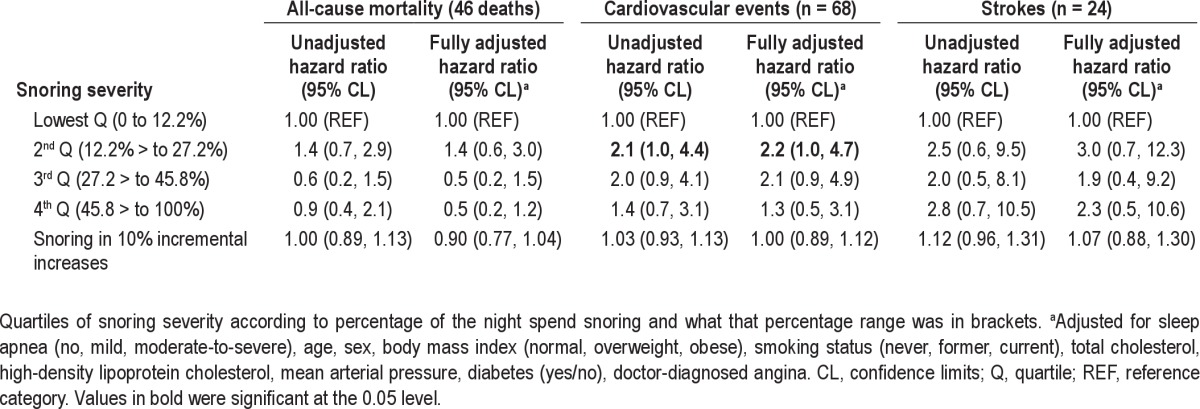
Kaplan-Meier plots comparing the quartiles of snoring exposure to mortality, incident cardiovascular disease and stroke (Figures 2–4) plus an associated log-rank test all indicated there was no discernable visual or statistical association between snoring and any of our outcomes. Table 1 shows the associations of risk factors with snoring duration expressed in quartiles. Table 2 shows the association between snoring severity (continuous and as categoric quartiles) and mortality, incident CVD, and stroke events before and after adjustment for OSA and other risk factors and confounders. No model we investigated indicated a significant association between snoring or loud snoring and any of these 3 outcomes. A sensitivity analysis of the CVD hospitalization data excluding peripheral arterial disease as a CVD event did not change our analysis because there was only 1 incident case. Inclusion of the 17 individuals with prevalent stroke or heart attack in models that statistically controlled for previous heart attack and stroke did not change the size or significance of the associations.
Figure 2.
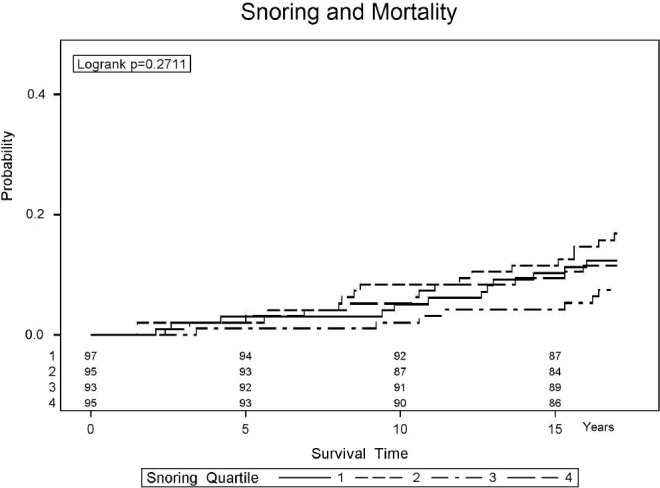
Kaplan-Meier estimation of the probability of mortality of participants with ascending quartiles of sleep time spent snoring. All 4 lines significantly overlap and none are statistically significantly different from any other line, suggesting the amount of time spent snoring has no relationship with mortality risk (see also Table 2 for multivariate confirmation).
Figure 3.
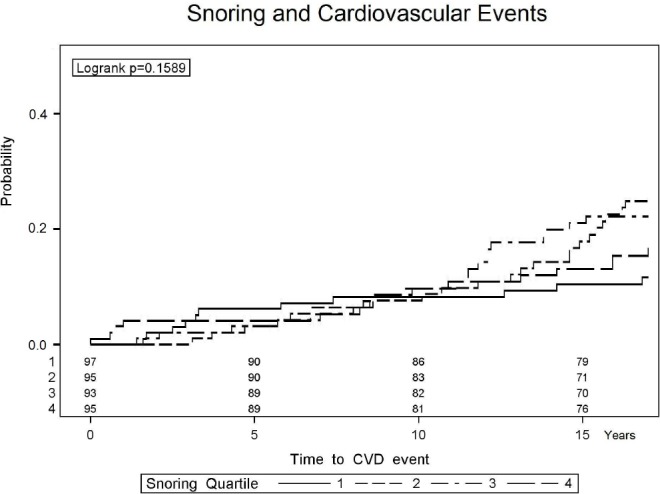
Kaplan-Meier estimation of the probability of incident cardiovascular disease in participants with ascending quartiles of sleep time spent snoring. All 4 lines significantly overlap and none are statistically significantly different from any other line, suggesting the amount of time spent snoring has no relationship with cardiovascular disease in general (see also Table 2 for multivariate confirmation).
Figure 4.

Kaplan-Meier estimation of the probability of incident stroke in participants with ascending quartiles of sleep time spent snoring. All 4 lines significantly overlap and none are statistically significantly different from any other line, but note that our analyses of stroke may be significantly underpowered (see also Table 2 and the Discussion section).
Table 2.
Independent association of snoring with mortality, cardiovascular events, and strokes before and after adjustment for obstructive sleep apnea and other risk factors
DISCUSSION
Snoring was not associated with all-cause mortality, incident CVD, or stroke over 17 yr in this community-based sample, irrespective of the multivariate model tested.
Questionnaire-based studies have regularly reported that snoring (or sleepy snoring27) is associated with mortality or CVD.1 Traditionally this association has been explained by the presence of people with occult sleep apnea being included in the snoring groups. The converse problem for ascertaining the effects of snoring on health has been that the cohort studies with objective measures of sleep apnea often have not measured snoring severity and/or have placed it within an ordinal scale of sleep disordered breathing severity.6
However, snoring may not belong in such a scale because snoring and sleep apnea are not completely separate phenomena. Snoring and OSA are certainly correlated (Figure 1; rho = 0.5). In the BHS, there are people with moderate-to-severe OSA who snore less than 40% of the night and others with no OSA who snore more than 80% of the night. Because the Sleep Study cohort21 of the BHS is one of the few community-based studies with objective and continuous severity measures of both sleep apnea and snoring, we were able to directly test the hypothesis that snoring itself, independent of the effects of sleep apnea and leading risk factors, is associated with premature mortality, CVD, or stroke. However, this was only a moderately sized cohort study and therefore only had power to detect moderate or larger effect sizes. Our cohort study with 17 yr of follow-up had power of 78%, 92%, and 51% for all-cause deaths, cardiovascular events, and stroke events, respectively, to detect a relative risk of at least 1.5 per SD increase in snoring percentage and power of 63%, 79%, and 36%, respectively, to detect a relative risk of 3.0 for pairwise quartile comparisons. Hence, our lack of an association for stroke events is less conclusive.
One potential limitation of the study was that snoring and sleep apnea were measured using the MESAM IV device rather than PSG. However, this device had very close agreement with standard PSG in terms of quantifying sleep apnea (Intraclass Correlation = 0.98) in the original prevalence study, as well as being validated by other research groups.21,28,29 Moreover, the prevalence estimate using this method in Busselton was in very close agreement (26% versus 24%) with the PSG-measured prevalence estimate from the widely accepted prevalence estimate from the Wisconsin Sleep Cohort.22 Finally, the ability of the MESAM IV device to predict OSA-associated mortality provides strong evidence that this is a valid OSA measurement technique in community-based epidemiologic investigations.
The quantification of snoring, on the other hand, has no agreed clear gold standard measurement in population studies. Our study may not necessarily measure the vibratory “load” that may be relevant to previous studies linking snoring to the coprevalence of carotid arteriosclerosis. It is possible that the methods used by Lee and colleagues10 captured snoring information that predicts risk that our measure did not. They used carotid and femoral artery ultrasound to quantify arteriosclerosis in 110 snoring and non-snoring volunteers who did not have sleep apnea or had mild OSA not severe enough to cause notable deoxygenation.10 A strong dose-dependent relationship was shown between snoring severity and the prevalence of carotid arteriosclerosis; 20% in mild snorers, 32% in moderate snorers, and 64% in heavy snorers. Importantly, snoring was not significantly associated with femoral arteriosclerosis in these same people, which argues that the convenience sample on which the study was built may not have been biased toward recruiting snorers with systemic arteriosclerosis. Snoring in association with carotid intima-media thickness and the presence of plaques in the carotids has also been reported by others.12 The general lack of association we observed may also have been falsely caused by bias if people who snored were more likely to receive effective CVD treatments.
The possibility that snoring itself, independent of any effects of sleep apnea, causes carotid arteriosclerosis or triggers stroke remains a biologically plausible hypothesis. We did not find statistically significant associations between snoring and stroke, but our study was not highly powered for stroke events. However, in one of the few cohorts where both snoring and sleep apnea have been objectively quantified, we were unable to see any discernable signal for an association between snoring and incident CVD or mortality.
ACKNOWLEDGMENTS
The authors thank Graham Meier for programming the data match that made these analyses possible. The Busselton Population Medical Research Foundation gave the authors access to the study data and the authors also thank the community of Busselton for their long-standing support. The authors also thank the Marburg Sleep Group (Wilma Althaus and Hartmut Schneider), Lyn Davies, and Jan Hedner for their pivotal role in baseline data collection. Research supported by Australian NHMRC grants 264598, 202916, and 571421 to Dr. Grunstein.
Footnotes
A commentary on this article appears in this issue on page 1193.
Dedication
This manuscript is dedicated to the memory of Dr. Helen Bearpark, who collected the baseline exposure data presented here with the expressed intention of longitudinal study. She was tragically killed in a road accident in December 1996.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Marshall, Wong and Grunstein have received funding and equipment from Fisher – Paykel Healthcare and in-kind support from Cephalon. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLOS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 6.Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 8.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 10.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Drager LF, Lorenzi-Filho G. Heavy snoring and carotid atherosclerosis: is there more than an association? Sleep. 2008;31:1335. [PMC free article] [PubMed] [Google Scholar]
- 12.Li YAN, Liu J, Wang WEI, et al. Association of self-reported snoring with carotid artery intima-media thickness and plaque. J Sleep Res. 2012;21:87–93. doi: 10.1111/j.1365-2869.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 13.Hedner J, Wilcox I, Sullivan CE. Speculations on the interaction between vascular disease and obstructive sleep apnoea. In: Saunders N, Sullivan C, editors. Sleep and breathing. New York: Dekker; 1994. pp. 823–46. [Google Scholar]
- 14.Amatoury J, Howitt L, Wheatley JR, Avolio AP, Amis TC. Snoring-related energy transmission to the carotid artery in rabbits. J Appl Physiol. 2006;100:1547–53. doi: 10.1152/japplphysiol.01439.2005. [DOI] [PubMed] [Google Scholar]
- 15.Cho J-G, Witting PK, Verma M, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34:751–7. doi: 10.5665/SLEEP.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice TR, Strollo PJ. A nuisance or nemesis: the adverse effects of snoring. Sleep. 2011;34:693–4. doi: 10.5665/SLEEP.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunstein R. Snorenheit 911: searching for the ‘truth’ about snoring. Sleep Med Rev. 2004;8:429–31. doi: 10.1016/j.smrv.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Young TB. Some methodologic and practical issues of reported snoring validity. Chest. 1991;99:531–2. doi: 10.1378/chest.99.3.531. [DOI] [PubMed] [Google Scholar]
- 19.Knuiman MW, James AL, Divitini ML, Bartholomew HC. Correlates of habitual snoring and witnessed apnoeas in Busselton, Western Australia. Aust NZ J Public Health. 2005;29:412–5. doi: 10.1111/j.1467-842x.2005.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 20.Rich J, Raviv A, Raviv N, Brietzke SE. An epidemiologic study of snoring and all-cause mortality. Otolaryngol Head Neck Surg. 2011;145:341–6. doi: 10.1177/0194599811402475. [DOI] [PubMed] [Google Scholar]
- 21.Bearpark H, Elliot L, Grunstein R, et al. Snoring and sleep apnea: a population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 22.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 23.Holman CD, Bass AJ, Rouse IL, Hobbs MST. Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust NZ J Public Health. 1999;23:453–9. doi: 10.1111/j.1467-842x.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 24.Magliano D, Liew D, Pater H, et al. Accuracy of the Australian National Death Index: comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust NZ J Public Health. 2003;27:649–53. doi: 10.1111/j.1467-842x.2003.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 25.Penzel T, Amend G, Meinzer K, Peter JH, Von Wichert P. Mesam: a heart rate and snoring recorder for detection of obstructive sleep apnea. Sleep. 1990;13:175–82. doi: 10.1093/sleep/13.2.175. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine Taskforce. Sleep-related breathing disorders in Adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 27.Lindberg E, Janson C, Svardsudd K, et al. Increased mortality among sleepy snorers: a prospective population based study. Thorax. 1998;53:631–7. doi: 10.1136/thx.53.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esnaola S, Duran J, Infante-Rivard C, Rubio R, Fernandez A. Diagnostic accuracy of a portable recording device (MESAM IV) in suspected obstructive sleep apnoea. Eur Resp J. 1996;9:2597–605. doi: 10.1183/09031936.96.09122597. [DOI] [PubMed] [Google Scholar]
- 29.Stoohs R, Guilleminault C. MESAM 4: an ambulatory device for the detection of patients at risk for obstructive sleep apnea syndrome (OSAS) Chest. 1992;101:1221–7. doi: 10.1378/chest.101.5.1221. [DOI] [PubMed] [Google Scholar]


