Abstract
Study Objectives:
Abnormal ventilatory drive may contribute to the pathophysiology of the childhood obstructive sleep apnea syndrome (OSAS). Concomitant with the obesity epidemic, more adolescents are developing OSAS. However, few studies have specifically evaluated the obese adolescent group. The authors hypothesized that obese adolescents with OSAS would have a blunted hypercapnic ventilatory response (HCVR) while awake and blunted ventilatory responses to carbon dioxide (CO2) during sleep compared with obese and lean adolescents without OSAS.
Design:
CVR was measured during wakefulness. During nonrapid eye movement (NREM) and rapid eye movement (REM) sleep, respiratory parameters and genioglossal electromyogram were measured during CO2 administration in comparison with room air in obese adolescents with OSAS, obese control study participants, and lean control study participants.
Setting:
Sleep laboratory.
Participants:
Twenty-eight obese patients with OSAS, 21 obese control study participants, and 37 lean control study participants.
Results:
The obese OSAS and obese control groups had a higher HCVR compared with the lean control group during wakefulness. During both sleep states, all 3 groups had a response to CO2; however, the obese OSAS group had lower percentage changes in minute ventilation, inspiratory flow, inspiratory time, and tidal volume compared with the 2 control groups. There were no significance differences in genioglossal activity between groups.
Conclusions:
HCVR during wakefulness is increased in obese adolescents. Obese adolescents with OSAS have blunted ventilatory responses to CO2 during sleep and do not have a compensatory prolongation of inspiratory time, despite having normal CO2 responsivity during wakefulness. Central drive may play a greater role than upper airway neuromotor tone in adapting to hypercapnia.
Citation:
Yuan H; Pinto SJ; Huang J; McDonough JM; Ward MB; Lee YN; Bradford RM; Gallagher PR; Shults J; Konstantinopoulou S; Samuel JM; Katz ES; Hua S; Tapia IE; Marcus CL. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. SLEEP 2012;35(9):1257–1267.
Keywords: Hypercapnic ventilatory response, CO2, genioglossus, REM sleep
INTRODUCTION
The prevalence of obesity among children and adolescents is rising,1 which significantly increases the risk of developing the obstructive sleep apnea syndrome (OSAS).2,3–4 Adolescence, the transition from childhood to adulthood, is a period of development known to be associated with major changes in sleep state organization5 and circadian rhythm.6 Obese adolescents have been shown to have an increased risk of developing OSAS compared with nonobese peers.7 However, few studies have specifically evaluated breathing during sleep in the adolescent age group, and the pathophysiologic mechanisms leading to OSAS in obese adolescents are poorly understood.
Both anatomic and functional factors (such as ventilatory drive and neuromotor tone) contribute to the pathogenesis of OSAS. The role of ventilatory drive in the pathophysiology of OSAS in adults and children with OSAS is not fully understood. Some studies have shown that adult patients with OSAS have a blunted ventilatory response to chemical stimulation,8,9 a normal ventilatory response,10,11 or an increased response.12,13 Studies in children with OSAS have demonstrated that the central ventilatory responses to hypoxia and hypercapnia are normal during wakefulness and sleep,14,15 although subtle abnormalities in ventilatory control are present.16 Additionally, studies have shown that children with OSAS have depressed spontaneous ventilation under anesthesia,17 or have a diminished ventilatory response to carbon dioxide (CO2).18 Previous studies suggested that an increase in chemical drive can elevate genioglossus activity before airway opening,19,20 indicating that upper airway muscles can respond to increasing chemical drive prior to arousal.21,22–23 However, the upper airway response to CO2 often occurs at a higher threshold than the ventilatory muscle response, leading to upper airway instability.24,25 In addition, ventilatory responses are also affected by sex. The HCVR during wakefulness has been shown to be greater in men than in women,26,27 although men have more obstructive apnea, perhaps due to ventilatory instability. Zhou et al. demonstrated that men were more susceptible to hypocapnic central apnea than women during nonrapid eye movement (NREM) sleep.28 Ventilatory responses are also affected by ventilatory drive. Some studies suggested that obese patients have decreased responses to CO229,30; another study of obese patients reported exaggerated respiratory chemosensitivity at high altitude.31 However, although the ventilatory drive is known to decrease during adolescence,32 data on ventilatory responses in obese adolescents with OSAS are not available. Because obese adolescents are at a higher risk for OSAS,3,4,7 which may be in part due to the development of the obesity hypoventilation syndrome, we hypothesized that obese adolescents with OSAS have blunted hypercapnic ventilatory responses (HCVR) during wakefulness, and a blunted ventilatory response to CO2 during sleep compared with obese and lean adolescents without OSAS, and male adolescents may be more susceptible. We also hypothesized that obese adolescents with OSAS have blunted upper airway neuromotor activity compared with obese and lean adolescent control groups.
METHODS
Study Design
Obese adolescents with OSAS, age- and body mass index (BMI)-matched obese control participants and age-matched lean control participants were studied. All study participants underwent anthropometric measurements, pulmonary function testing, and baseline polysomnography. They were classified as obese participants with OSAS, obese control participants, or lean control participants on the basis of their history, anthropometry, and polysomnography. On a separate occasion, HCVR testing was performed during wakefulness, and CO2 challenges were performed during NREM and rapid eye movement (REM) sleep. The study was approved by the Institutional Review Board of the Children's Hospital of Philadelphia. Written informed consent was obtained from the parents or legal guardians of each child, and assent obtained from the children.
Study Group
Study participants age 12-16 yr were recruited from the Sleep Center at Children's Hospital of Philadelphia (those with OSAS) or from the general community (obese and lean control participants) by means of advertisements. Participants were classified as obese if their BMI was > 95th percentile for age and sex, and lean if their BMI was < 85th percentile; those with a BMI between the 85th and 95th percentile were excluded.33 To limit overlap between groups, participants with OSAS were included only if their apnea-hypopnea index (AHI) was > 5/hr and control participants were included only if they were asymptomatic (i.e., no history of snoring) with an AHI < 1.5/hr.34,35,36–37 Individuals with craniofacial anomalies, neuromuscular disease, or significant medical conditions other than OSAS were excluded from the study.
Anthropometric Measurements
Weight was measured with a calibrated balance scale. Height was measured in centimeters with a stadiometer.
Baseline Polysomnography
Polysomnography studies were performed overnight. A Rembrandt polysomnography system (Embla, Broomfield, CO) recorded the following parameters: chest and abdominal wall motion using respiratory inductance plethysmography (Viasys Healthcare, Yorba Linda, CA), heart rate by electrocardiogram, arterial oxygen saturation (SpO2) by pulse oximetry (Masimo, Irvine, CA); end-tidal partial pressure of carbon dioxide (PETCO2), measured at the nose by infrared capnometry (Novametrix Medical System, Inc., Wallingford, CT), airflow using a 3-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA), nasal pressure by a pressure transducer (Pro-Tech Services, Inc., Walnut Cove, NC), electroencephalographic leads (C3/A2, C4/A1, F3A2, F4A1, O1/A2, O2/A1), left and right electrooculograms, submental electromyogram (EMG), and tibial EMG. Study participants were continuously observed by a polysomnography technician and were recorded on video with an infrared video camera. Studies were scored using standard pediatric sleep scoring criteria.38
Pulmonary Function Testing
Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines to ensure that there was no mechanical limitation affecting ventilatory response testing.39
Hypercapnic Ventilatory Response Testing
Ventilatory responses to hypercapnia were measured during wakefulness using a modification of the Read hyperoxic hypercapnic rebreathing technique,14,32,40,41,42,43–44 The study participants were seated comfortably, breathing through a mouthpiece. PETCO2 was measured by infrared capnometry (Novametrix Medical System, Inc., Wallingford, CT). Flow was measured using a heated pneumotachograph (Hans Rudolph, Inc., Shawnee, KS) and transducer (ADInstruments, Colorado Springs, CO). Minute ventilation was obtained by analog integration of the flow signal. All outputs were recorded on a PowerLab system (ADInstruments, Colorado Springs, CO). The study participants rebreathed from a bag filled with 70 ml/kg of a gas mixture with the initial composition of 95% oxygen (O2) and 5% CO2. The subjects breathed room air through the mouthpiece for several min to establish baseline values. The inhalation valve was then switched at the end of a normal expiration so that the study participant rebreathed the hypercapnic mixture. The study participants were asked to take 3 deep breaths after the valve was switched, and then breathe normally. They were encouraged to continue until PETCO2 reached 65 mm Hg. All tests were completed within 4 min so that significant respiratory acidosis would not occur.14 Ventilatory responses to hypercapnia were expressed as V̇E versus PETCO2.
CO2 Challenges During Sleep
After HCVR testing, the study participants underwent overnight polysomnography to determine the ventilatory response to CO2 during sleep. In addition to the routine polysomnographic leads, study participants wore a customized intraoral mouthpiece with electrodes to measure the genioglossal electromyogram (EMGgg).45,46 Study participants wore a full face mask (Philips Respironics, Murrysville, PA) attached to a heated pneumotachometer with a differential pressure transducer. The pneumotachometer was connected to a continuous positive airway pressure device (Philips Respironics, Murrysville, PA).45,47 CO2 was introduced via a port on the mask. Nasal pressure was measured at the mask using a pressure transducer with a demodulator. Transcutaneous partial pressure of carbon dioxide (PCO2) (Radiometer, Copenhagen) was primarily assessed rather than PETCO2 because accurate PETCO2 measurements could not be obtained during challenges due to the bias flow through the system. Signals were acquired on a PowerLab system and simultaneously displayed on a Rembrandt polysomnography system.
During NREM and REM sleep, trials were performed at a holding pressure defined as the nasal pressure just below the pressure at which flow limitation was abolished.48 Flow limitation was determined by the characteristic waveform pattern, consisting of increasing inspiratory flow followed by a midinspiratory plateau.49,50 The study participants breathed at holding pressure for at least 2 min before the challenge to obtain a stable baseline. CO2 was then administered at 1 L/min and increased as needed until the transcutaneous PCO2 increased by 3 mmHg from baseline, and was then maintained at that level for 1 min. Trials with changes of sleep state were excluded.
Data Analysis
For both HCVR and CO2 challenges during sleep, airflow, tidal volume (VT), inspiratory time (TI), and total respiratory cycle time (TTOT) were measured for each breath. The slope of the minute ventilation (V̇E)-PETCO2 curve was used to characterize the ventilatory response to hypercapnia during wakefulness. For CO2 challenge during sleep, these baseline ventilatory parameters were averaged over the 1 min preceding the CO2 challenge, and the last 1 min of the CO2 challenge. The changes in ventilatory parameters and EMGgg were expressed as the percentage of baseline values to account for variation in baseline EMGgg among individuals.45,51 Because TI increases in response to negative pressure challenges,48 even though the EMGgg activity per unit time may not change, the total EMGgg activity during inspiration increases. Therefore, EMGgg activity was corrected for TI, as described in the literature.45
Statistical Analysis
All results are expressed as mean ± standard deviation unless otherwise indicated. Statistical analyses were conducted using Stata 11.0 (StataCorp, College Station, TX), with 2-sided tests of hypotheses and a P < 0.05 as the criterion for statistical significance. Analyses were stratified between REM versus NREM sleep. One-way analysis of variance (ANOVA) was conducted to compare the mean levels of continuous variables between the 3 groups (obese OSAS, obese control, lean control); if significant differences were observed between groups, then groups were compared pairwise, with adjustment for multiple comparisons via the Bonferroni correction for multiple comparisons. Categorical variables were compared between groups with the chi-square test. For comparison of ventilatory responses during sleep among the 3 groups, in addition to the analysis via ANOVA, regression models were constructed that adjusted for both sex and holding pressure. The regression models were constructed by including indicator variables for the obese OSAS and obese control groups that take value 1 for participants in the obese OSAS or obese control group, and that take value 0 otherwise. The lean control group was therefore the reference group in this analysis. The regression models allowed for inclusion of additional variables in the model, to determine if the results of the analysis conducted by ANOVA would change after adjustment for additional variables such as sex and holding pressure.
RESULTS
Study Population
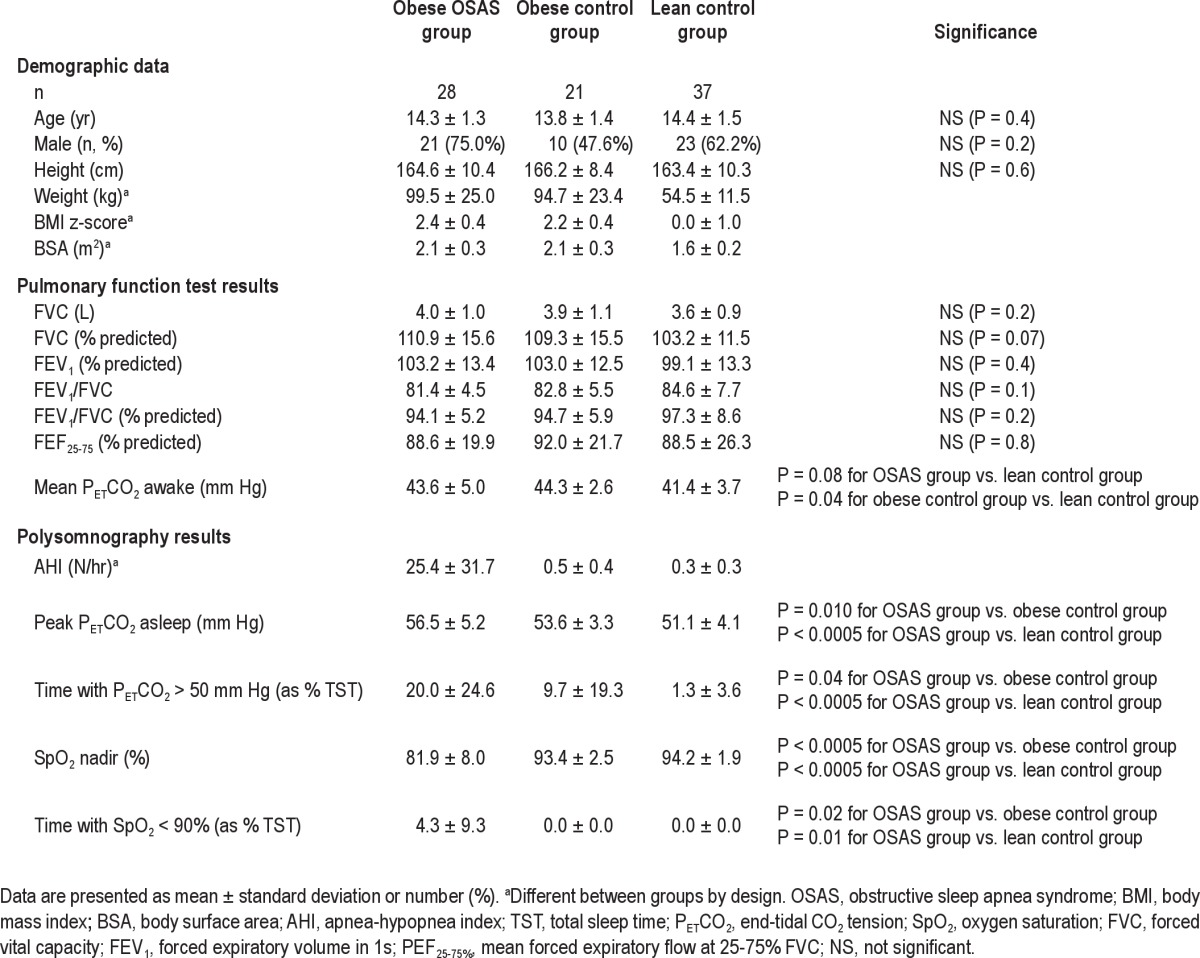
Twenty-eight study participants with OSAS, 21 in the obese control group, and 37 in the lean control group were recruited. Study population characteristics are shown in Table 1. As expected, those with OSAS had a higher AHI and the obese group had a higher BMI z-score. There were no significance differences in pulmonary function among the 3 groups. Obese groups had a higher PETCO2 than the lean control group during wakefulness, and those with OSAS had a higher peak PETCO2 and longer duration of hypoventilation (time with PETCO2 > 50 mm Hg) than the control groups when asleep.
Table 1.
Population characteristics and polysomnography results
HCVR Testing During Wakefulness
HCVR testing during wakefulness was performed successfully in 26 study participants with OSAS, 17 in the obese control group, and 36 in the lean control group. The results are shown in Figure 1 and Table 2. Those in the lean control group had a lower slope of HCVR compared with those with OSAS and the obese control group; however, no significant differences were found between the 2 obese groups, and there were no significant differences between any of the groups when HCVR was adjusted for weight or BMI. Typical examples of airflow and tidal volume changes during HCVR testing for obese OSAS, obese control, and lean control groups are shown in Figure 2. The values of PETCO2 at baseline prior to rebreathing test, immediately after the 3 big breaths and the final end-tidal CO2 values, as well as the range of change in the end-tidal CO2 values, are shown in Table 2. There was no significant difference among the 3 groups. Thus, challenges were very similar among the study participants
Figure 1.
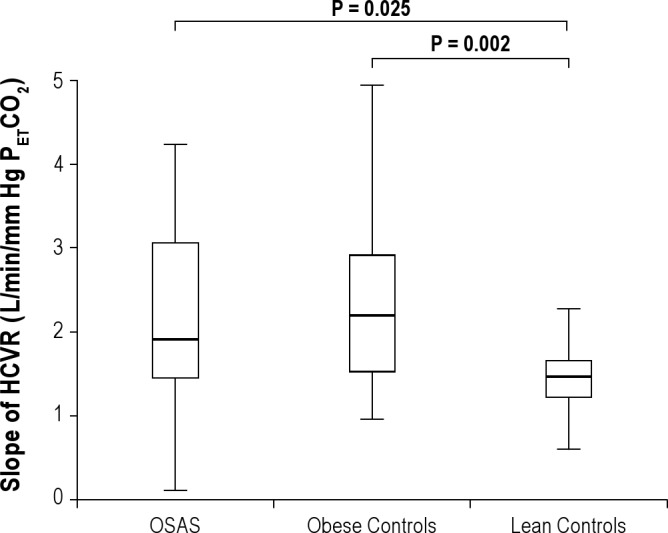
Slope of Hypercapnic Ventilatory Responses (HCVR). Box plots of the slope of the hypercapnic ventilatory responses (HCVR) are shown. The box represents the interquartile range that contains 50% of the values. The line across the box indicates the median. The whiskers extend from the box to the highest and lowest values. The HCVR was lower in the lean control group than in the obese OSAS and obese control groups.
Table 2.
Hypercapnic ventilatory response testing
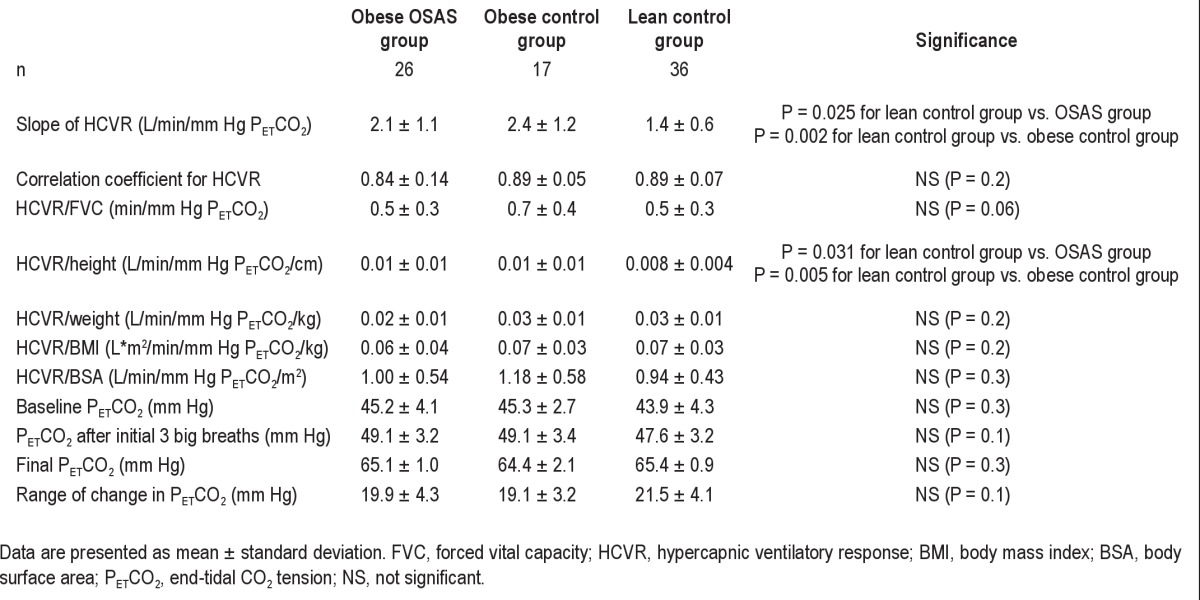
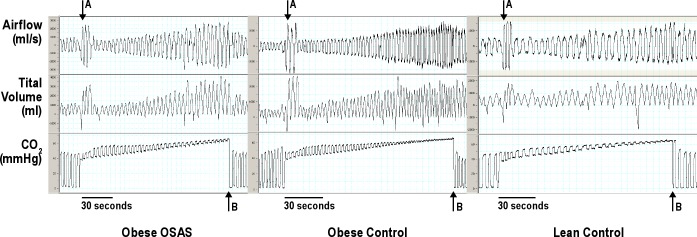
Figure 2.
Typical Examples of Airflow and Tidal Volume Changes During Hypercapnic Ventilatory Response Testing. Typical examples of air flow and tidal volume changes during hypercapnic ventilatory response testing from obese obstructive sleep apnea syndrome (OSAS), obese control, and lean control male study participants are shown. The time scale for each example is shown at the bottom of each plot. The arrow A designates the time point at which the study participant took 3 big breaths to equilibrate with the bag. The arrow B designates the time point at which end-tidal CO2 reached 65 mmHg.
The relationship between the slope of HCVR and the degree of polysomnographic abnormalities was compared for the OSAS group alone and for all study participants combined. No significant correlation was found between the AHI, SpO2 nadir, duration of desaturation, peak PETCO2, or duration of hypoventilation and the slope of HCVR.
Ventilatory Response to CO2 During Sleep
CO2 challenges during NREM sleep were performed in 21 study participants with OSAS, 17 in the obese control group, and 24 in the lean control group. Challenges were not performed in all study participants due to scheduling issues (a physician was required to be present overnight during the CO2 administration). Fewer study participants underwent CO2 challenge successfully during REM sleep due to the lower arousal threshold in this state compared with that in NREM sleep. Thus, 17 study participants with OSAS, 11 in the obese control group, and 17 in the lean control group underwent CO2 challenges successfully during REM sleep. A typical CO2 challenge for a lean control participant during NREM sleep is shown in Figure 3. The ventilatory responses to CO2 during NREM and REM sleep are shown in Table 3 and Figures 4 through 8. Results were similar after excluding those who did not complete both the sleep and wake parts of the protocol. The time from the beginning of each challenge to the 3 mm Hg rise in transcutaneous CO2 did not differ among the 3 groups (NREM: 4.94 ± 3.18, 3.89 ± 2.22 and 5.55 ± 2.44 min, P = 0.197; REM: 3.79 ± 1.90, 3.02 ± 2.26 and 4.46 ± 1.95 min, P = 0.189, for those in the obese OSAS, obese control, and lean control groups, respectively).
Figure 3.
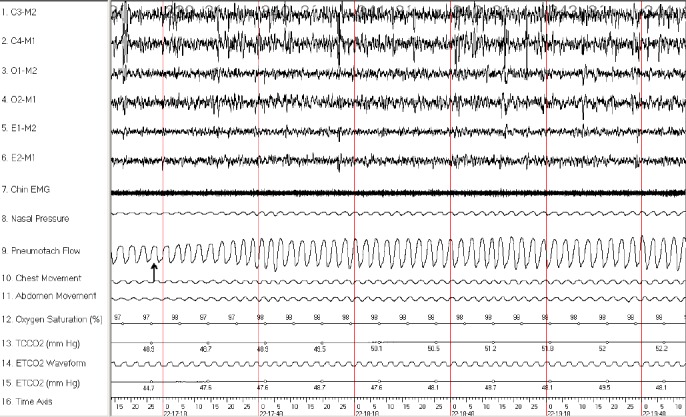
CO2 Challenge in a Lean Control Subject during NREM Sleep. A 3-min epoch from a 12-yr-old boy in the lean control group during non-rapid eye movement (NREM) sleep is shown. After administration of CO2 (arrow), the study participants had an increase in air flow. Y-axis parameters: C3-M2, C4-M1, O1-M2, and O2-M1 are electroencephalogram leads; E1-M2 and E2-M1 are left and right electrooculograms, respectively; Chin electromyogram (EMG), submental EMG signal; pneumotach flow, airflow measured with a heated pneumotachometer; ETCO2, end-tidal PCO2 value; TCCO2, transcutaneous PCO2; time axis, clock time (in sec).
Table 3.
Percentage changes in ventilatory responses to hypercapnia during nonrapid eye movement and rapid eye movement sleep
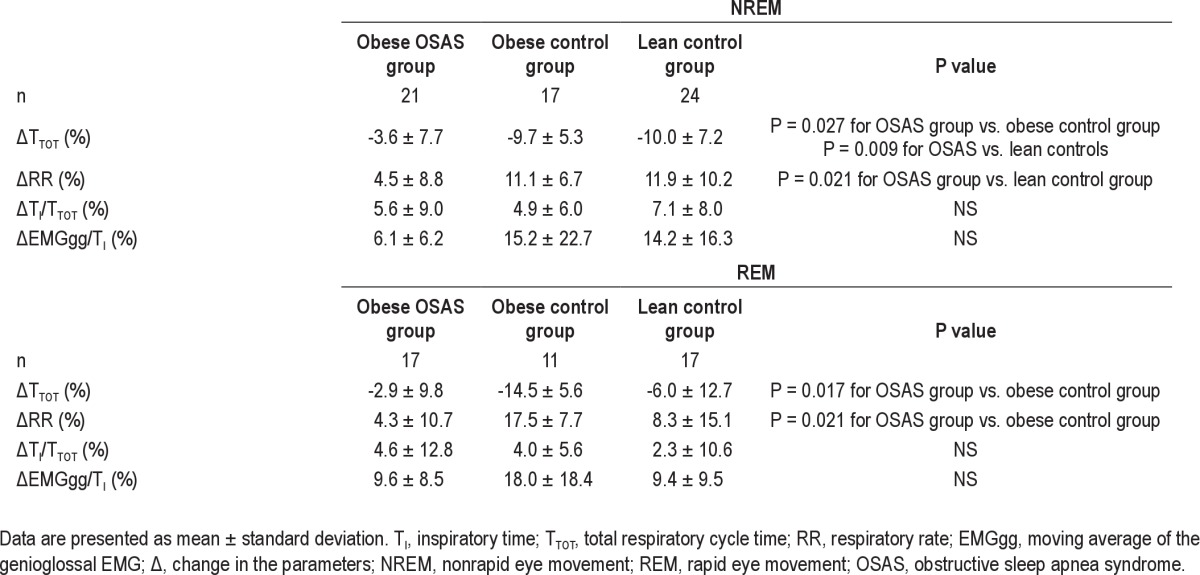
Figure 4.
Percentage change in minute ventilation during NREM and REM sleep. The percentage change in minute ventilation (V̇E) during nonrapid eye movement (NREM) and rapid eye movement (REM) sleep is shown for the 3groups. See Figure 1 for a description of the box plots. During NREM and REM sleep, study participants with obstructive sleep apnea syndrome (OSAS) had a lower percentage change in V̇E in response to CO2 than those in the control groups.
Figure 8.
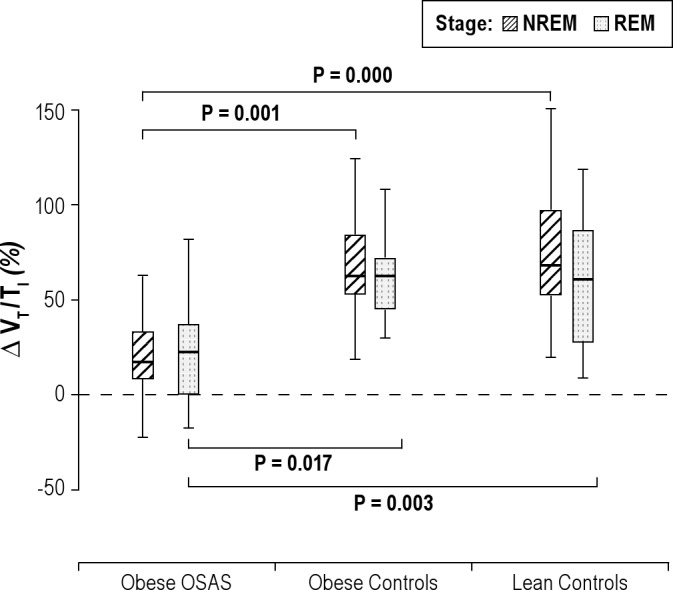
Percentage change in VT/TI during NREM and REM sleep. The percentage change in the tidal volume to inspiratory time ratio (VT/TI) during nonrapid eye movement (NREM) and rapid eye movement (REM) sleep is shown for the 3 groups. See Figure 1 for a description of the box plots. During NREM and REM sleep, study participants with OSAS had a lower percentage change in VT/TI in response to CO2 than those in the control groups.
Ventilatory Responses to CO2 During NREM Sleep
The changes in minute ventilation (V̇E), inspiratory flow, and tidal volume during the CO2 challenge compared with baseline (expressed as a percent change of the baseline values) are shown in Figures 4 through 6. V̇E, inspiratory flow, and VT all increased in response to CO2 in all 3 groups. However, OSAS had smaller changes in V̇E, flow, and tidal volume in response to CO2 compared with the control groups.
Figure 6.
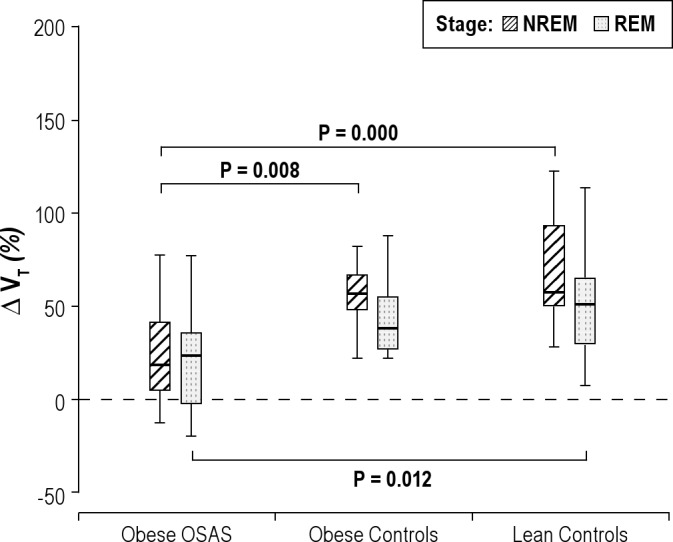
Percentage change in tidal volume during NREM and REM sleep. The percentage change in tidal volume (VT) during nonrapid eye movement (NREM) and rapid eye movement (REM) sleep is shown for the 3 groups. See Figure 1 for a description of the box plots. During NREM sleep, study participants with obstructive sleep apnea syndrome (OSAS) had a lower percentage change in VT in response to CO2 than those in the control groups. During REM sleep, those in the OSAS group had a lower percentage change in VT than those in the lean control group.
In the 2 control groups, TI decreased during the CO2 challenge (−5.35 ± 5.82% and −3.92 ± 6.88% for obese control and lean control groups, respectively). In contrast, there was virtually no change in TI in the OSAS group (1.55 ± 8.65 %) (Figure 7). VT/TI increased in all 3 groups, with a greater increase in the 2 control groups than in the OSAS group (Figure 8).
Figure 7.
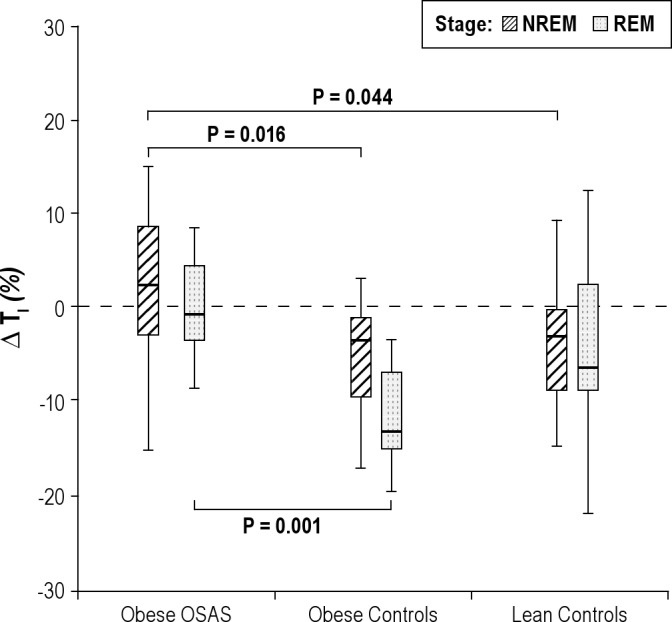
Percentage change in inspiratory time during NREM and REM sleep. The percentage change in inspiratory time (TI) during nonrapid eye movement (NREM) and rapid eye movement (REM) sleep is shown for the 3groups. See Figure 1 for a description of the box plots. During NREM sleep, study participants with obstructive sleep apnea syndrome (OSAS) had a lower percentage change in TI in response to CO2 than those in the control groups. During REM sleep, study participants with OSAS had a lower percent change in TI than those in the obese control group.
Changes in TTOT, respiratory rate (RR), and TI/TTOT are shown in Table 3. TTOT decreased in the 3 groups during the CO2 challenge, with a smaller decrease in the OSAS group in comparison with the 2 control groups. No significant difference in TI/TTOT was found among the 3 groups. RR increased in all 3 groups during the CO2 challenge; this increase was significantly greater in the lean control group than in the OSAS group, with a trend for a greater increase in the obese control group compared with the OSAS group.
The correlation between the degree of polysomnographic abnormalities and the ventilatory response parameters during NREM sleep was evaluated for the OSAS group. A negative correlation was found between AHI and change in TI (P = 0.028), AHI and change in inspiratory flow (P = 0.015), the percentage of total sleep time with SpO2 less than 90% and change in VT (P = 0.021), respectively. A positive correlation was found between SpO2 nadir and change in flow (P = 0.031). No correlation was found between other parameters.
Ventilatory Responses to CO2 During REM Sleep
In REM sleep, similar to the findings in NREM sleep, V̇E, inspiratory flow, and VT/TI all increased in response to CO2 in the 3 groups, with a smaller change in the OSAS group compared with the control groups (Figures 4, 5, and 8). For VT, the percent increase in the OSAS group was lower than that in the lean control group, with a trend for a lower increase in the OSAS group compared with the obese control group (Figure 6). For TI, similar to NREM sleep, almost no change was found in the OSAS group, although TI decreased in the 2 control groups (Figure 7). However, significant differences in TI, TTOT, and RR were found only between the OSAS and obese control groups, with no significant differences between the OSAS and lean control groups. There were no significant differences between groups in the change in TI/TTOT (Table 3).
Figure 5.
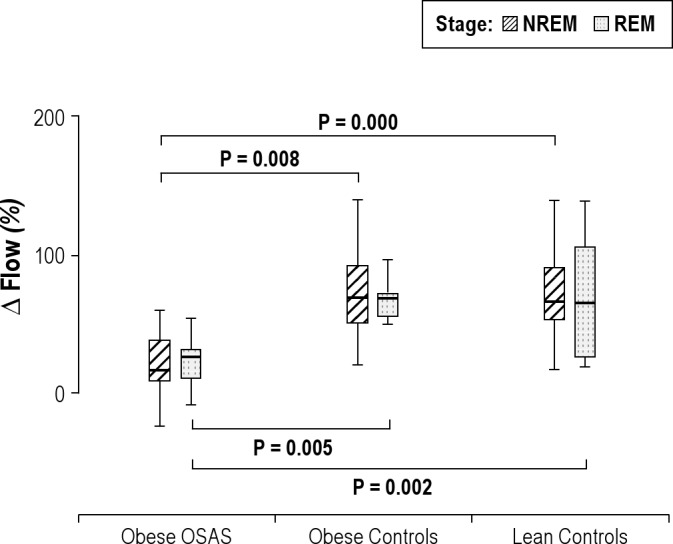
Percentage change in inspiratory flow during NREM and REM sleep. The percentage change in inspiratory airflow during nonrapid eye movement (NREM) and rapid eye movement (REM) sleep is shown for the 3groups. See Figure 1 for a description of the box plots. During NREM and REM sleep, study participants with obstructive sleep apnea syndrome (OSAS) had a lower percentage change in inspiratory flow in response to CO2 than those in the control groups.
In the OSAS group, no correlation between the degree of polysomnographic abnormalities and the ventilatory response parameters during REM sleep was found.
Genioglossal EMG Activity During Sleep
The changes in EMGgg activity during the CO2 challenge compared with baseline (expressed as a percent change of the baseline values, corrected for TI) are shown in Table 3. There was a trend for an increase in EMGgg activity in response to CO2 in all 3 groups during NREM and REM sleep compared with the baseline level, but changes were not statistically significant and did not differ between groups.
Effects of Sex and Holding Pressure on the Ventilatory Responses During Sleep
There were differing percentages and numbers of males among the 3 study groups, although the difference was not significant. Regression analysis after ANOVA indicated that there was no significant effect of sex on HCVR or the ventilatory responses during sleep.
All trials had a positive holding pressure during sleep; however, most of the trials from the OSAS group had a higher holding pressure than the control groups (during NREM, holding pressure was 8.2 ± 5.3, 2.6 ± 0.9 and 3.3 ± 1.8 cm H2O for OSAS, obese control, and lean control groups, respectively, P < 0.0005; during REM, holding pressure was 9.2 ± 5.4, 2.0 ± 0.5 and 3.5 ± 1.8 cm H2O for OSAS, obese control, and lean control groups, respectively, P < 0.0005). Therefore, the effects of holding pressure were evaluated. Holding pressure had no statistically significant effect on the ventilatory responses in the regression model.
DISCUSSION
This study investigated the ventilatory responses to CO2 during wakefulness and sleep in obese adolescents with OSAS compared with obese and lean control groups. During wakefulness, the HCVR was higher in obese OSAS and obese control groups compared with the lean control group. During NREM and REM sleep, obese adolescents with OSAS had a blunted ventilatory response to hypercapnia despite being on a holding pressure that eliminated flow limitation. In both sleep states, for all 3 groups, V̇E, inspiratory flow, and VT increased in response to CO2. However, the responses were greater in the 2 control groups compared with the OSAS group. Control groups were able to decrease TI and TTOT with a resultant increase in RR, thus V̇E increased; however, in the OSAS group, TI did not decrease and decreases in TTOT were lower than that in the control groups during both NREM and REM sleep. Ventilatory responses to CO2 during sleep were similar between obese and lean control groups.
Ventilatory Drive During Wakefulness
The role of ventilatory drive in the pathophysiology of OSAS is controversial. Several studies in adults have assessed the ventilatory response to hypercapnia in patients with OSAS, but the results have been inconsistent. The ventilatory response to hypercapnia during wakefulness in adults with OSAS has been reported to be higher,12,13 lower,9,52 and the same,11,53,54 when compared with control groups. A study showed no difference in hypercapnic ventilatory sensitivity between patients with OSAS and age-, sex- and BMI-matched control groups.11 For studies in children, it has been shown that there is no difference in the ventilatory response to hypercapnia during either wakefulness or sleep in children with OSAS compared with control groups.14,15 Similarly, studies have shown that the occlusion pressure in 100 milliseconds (P0.1), a measurement of ventilatory drive independent of upper airway resistance, does not correlate with measures of upper airway collapsibility during wakefulness (although there is a correlation during sleep).55 A study on children age 6-12 yr with OSAS showed that the hypercapnic P0.1 during wakefulness was not significantly related to the AHI, but the hypoxic P0.1 was related to the AHI. Moreover, during wakefulness, a positive correlation was found between resting end-tidal CO2 and OSAS severity.56 In addition, children with OSAS have been shown to have blunted ventilatory and respiratory timing responses to repeated brief, hypercapnic challenges during wakefulness compared with a control group of children.16 These studies suggest that subtle abnormalities in ventilatory control may exist in children with OSAS, even during wakefulness.
In the current study, during wakefulness, HCVR was increased in both the obese OSAS and obese control groups compared with the lean control group. The reason for an increase in chemical drive in OSAS is unclear. Adults with OSAS and normocapnia were found to have a higher hypercapnic response to CO2 in comparison with control groups. An increased ventilatory drive may contribute to obstructive breathing during sleep by causing ventilatory instability.57,58–59 An increase in the adrenosympathetic response to stress can also lead to an increase in the hypercapnic ventilatory response.60 However, in the current study, the obese control group also showed a higher response to CO2 compared with the lean control group, and no significant difference was found between the 2 obese groups. Thus, it is possible that obesity played a role in the mechanism. Obesity has been thought to result in a decreased ventilatory response to CO2 due to changes in pulmonary mechanics, so this increased HCVR is unexplained.31 In patients with OSAS in whom both obesity and awake hypercapnia are present, the sensitivity to CO2 is probably reduced. The current study evaluated HCVR using a hyperoxic hypercapnic gas mixture. It is possible that obese patients have different responses to hyperoxia compared with lean patients. Future studies that include measurement of the hypoxic ventilatory response may shed light on this. In addition, it is controversial as to whether HCVR should be adjusted for body size.26,32,61,62 However, recent animal data suggest that the absolute value of the ventilatory response should be used.51
Ventilatory Drive During Sleep
During sleep, inspiratory airflow, VT, RR, and V̇E increased in response to CO2 in all study participants. Ventilatory responses to CO2 were greater in the 2 control groups than in the OSAS group. This indicated that activation of the central nervous system by CO2 occurred in both OSAS and control groups during sleep. However, in the OSAS group, a blunted response to CO2 was found during sleep, with less of an increase in inspiratory airflow, VT, and V̇E than the control groups. This was not due to body weight as the response to CO2 during sleep in the OSAS group differed from the BMI-matched obese control group. There are several possible reasons for the blunted CO2 response in the OSAS group. (1) The lower ventilatory response to CO2 during sleep compared with that during wakefulness could be due to a decrease in central ventilatory drive during sleep. Although the OSAS group had a normal HCVR during wakefulness, it has previously been shown that ventilatory drive is sleep state-specific. A correlation between P0.1 and upper airway collapsibility during sleep has been reported, but such a correlation was not found during wakefulness.55 Furthermore, studies have shown that intubated children with OSAS have a higher end-tidal CO2 and depressed spontaneous ventilation during halothane anesthesia compared with control groups.17 (2) The blunted response to CO2 during sleep could also be due to increased upper airway collapsibility; i.e., patients with OSAS may have a normal central ventilatory drive, but ventilation may be impeded by increased upper airway resistance and/or collapse. It has previously been shown that children who do not have OSAS have decreased VT and V̇E in response to inspiratory resistive loading during sleep.63 (3) Patients with OSAS may have a blunted cerebrovascular response to CO2 due to chronic nocturnal hypercapnia and hypoxemia, or to inflammation related to OSAS.64,65 For example, adults and children with OSAS have increased brachial artery hyperactivity. (4) It is unlikely that the blunted ventilatory response in the obese OSAS group was due to pulmonary mechanical limitation because these patients had a normal HCVR during wakefulness and normal pulmonary function tests.
In the current study, we found that in obese adolescents with OSAS, there was a higher sensitivity to CO2 during wakefulness compared with the lean control group, but a decreased sensitivity during sleep. This finding has important implications. The blunted response during sleep could result in prolonged respiratory events; the enhanced response during wakefulness might lead to an inappropriately high ventilatory response upon arousal from an apnea. This inappropriate response could result in the reduction of CO2 to a point that induces apnea upon the return to sleep; which would be prolonged subsequently because of the blunted response, thus leading to ventilatory instability.
Upper Airway Neuromuscular Tone
In this study, genioglossus activity did not increase significantly in response to CO2 in any of the groups. This was unexpected as an increase in chemical drive and in negative pharyngeal pressure are the main triggers to activate pharyngeal dilators.66 Studies have shown elevated genioglossus activity prior to airway opening,19,20 indicating that upper airway muscles can respond to increasing chemical drive prior to arousal.21,22–23 Although the current study used intraoral electrodes rather than needle electrodes to measure EMGgg, this noninvasive technique is adequate to capture upper airway muscular responses to subatmospheric pressure,45,46 The possible reason for the lack of EMG response to CO2 despite the active ventilatory response is that the central ventilatory response to CO2 occurs at a lower threshold than the upper airway neuromuscular response to CO2. Moreover, a recent study has shown that there is an individual chemical threshold to genioglossus activation.66 This may explain the variable EMGgg response to the CO2 challenge in the current study.
Effect of Sex
Several studies have reported a sex difference in the hypercapnic ventilatory response. The HCVR has been shown to be greater in men than women,26,27 which may be in part due to the greater forced vital capacity in males; van Klaveren and Demedts found differing HCVR responses but similar P0.1 in males compared with females.67 Zhou et al. demonstrated that women were less susceptible to hypocapnic central apnea during NREM sleep than men.28 These results suggest that ventilatory stability in men may be more susceptible to the influence of chemical factors than in women. In the current adolescent study, sex was not associated with any differences in ventilatory responses during sleep or wakefulness. This may be because we had more male than female patients, although results were controlled for sex. Future studies using close sex matching are desirable. In the current study, identification and recruitment of adolescent girls with OSAS was difficult, which may have been due to a low prevalence of OSAS in adolescent girls. The prevalence of OSAS in adolescent girls compared with that in boys is unknown, although a clinic-based study suggests a much higher prevalence in males.68 The prevalence of OSAS is higher in adult men than in premenopausal women69; in prepubertal children, data suggest there may also be a male preponderance.70 Population-based studies are needed to determine the sex distribution of OSAS in adolescents.
Effect of Holding Pressure
In this study, all the trials from the 3 groups had a positive holding pressure. However, as expected, the holding pressure was higher in the OSAS group. Studies in both animals71 and children72 have shown that positive nasal pressure causes hypotonia of upper airway muscles. To determine whether the ventilatory responses during sleep were affected by the different holding pressure levels among the groups, a regression model was used and found no statistically significant effects of holding pressure levels on ventilatory parameters. Thus, we believe that the ventilatory responses between the OSAS and control groups were due to differences in ventilatory drive rather than differences in holding pressures.
Limitations
The hypercapnic responses measured during wakefulness cannot be compared directly with those measured during sleep. During HCVR, a different level of hypercapnia was used, CO2 was delivered in a hyperoxic gas mixture, and the patient was seated and breathing through a mouthpiece rather than lying down and breathing through a mask. Nevertheless, the most important finding of this study was that obese patients with OSAS had a blunted response to CO2 during sleep, but an increased HCVR during wakefulness, whereas obese control patients tested with the same methodology had an increased response to CO2 during both wakefulness and sleep.
CONCLUSIONS
In contrast with our original hypothesis, this study has shown that ventilatory responses to hypercapnia during wakefulness are higher in the obese OSAS and obese control groups compared with the lean control group. However, the results of the study support the hypothesis that adolescents with OSAS have blunted responses to CO2 during NREM and REM sleep. This suggests that state-specific abnormalities in central ventilatory drive play a role in the pathophysiology of OSAS in obese adolescents.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Marcus has received equipment and study funding from Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by NIH grants U54 RR023567 and R01 HL58585. Haibo Yuan is a recipient of the state scholarship from the China Scholarship Council. Philips Respironics, Inc. provided the airway pressure device. The authors thank all of the Children's Hospital of Philadelphia sleep laboratory technologists who helped conduct this study. The authors are grateful to the children and their families for their enthusiastic participation in this study.
REFERENCES
- 1.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12:25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 3.Rudnick EF, Walsh JS, Hampton MC, Mitchell RB. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg. 2007;137:878–82. doi: 10.1016/j.otohns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev. 2006;7:247–59. doi: 10.1016/j.prrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Kohler MJ, Thormaehlen S, Kennedy JD, et al. Differences in the association between obesity and obstructive sleep apnea among children and adolescents. J Clin Sleep Med. 2009;5:506–11. [PMC free article] [PubMed] [Google Scholar]
- 8.Verbraecken J, De Backer W, Willemen M, De Cock W, Wittesaele W, Van de H. Chronic CO2 drive in patients with obstructive sleep apnea and effect of CPAP. Respir Physiol. 1995;101:279–87. doi: 10.1016/0034-5687(95)00037-e. [DOI] [PubMed] [Google Scholar]
- 9.Garay SM, Rapoport D, Sorkin B, Epstein H, Feinberg I, Goldring RM. Regulation of ventilation in the obstructive sleep apnea syndrome. Am Rev Respir Dis. 1981;124:451–7. doi: 10.1164/arrd.1981.124.4.451. [DOI] [PubMed] [Google Scholar]
- 10.Breskovic T, Valic Z, Lipp A, et al. Peripheral chemoreflex regulation of sympathetic vasomotor tone in apnea divers. Clin Auton Res. 2010;20:57–63. doi: 10.1007/s10286-009-0034-1. [DOI] [PubMed] [Google Scholar]
- 11.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Ventilatory and cerebrovascular responses to hypercapnia in patients with obstructive sleep apnoea: effect of CPAP therapy. Respir Physiol Neurobiol. 2009;165:73–81. doi: 10.1016/j.resp.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Makinodan K, Yoshikawa M, Fukuoka A, et al. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration. 2008;75:257–64. doi: 10.1159/000112471. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Grunstein RR, Teichtahl H. Association between ventilatory response to hypercapnia and obstructive sleep apnea-hypopnea index in asymptomatic subjects. Sleep Breath. 2007;11:103–8. doi: 10.1007/s11325-006-0090-x. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Gozal D, Arens R, et al. Ventilatory responses during wakefulness in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1994;149:715–21. doi: 10.1164/ajrccm.149.3.8118641. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol. 1998;84:1926–36. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 16.Gozal D, Arens R, Omlin KJ, et al. Ventilatory response to consecutive short hypercapnic challenges in children with obstructive sleep apnea. J Appl Physiol. 1995;79:1608–14. doi: 10.1152/jappl.1995.79.5.1608. [DOI] [PubMed] [Google Scholar]
- 17.Waters KA, McBrien F, Stewart P, Hinder M, Wharton S. Effects of OSA, inhalational anesthesia, and fentanyl on the airway and ventilation of children. J Appl Physiol. 2002;92:1987–94. doi: 10.1152/japplphysiol.00619.2001. [DOI] [PubMed] [Google Scholar]
- 18.Strauss SG, Lynn AM, Bratton SL, Nespeca MK. Ventilatory response to CO2 in children with obstructive sleep apnea from adenotonsillar hypertrophy. Anesth Analg. 1999;89:328–32. doi: 10.1097/00000539-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Berry RB, McNellis MI, Kouchi K, Light RW. Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. Am J Respir Crit Care Med. 1997;156:127–32. doi: 10.1164/ajrccm.156.1.9608037. [DOI] [PubMed] [Google Scholar]
- 20.Kuna ST, Smickley J. Response of genioglossus muscle activity to nasal airway occlusion in normal sleeping adults. J Appl Physiol. 1988;64:347–53. doi: 10.1152/jappl.1988.64.1.347. [DOI] [PubMed] [Google Scholar]
- 21.Carlson DM, Onal E, Carley DW, Lopata M, Basner RC. Palatal muscle electromyogram activity in obstructive sleep apnea. Am J Respir Crit Care Med. 1995;152:1022–7. doi: 10.1164/ajrccm.152.3.7663778. [DOI] [PubMed] [Google Scholar]
- 22.Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–73. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- 23.Okabe S, Chonan T, Hida W, Satoh M, Kikuchi Y, Takishima T. Role of chemical drive in recruiting upper airway and inspiratory intercostal muscles in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:190–5. doi: 10.1164/ajrccm/147.1.190. [DOI] [PubMed] [Google Scholar]
- 24.Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am Rev Respir Dis. 1987;135:378–82. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- 25.Borel JC, Melo-Silva CA, Gakwaya S, Series F. Influence of CO2 on upper airway muscles and chest wall/diaphragm corticomotor responses assessed by transcranial magnetic stimulation in awake healthy subjects. J Appl Physiol. 2012;112:798–805. doi: 10.1152/japplphysiol.00713.2011. [DOI] [PubMed] [Google Scholar]
- 26.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol. 1983;54:874–9. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- 27.Regensteiner JG, Woodard WD, Hagerman DD, et al. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol. 1989;66:808–13. doi: 10.1152/jappl.1989.66.2.808. [DOI] [PubMed] [Google Scholar]
- 28.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol. 2000;89:192–9. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–10. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zavorsky GS, Murias JM, Kim dJ, Gow J, Christou NV. Poor compensatory hyperventilation in morbidly obese women at peak exercise. Respir Physiol Neurobiol. 2007;159:187–95. doi: 10.1016/j.resp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Ge RL, Stone JA, Levine BD, Babb TG. Exaggerated respiratory chemosensitivity and association with SaO2 level at 3568 m in obesity. Respir Physiol Neurobiol. 2005;146:47–54. doi: 10.1016/j.resp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Glomb WB, Basinski DJ, Davidson SL, Keens TG. Developmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthood. J Appl Physiol. 1994;76:314–20. doi: 10.1152/jappl.1994.76.1.314. [DOI] [PubMed] [Google Scholar]
- 33.Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Am J Clin Nutr. 1994;59:307–16. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- 34.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 35.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 36.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 37.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 38.Iber C, Ancoli-Israel Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. [Google Scholar]
- 39.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 40.Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med. 1967;16:20–32. doi: 10.1111/imj.1967.16.1.20. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Marcus CL, Bandla P, et al. Cortical processing of respiratory occlusion stimuli in children with central hypoventilation syndrome. Am J Respir Crit Care Med. 2008;178:757–64. doi: 10.1164/rccm.200804-606OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Colrain IM, Panitch HB, et al. Effect of sleep stage on breathing in children with central hypoventilation. J Appl Physiol. 2008;105:44–53. doi: 10.1152/japplphysiol.01269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcus CL, Livingston FR, Wood SE, Keens TG. Hypercapnic and hypoxic ventilatory responses in parents and siblings of children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1991;144:136–40. doi: 10.1164/ajrccm/144.1.136. [DOI] [PubMed] [Google Scholar]
- 44.Glomb WB, Marcus CL, Keens TG, Ward SL. Hypercapnic and hypoxic ventilatory and cardiac responses in school-aged siblings of sudden infant death syndrome victims. J Pediatr. 1992;121:391–7. doi: 10.1016/s0022-3476(05)81791-3. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Pinto SJ, Allen JL, et al. Upper airway genioglossal activity in children with sickle cell disease. Sleep. 2011;34:773–8. doi: 10.5665/SLEEP.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–60. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 47.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J, Karamessinis LR, Pepe ME, et al. Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep. 2009;32:1173–81. doi: 10.1093/sleep/32.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 50.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 51.Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- 52.Osanai S, Akiba Y, Fujiuchi S, et al. Depression of peripheral chemosensitivity by a dopaminergic mechanism in patients with obstructive sleep apnoea syndrome. Eur Respir J. 1999;13:418–23. doi: 10.1183/09031936.99.13241899. [DOI] [PubMed] [Google Scholar]
- 53.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–9. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 54.Rajagopal KR, Abbrecht PH, Tellis CJ. Control of breathing in obstructive sleep apnea. Chest. 1984;85:174–80. doi: 10.1378/chest.85.2.174. [DOI] [PubMed] [Google Scholar]
- 55.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 56.Fregosi RF, Quan SF, Jackson AC, et al. Ventilatory drive and the apnea-hypopnea index in six-to-twelve year old children. BMC Pulm Med. 2004;4:4. doi: 10.1186/1471-2466-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol. 2007;581:291–8. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engel LA, Ritchie B. Ventilatory response to inhaled carbon dioxide in hyperthyroidism. J Appl Physiol. 1971;30:173–7. doi: 10.1152/jappl.1971.30.2.173. [DOI] [PubMed] [Google Scholar]
- 61.Hirshman CA, McCullough RE, Weil JV. Normal values for hypoxic and hypercapnic ventilatory drives in man. J Appl Physiol. 1975;38:1095–8. doi: 10.1152/jappl.1975.38.6.1095. [DOI] [PubMed] [Google Scholar]
- 62.Patrick JM, Howard A. The influence of age, sex, body size and lung size on the control and pattern of breathing during CO 2 inhalation in Caucasians. Respir Physiol. 1972;16:337–50. doi: 10.1016/0034-5687(72)90063-1. [DOI] [PubMed] [Google Scholar]
- 63.Marcus CL, Moreira GA, Bamford O, Lutz J. Response to inspiratory resistive loading during sleep in normal children and children with obstructive apnea. J Appl Physiol. 1999;87:1448–54. doi: 10.1152/jappl.1999.87.4.1448. [DOI] [PubMed] [Google Scholar]
- 64.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–e569. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 65.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans CO, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–14. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 66.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34:1061–73. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Klaveren RJ, Demedts M. Determinants of the hypercapnic and hypoxic response in normal man. Respir Physiol. 1998;113:157–65. doi: 10.1016/s0034-5687(98)00057-7. [DOI] [PubMed] [Google Scholar]
- 68.Accardo JA, Shults J, Leonard MB, Traylor J, Marcus CL. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep. 2010;33:1333–9. doi: 10.1093/sleep/33.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–70. [PubMed] [Google Scholar]
- 70.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on respiratory frequency. Respir Physiol. 1982;49:223–33. doi: 10.1016/0034-5687(82)90075-5. [DOI] [PubMed] [Google Scholar]
- 72.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]