Abstract
Study Objectives:
Sleep disordered breathing (SDB) occurs at an increased incidence in children with Down Syndrome (DS) compared to the general pediatric population. We hypothesized that, compared with typically developing (TD) children with SDB, children with DS have a reduced cardiovascular response with delayed reoxygenation after obstructive respiratory events, and reduced sympathetic drive, providing a potential explanation for their increased risk of pulmonary hypertension.
Design:
Beat-by-beat heart rate (HR) was analyzed over the course of obstructive events (pre, early, late, post-event) and compared between groups. Also compared were the time for oxygen resaturation post-event and overnight urinary catecholamines.
Setting:
Pediatric sleep laboratory.
Patients:
Sixty-four children aged 2-17 y referred for investigation of SDB (32 DS; 32 TD) matched for age and obstructive apnea/hypopnea index.
Measurement and Results:
Children underwent overnight polysomnography with overnight urine collection. Compared to TD children, those with DS had significantly reduced HR changes post-event during NREM (DS: 21.4% ± 1.8%, TD: 26.6% ± 1.6%, change from late to post-event, P < 0.05). The time to resaturation post-event was significantly increased in the DS group (P < 0.05 for both NREM and REM sleep). Children with DS had significantly reduced overnight urinary noradrenaline (P < 0.01), adrenaline (P < 0.05) and dopamine levels (P < 0.01) compared with TD children.
Conclusion:
Children with DS and SDB exhibit a compromised acute cardio-respiratory response and dampened sympathetic response to SDB compared with TD children with SDB. These data may reflect autonomic dysfunction in children with DS that may place them at increased risk for cardiovascular complications such as pulmonary hypertension.
Citation:
O’Driscoll DM; Horne RSC; Davey MJ; Hope SA; Anderson V; Trinder J; Walker AM; Nixon GM. Cardiac and sympathetic activation are reduced in children with down syndrome and sleep disordered breathing. SLEEP 2012;35(9):1269-1275.
Keywords: Down syndrome, sleep disordered breathing, apnea, hypopnea, heart rate, sympathetic, oxygen saturation
INTRODUCTION
Down Syndrome (DS), caused by an extra copy of chromosome 21, has an incidence of approximately 1 in 800 births. The syndrome affects multiple systems, resulting in both physical and cognitive problems. In addition, there is an increased incidence of obstructive sleep apnea (OSA), reported in 60% of children with DS1 compared with 1% to 3% in the general pediatric population.2 OSA has been shown to be associated with cardiovascular sequelae such as hypertension3 and left ventricular hypertrophy4 in typically developing (TD) children (i.e., without DS). Given the increased incidence of OSA in DS, these children can be considered to be at high risk for cardiovascular complications, but the way they respond to this condition has not previously been studied.
Cardiovascular activity undergoes considerable change during and after episodes of airway obstruction during sleep. At the onset of an obstructive apnea, blood pressure (BP) and heart rate (HR) initially decrease, then increase as hypoxia develops. Finally, there is a large surge in sympathetic activity, BP and HR at the termination of the apnea and resumption of ventilation, often concomitant with arousal from sleep.5 Acutely, augmented cardiovascular and ventilatory responses at apnea termination serve to re-establish airway patency and restore oxygenation, providing important protection against hypoxemia. Chronic OSA in adults is associated with cardiovascular diseases including systemic hypertension arising from these frequent cardiovascular perturbations.6 This transient augmentation of cardiovascular activity, however, can be considered to be the appropriate response when faced with the challenges of hypercapnia, hypoxia, and increasing ventilatory effort that occur during an obstructive apnea.
Interestingly, there is evidence that individuals with DS exhibit autonomic dysfunction. Studies during wakefulness have reported that adults with DS have an attenuated cardiovascular response to exercise tests, isometric handgrips, cold pressor tests, and orthostatic challenges.7–10 Importantly, exercise testing in individuals with DS during wakefulness has shown that the reduced cardiovascular response accompanies a reduced ventilatory response.8,11,12 In addition, we have recently shown that the cardiovascular response to spontaneous arousal from sleep is reduced in children with DS and sleep disordered breathing (SDB).13 These reduced responses in DS may be due to reduced sympathetic activation or blunted vagal withdrawal. If the cardiorespiratory response at the termination of an apnea is similarly reduced, children with DS may be somewhat protected from developing systemic hypertension, but they may be at increased risk for hypoxia. In essence, the dampened sympathetic activity in DS may exacerbate acute hypoxic exposure, in that cardiorespiratory compensations are limited, reoxygenation is slower, and arterial hypoxemia is prolonged following apnea termination. As children with DS have a higher propensity to develop pulmonary hypertension than TD children,14 an inefficient cardiorespiratory response to apnea, and therefore increased hypoxia, might be an important mediator in this relationship.15,16
The aim of this study was to test the hypotheses that (1) the cardiovascular response at obstructive event termination in children with DS is reduced compared with TD children; (2) that this is reflected in more prolonged oxygen desaturation following obstructive events; and (3) that overnight sympathetic activity is reduced in children with DS compared with TD children.
METHODS
The Monash University and Southern Health Human Research Ethics Committees granted ethical approval for this project. Written informed consent was obtained from parents and the project explained verbally to the children prior to commencement of the study; no monetary incentive was provided for participation.
Subjects
Thirty-two children with DS (18M) and 32 TD children (23M) referred for investigation of SDB took part in this study. Children were aged between 2-17 y. Children with DS were recruited from consecutive referrals to the Melbourne Children’s Sleep Centre over an 18-month period. The TD children were identified from a larger cohort of children taking part in a study investigating cardiovascular and neurocognitive outcomes in SDB.3 An individual match was located for each child with DS based on age (mean difference 0.9 ± 0.3 y) and severity of SDB (see below for definitions). Of the children with DS, 3 were taking thyroid hormone supplementation for hypothyroidism; 15 had a history of congenital heart disease (CHD) resolved in infancy (3 requiring surgery); 3 had a history of gastrointestinal obstruction in infancy; and 11 had previously undergone adenotonsillectomy. All children, both with and without DS, were well at the time of the study. Five children with DS and 4 TD children had a diagnosis of asthma (not requiring bronchodilator use on the day of the study).
Protocol
All children underwent routine overnight polysomnography (PSG) using a commercially available PSG system (Series E Sleep System, Compumedics, Melbourne, Australia). Electroencephalograms (EEG: C4/A1, O2/A1), electroculograms (EOG: left and right outer canthus), an electromyogram (EMG: submentalis muscle), electrocardiogram (ECG), left and right leg EMG, and body position were recorded. Oxygen saturation (SpO2) was measured by pulse oximetry (Masimo Radical SET, Masimo Corp, CA, USA, or Bitmos GmbH, Bitmos, Dusseldorf, Germany, both of which use Masimo signal extraction technology (SET) for signal processing). All oximeters were set to 2-s averaging time. Thoracic and abdominal breathing movements were recorded via uncalibrated respiratory inductance plethysmography (z-RIP belts, Pro-Tech Services Inc., Mukilteo, WA, USA). Both end-tidal and transcutaneous carbon dioxide (PetCO2: Capnocheck Plus, BCI Inc., Waukesha, WI, USA; TCO2: TCM3, Radiometer, Copenhagen, Denmark) were recorded, and airflow was measured via nasal pressure and oronasal thermistor (Compumedics, Melbourne, Australia). Immediately prior to “lights out,” the subjects were asked to lie still with their eyes open for 5 min to record baseline HR during wake. Following the PSG study, data were transferred via European data format to data analysis software (Chart 5, ADInstruments, Sydney, Australia) for detailed cardiovascular analysis. Prior to PSG, height and weight were recorded, and body mass index (BMI) calculated. To account for the effect of age and gender, BMI was converted to a z-score according to published criteria.17
Data Analysis
As data collection preceded the introduction of the American Academy of Sleep Medicine criteria for sleep in 2007,18 earlier guidelines differing only in minor areas from those criteria were used. Sleep was scored from the EEG, EOG, and chin EMG channels in 30-s epochs according to standard criteria.19 Respiratory events ≥ 2 respiratory cycles in duration were scored. Obstructive apneas, mixed apneas, and hypopneas were defined according to standard criteria (hypopnea desaturation criterion ≥ 3%).20 An obstructive apnea hypopnea index (OAHI) was calculated, defined as the total number of obstructive apneas, mixed apneas, and obstructive hypopneas per hour of total sleep time. Diagnostic criteria for the classification of OSA severity followed current clinical practice: children were categorized as having primary snoring (PS, OAHI < 1 event/h); mild OSA (OAHI between 1-5 events/h), or moderate/severe OSA (OAHI > 5 events/h).
Arousals were scored according to the guidelines of the American Sleep Disorders Association (ASDA)21—i.e., an abrupt shift in EEG frequency lasting > 3 s (including any combination of theta, alpha, or other activity > 16 Hz, but not spindle or delta frequencies). Subcortical activations were also scored when ≥ 2 of the following events occurred and met criteria described by Mograss et al.22: an increase in EMG, an increase in HR, or a body movement (i.e., autonomic arousals not meeting ASDA criteria). Arousals and subcortical activations were combined to give a total arousal index.
To ensure a stable baseline (i.e., no arousals or respiratory events were present), only obstructive respiratory events preceded by a minimum of 30 s prior stable sleep were included in the cardiovascular analysis. Beat-by-beat HR was analyzed and averaged for the following periods: pre-event (10 s immediately preceding the apnea/hypopnea), early-event (first half of apnea/hypopnea), late-event (last half of apnea/hypopnea) and post-event (average of the 3 consecutive peak measurements within 15 s of event termination) as described previously.25 An advantage of this method was that cardiovascular data during events of different durations could be grouped and compared.26 The time for oxygen resaturation post event was measured in seconds from the nadir saturation to re-establishment of the baseline SpO2 (i.e., the level prior to the respiratory event).
Urine Collection and Analysis
Twelve-hour timed overnight urine collection (approximately 19:00-07:00) was performed in 18 children with DS and their respective 18 TD matches. Children were asked to void upon arrival at the sleep laboratory, and all urine passed thereafter was collected until morning, including the first morning void. Urine was acidified with sulfamic acid to a pH between 2 and 3, and stored at 4°C until analysis. Urinary catecholamine levels were determined using high performance liquid chromatography with electrochemical detection.27 Noradrenaline, adrenaline, and dopamine levels were measured and divided by the urine concentration of creatinine.
In a subset of children who wore nappies overnight (n = 12 DS, n = 2 TD), a representative overnight spot sample of urine was collected by extracting urine from a cotton pad insert placed within the nappy as described previously.28 Briefly, total urinary output over 12 h was calculated by subtracting the final weight of the used nappy and insert from the weight before the study. In the morning, urine was squeezed from the cotton pad insert using a hydraulic press applying a maximum pressure of 4 tons.
Statistical Analysis
Statistical analysis was performed using Sigma Plot (Version 11, Systat Software Inc) and Intercooled Stata (Version 10, Stata Corp). Subject demographics, polysomnographic characteristics, and urinary catecholamines were compared between groups using Student paired t-tests, or Wilcoxon signed rank tests for nonparametric data. For HR, within-subject values were averaged for respiratory events in each sleep state for each group to ensure that each subject contributed only once to the group mean. The effect of sleep state on cardiovascular responses by phase of events were assessed using a 2-way ANOVA with Student-Newman-Keuls post hoc analysis to assess both the actual HR and the % HR changes across the obstructive event (pre-event deemed 100%). Multivariate cluster-wise linear regression was used to determine associations between % change in HR from late-event to post-event and the following variables: group (DS versus TD), sleep state (NREM versus REM), category of arousal from sleep (none, subcortical activation, ASDA arousal), SpO2 desaturation (%), and length of obstructive event (s). For this part of the analysis, data were entered into the model as individual events and clustered by subject to account for repeated observations on individuals. The proportion of events associated with oxygen desaturation was compared between groups using a z-test. Desaturation and resaturation variables were compared between groups and across sleep states using one way ANOVA with Dunn post hoc testing. In addition multivariate cluster-wise linear regression was used to determine associations between time to resaturation and the following variables: group (DS versus TD), sleep state (NREM versus REM), baseline SpO2 (%), length of obstructive event (s), and SpO2 desaturation (%). Linear regressions were used to examine the relationship between the % Δ HR between late and post-event in each sleep state and the catecholamines for both groups. Data are presented as mean ± SEM or median (interquartile range) where appropriate. Statistical significance was taken at the P < 0.05 level.
RESULTS
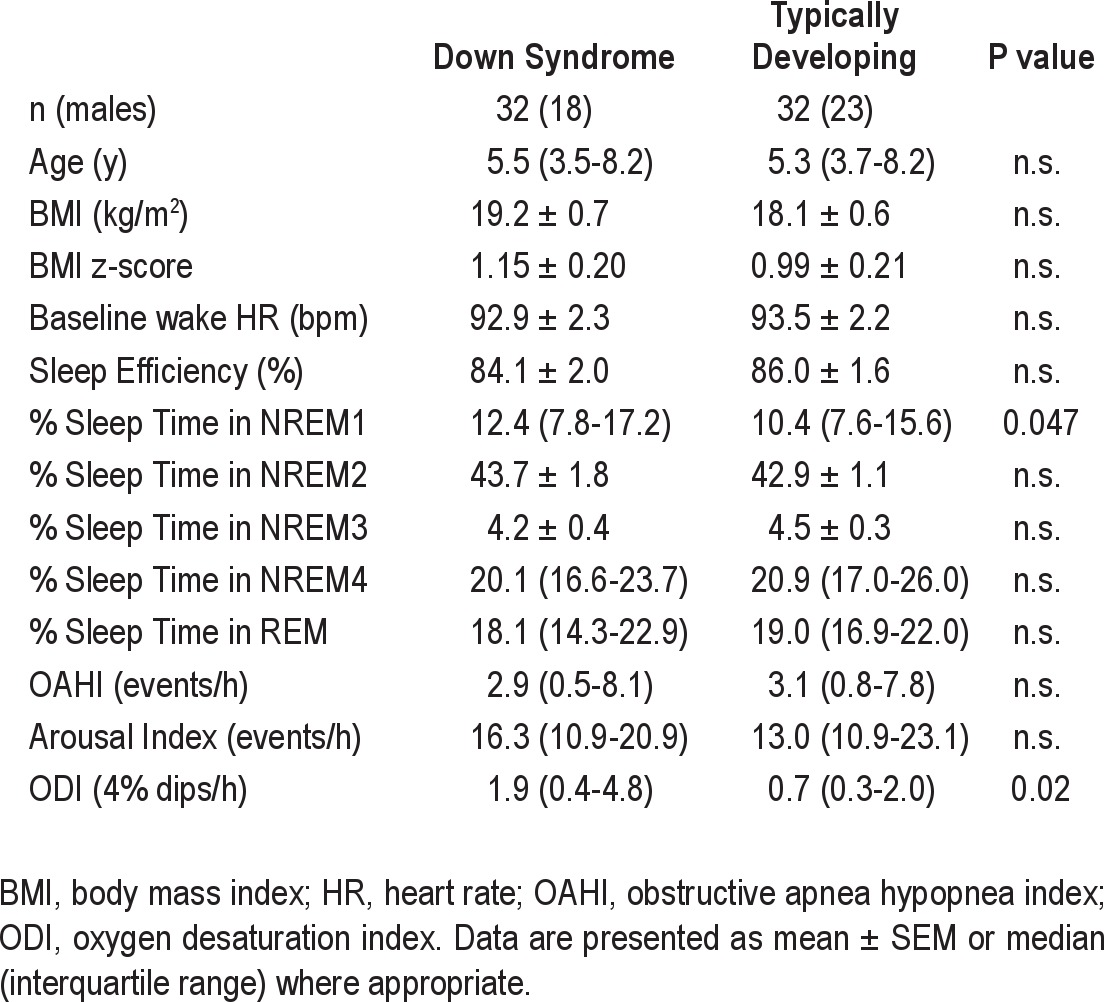
Subject demographics and polysomnographic characteristics are presented in Table 1. For both groups, 11 subjects were diagnosed with PS, 10 with mild OSA, and 11 with moderate/severe OSA. Statistical comparison confirmed there was no difference between the groups for age or BMI. The only polysomnographic characteristics that differed were % sleep time in NREM1 and 4% oxygen desaturation index (ODI), both of which were significantly increased in the DS group.
Table 1.
Patient demographics and polysomnographic characteristics
Changes in Heart Rate during Obstructive Events
For cardiovascular analysis, 8 children with PS were excluded as they had no obstructive events (4 with DS, 4 TD). A total of 200 events were analyzed from children with DS (141 in NREM and 59 in REM); a total of 213 events were analyzed in the TD group (155 in NREM and 58 in REM). The majority of events were classified as hypopneas (171 in children with DS and 185 in TD children). As we have previously shown no difference in the cardiovascular profile between event types (apnea versus hypopnea),25 both event types were grouped for analysis.
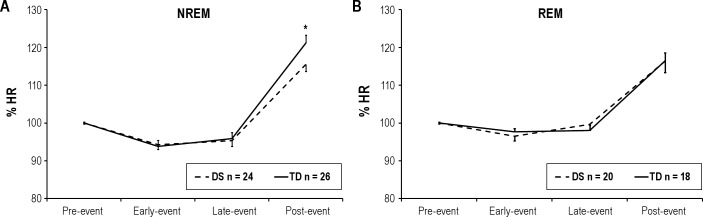
Fifty children (24 DS, 26 TD) had obstructive events in NREM and 38 children (20 DS, 18 TD) had obstructive events in REM available for analysis. There was no difference in pre-event HR between the groups in either sleep state (NREM: TD, 86 ± 3 bpm; DS, 89 ± 3 bpm; P > 0.05. REM: TD, 87 ± 3 bpm; DS, 91 ± 3 bpm; P > 0.05). For both the TD and DS groups, the change in HR expressed as a % of the pre-event level (% HR), was significantly greater post-event compared to pre-event, early-event, and late-event in NREM (P < 0.05 for all, Figure 1A). In addition, % HR significantly decreased from pre-event to early and late-event (P < 0.05 for all). During NREM, % HR was significantly higher in the TD group than the DS group post-event (P < 0.05), but there was no difference in % HR during other event phases. During REM sleep, for both the TD and DS groups, % HR was significantly greater post-event than pre-event, early-event, and late-event (P < 0.05 for all, Figure 1B). During REM, there was no significant difference in % HR at any time point during obstructive events between the two groups.
Figure 1.
Mean group data of HR (%) during obstructive respiratory events in children with Down Syndrome (DS) and typically developing (TD) children during (A) NREM and (B) REM. Pre-event HR normalized to 100%. *P < 0.05 TD compared to DS.
When the changes in HR between late and post-event were expressed as percentage change (Figure 2), ΔHR was significantly greater during obstructive events in the TD group compared to the DS group in NREM (TD: 26.6% ± 1.6%, DS: 21.4% ± 1.8%, P < 0.05). A similar trend appeared in REM; however, this failed to reach statistical significance (TD: 19.0% ± 1.9%, DS: 16.7% ± 2.1%, P > 0.05). To explore the effect of a history of CHD within the DS group, we compared ΔHR between those with and without and found no difference in either sleep state (NREM: 22.6% ± 2.6% vs. 20.4% ± 2.4%, P > 0.05. REM: 21.5% ± 3.4% vs. 13.4% ± 2.2%, P > 0.05). Similarly, to explore the effect of adenotonsillectomy within the DS group, we compared ΔHR between those with a history of surgery and those without and found no difference in either sleep state (NREM: 18.4% ± 1.4% vs. 21.9% ± 2.1%, P > 0.05. REM: 15.9% ± 5.0% vs. 18.2% ± 1.9%, P > 0.05).
Figure 2.
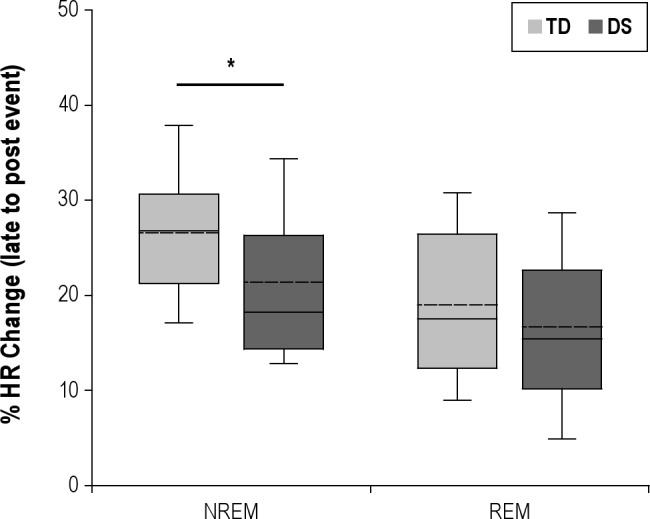
Boxplots of % change in HR at the termination of obstructive events (late-event to post-event) in children with Down Syndrome (DS) and typically developing (TD) children during NREM and REM. The boundary of the box closest to zero indicates the 25th percentile, the solid line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. The dotted line within each box represents the mean. *P < 0.05.
Determinants of the Change in Heart Rate Post Event
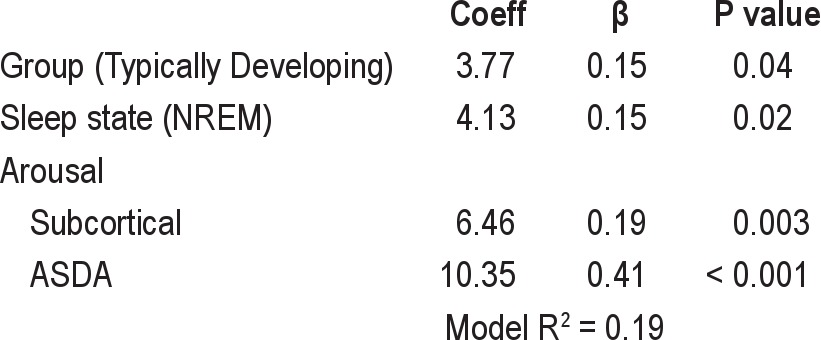
While a similar proportion of obstructive events were terminated with ASDA defined arousals between the 2 groups (TD 57%, DS 59%, P > 0.05), significantly more events in the TD group were terminated with subcortical activation (TD 26%, DS 7%, P < 0.001). Stepwise multivariate clusterwise regression for the % change in HR from late-event to post-event is presented in Table 2. The TD group (P = 0.04), the NREM sleep state (P = 0.02), and arousal from sleep (both subcortical activation [P = 0.003] and ASDA arousal [P < 0.001]) were all significant independent predictors of a larger % change in HR from late-event to post-event. Oxygen desaturation and length of respiratory event were not significant independent predictors of the change in HR and were not included in the final equation.
Table 2.
Predictor variables of the % HR change from late-event to post-event: stepwise multivariate clusterwise linear regression
Oxygen Desaturation and Resaturation
Significantly more events in the DS group were associated with oxygen desaturation compared to the TD group (60% versus 38%, P < 0.001). There was no significant difference between groups in either sleep state for baseline SpO2 (NREM: DS 98.1% ± 0.2%, TD 97.4% ± 0.2%; REM: DS 98.4% ± 0.2%, TD 98.6% ± 0.2%; P > 0.05), length of event (NREM: DS 15.5 ± 0.9 s, TD 16.3 ± 1.0 s; REM: DS 15.8 ± 1.5 s, TD 15.8 ± 1.4 s; P > 0.05), or average event-associated desaturation (NREM: DS 4.4% ± 0.2%, TD 4.0% ± 0.3%; REM: DS 4.7% ± 0.4%, TD 4.3% ± 0.3%; P > 0.05). However, the time to resaturation (Figure 3) was significantly increased in the DS group for both sleep states (NREM: TD 4.9 ± 0.4 s, DS 9.9 ± 0.7 s, P < 0.05; REM: TD 5.3 ± 0.7 s, DS 9.5 ± 0.8 s, P < 0.05). These data were confirmed using stepwise multivariate clusterwise regression, where the only significant independent predictor of increased time to resaturation was the DS grouping (Coeff = 4.84, β = 0.43, R2 = 0.18, P < 0.001)
Figure 3.
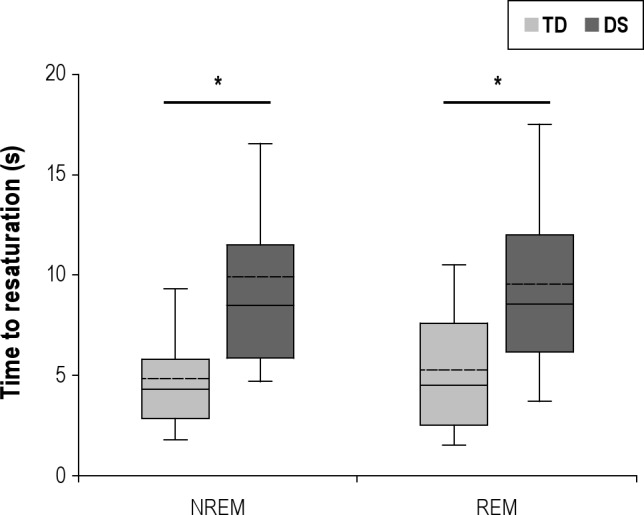
Boxplots of the time to oxygen resaturation post-event in children with Down Syndrome (DS) and typically developing (TD) children during NREM and REM. The boundary of the box closest to zero indicates the 25th percentile, the solid line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. The dotted line within each box represents the mean. *P < 0.05.
Urinary Catecholamines
The mean overnight urine output was 213 ± 19 mL. Urinary noradrenaline, adrenaline, and dopamine were all significantly reduced in the children with DS compared to the TD children (noradrenaline, 24.4 ± 3.7 vs. 37.2 ± 3.3 μmol/molCR, P < 0.01; adrenaline, 2.9 ± 0.6 vs. 5.0 ± 0.9 μmol/molCR, P < 0.05; dopamine, 190.9 ± 27.3 vs. 324.7 ± 25.8 μmol/molCR, P < 0.01. Figure 4). Linear regressions revealed no significant associations between the % Δ HR between late and post-event in NREM or REM and any of the catecholamines in the DS group. Similarly, no significant association was found in the TD group; however, the association between % Δ HR between late and post-event in NREM and adrenaline approached significance (R = 0.49, P = 0.08).
Figure 4.
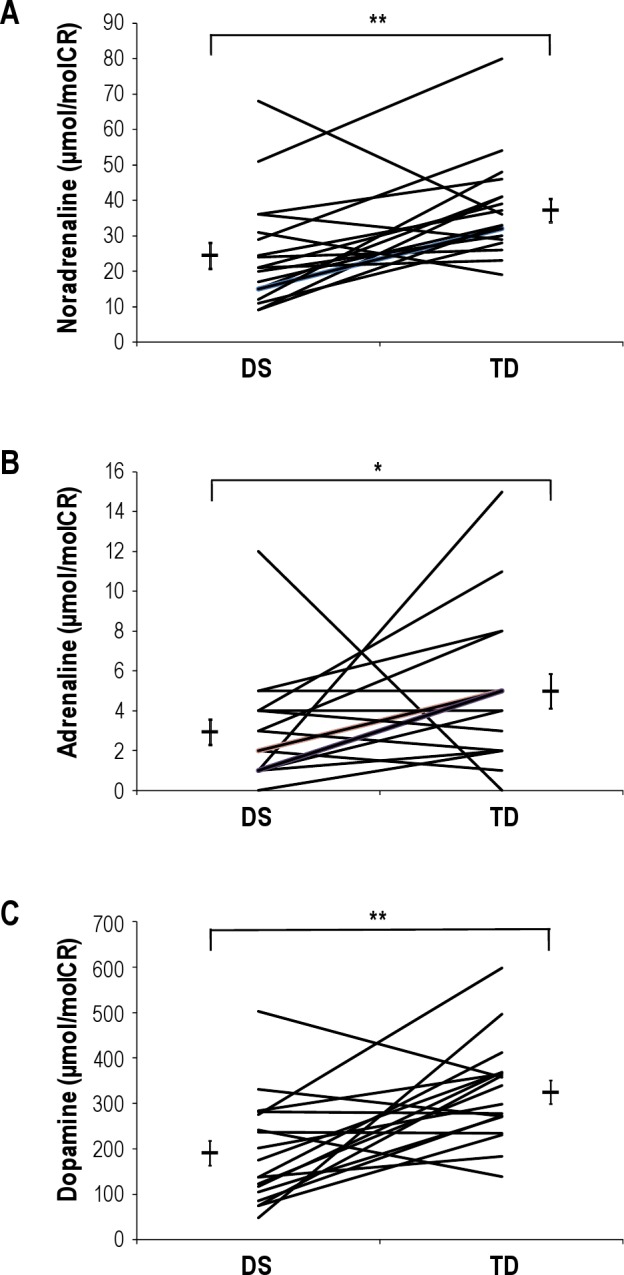
Display of overnight urinary noradrenaline (A), adrenaline (B), and dopamine (C), corrected for creatinine excretion, in children with Down Syndrome (DS) and their typically developing (TD) matched pair. Group mean data and SEM also shown. *P < 0.05, **P < 0.01.
DISCUSSION
The major findings of this study are that in children with DS compared with TD children: (1) the HR response at the termination of an obstructive event is dampened in NREM, (2) reoxygenation following an obstructive event is delayed, and (3) overnight sympathetic activation (urinary catecholamines) is reduced. Combined, these markedly reduced cardiovascular and ventilatory responses of children with DS may exacerbate acute hypoxic exposure arising from obstructive events.
We have previously shown that children with DS have a reduced HR response to spontaneous arousal from sleep.13 In support of that study, we now show that children with DS have a reduced HR response at respiratory event termination. Furthermore, our current data confirm that other independent predictors of the HR response post-event in children are the NREM sleep state and arousal from sleep (both subcortical and ASDA defined), also supporting our previous findings in healthy children.25 This finding that the cardiovascular change post-event increased with the increasing levels of arousal is consistent with reports linking the duration of the arousal from sleep to the associated autonomic response.29 Importantly, the TD group itself was also an independent predictor of the HR response; therefore our data showing a dampened HR response in DS are not merely a reflection of the reduced proportion of events terminated with subcortical activations in that group.
Our finding of increased hypoxic exposure time post event in children with DS supports our hypothesis of a reduced ventilatory response concomitant with the reduced cardiovascular response. Importantly, increased hypoxic exposure may increase the risk for this group developing pulmonary hypertension, as pulmonary vasoconstriction is primarily related to local oxygen concentration and not elevated sympathetic activity. Pulmonary hypertension occurs at a much higher rate in children with DS with or without CHD.14 As children with DS are at much higher risk of OSA (primarily as a result of relative macroglossia30) and have a higher propensity to develop pulmonary hypertension than healthy children, increased hypoxic exposure time in OSA might be an important contributor to cardiovascular morbidity in this group.15,16 In support of this idea, relief of airway obstruction by intubation in children with DS has been shown to reverse pulmonary hypertension.31 Furthermore, case reports of children with DS and cor pulmonale have described improvement in cardiovascular status following surgical treatment of OSA.15,16 Interestingly, in our data set, obstructive respiratory events in children with DS result more often in oxygen desaturation than TD children. While this study was not designed to examine the frequency of this outcome, it is possible that children with DS are more likely to desaturate with apnea as a result of known respiratory abnormalities, such as reduced functional residual capacity due to reduced tone or pulmonary hypoplasia.32 Structural abnormalities in pulmonary development in DS include a diminished number of alveoli, enlarged alveoli and alveolar ducts, and a smaller alveolar surface area.33 Importantly, the smaller alveolar surface area, accompanied by loss of capillary surface area, has also been hypothesised as a mediator of pulmonary hypertension in DS,33 and together with a history of CHD, is likely to be a major contributor to delayed resaturation.
Conversely, our data may also support a hypothesis for protection from systemic hypertension in children with DS and OSA. One of the mechanisms postulated for the development of systemic hypertension in TD children and adults with OSA is the large, repetitive surges in HR and BP at apnea termination.6 Indeed we have previously shown that the cardiovascular surges in children with OSA are of the same magnitude as those seen in adults.25 These surges in cardiovascular activity may elevate systemic BP by resetting the baroreceptors or causing local endothelial damage.34 As our data have shown a dampened HR response at apnea termination in children with DS, it may then be possible that the risk of systemic hypertension associated with OSA is similarly reduced. While we do not have a measure of BP in our group, a recent study investigating the prevalence of OSA in adults with DS reported that while 14/16 had an apnea/hypopnea index greater than 15 events/h (most with severe OSA), none of the subjects were hypertensive.35 In fact, all adults in the study had low BP, supporting the hypothesis that OSA in DS is not associated with systemic hypertension.
Mirroring our finding of reduced HR responsiveness, we also found reduced overnight urinary catecholamines in children with DS and OSA compared with TD children, indicating dampened sympathetic activation. OSA itself has been shown to be associated with increased urinary catecholamines in both healthy adults36 and children28 due to intermittent hypercapnia, hypoxia, and arousal, each increasing sympathetic outflow.5 We now report for the first time that catecholamine concentrations are significantly reduced in children with DS in response to OSA compared with their TD peers. Reduced catecholamine concentration has previously been reported in DS in response to exercise. Eberhard et al.37 reported little change in adrenaline concentrations in response to high-intensity cycling in adolescents with DS. Additionally, Fernhall et al.38 demonstrated that peak aerobic exercise did not result in any change of adrenaline concentrations in individuals with DS, and only a very slight increase of noradrenaline. Combined, these previous studies during wakefulness and our data during sleep support the concept of sympathetic dysfunction through altered catecholamine responsiveness in DS.
Limitations
The main limitation of this study was the heterogeneous medical history of the DS group, in particular that almost half had a history of CHD. CHD is common in DS,39 and although all cases in our subject group were resolved in infancy, this may be a potential confounder, given the primary outcome of HR. In addition, one-third of the DS group had previously undergone adenotonsillectomy; the pathogenesis in subjects who still have OSA after adenotonsillectomy may be different from those in whom the condition has resolved. However, as we found no difference in the HR response post-event between those with and without CHD in the DS group, nor between those with a history of adenotonsillectomy and without, we are confident that our finding of a dampened response in children with DS compared to TD children remains valid. Secondly, we do not have a quantitative measure of ventilation to confirm the reduced ventilatory response at apnea termination in our subjects with DS, as this measurement is challenging and not likely to be feasible in children with DS. Therefore the lack of ventilatory compensation post-event in this group is inferred. However, previous studies showing a reduced ventilatory response during exercise testing in individuals with DS,8,11,12 as well as our data showing delayed hypoxic recovery, support a reduced ventilatory response.
In conclusion, our study demonstrates that children with DS and SDB have a reduced HR response and delayed reoxygenation post-event, together with reduced overnight urinary catecholamines, compared with TD children with SDB. These data showing a compromised acute cardiorespiratory response and dampened sympathetic response to SDB may be a reflection of autonomic dysfunction in children with DS, which may place this group uniquely at risk for cardiovascular complications, namely pulmonary hypertension. Early detection and treatment of SDB may therefore be necessary to minimize cardiovascular risk in children with DS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. O’Driscoll is the recipient of a TSANZ/Allen and Hanbury’s Respiratory Research Fellowship. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the staff of the Melbourne Children’s Sleep Centre and all the children and their parents who participated in this study. This project was supported by a Windermere Foundation Special Grant (SG130-08), National Health and Medical Research Council of Australia Project Grants (384142 and 491001), and the Victorian Government’s Operational Infrastructure Support Program.
REFERENCES
- 1.Ng DK, Hui HN, Chan CH, et al. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006;47:774–9. [PubMed] [Google Scholar]
- 2.Ebert CS, Jr., Drake AF. The impact of sleep-disordered breathing on cognition and behavior in children: a review and meta-synthesis of the literature. Otolaryngol Head Neck Surg. 2004;131:814–26. doi: 10.1016/j.otohns.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128:e85–92. doi: 10.1542/peds.2010-3431. [DOI] [PubMed] [Google Scholar]
- 4.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 5.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Driscoll DM, Morrell MJ. The interaction between respiratory and autonomic function during sleep-related changes in pharyngeal airway patency. Auton Neurosci. 2005;120:18–25. doi: 10.1016/j.autneu.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Fernhall B, Figueroa A, Collier S, Baynard T, Giannopoulou I, Goulopoulou S. Blunted heart rate response to upright tilt in people with Down syndrome. Arch Phys Med Rehabil. 2005;86:813–8. doi: 10.1016/j.apmr.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Guerra M, Llorens N, Fernhall B. Chronotropic incompetence in persons with down syndrome. Arch Phys Med Rehabil. 2003;84:1604–8. doi: 10.1053/s0003-9993(03)00342-3. [DOI] [PubMed] [Google Scholar]
- 9.Fernhall B, Otterstetter M. Attenuated responses to sympathoexcitation in individuals with Down syndrome. J Appl Physiol. 2003;94:2158–65. doi: 10.1152/japplphysiol.00959.2002. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa A, Collier SR, Baynard T, Giannopoulou I, Goulopoulou S, Fernhall B. Impaired vagal modulation of heart rate in individuals with Down syndrome. Clin Auton Res. 2005;15:45–50. doi: 10.1007/s10286-005-0235-1. [DOI] [PubMed] [Google Scholar]
- 11.Baynard T, Pitetti KH, Guerra M, Fernhall B. Heart rate variability at rest and during exercise in persons with Down syndrome. Arch Phys Med Rehabil. 2004;85:1285–90. doi: 10.1016/j.apmr.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Fernhall B, Pitetti KH, Rimmer JH, et al. Cardiorespiratory capacity of individuals with mental retardation including Down syndrome. Med Sci Sports Exerc. 1996;28:366–71. doi: 10.1097/00005768-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 13.O’Driscoll DM, Horne RS, Davey MJ, Hope SA, Walker AM, Nixon GM. The heart rate response to spontaneous arousal from sleep is reduced in children with Down syndrome referred for evaluation of sleep-disordered breathing. Am J Physiol Heart Circ Physiol. 2010;298:H1986–90. doi: 10.1152/ajpheart.00701.2009. [DOI] [PubMed] [Google Scholar]
- 14.Shah PS, Hellmann J, Adatia I. Clinical characteristics and follow up of Down syndrome infants without congenital heart disease who presented with persistent pulmonary hypertension of newborn. J Perinat Med. 2004;32:168–70. doi: 10.1515/JPM.2004.030. [DOI] [PubMed] [Google Scholar]
- 15.Levine OR, Simpser M. Alveolar hypoventilation and cor pulmonale associated with chronic airway obstruction in infants with Down syndrome. Clin Pediatr (Phila) 1982;21:25–9. doi: 10.1177/000992288202100104. [DOI] [PubMed] [Google Scholar]
- 16.Rowland TW, Nordstrom LG, Bean MS, Burkhardt H. Chronic upper airway obstruction and pulmonary hypertension in Down’s syndrome. Am J Dis Child. 1981;135:1050–2. doi: 10.1001/archpedi.1981.02130350050016. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st Ed. Westchester, IL: American Academy of Sleep Medicine 2007; The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 20.ATS. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 21.ASDA. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 22.Mograss MA, Ducharme FM, Brouillette RT. Movement/arousals. Description, classification, and relationship to sleep apnea in children. Am J Respir Crit Care Med. 1994;150:1690–6. doi: 10.1164/ajrccm.150.6.7952634. [DOI] [PubMed] [Google Scholar]
- 23.Foo JY. Pulse transit time in paediatric respiratory sleep studies. Med Eng Phys. 2007;29:17–25. doi: 10.1016/j.medengphy.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Foo JY, Lim CS. Difference in pulse transit time between populations: a comparison between Caucasian and Chinese children in Australia. J Med Eng Technol. 2008;32:162–6. doi: 10.1080/03091900600632694. [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll DM, Foster AM, Ng ML, et al. Acute cardiovascular changes with obstructive events in children with sleep disordered breathing. Sleep. 2009;32:1265–71. doi: 10.1093/sleep/32.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med. 2002;165:61–6. doi: 10.1164/ajrccm.165.1.2009062. [DOI] [PubMed] [Google Scholar]
- 27.Peaston RT. Routine determination of urinary free catecholamines by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1988;424:263–72. doi: 10.1016/s0378-4347(00)81103-2. [DOI] [PubMed] [Google Scholar]
- 28.O’Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12:483–8. doi: 10.1016/j.sleep.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 29.O’Driscoll DM, Meadows GE, Corfield DR, Simonds AK, Morrell MJ. Cardiovascular response to arousal from sleep under controlled conditions of central and peripheral chemoreceptor stimulation in humans. J Appl Physiol. 2004;96:865–70. doi: 10.1152/japplphysiol.00749.2003. [DOI] [PubMed] [Google Scholar]
- 30.Uong EC, McDonough JM, Tayag-Kier CE, et al. Magnetic resonance imaging of the upper airway in children with Down syndrome. Am J Respir Crit Care Med. 2001;163:731–6. doi: 10.1164/ajrccm.163.3.2004231. [DOI] [PubMed] [Google Scholar]
- 31.Loughlin GM, Wynne JW, Victorica BE. Sleep apnea as a possible cause of pulmonary hypertension in Down syndrome. J Pediatr. 1981;98:435–7. doi: 10.1016/s0022-3476(81)80716-0. [DOI] [PubMed] [Google Scholar]
- 32.Antonarakis SE, Epstein CJ. The challenge of Down syndrome. Trends Mol Med. 2006;12:473–9. doi: 10.1016/j.molmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Cooney TP, Thurlbeck WM. Pulmonary hypoplasia in Down’s syndrome. N Engl J Med. 1982;307:1170–3. doi: 10.1056/NEJM198211043071902. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–6. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 35.Trois MS, Capone GT, Lutz JA, et al. Obstructive sleep apnea in adults with Down syndrome. J Clin Sleep Med. 2009;5:317–23. [PMC free article] [PubMed] [Google Scholar]
- 36.Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest. 2003;123:1119–26. doi: 10.1378/chest.123.4.1119. [DOI] [PubMed] [Google Scholar]
- 37.Eberhard Y, Eterradossi J, Therminarias A. Biochemical changes and catecholamine responses in Down’s syndrome adolescents in relation to incremental maximal exercise. J Ment Defic Res. 1991;35:140–6. doi: 10.1111/j.1365-2788.1991.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernhall B, Baynard T, Collier S R, et al. Catecholamine response to maximal exercise in persons with Down syndrome. Am J Cardiol. 2009;103:724–6. doi: 10.1016/j.amjcard.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Pueschel SM. Clinical aspects of Down syndrome from infancy to adulthood. Am J Med Genet Suppl. 1990;7:52–6. doi: 10.1002/ajmg.1320370708. [DOI] [PubMed] [Google Scholar]