Abstract
Aortic valve sparing operations were developed to preserve the native aortic valve during surgery for aortic root aneurysm as well as surgery for ascending aortic aneurysms with associated aortic insufficiency. There are basically two types of aortic valve sparing oprations: remodeling of the aortic root and reimplantation of the aortic valve. These operations have been performed for over two decades and the clinical outcomes have been excellent in experienced hands. Although remodeling of the aortic root is physiologically superior to reimplantation of the aortic valve, long-term follow-up suggests that the latter is associated with lower risk of developing aortic insufficiency. Failure of remodeling of the aortic root is often due to dilatation of the aortic annulus. Thus, this type of aortic valve sparing should be reserved for older patients with ascending aortic aneurysm and normal aortic annulus whereas reimplantation of the aortic valve is more appropriate for young patients with inherited disorders that cause aortic root aneurysms. This article summarizes the published experience with these two operations. They are no longer experimental procedures and should be part of the surgical armamentarium to treat patients with aortic root aneurysm and ascending aortic aneurysms with associated aortic insufficiency.
Keywords: Aortic root aneurysm, Aortic insufficiency, Aortic valve sparing
BACKGROUND
The term "aortic valve sparing operation" was first used in one of our publications that described a new surgical procedure for reconstruction of the aortic root with preservation of the native aortic valve in patients with aortic root aneurysm with or without aortic insufficiency (AI) [1]. That operation consisted in excising the aortic sinuses along with the coronary arteries and reimplanting the aortic valve and coronary arteries inside a cylindrical Dacron graft. A prerequisite was that the aortic cusps should be normal or have minimal anatomic changes. One year later, Sarsam and Yacoub [2] published a study titled "remodeling of the aortic valve annulus" whereby the aortic sinuses were excised and replaced with tailored tubular Dacron graft with three neo-aortic sinuses. In 1995 we pusblished a study on 45 patients with aortic root aneurysm and ascending aortic aneurysms with AI and described an array of different operative procedures that ranged from simple adjustment of the sinotubular junction in patients with ascending aortic aneurysm and AI due to dilation of the sinotubular junction to replacement of one, two, or three aortic sinuses with a tailored tubular Dacron graft to reimplantation of the aortic valve into a cylindrical Dacron graft [3]. We referred to the latter operation as "reimplantation of the aortic valve" and to the others as "remodeling of the aortic root" [4]. Many surgeons have described modifications to the original techniques of reimplantation of the aortic valve and remodeling of the aortic root [5-11]. In this paper we will review only the information of the original techniques because there are no long-term data on the newer modifications.
PATIENTS' SELECTION
Aortic valve sparing operations were developed to preserve the aortic valve in patients with aortic root aneurysm with or without AI and in patients with ascending aortic aneurysm and AI secondary to dilation of the sinotubular junction [4]. In both instances the cusps have to be reasonably normal. Trans-esophageal echocardiography remains the best diagnostic tool to select patients for these operations. All the components of the aortic root must be carefully interrogated, particularly the aortic cusps since they are the most important determinant of repairability. Calcified, sclerotic, and stiff cusps are not suitable for repair. Neither are pliable cusps with thickened free margins due to longstanding regurgitation when the cusps heights are reduced. Prolapse of one or more cusps does not preclude a good repair if the cusp tissue is found to be of good strength at surgery. The free margins of the cusps can be shortened by plication or by a triangular resection along the nodule of Arantius. Cusps with large fenestrations can be reinforced with a double layer of a fine suture of expanded polytetrafluoroethylene. Cusp repair does not seem to alter the long-term results in series of cases done by experienced surgeons [12,13]. In addition to the aortic cusps, anatomic, and geometric information regarding the aortic annulus, aortic sinuses and sinotubular junction must be obtained by trans-esophagel echocardiography to aid the surgeon to plan the procedure. The diameter of the aortic annulus is surprisingly small in patients with normal aortic valves [14] and surgeons must have this knowledge to determine when the aortic annulus is dilated.
OPERATIVE TECHNIQUES
The operative techniques of reimplantation of the aortic valve and remodeling of the aortic root have been extensively published [1,3,4,15,16]. We continue to use both techniques and try to match the procedure to the pathology of the aortic root.
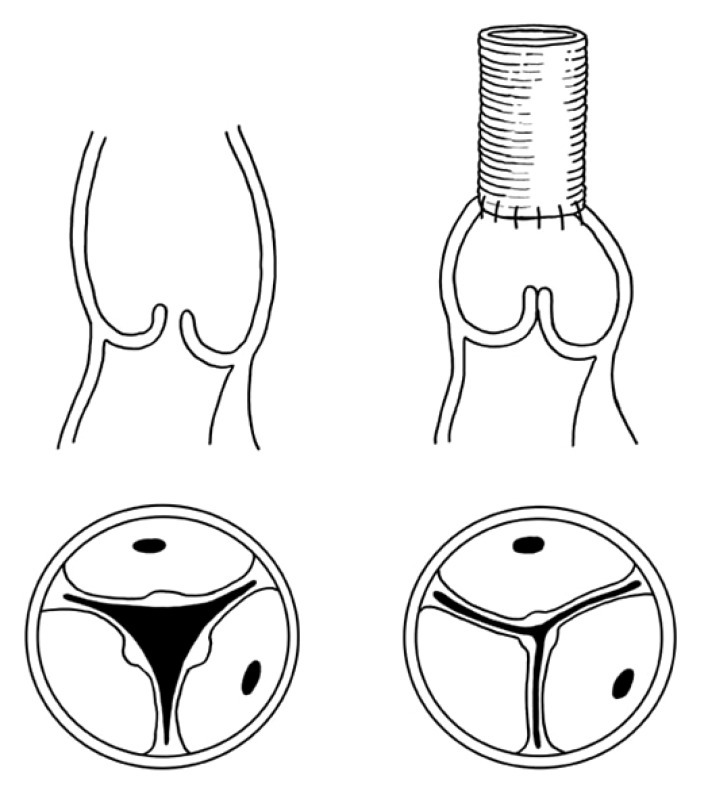
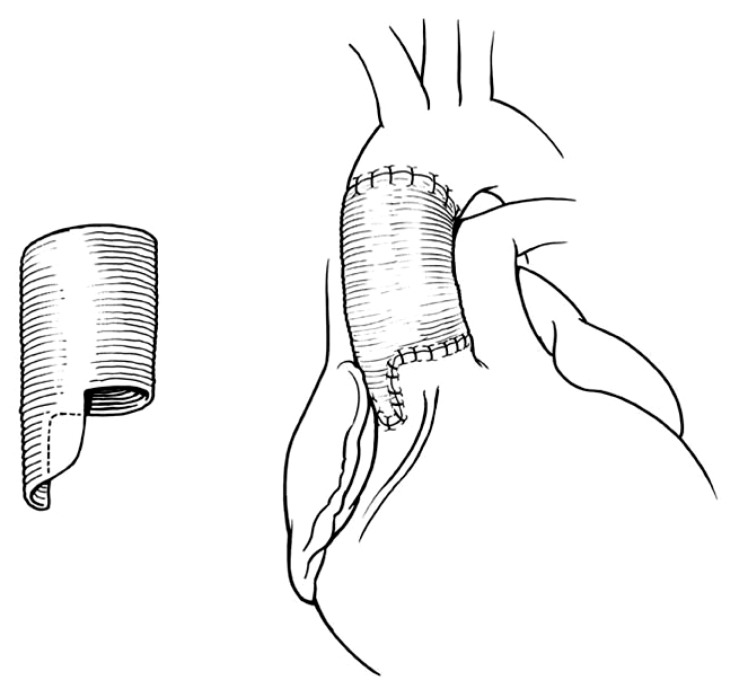
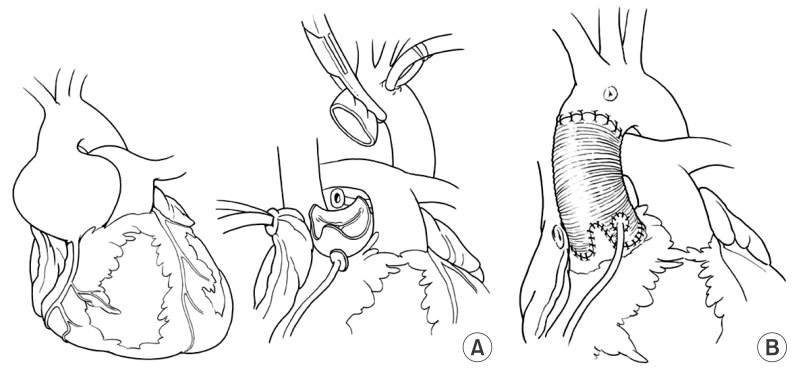
Older patients (e.g., age >50 years) with ascending aortic aneurysm and dilated sinotubular junction with normal cusps and normal aortic annulus and moderate or severe AI can have simple replacement of the ascending aorta with reduction of the diameter of the sinotubular junction to allow the cusps to coapt centrally [17,18]. This type of remodeling of the aortic root is illustrated in Fig. 1. It is relatively simple and the results have been excellent [17,18]. One or more cusps may need shortening of the free margin if they are prolapsing [18]. The non-coronary aortic sinus may be dilated and thinned out in some patients and it may be safer to replace it by tailoring the end of the Dacron to create a neo-aortic sinus [4]. This procedure is illustrated in Fig. 2. If all 3 aortic sinuses are dilated a complete remodeling is needed [4,15,16], as shown in Fig. 3.
Fig. 1.
Replacement of the ascending aorta with adjustment of diameter of the sinotubular junction to correct aortic insufficiency. Dilatation of the sinotubular junction prevents the cups from coapting centrally. Replacement of the ascending aorta with a graft of appropriate diameter and sutured to the aortic root at the level of the sinotubular junction restores valve competence.
Fig. 2.
Replacement of the ascending aorta with adjustment of diameter of the sinotubular junction and replacement of the non-coronary aortic sinus. When the non-coronary sinus is dilated, the graft selected to correct the dilatation of the sinotubular junction can be tailored to replace one or more sinues.
Fig. 3.
Complete remodeling of the aortic root with replacement of all three aortic sinuses and reimplantation of the coronary arteries. (A) The three aortic sinuses are excised leaving 4 to 5 mm of sinus wall attached to the aortic annulus. (B) A tubular Dacron graft is tailored to create 3 neo-aortic sinuses. The coronary arteries are reimplanted.
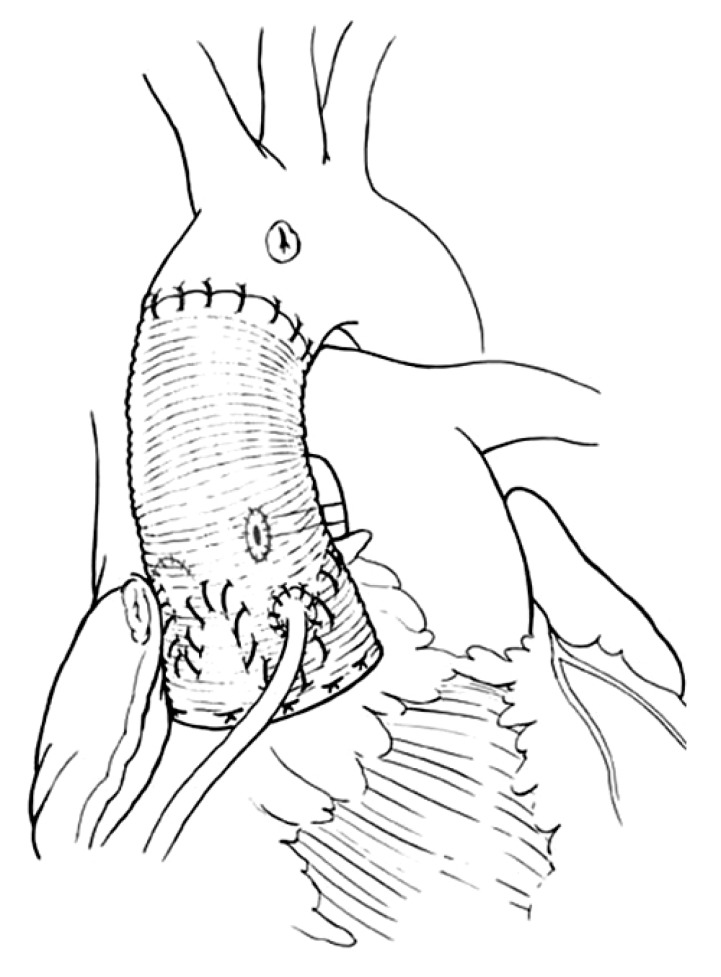
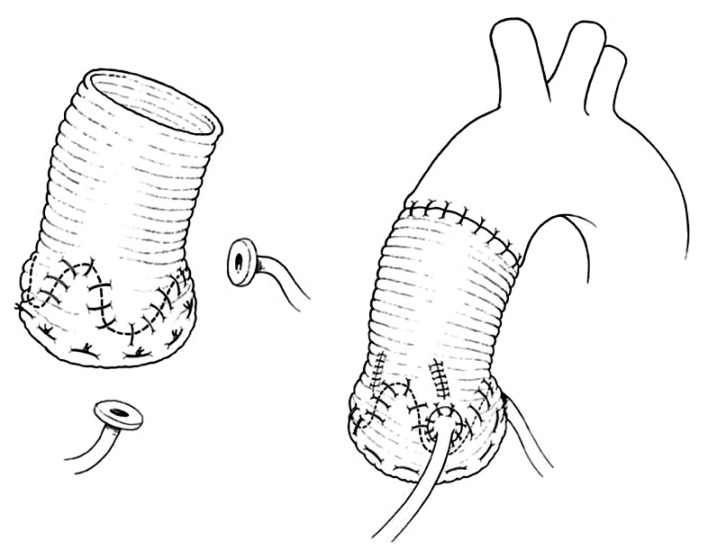
Younger patients with inherited aortic root aneurysms such as in Marfan syndrome, Loyes-Dietz syndrome, familial aneurysm, and others may have associated annuloaortic ectasia or develop dilatation of the aortic annulus years after the remodeling of the aortic root and have an increased risk of late AI [19-23]. Thus, these patients should have reimplantation of the aortic valve to permanently stabilize the aortic annulus. Reimplantation of the aortic valve into a cylindrical graft has been used extensively and the long-term results have been excellent [16,24,25]. This operation is illustrated in Fig. 4. The diameter of the graft is determined either by estimating the ideal diameter of the sinotubular or by measuring the height of the cusps [1,15,16]. We often measure the diameter of the aortic annulus, the height of the cusps and estimate the ideal diameter of the sinotubular junction before choosing a graft. The latter can be estimated by pulling on the three commissures upward and approximating them until the cusps touch each other in the center. The diameter of the circle that includes all three commissures is the estimated sinotubular junction. De Kerchove et al. [26] determine the diameter of the graft by measuring the height of the commissure between the non-coronary cusp and the left coronary cusp. Patients with aortic root aneurysm may have cusps larger than normal [27] and dilated aortic annulus and no single measurement is adequate to select the diameter of the graft in all cases. If the selected graft is too large it may not reduce the diameter of the aortic annulus to allow for adequate cusp coaptation, and if the graft is too small the cusps may touch the graft during systole with consequent cusp abrasion. In our experience most patients with aortic root aneurysm need grafts of 26 to 34 mm. It has been shown that the presence of aortic sinuses is important for normal cusp motion and reduction of cusp stress [28-31]. There is echocardiographic evidence that opening and closure velocity of the cusps are increased when the valve is reimplanted into a cylindrical graft without neo-aortic sinuses and creation of neo-aortic sinuses reduces the velocity [30,31]. In addition to the aortic sinuses, compliance of aortic root is also important to modulate mechanical stresses on the cusps [32]. From this viewpoint, remodeling of the aortic root is physiologically superior to reimplantation of the aortic valve because postoperatively the aortic annulus movements closely resemble the normal [28]. However, as stated above remodeling of the aortic root does not correct or prevent annular dilatation and development of late AI is a serious drawback in young patients [19-23]. Thus, reimplantation remains the procedure of choice to treat patients with inherited aortic root aneurysms. Whether creation of neo-aortic sinuses is important remains to be proven because the longest follow-up on this operation is on patients who had a cylindrical graft and the results have been excellent up to 15 years [16,24,25]. There are two commercially available grafts with aortic sinuses that can be used for the reimplanation procedure [6,7]. The initial results with the Valsalva Graft (Vascutek Ltd., Renfreshsire, Scotland) appear satisfactory [33,34]. Our reservation regarding that graft is its shape. The sinuses are spherical and in the normal aortic root the aortic annulus evolves along a cylinder with three sinuses in between the annulus. The graft described by Richardt et al. [7] is anatomically correct but it is not yet widely used. It is also possible to create neo-aortic sinuses with a tubular Dacron graft by selecting a graft 2 to 4 mm larger than what is needed for the reimplantation and then plicating the areas of the graft that correspond to the nadir of the aortic annulus and the spaces in between commissures [15,16]. This technique is illustrated in Fig. 5. That is the technique we have used during the past decade since we do not have access to the Richardt's graft [7] due to regulatory issues in Canada.
Fig. 4.
Reimplantation of the aortic valve into a cylindrical Dacron graft. The aortic sinuses are excised as in Fig. 3A. The aortic valve is sutured inside a tubular Dacron graft of diameter equal to the sinotubular junction or equal to two-thirds of the double of the cusps heights. The graft is secured beneath the aortic annulus and the remnants of the aortic sinuses are sutured to the Dacron graft. The coronary arteries are reimplanted.
Fig. 5.
Reimplanation of the aortic valve into a cylindrical Dacron graft with creation of neo-aortic sinuses. A tubular Dacron graft 4 mm larger than needed is used for reimplantation of the aortic valve and the spaces in between commissure are plicated to create neo-aortic sinuses.
Whether the remodeling of the aortic root or reimplantation of the aortic valve is used, at the end of the procedure the cusps must coapt within the reconstructed aortic root and the cusps must coapt for several millimeters [35,36]. In order to accomplish that the free margin of the cusps may have to be shortened by plication along the nodule of Arantius or with reinforcement of the free margin with fine expanded polytetrafluoroethylene sutures [12,13].
Bicuspid aortic valve may be associated with aortic root aneurysm and if the cusps are of reasonable quality they can be preserved and provide satisfactory results. Since dilatation of the aortic annulus is often present in patients with incompetent bicuspid aortic valves, the technique of reimplantation of the aortic valve is probably better than other techniques [37]. Cusp area enhancement with glutaraldehyde fixed pericardial patch shortens the durability of repair [38]. In addition, truly bicuspid aortic valves (type 0 in Sievers and Schmidtke's classification [39]) and those with type 1 or 2 but anatomically similar to type 0 (cusps arrangement close to 180°) seem do provide more stable long-term results [38].
An occasional patient with aortic root aneurysm, particularly those with bicuspid aortic valve, will have a coronary artery orifice too close to a commissure to be safely detached and then reimplanted [40]. In this anatomic situation we leave the coronary artery attached to the aortic root and tailor the Dacron graft in such way as to support the aortic annulus from the outside and create an orifice in the graft to accommodate the anomalously inserted coronary artery [40].
OPERATIVE OUTCOMES
The operative mortality of aortic valve sparing procedures is reportedly low. In Yacoub's series [41] of 158 patients who had remodeling of the aortic root from 1979 through 1997, the overall mortality was 4.6% but included 49 patients with acute aortic dissection. In our series [16] of 289 patients (228 reimplantation and 61 remodeling) operated from 1988 through 2007, the operative mortality was 1.7%. The operative mortality was 2.8% among 144 patients enrolled in a multicenter trial on the remodeling procedure and aortic annuloplasty reported by Lansac et al. [42]. In a multicenter trial on the reimplantation technique using the Valsalva Graft reported by De Paulis et al. [33] the operative mortality was 1.8% among 278 patients.
Aortic valve sparing operations are complex operative procedures and require long cardiopulmonary bypass and aortic clamping times, which often increase the risk of post-pump coagulopathy. In addition, there are numerous areas where bleeding can occur. Thus, postoperative bleeding and the need for re-exploration of the mediastinum is a common complication of these operations [16]. Other postoperative problems are similar to those associated with aortic root surgery: complete heart block, myocardial infarction, infection, dysrhythmias, respiratory failure, renal failure, etc, but they are all uncommon.
LONG TERM RESULTS
The mean age of patients who has had these operations varies widely among reported series [33,41-43]. Many series do not distinguish patients with primary aortic root aneurysm from those with ascending aortic aneurysm and secondary dilatation of the aortic sinuses, and since the latter is more common in older patients, the mean age of their patients will be higher than in those series that include large numbers of patients with inherited disorders of the aortic root such as Marfan syndrome. Regardless of the patients' mix, the long term survival following aortic valve sparing operations is excellent [9,16,33,41-44]. The survival is similar to that of the general population in patients without aortic dissection [41,43,44].
Valve-related complications such as thromboembolism and infective endocarditis are relatively rare and the rates are certainly much lower than after replacement of the aortic root with prosthetic devices [34,45-48].
The main problem after aortic valve sparing operations is the development of aortic insufficiency. It has been established that in young patients with inherited connective tissues disorder reimplantation of the aortic valve provides more stable aortic valve function than remodeling of the aortic of the aortic root [20,23,24,47,48]. The main cause of early failure of aortic valve sparing operations is technical errors [49] and probably lack of recognition of cusp prolapse. As mentioned above, regardless of the technique used to repair the dilated aortic root, the cusps must coapt above the level of the nadir of the aortic annulus and the coaptation length must be of at least 4 mm in the central part. The main cause of late failure is probably degeneration of the aortic cusps but more information is needed to confirm this observation. It has been postulated that a rigid aortic root may accelerate degenerative changes in the aortic cusps [32]. Interestingly, we have found that age had a protective effect against the development of late aortic insufficiency after reimplantation of the aortic valve [16], suggesting that elastic aortic cusps probably have greater adaptability to a rigid root than the more sclerotic ones often seen in older patients.
Some surgeons found that preoperative aortic insufficiency and the need for cusp repair at the time of surgery are predictive of postoperative aortic insufficiency [9,23], whereas others do not believe that incompetent aortic valves before surgery or cusp repair has deleterious effects on long term outcomes [12,16,50,51]. This difference is probably due to patients' selection or incorrect matching of the procedure to the pathology [13].
We believe the long term results of remodeling of the aortic root are equally good to reimplantation of the aortic valve when performed in the correct patient. Older patients with normal aortic annulus and dilated sinuses are not likely to develop annulo-aortic ectasia and the remodeling procedure provides excellent long term valve function [16]. Younger patients, particularly those with Marfan syndrome, Loyes-Dietz syndrome and familial aneurysms do better with reimplantation of the aortic valve. In a recent review of our experience with reimplantation of the aortic valve in 296 patients whose mean age was 47 years (range, 11 to 79 years), and followed up to 22 years, mean of 6.4 years, only 3 patients required reoperation on the aortic valve and 11 developed moderate or severe aortic insufficiency [25]. In that series, the freedom from reoperation at 15 years was 98% and the freedom from moderate or severe aortic insufficiency was 89%. These results are excellent and certainly better than those obtained after mitral valve repair, the gold standard approach to treat mitral regurgitation due to degenerative diseases of the mitral valve.
Patients with Marfan syndrome and other inherited aortic root aneurysm seem to benefit for reimplantation of the aortic root. In recent review of our experience we found that Marfan syndrome was protective against the development of late aortic insufficiency [25].
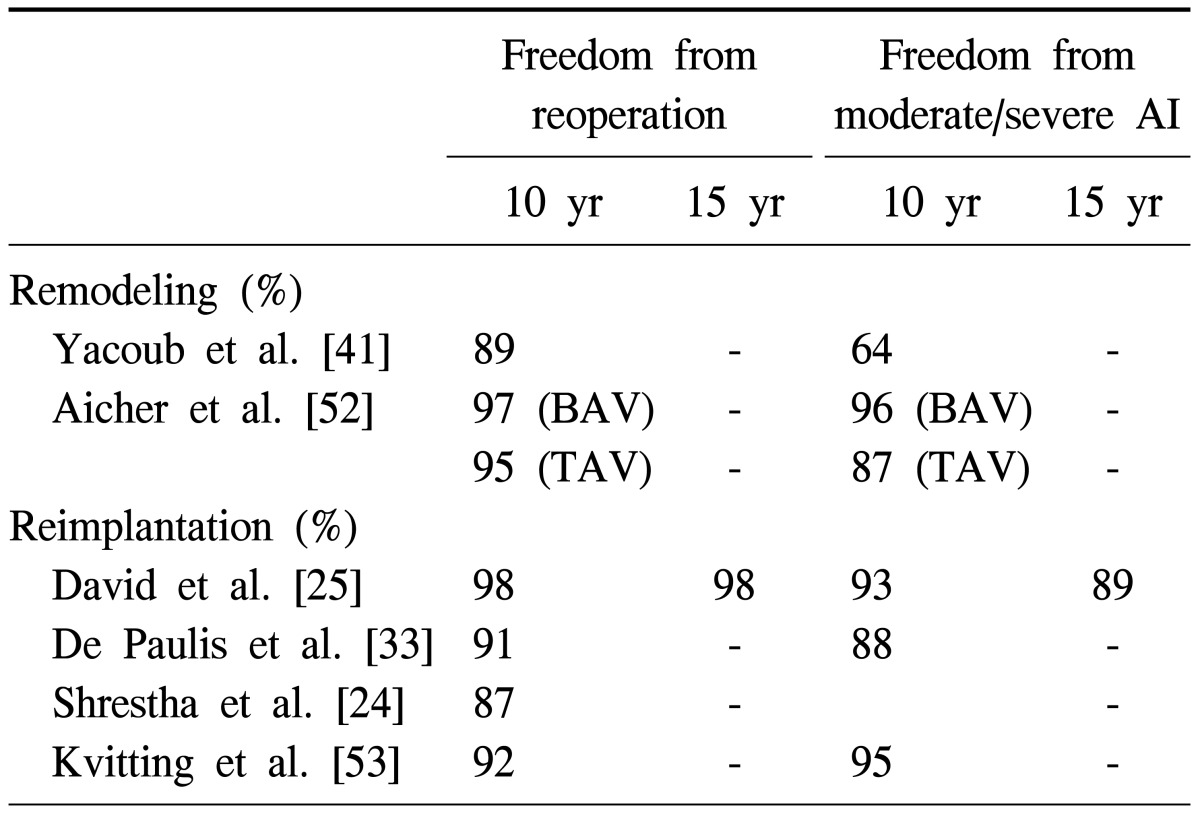
Several centers have accumulated sizeable experience with aortic valve sparing operations [23,33,34,37,53-55]. Table 1 summarizes freedom from reoperation on the aortic valve and freedom from moderate or severe aortic insufficiency in large series with follow-up of at least a decade.
Table 1.
Freedom from reoperation and freedom from moderate and severe aortic insufficiency after aortic valve sparing operations
AI, aortic insufficiency; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve.
SUMMARY
Aortic valve sparing operations to treat patients with aortic root aneurysm with or without aortic insufficiency and patients with ascending aortic aneurysm and aortic insufficiency are no longer "experimental" procedures and the principles are well established. Thus, patients with bicuspid or tricuspid aortic valves and an aneurysm can be successfully treated with these procedures. Reimplantation of the aortic valve should be employed in patients with inherited connective tissue disorders. The role of neo-aortic sinuses in the durability of this operation remains to be determined with further clinical follow-up. Remodeling of the aortic root is ideal for older patients with normal aortic annulus and primarily ascending aortic aneurysms. The long term results of these operations very been excellent and justify their inclusion in the surgical armamentarium to treat patients with aortic root and ascending aortic aneurysms.
References
- 1.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617–621. [PubMed] [Google Scholar]
- 2.Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg. 1993;105:435–438. [PubMed] [Google Scholar]
- 3.David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg. 1995;109:345–351. doi: 10.1016/S0022-5223(95)70396-9. [DOI] [PubMed] [Google Scholar]
- 4.David TE. Remodeling the aortic root and preservation of the native aortic valve. Oper Tech Card Thorac Surg. 1996;1:44–56. [Google Scholar]
- 5.Cochran RP, Kunzelman KS, Eddy AC, Hofer BO, Verrier ED. Modified conduit preparation creates a pseudosinus in an aortic valve-sparing procedure for aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1995;109:1049–1057. doi: 10.1016/S0022-5223(95)70187-7. [DOI] [PubMed] [Google Scholar]
- 6.De Paulis R, De Matteis GM, Nardi P, et al. One-year appraisal of a new aortic root conduit with sinuses of Valsalva. J Thorac Cardiovasc Surg. 2002;123:33–39. doi: 10.1067/mtc.2002.119066. [DOI] [PubMed] [Google Scholar]
- 7.Richardt D, Karluss A, Schmidtke C, Sievers HH, Scharfschwerdt M. A new sinus prosthesis for aortic valve-sparing surgery maintaining the shape of the root at systemic pressure. Ann Thorac Surg. 2010;89:943–946. doi: 10.1016/j.athoracsur.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 8.Lansac E, Di Centa I, Raoux F, et al. An expansible aortic ring for a physiological approach to conservative aortic valve surgery. J Thorac Cardiovasc Surg. 2009;138:718–724. doi: 10.1016/j.jtcvs.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Urbanski PP, Zhan X, Hijazi H, Zacher M, Diegeler A. Valve-sparing aortic root repair without down-sizing of the annulus. J Thorac Cardiovasc Surg. 2012;143:294–302. doi: 10.1016/j.jtcvs.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 10.Pepper J, Golesworthy T, Utley M, et al. Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact Cardiovasc Thorac Surg. 2010;10:360–365. doi: 10.1510/icvts.2009.220319. [DOI] [PubMed] [Google Scholar]
- 11.Hess PJ, Jr, Harman PK, Klodell CT, et al. Early outcomes using the Florida sleeve repair for correction of aortic insufficiency due to root aneurysms. Ann Thorac Surg. 2009;87:1161–1168. doi: 10.1016/j.athoracsur.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.David TE, Armstrong S. Aortic cusp repair with Gore-Tex sutures during aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2010;139:1340–1342. doi: 10.1016/j.jtcvs.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Boodhwani M, de Kerchove L, Watremez C, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg. 2011;141:917–925. doi: 10.1016/j.jtcvs.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Capps SB, Elkins RC, Fronk DM. Body surface area as a predictor of aortic and pulmonary valve diameter. J Thorac Cardiovasc Surg. 2000;119:975–982. doi: 10.1016/S0022-5223(00)70092-4. [DOI] [PubMed] [Google Scholar]
- 15.David TE. How I do aortic valve sparing operations to treat aortic root aneurysm. J Card Surg. 2011;26:92–99. doi: 10.1111/j.1540-8191.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- 16.David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S14–S19. doi: 10.1016/j.jtcvs.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 17.David TE, Feindel CM, Armstrong S, Maganti M. Replacement of the ascending aorta with reduction of the diameter of the sinotubular junction to treat aortic insufficiency in patients with ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2007;133:414–418. doi: 10.1016/j.jtcvs.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 18.Asano M, Kunihara T, Aicher D, El Beyrouti H, Rodionycheva S, Schafers HJ. Mid-term results after sinutubular junction remodelling with aortic cusp repair. Eur J Cardiothorac Surg. 2012 Apr 14; doi: 10.1093/ejcts/ezs120. [Epub] [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira NC, David TE, Ivanov J, et al. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2003;125:789–796. doi: 10.1067/mtc.2003.57. [DOI] [PubMed] [Google Scholar]
- 20.Birks EJ, Webb C, Child A, Radley-Smith R, Yacoub MH. Early and long-term results of a valve-sparing operation for Marfan syndrome. Circulation. 1999;100(19 Suppl):II29–II35. doi: 10.1161/01.cir.100.suppl_2.ii-29. [DOI] [PubMed] [Google Scholar]
- 21.Leyh RG, Fischer S, Kallenbach K, et al. High failure rate after valve-sparing aortic root replacement using the "remodeling technique" in acute type A aortic dissection. Circulation. 2002;106(12 Suppl 1):I229–I233. [PubMed] [Google Scholar]
- 22.Bethea BT, Fitton TP, Alejo DE, et al. Results of aortic valve-sparing operations: experience with remodeling and reimplantation procedures in 65 patients. Ann Thorac Surg. 2004;78:767–772. doi: 10.1016/j.athoracsur.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 23.Hanke T, Charitos EI, Stierle U, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg. 2009;137:314–319. doi: 10.1016/j.jtcvs.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha M, Baraki H, Maeding I, et al. Long-term results after aortic valve-sparing operation (David I) Eur J Cardiothorac Surg. 2012;41:56–61. doi: 10.1016/j.ejcts.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David TE, Armstrong S, Manlhiot C, McCrindle BW, Feindel CM. Long term results of aortic root repair using the reimplantion technique. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.11.075. in press. [DOI] [PubMed] [Google Scholar]
- 26.de Kerchove L, Boodhwani M, Glineur D, Noirhomme P, El Khoury G. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg. 2011;92:749–751. doi: 10.1016/j.athoracsur.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Thubrikar MJ, Labrosse MR, Zehr KJ, Robicsek F, Gong GG, Fowler BL. Aortic root dilatation may alter the dimensions of the valve leaflets. Eur J Cardiothorac Surg. 2005;28:850–855. doi: 10.1016/j.ejcts.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Leyh RG, Schmidtke C, Sievers HH, Yacoub MH. Opening and closing characteristics of the aortic valve after different types of valve-preserving surgery. Circulation. 1999;100:2153–2160. doi: 10.1161/01.cir.100.21.2153. [DOI] [PubMed] [Google Scholar]
- 29.Grande-Allen KJ, Cochran RP, Reinhall PG, Kunzelman KS. Re-creation of sinuses is important for sparing the aortic valve: a finite element study. J Thorac Cardiovasc Surg. 2000;119(4 Pt 1):753–763. doi: 10.1016/S0022-5223(00)70011-0. [DOI] [PubMed] [Google Scholar]
- 30.De Paulis R, De Matteis GM, Nardi P, Scaffa R, Buratta MM, Chiariello L. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg. 2001;72:487–494. doi: 10.1016/s0003-4975(01)02747-3. [DOI] [PubMed] [Google Scholar]
- 31.Aybek T, Sotiriou M, Wohleke T, et al. Valve opening and closing dynamics after different aortic valve-sparing operations. J Heart Valve Dis. 2005;14:114–120. [PubMed] [Google Scholar]
- 32.Fokin AA, Robicsek F, Cook JW, Thubrikar MJ, Schaper J. Morphological changes of the aortic valve leaflets in non-compliant aortic roots: in-vivo experiments. J Heart Valve Dis. 2004;13:444–451. [PubMed] [Google Scholar]
- 33.De Paulis R, Scaffa R, Nardella S, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S23–S27. doi: 10.1016/j.jtcvs.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 34.Patel ND, Weiss ES, Alejo DE, et al. Aortic root operations for Marfan syndrome: a comparison of the Bentall and valve-sparing procedures. Ann Thorac Surg. 2008;85:2003–2010. doi: 10.1016/j.athoracsur.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Pethig K, Milz A, Hagl C, Harringer W, Haverich A. Aortic valve reimplantation in ascending aortic aneurysm: risk factors for early valve failure. Ann Thorac Surg. 2002;73:29–33. doi: 10.1016/s0003-4975(01)03312-4. [DOI] [PubMed] [Google Scholar]
- 36.Kunihara T, Aicher D, Rodionycheva S, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg. 2012;143:1389–1395. doi: 10.1016/j.jtcvs.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 37.de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142:1430–1438. doi: 10.1016/j.jtcvs.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Aicher D, Kunihara T, Abou Issa O, Brittner B, Graber S, Schafers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123:178–185. doi: 10.1161/CIRCULATIONAHA.109.934679. [DOI] [PubMed] [Google Scholar]
- 39.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh AM, David TE. Aortic valve-sparing operations: dealing with the coronary artery that is too close to the aortic annulus. Ann Thorac Surg. 2009;88:1026–1028. doi: 10.1016/j.athoracsur.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 41.Yacoub MH, Gehle P, Chandrasekaran V, Birks EJ, Child A, Radley-Smith R. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg. 1998;115:1080–1090. doi: 10.1016/S0022-5223(98)70408-8. [DOI] [PubMed] [Google Scholar]
- 42.Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring to standardise aortic valve repair: preliminary results of a prospective multicentric cohort of 144 patients. Eur J Cardiothorac Surg. 2010;38:147–154. doi: 10.1016/j.ejcts.2010.01.041. [DOI] [PubMed] [Google Scholar]
- 43.David TE, Armstrong S, Maganti M, Colman J, Bradley TJ. Long-term results of aortic valve-sparing operations in patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2009;138:859–864. doi: 10.1016/j.jtcvs.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 44.David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg. 2006;132:347–354. doi: 10.1016/j.jtcvs.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 45.Benedetto U, Melina G, Takkenberg JJ, Roscitano A, Angeloni E, Sinatra R. Surgical management of aortic root disease in Marfan syndrome: a systematic review and meta-analysis. Heart. 2011;97:955–958. doi: 10.1136/hrt.2010.210286. [DOI] [PubMed] [Google Scholar]
- 46.Bernhardt AM, Treede H, Rybczynski M, et al. Comparison of aortic root replacement in patients with Marfan syndrome. Eur J Cardiothorac Surg. 2011;40:1052–1057. doi: 10.1016/j.ejcts.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Rahnavardi M, Yan TD, Bannon PG, Wilson MK. Aortic valve-sparing operations in aortic root aneurysms: remodeling or reimplantation? Interact Cardiovasc Thorac Surg. 2011;13:189–197. doi: 10.1510/icvts.2011.267401. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Wang W, Wang X, Tian C, Meng YH, Chang Q. Reimplantation versus remodeling: a meta-analysis. J Card Surg. 2011;26:82–87. doi: 10.1111/j.1540-8191.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 49.Oka T, Okita Y, Matsumori M, et al. Aortic regurgitation after valve-sparing aortic root replacement: modes of failure. Ann Thorac Surg. 2011;92:1639–1644. doi: 10.1016/j.athoracsur.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 50.de Kerchove L, Boodhwani M, Glineur D, et al. Effects of preoperative aortic insufficiency on outcome after aortic valve-sparing surgery. Circulation. 2009;120(11 Suppl):S120–S126. doi: 10.1161/CIRCULATIONAHA.108.841445. [DOI] [PubMed] [Google Scholar]
- 51.de Kerchove L, Boodhwani M, Glineur D, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg. 2009;88:455–461. doi: 10.1016/j.athoracsur.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 52.Aicher D, Langer F, Lausberg H, Bierbach B, Schafers HJ. Aortic root remodeling: ten-year experience with 274 patients. J Thorac Cardiovasc Surg. 2007;134:909–915. doi: 10.1016/j.jtcvs.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 53.Kvitting JPE, Kari FA, Fischbein MP, et al. Tirone David valve-sparing aortic root replacement: equivalent long-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.09.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka H, Ogino H, Matsuda H, Minatoya K, Sasaki H, Iba Y. Midterm outcome of valve-sparing aortic root replacement in inherited connective tissue disorders. Ann Thorac Surg. 2011;92:1646–1649. doi: 10.1016/j.athoracsur.2011.06.090. [DOI] [PubMed] [Google Scholar]
- 55.Patel ND, Arnaoutakis GJ, George TJ, et al. Valve-sparing aortic root replacement in Loeys-Dietz syndrome. Ann Thorac Surg. 2011;92:556–560. doi: 10.1016/j.athoracsur.2011.04.003. [DOI] [PubMed] [Google Scholar]