Abstract
Cyclosporin is an immunosuppressant that has recently been proposed as a treatment to prevent reperfusion injury in acute myocardial infarction (MI). We aimed to determine the overall efficacy of cyclosporin in experimental studies of acute reperfused MI. We conducted a systematic review and stratified meta-analysis of published studies describing the efficacy of cyclosporin in experimental models of acute reperfused MI. We included all in vivo publications of cyclosporin where infarct size was measured. A literature search identified 29 potential studies of which 20 fulfilled the eligibility criteria. In these studies (involving four species of animals), cyclosporin reduced myocardial infarct size by a standardized mean (95% confidence interval) difference of −1.60 (−2.17, −1.03) compared with controls. Cyclosporin failed to demonstrate a convincing benefit in studies involving pigs. Despite this observation, the overall efficacy of cyclosporin did not differ across species (P= 0.358). The dose of cyclosporin given did not affect final infarct size (P= 0.203). Funnel plots of these data suggested heterogeneity among the studies. Cyclosporin had variable effects on infarct size compared with placebo. Cyclosporin had no effect on myocardial infarct size in swine, raising a question over the potential cardioprotective effects of cyclosporin in man.
Keywords: review article, cardiovascular pharmacology, methods and techniques
Introduction
Cyclosporin is a cyclic decapeptide metabolite of soil fungus and a potent immunosuppressant drug (Martindale, 1993). Cyclosporin inhibits immunocompetent T-cell activation by binding the cytosolic protein cyclophilin leading to inhibition of calcineurin, which is required for activation of IL-2 transcription. In fact, cyclosporin has pleiotropic effects not only on immune cell activation, but also on the mitochondria where it inhibits mitochondrial permeability transition pore opening leading to inhibition of cytochrome c release and reduced apoptosis (Duchen et al., 1993).
Beyond its established use as an anti-rejection drug after allogeneic organ transplantation, cyclosporin has recently been proposed as a treatment to prevent reperfusion injury in the heart (Piot et al., 2008). During acute myocardial infarction (MI), anaerobic glycolysis increases the abundance of cytosolic Ca2+. At the onset of reperfusion, the release of oxygen-derived free radicals coupled with cytosolic Ca2+ triggers the opening of the mitochondrial permeability transition pores (mPTP). The opening of these channels results in a sudden change in osmotic forces leading to rupture of the outer mitochondrial membrane and the release of molecules that promote apoptosis into the cytosol leading in turn to cell death (Duchen et al., 1993; Di Lisa et al., 2003; Gomez et al., 2009). Cyclosporin is postulated to prevent reperfusion injury in the heart and other tissues through inhibition of mPTPs and so enhancing cell survival.
Based on findings from studies in experimental acute MI, clinical trials are currently underway to determine the cardioprotective effects of cyclosporin in acute MI patients (Hausenloy and Yellon, 2008). Consequently, the results of the experimental studies with cyclosporin in experimental MI become very important, both in terms of the overall effect of cyclosporin in these studies and also their strengths and potential weaknesses, and their applicability to patients with acute MI is critically important.
However, some but not all (Dow and Kloner, 2007; Leshnower et al., 2008; Pagel and Krolikowski, 2009; Boengler and Hilfiker-Kleiner, 2010; Karlsson et al., 2010; Lie et al., 2010; Matsubara et al., 2010; Skychally et al., 2010) studies have described a cardioprotective effect for cyclosporin, giving rise to uncertainty with regard to the overall effect of this drug in acute MI. Furthermore, the quality of these studies has not been described. Therefore, our purpose was to systematically detect and review publications in which cyclosporin has been studied as a cardioprotective agent in experimental models of acute reperfused MI. The rationale for our study is in line with the recent guidelines for reporting in vivo experiments published by the British Journal of Pharmacology (Kilkenny et al., 2010; McGrath et al., 2010).
Our first aim was to determine the overall efficacy (if any) of cyclosporin to reduce infarct size compared with placebo. Our second aim was to critically appraise the quality of these studies in order to determine any relationships between study quality and the efficacy of cyclosporin.
Methods
Literature search
We searched for published abstracts and full papers in which cyclosporin or placebo was used in vivo to limit reperfusion injury in an in vivo animal model of acute MI. The inclusion criterion for outcome was infarct size measured in vivo[e.g. by a biochemical method (such as serial troponin) or by imaging (MRI or single photon emission computed tomography)] or ex vivo with histological methods. Data from in vitro studies with cyclosporin and studies with other mPTP inhibitors other than cyclosporin were not included. All of these criteria were pre-determined before the search was carried out.
Two electronic searches on the Web of Knowledge comprising Medline, Web of Science with conference proceeding, BIOSIS and CABI were carried out. The first search was performed between 14 November 2009 and 10 December 2009. An updated search using the same terms were performed on 4 January 2011. The search terms used were: (myocardial reperfusion OR MI) AND (cyclosporin OR mitochondrial permeability transition pore) AND (animals or animal) NOT (cerebral OR stroke OR hepatic*). No language constraints were applied in the search, but full papers required an English language version. All ‘Reviews’, ‘Editorials’, ‘Books’, ‘Case reports’ and ‘Letters’ were excluded.
In order to determine the quality of these studies, we used the ARRIVE guidelines (Table 1; Kilkenny et al., 2010; McGrath et al., 2010) and a validated 10-item quality score (Table 2; Macleod et al., 2005, 2008).
Table 1.
Study quality base on ARRIVE guidelines
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karlsson et al. (2010) | + | + | + | + | + | + | − | − | − | − | + | + | − | + | + | + | − | + | + | − | 13 |
| Lie et al. (2010) | + | + | + | + | + | + | − | + | − | + | + | + | − | − | + | + | − | + | + | − | 14 |
| Boengler and Hilfiker−Kleiner (2010) | − | − | + | − | + | − | − | − | − | − | − | − | − | − | + | + | − | + | + | − | 6 |
| Skychally et al. (2010) | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − | 4 |
| Matsubara et al. (2010) | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | + | + | 8 |
| Dow et al. (2009) | + | − | + | + | − | − | − | − | − | + | − | + | − | − | − | + | − | − | + | − | 7 |
| Pagel and Krolikowski (2009) | + | + | + | + | + | − | − | − | − | − | − | + | − | − | + | − | − | + | + | − | 9 |
| Gomez et al. (2008) | + | + | + | + | + | + | − | + | − | − | + | + | − | + | + | + | − | − | + | − | 13 |
| Fang et al. (2008) | + | + | + | + | − | + | − | − | − | − | + | + | − | − | − | − | − | + | + | − | 9 |
| Leshnower et al. (2008) | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | + | − | − | + | + | 9 |
| Huhn et al. (2008) | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | 8 |
| Lim et al. (2007) | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | + | − | − | + | − | 8 |
| Xie and Yu (2007) | + | + | + | + | + | − | − | − | − | − | + | + | − | − | − | − | − | − | + | + | 9 |
| Ikeda et al. (2006) | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | + | − | + | + | + | 10 |
| Wang et al. (2006) | + | + | + | + | + | + | − | − | − | − | + | + | − | + | − | − | − | + | + | − | 11 |
| Argaud et al. (2005a) | + | + | + | + | + | + | − | − | − | − | + | + | − | − | − | − | − | − | + | − | 9 |
| Krolikowski et al. (2005) | + | + | + | + | + | + | − | − | − | − | + | + | − | + | − | − | − | + | + | − | 11 |
| Argaud et al. (2004) | + | + | + | + | + | + | − | − | − | − | + | + | − | − | − | + | − | − | + | − | 10 |
| Niemann et al. (2002) | + | + | + | + | + | + | + | + | − | − | + | + | − | − | − | − | − | − | + | − | 11 |
| Squadrito et al. (1999) | + | + | + | + | + | + | − | − | − | − | + | + | − | − | − | − | − | − | + | − | 9 |
Study quality items are: (1) Title; (2) Abstract; (3) Background; (4) Objectives; (5) Ethical statement; (6) Study design; (7) Experimental procedures; (8) Experimental animals; (9) Housing and husbandry; (10) Sample size; (11) Allocating animals to experimental group; (12) Experimental outcomes; (13) Statistical methods; (14) Baseline data; (15) Numbers analysed; (16) Outcomes and estimation; (17) Adverse events; (18) Interpretation/scientific implications; (19) Generalizability/translation; and (20) Funding.
Table 2.
Study quality report
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karlsson et al. (2010) | + | + | + | − | + | − | + | − | + | + | 7 |
| Lie et al. (2010) | + | + | + | − | + | + | + | + | − | + | 8 |
| Boengler and Hilfiker-Kleiner (2010) | + | − | − | − | + | − | + | − | − | − | 3 |
| Skychally et al. (2010) | + | + | − | − | − | − | + | − | − | − | 3 |
| Matsubara et al. (2010) | + | − | − | − | + | + | + | − | − | − | 4 |
| Dow and Kloner (2007) | + | + | − | − | − | − | − | + | − | − | 3 |
| Pagel and Krolikowski (2009) | + | + | − | − | + | + | − | − | − | − | 4 |
| Gomez et al. (2008) | + | + | − | − | + | + | + | − | − | + | 6 |
| Fang et al. (2008) | + | + | − | − | + | − | + | − | − | − | 4 |
| Leshnower et al. (2008) | + | − | − | − | + | + | + | − | − | − | 4 |
| Huhn et al. (2008) | + | − | − | − | + | − | + | − | − | − | 3 |
| Lim et al. (2007) | + | − | − | − | + | + | + | − | − | − | 4 |
| Xie and Yu (2007) | + | + | − | − | − | + | + | − | − | − | 4 |
| Ikeda et al. (2006) | + | + | − | − | + | − | + | − | − | − | 4 |
| Wang et al. (2006) | + | + | − | − | + | + | − | − | − | − | 4 |
| Argaud et al. (2005a) | + | + | − | − | + | − | + | − | − | − | 4 |
| Krolikowski et al. (2005) | + | + | − | − | + | + | − | − | − | − | 4 |
| Argaud et al. (2004) | + | + | − | − | + | − | + | − | − | − | 4 |
| Niemann et al. (2002) | + | + | − | − | + | − | − | − | − | − | 3 |
| Squadrito et al. (1999) | + | + | − | − | − | − | − | − | − | − | 2 |
Study quality items are: (1) Publication in a peer-reviewed journal; (2) Randomization to either treatment with cyclosporin or placebo control; (3) Blinded assessment of outcome; (4) Use of animal models with co-morbidity; (5) Statement of compliance with regulatory requirement; (6) Method of confirmation of ischemia; (7) Statement of control of temperature; (8) Sample size calculation; (9) Blinded induction of ischaemia; and (10) Statement of conflict of interest.
We aimed to quantify the effect of cyclosporin on infarct size compared with placebo and also explored the relationships between measures of study quality and the overall effect of cyclosporin on infarct size.
Statistics
As the outcomes were reported on different scales, unbiased standardized mean differences [SMD, no units (Table 3)] had to be used to compare the results of the different studies in a meta-analysis.
Table 3.
Standardized mean differences between intervention and control group
| Intervention group | Control group | Standardized mean difference (95% CI) | Unbiased standardized mean difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Study | N | Mean | SD | N | Mean | SD | ||
| Pagel and Krolikowski (2009) | 6 | 42 | 5.00 | 6 | 46 | 5.00 | −0.80 (−1.98, 0.38) | −0.74 (−1.82, 0.35) |
| Gomez et al. (2008) | 8 | 35 | 14.14 | 9 | 58 | 15.00 | −1.57 (−2.66, −0.49) | −1.49 (−2.53, −0.46) |
| Gomez et al. (2008) | 7 | 36 | 18.52 | 9 | 66 | 21.00 | −1.50 (−2.62, −0.39) | −1.42 (−2.48, −0.36) |
| Fang et al. (2008) | 12 | 24.9 | 3.60 | 12 | 47.5 | 4.20 | −5.78 (−7.60, −3.96) | −5.58 (−7.34, −3.82) |
| Leshnower et al. (2008) | 12 | 39 | 10.39 | 15 | 60 | 7.75 | −2.33 (−3.31, −1.35) | −2.26 (−3.21, −1.31) |
| Huhn et al. (2008) | 9 | 31.8 | 7.70 | 9 | 51.4 | 5.00 | −3.02 (−4.37, −1.67) | −2.88 (−4.16, −1.59) |
| Lim et al. (2007) | 7 | 32 | 7.94 | 9 | 48 | 12.00 | −1.53 (−2.65, −0.41) | −1.45 (−2.51, −0.39) |
| Xie and Yu (2007) | 6 | 30.3 | 2.70 | 6 | 48.8 | 5.50 | −4.27 (−6.32, −2.22) | −3.94 (−5.83, −2.05) |
| Ikeda et al. (2006) | 5 | 27.4 | 9.84 | 8 | 45 | 9.90 | −1.78 (−3.09, −0.47) | −1.66 (−2.88, −0.44) |
| Wang et al. (2006) | 8 | 24 | 2.00 | 8 | 44 | 4.00 | −6.32 (−8.73, −3.92) | −5.98 (−8.25, −3.71) |
| Argaud et al. (2005a) | 10 | 24 | 12.65 | 10 | 60 | 18.97 | −2.23 (−3.35, −1.12) | −2.14 (−3.21, −1.07) |
| Argaud et al. (2005a) | 10 | 24 | 12.65 | 10 | 60 | 18.97 | −2.23 (−3.35, −1.12) | −2.14 (−3.21, −1.07) |
| Krolikowski et al. (2005) | 7 | 43 | 6.00 | 8 | 42 | 7.00 | 0.15 (−0.86, 1.17) | 0.14 (−0.81, 1.10) |
| Krolikowski et al. (2005) | 7 | 24 | 3.00 | 8 | 42 | 7.00 | −3.26 (−4.80, −1.71) | −3.07 (−4.52, −1.61) |
| Argaud et al. (2004) | 12 | 24 | 13.86 | 12 | 55 | 27.71 | −1.41 (−2.31, −0.52) | −1.37 (−2.23, −0.50) |
| Argaud et al. (2004) | 9 | 26 | 18.00 | 12 | 55 | 27.71 | −1.20 (−2.14, −0.27) | −1.15 (−2.06, −0.25) |
| Argaud et al. (2004) | 7 | 24 | 15.87 | 12 | 55 | 27.71 | −1.28 (−2.30, −0.26) | −1.22 (−2.20, −0.25) |
| Argaud et al. (2004) | 7 | 25 | 13.23 | 12 | 55 | 27.71 | −1.27 (−2.28, −0.25) | −1.21 (−2.18, −0.24) |
| Niemann et al. (2002) | 4 | 35.08 | 25.84 | 4 | 58.15 | 11.08 | −1.16 (−2.66, 0.34) | −1.01 (−2.31, 0.29) |
| Niemann et al. (2002) | 4 | 23.54 | 27.24 | 4 | 58.15 | 11.08 | −1.66 (−3.27, −0.06) | −1.45 (−2.85, −0.05) |
| Niemann et al. (2002) | 4 | 13.62 | 11.08 | 4 | 58.15 | 11.08 | −4.02 (−6.43, −1.61) | −3.49 (−5.59, −1.40) |
| Niemann et al. (2002) | 4 | 16.62 | 14.30 | 4 | 58.15 | 11.08 | −3.25 (−5.36, −1.14) | −2.82 (−4.66, −0.99) |
| Squadrito et al. (1999) | 6 | 12 | 4.00 | 6 | 57 | 7.00 | −7.89 (−11.25, −4.54) | −7.29 (−10.38, −4.19) |
| Karlsson et al. (2010) | 12 | 49.2 | 13.89 | 15 | 41.14 | 15.94 | 0.53 (−0.24, 1.31) | 0.52 (−0.23, 1.27) |
| Lie et al. (2010) | 19 | 47.3 | 15.70 | 19 | 51.4 | 16.50 | −0.25 (−0.89, 0.38) | −0.25 (−0.87, 0.38) |
| Boengler and Hilfiker-Kleiner (2010) | 10 | 25.4 | 6.96 | 7 | 25.5 | 4.76 | −0.02 (−0.98, 0.95) | −0.02 (−0.93, 0.90) |
| Skychally et al. (2010) | 4 | 24.52 | 6.82 | 4 | 35.15 | 7.34 | −1.50 (−3.07, 0.07) | −1.30 (−2.67, 0.06) |
| Dow and Kloner, 2007 | 4 | 42 | 6.00 | 10 | 27 | 12.65 | 1.32 (0.06, 2.58) | 1.24 (0.06, 2.41) |
| Dow and Kloner, 2007 | 8 | 38 | 11.31 | 10 | 27 | 12.65 | 0.91 (−0.07, 1.89) | 0.87 (−0.06, 1.80) |
| Matsubara et al. (2010) | 6 | 39.1 | 4.16 | 7 | 53.4 | 23.81 | −0.80 (−1.94, 0.33) | −0.75 (−1.80, 0.31) |
| Matsubara et al. (2010) | 4 | 39.6 | 3.60 | 7 | 53.4 | 23.81 | −0.71 (−1.97, 0.56) | −0.65 (−1.80, 0.51) |
CI = confidence interval.
The overall effect and the effect of different moderator variables (species, dose, reperfusion time and quality score) were analysed in random effects meta-regression models.
For one experiment, the observed SDs (σ) in Groups 1 and 2 (intervention and control), respectively, are
and
The unit for this is the original unit, which was reduction of infarct size as percentage of area at risk. The pooled SD of both groups (intervention and control) is
The unit for this is still the original unit and the SMD is
therefore, the numerator and the denominator are both in the original unit, which means that the SMD has no unit.
All analyses have been carried out in R version 2.11.0. The MAd package version 0.8 (http://rwiki.sciviews.org/doku.php?id=packages:cran:ma_meta-analysis) was used for the meta-regression. P-values are not adjusted for multiple testing and have to be considered as descriptive.
Results
Our search identified 588 ‘hits’ (Medline 241, BIOSIS 301, Web of Science 43, CABI 3). After screening the electronic abstracts, 29 were considered potentially relevant and full publications retrieved. Of these publications, 9 (31.0%) were excluded for the following reasons, two (6.9%) reports were meeting abstracts that were subsequently published in full (Niemann et al., 2002; Leshnower et al., 2005), two (6.9%) studies described in vitro experiments (Massoudy et al., 1997; Jiao et al., 2001), two (6.9%) studies used different mPTP inhibitors and not cyclosporin (Argaud et al., 2005b; Gomez et al., 2007), one (3.4%) paper was written in Spanish (Edmundo et al., 2007), one (3.4%) study used cyclosporin in combination with another agent (Pagel et al., 2006) and one (3.4%) study reported the effect of cyclosporin on mortality but not infarct size (Laudi et al., 2006). Therefore, 20 papers (69.0%) involving in vivo models of experimental MI in four species (mice, rats, rabbits and pigs), were included (Table 4).
Table 4.
Study characteristics
| Reference | Species | n | Dose (mg/kg) | Timing of cyclosporin administration | Reperfusion time | Route | Assessment of infarct size | Infarct size |
|---|---|---|---|---|---|---|---|---|
| Karlsson et al. (2010) | Pigs | 27 | 10 | 3 min R | 120 min | IV | Pathology | %AAR |
| Lie et al. (2010) | Pigs | 38 | 10 | 5 min R | 180 min | IV | Pathology | %AAR |
| Troponin | ||||||||
| Boengler and Hilfiker-Kleiner (2010) | Mouse | 17 | 10 | 5 min R | 120 min | IV | Pathology | %AAR |
| Skychally et al. (2010) | Pigs | 8 | 5 | 5 min R | 120 min | IV | Pathology | %AAR |
| Matsubara et al. (2010) | Rabbits | 17 | 25 | 1 h I | 180 min | IV | Pathology | |
| 0 min R | ||||||||
| Dow et al. (2009) | Rats | 22 | 5 | 3 min R | 120 min | IV | Pathology | %AAR |
| 10 | ||||||||
| Pagel and Krolikowski (2009) | Rabbit | 12 | 5 | 5 min R | 180 min | IV | Pathology | %AAR |
| Gomez et al. (2008) | Mouse | 33 | 10 | 5 min R | 24 h | IV | Pathology | %AAR |
| Planimetry | ||||||||
| Fang et al. (2008) | Rats | 24 | 10 | 5 min R | 120 min | IV | Pathology | %AAR |
| Planimetry | ||||||||
| Leshnower et al. (2008) | Rabbit | 27 | 25 | 0 I | 3 h | IV | Pathology | %AAR |
| Planimetry | ||||||||
| Huhn et al. (2008) | Rats | 9 | 5 | 5 min R | 120 min | IV | Pathology | %AAR |
| Planimetry | ||||||||
| Lim et al. (2007) | Mouse | 7 | 10 | 0 min R | 120 min | IV | Pathology | %AAR |
| Planimetry | ||||||||
| Xie and Yu (2007) | Rats | 12 | 10 | 10 min I | 180 min | IV | Pathology | %LVA |
| EM | ||||||||
| Ikeda et al. (2006) | Rats | 13 | 5 | 15 I | 2 h | IV | Pathology | %AAR |
| Wang et al. (2006) | Rabbit | 16 | 10 | 5 min R | 180 min | IV | Pathology | %AAR |
| Argaud et al. (2005a) | Rabbit | 30 | 10 | 10 min I | 4 h | IV | Pathology | %LVW |
| 1 min R | Planimetry | |||||||
| Krolikowski et al. (2005) | Rabbit | 22 | 5 | 5 min R | 3 h | IV | Pathology | %AAR |
| 10 | ||||||||
| Argaud et al. (2004) | Rabbit | 47 | 10 | 15 min I | 4 h | IV | Pathology | %LVW |
| Planimetry | ||||||||
| Niemann et al. (2002) | Rat | 24 | 5 | 3 days I | 24 h | PO | Pathology | %AAR |
| Planimetry | ||||||||
| Squadrito et al. (1999) | Rats | 12 | 0.25 | 5 min A | 48 h | IV | Pathology | %AAR |
| 0.5 | ||||||||
| 1 | %LVA |
Cyclosporin was given either before ischemia (I), during ischemia and before reperfusion (R) or after ischaemia (A).
AAR = myocardial area at risk; EM = electron microscopy; IV = intravenous; LVA = total left ventricular area; LVW = left ventricular weight; PO = per oral.
Design and quality of papers
All of the papers were published in a peer-reviewed journal (Table 4). Of the 20 papers, 16 (80%) reported a statement of compliance with regulatory requirement, 15 (75%) reported random allocation, 14 (70%) reported control of body temperature and 9 (45%) described a method of confirmation. Three (15%) studies had a statement on the authors' conflict of interest, two (10%) studies reported a blinded assessment of outcome and/or described a sample size calculation and only one study (5%) reported blinded induction of ischaemia. None of the studies reported the use of animals with co-morbidities. The median quality score calculated as the sum of quality items in each study was four (range, 2–8) out of a possible 10.
Cyclosporin and infarct size
Thirty-one groups of experiments involving a total of 417 animals were reported (Table 2). Overall, cyclosporin reduced infarct size volume by a standardized mean (95 % confidence interval) difference of −1.60 (−2.17, −1.03) units (Figure 1).
Figure 1.
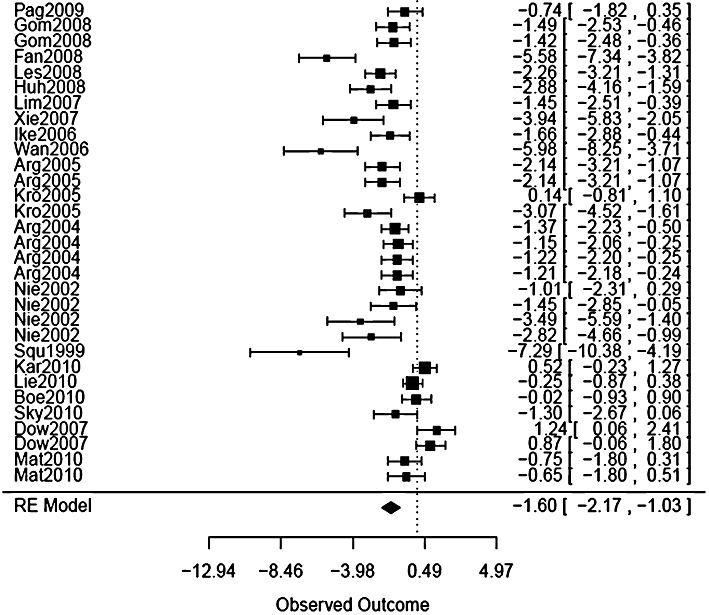
Forest plot of the size of effect of cyclosporin on infarct size. A forest plot illustrates the relative strength of treatment effects in individual scientific studies that address the same question and so graphically represents a ‘meta-analysis’ of a group of these studies.
A funnel plot of infarct size (Figure 2) revealed heterogeneity in the effect of cyclosporin on infarct size across the studies. In nine studies involving 11 (36%) sets of experiments, cyclosporin had no statistically significant effect on infarct size (Niemann et al., 2002; Dow and Kloner, 2007; Leshnower et al., 2008; Pagel and Krolikowski, 2009; Boengler and Hilfiker-Kleiner, 2010; Karlsson et al., 2010; Lie et al., 2010; Matsubara et al., 2010; Skychally et al., 2010).
Figure 2.
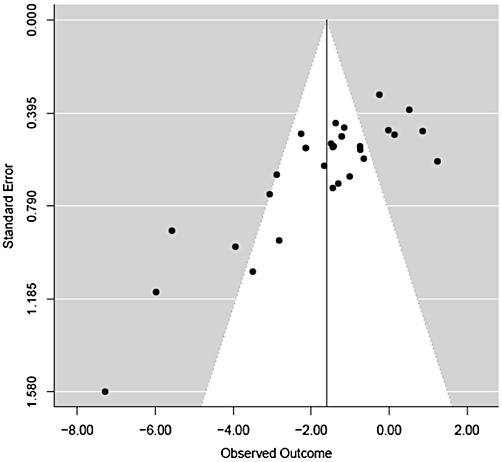
Funnel plot of all experiments. A funnel plot is a scatter plot of treatment effect against a measure of study size. It is used primarily as a visual aid to assess for bias or heterogeneity. An inverted funnel shape arises from a data set in which bias or heterogeneity is unlikely. An asymmetric funnel indicates a relationship between treatment effect and sample size indicating the possibility of bias or a systematic difference between smaller and larger studies (‘small study effects’). Asymmetry can also arise from use of an inappropriate effect measure (Egger et al., 1997). The funnel plot in this figure indicates there is heterogeneity in the effect of cyclosporin on infarct size across the studies.
The moderators taken into account in the regression analyses were species, dose, reperfusion time and quality score. Quality items 1 and 4 were omitted as there was no variation among the studies for these criteria (item 1 was fulfilled in all studies, item 4 in none). The results of the regression analyses are shown in Table 5.
Table 5.
Regression results for mixed models
| Variable | Effect estimate | 95% confidence interval | P-value | P-value |
|---|---|---|---|---|
| Intercept | −1.602 | (−2.173, −1.030) | <0.001 | – |
| Intercept | −0.977 | (−2.802, 0.847) | =0.294 | =0.358 |
| Mouse special | −0.443 | (−4.106, 3.221) | =0.813 | |
| Pig | 0.673 | (−1.900, 3.245) | =0.608 | |
| Rabbit | −0.647 | (−2.677, 1.383) | =0.532 | |
| Rat | −1.252 | (−3.347, 0.842) | =0.241 | |
| Intercept | −1.596 | (−2.786, −0.406) | =0.009 | – |
| Dose | −0.001 | (−0.098, 0.095) | =0.982 | |
| Intercept | −1.145 | (−1.843, −0.448) | =0.001 | – |
| Reperfusion hours | −0.059 | (−0.115, −0.003) | =0.040 | |
| Intercept | −2.943 | (−4.892, −0.993) | =0.003 | – |
| Quality score | 0.330 | (−0.128, 0.787) | =0.158 | |
| Intercept | −1.318 | (−2.613, −0.023) | =0.046 | – |
| Quality item 2 | −0.364 | (−1.815, 1.086) | =0.622 | |
| Intercept | −1.727 | (−2.299, −1.155) | <0.001 | – |
| Quality item 3 | 1.858 | (−0.248, 3.964) | =0.084 | |
| Intercept | −1.453 | (−2.975, 0.069) | =0.061 | – |
| Quality item 5 | −0.180 | (−1.827, 1.467) | =0.830 | |
| Intercept | −1.575 | (−2.324, −0.826) | <0.001 | – |
| Quality item 6 | −0.085 | (−1.281, 1.111) | =0.889 | |
| Intercept | −1.740 | (−2.763, −0.717) | =0.001 | – |
| Quality item 7 | 0.195 | (−1.052, 1.443) | =0.759 | |
| Intercept | −1.816 | (−2.345, −1.286) | <0.001 | – |
| Quality item 8 | 2.398 | (0.783, 4.014) | =0.004 | |
| Intercept | −1.675 | (−2.246, −1.105) | <0.001 | – |
| Quality item 9 | 2.194 | (−0.799, 5.187) | =0.151 | |
| Intercept | −1.758 | (−2.368, −1.147) | <0.001 | – |
| Quality item 10 | 1.121 | (−0.500, 2.743) | =0.175 | |
| Intercept | −2.129 | (−3.708, −0.551) | =0.008 | – |
| Total N | 0.032 | (−0.058, 0.121) | =0.485 |
The models are based on the assumption that the correct error type was provided.
Cyclosporin had no effect on infarct size in pigs (sus scrofa domesticus) and species type was not associated with cyclosporin efficacy (Table 5). The dose of cyclosporin administered (P= 0.98) had no significant association with infarct size. The duration of reperfusion before infarct size assessment (or euthanasia) was inversely related to infarct size (P= 0.04).
Relationship between quality score and cyclosporin effect on infarct size
We studied the individual components that made up the Quality Score to determine their relationship with final infarct size. Compared with the magnitude of cyclosporin treatment effect [−1.82 (SMD)] in papers that did not describe a sample size calculation, cyclosporin had a smaller reduction in infarct size [0.57 (SMD)] in papers in which there was no calculation of sample size reported (P= 0.004). Overall Quality Score was not related to cyclosporin efficacy or infarct size (P= 0.16).
Discussion and conclusions
The main findings of our study are, firstly, the meta-analysis confirmed that cyclosporin reduces infarct size when used in experimental models of acute reperfused MI. Secondly, the efficacy of cyclosporine was unrelated to the species studied and thirdly, the presence of one of the measures of study quality, a sample size calculation, was associated with a smaller effect of cyclosporin on infarct size.
Clinical studies have shown that limiting infarct size translates to improve clinical outcome in the longer term (Burns et al., 2002; Gibbons et al., 2004). In our meta-analysis of pre-clinical studies, we demonstrated that although overall cyclosporin reduced infarct size in animal models of reperfused MI, cyclosporin had no effect on infarct size in just over one-third of the experiments (11 out of 31 experiments). Cyclosporin had mixed effects on infarct size in a swine model of acute reperfused MI, with a reduction in infarct size being observed in one study (Skychally et al., 2010) and no effect in three other investigations (Karlsson et al., 2010, 2011; Lie et al., 2010). Because the hearts of swine and man are similar (e.g. in terms of coronary anatomy, few collaterals, myocardial mass), the lack of a cyclosporin treatment effect in swine raises concern as to whether or not cyclosporin may be cardioprotective in man. Experimental conditions (e.g. duration of ischaemia, sample size, dose of cyclosporin) may be relevant for whether or not cyclosporin might attenuate reperfusion injury in this model. In fact, the plasma concentrations of cyclosporin achieved for similar bolus doses differ between swine and man (Karlsson et al., 2011) and there is a narrow therapeutic window to achieve the target concentration of 0.2 µmol·L−1 (Griffiths and Halestrap, 1993), limiting the chances of a true beneficial effect of the drug in patients with acute MI in clinical practice. There is also the possibility that cyclosporin might not just have no beneficial effect on infarct size, but instead, as described by Dow and Kloner (2007), cyclosporin therapy might be associated with an increase in infarct size. This possibility is very important because the purpose of therapeutic evaluations in pre-clinical animal models is to provide information on both safety and efficacy.
We showed that infarct size was inversely related to the reperfusion time where reperfusion over a longer period was associated with a smaller infarct size. This observation is likely independent of cyclosporine and probably reflects the cardioprotective effect of repair responses, such as collateral artery recruitment (Berry et al., 2007) and endogenous reperfusion injury salvage kinases (Hausenloy and Yellon, 2007).
Using a previously published quality score (Macleod et al., 2008), we found that the majority [17 (55%)] of the studies had a quality score of 4 out of 10. Only four studies (12.9%) had a score above 5 and around one-third of the studies [10 (32%)] had a score below 4. Most of the studies [26 (84%)] had a quality score of 3 and 4. This observation, coupled with the modest number of animals studied overall, resulted in insufficient power to discount the possibility that a higher quality score might have resulted in a smaller infarct size. One explanation for the similarities in quality score is that similar methods and reporting styles were adopted by different investigators. The only study quality index that showed a significant result was ‘calculation of sample size’ (P= 0.004). Sample size calculation is standard practice in clinical trials and it helps to ensure that the study is sufficiently powered to investigate the question asked (i.e. avoid a false negative result). One potentially contentious reason for why studies without an initial sample size calculation might be associated with a small effect size is that a study may be allowed to continue until such times as an effect, albeit small, might be observed. Some of the other indices might have been available or could have been adopted to enhance the strength of the study. We also hope the inclusion of both ‘negative’ and ‘positive’ papers in our analysis should provide some reassurance against reporting bias. However, we cannot exclude the possibility that some ‘negative’ studies may either have been rejected by peer review or may not have ever even submitted.
Study quality is important because the findings of preclinical drug development studies may be used to support the transition of candidate drugs, such as cyclosporin, into clinical studies in man. A study with false positive results may lead to an overestimation of the efficacy of a drug potentially leading to inappropriate testing in humans. We wondered whether some of the error terms that were reported as SDs were actually SEMs. There was a clear separation in SDs for the control groups of all experiments (calculated from the SEMs in studies that report SEMs), with SDs in studies that claim to report SEMs being considerably larger than SDs in the studies that claimed to report SDs. Additionally, there was a wide variation in study quality meaning an overestimation or underestimation of the treatment effect cannot be discounted.
Cyclosporin has a propensity to cause adverse effects including infection and cancer related to immunosuppression. However, these effects are mainly related to chronic therapy. A single intravenous dose of cyclosporin, as administered by Piot et al. (2008), is less likely to cause safety concerns. We did not include studies of cyclosporin in isolated perfused hearts because our focus was on in vivo studies in animal models. While isolated perfused heart studies can provide invaluable mechanistic information on pathways involved in ischaemia and reperfusion, our focus was on in vivo models that most closely mimic human MI.
Limitations
We cannot discount the possibility of a negative publication bias against studies that had negative results. However, not all of the studies that we have identified had positive results and importantly, the large animal studies in swine (Karlsson et al., 2010; Lie et al., 2010) had negative results that were considered in our overall analysis.
Conclusions
In conclusion, in our meta-analysis of 20 in vivo experimental studies in animal models (involving four species) reperfused MI, we found that, overall, cyclosporin reduced infarct size but there was considerable heterogeneity of effect across studies. However, the negative studies in porcine hearts raise a concern about the potential cardioprotective effects of cyclosporin in man. We did not show an association between study quality and infarct size, which may have been due to the similar reporting styles adopted in these investigations. Given the critical importance of in vivo experimental studies for therapeutic drug development in man, we support the recent ARRIVE guidelines and Gold Standard Publication Checklist (Hooijmans et al., 2011) for experimental research recently published in the British Journal of Pharmacology.
Acknowledgments
This study was funded by the University of Glasgow. Professor Berry is supported by a Senior Fellowship from the Scottish Funding Council and receives grant funding from the British Heart Foundation, Chief Scientist Office, Medical Research Council and Medical Research Scotland.
Glossary
- AAR
area at risk
- EM
electron microscopy
- IV
intravenous
- LVA
left ventricular area
- LVW
left ventricular weight
- MI
myocardial infarction
- mPTP
mitochondrial permeability transition pore
- PO
per oral
- SMD
standardized mean differences
- SPECT
single photon emission computed tomography
Conflict of interest
None.
References
- Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, et al. Preconditioning delays Ca2+ induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. doi: 10.1016/j.cardiores.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion. J Mol Cell Cardiol. 2005a;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize R. Post-conditioning inhibits mitochondrial permeability transition. Circulation. 2005b;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- Berry C, Balachandran KP, L'Allier PL, Lespérance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. Eur Heart J. 2007;28:278–291. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- Boengler K, Hilfiker-Kleiner D. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischaemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationship of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following acute myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Canton M, Menabo R, Dodoni G, Bernardi P. Mitochondria and reperfusion injury: the role of permeability transition. Basic Res Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- Dow J, Kloner RA. Post-conditioning does not reduce myocardial infarct size in an in vivo regional ischaemia rodent model. J Cardiovasc Pharmacol Ther. 2007;12:153–163. doi: 10.1177/1074248407300897. [DOI] [PubMed] [Google Scholar]
- Dow J, Bhandari A, Kloner RA. The mechanism by which ischemic postconditioning reduces reperfusion arrhythmias in rats remains elusive. J Cardiovasc Pharmacol Ther. 2009;14:99–103. doi: 10.1177/1074248408329606. [DOI] [PubMed] [Google Scholar]
- Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochorndial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- Edmundo C, Garcia N, Pavon N. Inhibition of permeability transition and myocardial reperfusion damage by cyclosporine A. Arch Cardiol Mex. 2007;77(Suppl. 4):s77–s81. [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–624. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Wu L, Chen L. Post-conditioning attenuates cardiocyte ultrastructure injury and apoptosis by blocking mitochondrial permeability transition in rats. Acta Cardiol. 2008;63:377–387. doi: 10.2143/AC.63.3.1020316. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–1542. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, et al. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by post-conditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- Gomez L, Li B, Mewton N, Sanchez I, Piot C, Elbaz M, et al. Inhibition of mitochondrial permeability transition pore opening: translation to patients. Cardiovasc Res. 2009;83:226–233. doi: 10.1093/cvr/cvp063. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Protection by cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359:518–520. doi: 10.1056/NEJMe0803746. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, de Vries R, Leenaars M, Curfs J, Ritskes-Hoitinga M. Improving planning, design, reporting and scientific quality of animal experiments by using the Gold Standard Publication Checklist, in addition to the ARRIVE guidelines. Br J Pharmacol. 2011;162:1259–1260. doi: 10.1111/j.1476-5381.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn R, Hienen A, Weber C, Hollmann MW, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditionig in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–471. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Miura T, Sakamoto J, Miki T, Tanno M, Kobayashi H, et al. Activation of ERK and suppression of calcineurin are interacting mechanisms of cardioprotection afforded by delta-opioid receptor activation. Basic Res Cardiol. 2006;2006:418–426. doi: 10.1007/s00395-006-0595-2. [DOI] [PubMed] [Google Scholar]
- Jiao Z, Gorodnya OM, Yang XM, Fan THM. Cyclosporine A inhibits cytochrome C release and prevents myocardial cell death induced by ischaemia/reperfusion. J Am Coll Cardiol. 2001;37:346A. (Abstract) [Google Scholar]
- Karlsson LO, Zhou AX, Larsson E, Astrom-Olsson K, Mansson C, Akyurek LM, et al. Cyclosporine does not reduce myocardial infarct size in a porcine ischaemia-reperfusion model. J Cardiovasc Pharmacol Ther. 2010;15:182–189. doi: 10.1177/1074248410362074. [DOI] [PubMed] [Google Scholar]
- Karlsson LO, Bergh N, Grip L. Cyclosporine A, 2.5 mg/kg, does not reduce myocardial infarct size in a porcine model of ischemia and reperfusion. J Cardiovasc Pharmacol Ther. 2011 doi: 10.1177/1074248411407636. doi: 10.1177/1074248411407636. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Brown W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolikowski JG, Bienengraeber M, Weihrauch DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005;101:1590–1596. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- Laudi S, Schmitz V, Trump S, Christians U, Steudel W. Preconditioning with cyclosporine alters survival in murine myocardial ischemia-reperfusion-injury. J Heart Lung Transplant. 2006;25:s168, 363. (Abstract 363) [Google Scholar]
- Leshnower BG, Sakamoto H, Kanemoto S, Hamamoto H, Zeeshan A, Hinmon R, et al. Inhibition of mitochondrial permeability transition produces reperfusion induced myocyte apoptosis and improves early post-MI function. Circulation. 2005;112(Suppl. S):U501–U502. 78th Annual Scientific Session of the AHA. [Google Scholar]
- Leshnower BG, Kanemoto S, Matsubara M, Sakamoto H, Hinmon R, Gorman JH. Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion dependent of calcineurin inhibition. Ann Thorac Surg. 2008;86:1286–1292. doi: 10.1016/j.athoracsur.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie RH, Stoettrup N, Sloth E, Hasenkam M, Kroyer R, Nielsen TT. Post-conditioning with cyclosporine A fails to reduce infarct size in an in vivo porcine model. Acta Anaesthesiol Scand. 2010;54:804–813. doi: 10.1111/j.1399-6576.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Ebrahim S, Roberts I. Surveying the literature from animal experiments: systematic review and meta-analysis are important contributions. BMJ. 2005;331:110. doi: 10.1136/bmj.331.7508.110-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Van der Worp B, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Martindale. The Extra Pharmacopoeia. 30th edn. London: Pharmaceutical Press; 1993. p. 467. [Google Scholar]
- Massoudy P, Zahler S, Kupatt C, Reder C, Becker BF, Gerlach E. Cardioprotection by cyclosporine A in experimental ischemia and reperfusion – evidence of nitric oxide dependent mechanism mediated by endothelin. J Mol Cell Cardiol. 1997;29:535–544. doi: 10.1006/jmcc.1996.0297. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Ranji M, Leshnower BG, Noma M, Ratcliffe SJ, Chance B, et al. In vivo fluorometic assessment of cyclosporine on mitochondria function during myocardial ischaemia and reperfusion. Ann Thorac Surg. 2010;89:1532–1537. doi: 10.1016/j.athoracsur.2010.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann CU, Saeed M, Akbari H, Jacobsen W, Benet LZ, Christians U, et al. Close association between the reduction in myocardial energy metabolism and infarct size: dose-response assessment of cyclosporine. J Pharmacol Exp Ther. 2002;302:1123–1128. doi: 10.1124/jpet.102.036848. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: restoration of cardioprotection by cyclosporine A in rabbits. Anesth Analg. 2009;108:1076–1082. doi: 10.1213/ane.0b013e318193e934. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Neff DA, Weihrauch D, Bienengraeber M, Kersten JR, et al. Inhibition of glycogen synthase kinase enhances isoflurane induced protection against myocardial infarction during early reperfusion in vivo. Anesth Analg. 2006;102:1348–1354. doi: 10.1213/01.ane.0000202379.61338.37. [DOI] [PubMed] [Google Scholar]
- Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Eng J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- Skychally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 2010;24:85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Squadrito G, Saitta A, Campo GM, Arlotta GM. Cyclosporine-A reduces leukocyte accumulation and protects against myocardial ischaemia reperfusion injury in rats. Eur J Pharmacol. 1999;364:159–168. doi: 10.1016/s0014-2999(98)00823-1. [DOI] [PubMed] [Google Scholar]
- Wang C, Neff DA, Krolikowski JG, Weihrauch D, Bienengraeber M, Warltier DC, et al. The influence of B-cell lymphoma 2 protein, an anti-apoptic regulator of mitochondrial permeability transition, on isoflurane-induced and ischemic preconditioning in rabbits. Anesth Analg. 2006;102:1355–1360. doi: 10.1213/01.ane.0000202463.28618.64. [DOI] [PubMed] [Google Scholar]
- Xie JR, Yu LN. Cardioprotective effects of cyclosporine A in an in vivo model of myocardial ischemia and reperfusion. Acta Anaesthesiol Scand. 2007;51:909–913. doi: 10.1111/j.1399-6576.2007.01342.x. [DOI] [PubMed] [Google Scholar]


