Abstract
Development of novel cell migration modulators for anti-inflammatory and cardiovascular therapy is a complex task since any modulator will necessarily interfere with a balanced system of physiological regulators directing proper positioning of diverse immune cell types within the body. Whereas this shall serve efficient pathogen elimination, lack of proper control over these processes may result in counterproductive chronic inflammation and progressive tissue injury instead of healing. Prediction of the therapeutic potential or side effects of any migration modulator is not possible based on theoretical considerations alone but needs to be experimentally evaluated in preclinical disease models and by clinical studies. Here, we briefly summarize basic mechanism of cell migration, and groups of synthetic drugs currently in use for migration modulation. We then discuss one fundamental problem encountered with single-target approaches that arises from the complexity of any inflammation, with multiple interacting and often redundant factors being involved. This issue is likely to arise for any class of therapeutic agent (small molecules, peptides, antibodies, regulatory RNAs) addressing a single gene or protein. Against this background of studies on synthetic migration modulators addressing single targets, we then discuss the potential of endogenous proteins as therapeutic migration modulators, or as parent compounds for the development of mimetic drugs. Regulatory proteins of this type commonly address multiple receptors and signalling pathways and act upon the immune response in a phase-specific manner. Based on recent evidence, we suggest investigation of such endogenous migration modulators as novel starting points for anti-inflammatory and cardiovascular drug development.
Keywords: inflammatory diseases, cardiovascular diseases, cell migration, chemotaxis, anti-inflammatory therapy, cardiovascular therapy, drug development
Basic considerations in cell migration modulation
Pharmaceutical companies have targeted a broad spectrum of molecules for the treatment of inflammatory diseases. A number of immune cell migration-modulating drugs were highly efficient in animal models of inflammatory disorders, and some of these were also successful in clinical trials. The search for novel mechanistic principles and approaches in cell migration modulation continues, with increasing attention to the fact that single-target drugs have inherent limitations due to the complexity of inflammatory processes with multiple interacting and often functionally redundant factors being involved (Mackay, 2008).
The development of novel immune cell migration modulators for anti-inflammatory and cardiovascular therapy is a complex task, since they will necessarily interfere with a delicately balanced system of multiple migration regulators that direct proper positioning of diverse immune cell types within the body (Luster et al., 2005). In principle, this shall serve efficient pathogen elimination and resolution either by background immune surveillance or by non-destructive short-term inflammation (Wolf et al., 2003) (Figure 1). On the other hand, lack of control over these primarily beneficial processes may result in ‘counterproductive’ chronic inflammation associated with insidious chronic tissue injury, instead of proper healing within a short time period. It should be emphasized that it is impossible to predict, based on theoretical considerations alone, the therapeutic potential or side effects of any new immune cell migration modulator for different disease settings. Instead, its overall effects need to be investigated experimentally in detail (e.g. application route, dosage regime) for every envisaged target disease separately. The necessity to conduct detailed in vivo studies addressing, for example drug dose issues, is illustrated by the paradoxical stimulation of malignant tumour growth by low concentrations of a RGD-mimetic integrin inhibitor primarily developed as an anti-tumour agent (Reynolds et al., 2009). Nevertheless, the identification of basic in vitro effects of a new agent may serve as a guideline for the design of in vivo experiments in disease models, that is which cell functions should to be measured during treatment and which side effects might be anticipated.
Figure 1.
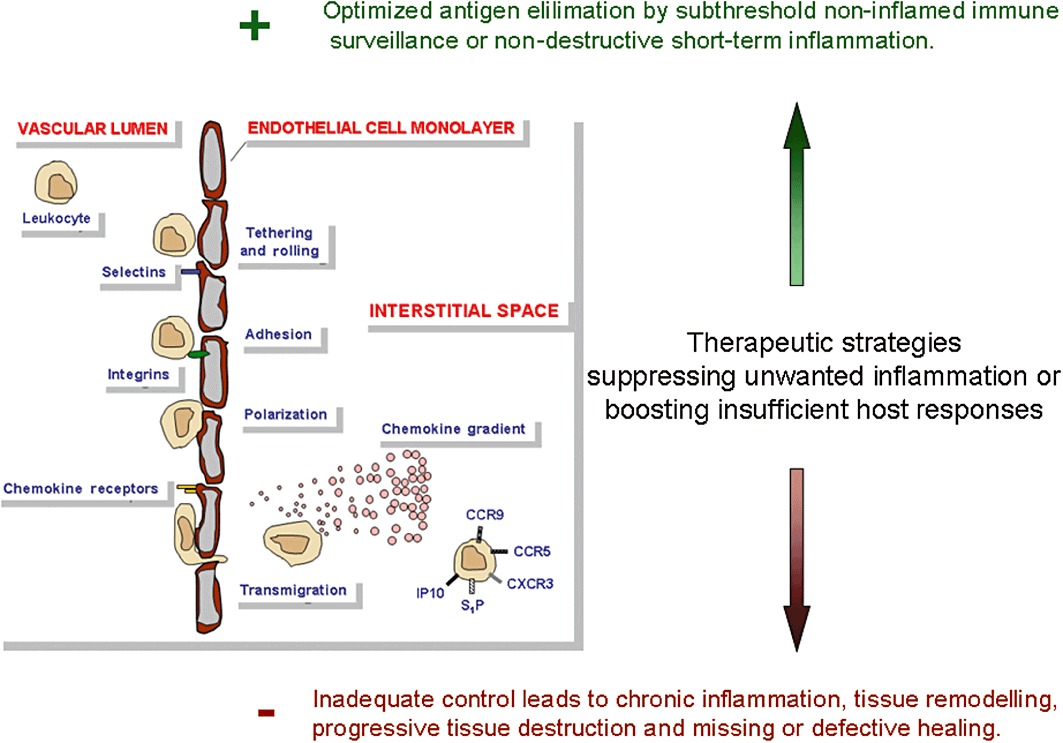
Immune cell migration and its therapeutic modulation: Addressing a delicate balance between efficient pathogen elimination with non-destructive and short-term inflammation versus chronic inflammation associated with progressive and often insidious tissue injury.
Molecular mechanisms of cell migration
Our knowledge about the fundamental molecular mechanism of cell migration (Figure 2) and their regulatory factors has been greatly expanded by numerous groundbreaking studies (Schulz et al., 2009; Mempel et al., 2006; Alvarez et al., 2008; Laird et al., 2008; Mora and von Andrian, 2008) and novel analytical methods (Wolf et al., 2003; Sumen et al., 2004; Halin et al., 2005; Wolf et al., 2009) during the last decade. This includes the molecular mechanisms of amoeboid migration of leucocytes including intracellular polarization processes, influences from the microenvironment of the migrating cells and the balance between immigration and egress (Matloubian et al., 2004) versus local confinement and residency. Live-cell microscopy has refined the conception of immune processes, from abstract models of leucocyte trafficking, into a more immediate understanding of how and in which sequence immune cells interact with each other, and eliminate pathogens or repair tissues. We only briefly summarize basic cell migration mechanisms since excellent reviews on this topic are available (Friedl and Weigelin, 2008; Wong et al., 2010).
Figure 2.
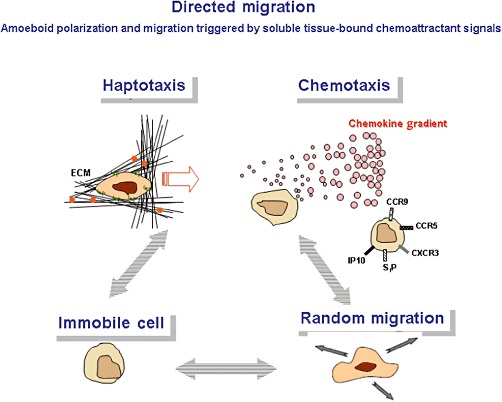
Directional motility: Haptotaxis is the directional movement along a gradient of cellular adhesion sites or substrate-bound chemoattractants as present in the extracellular matrix. Chemotaxis is the cell movement following a gradient of soluble molecules such as chemokines.
Migration modulators may be roughly classified according to a partial process that is strongly affected (Figure 3), but any modulator may have different biological effects in immune cell subtypes, and the overall effect therefore needs to be determined in relevant disease models in vivo. Commonly, the effect of a new migration modulator is characterized for individual leucocyte subclasses only. Because of their important role in mediating inflammatory reactions, interstitial cell migration offers interesting therapeutic targets, both for the attenuation of uncontrolled inflammation or the enhancement of inadequate host immune responses. Although a number of fundamental molecular mechanisms of cell migration are active in any type of cells and tissues, beyond this common basis, there are significant differences in the trafficking behaviour of leucocytes to and from distinct organs. The molecular foundations for these differences are incompletely understood. For obvious reasons, it would be preferable, however, to have tissue-specific cell migration inhibitors (Marsolais and Rosen, 2009) targeting the diseased organ only, instead of modulating migration throughout the body. An example for tissue-specific migration inhibition in late-stage clinical evaluation for Crohn's disease is the CCR9 inhibitor CCX282 (Mackay, 2008).
Figure 3.
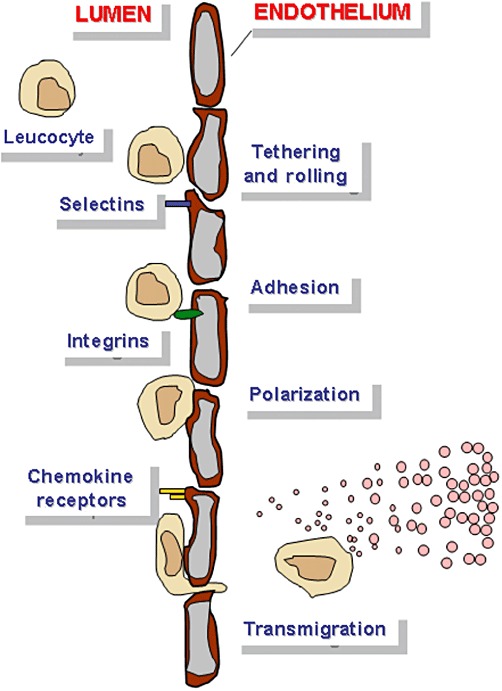
Checkpoints to target migration: Molecular targets of drugs in clinical development include selectins (TBC1269, Revotar by Texas Biotechnology), integrins (Eftaluzimab by MerckSerono, Natalizumab by Elan and Biogen Idec, Cilengitide by Merck) and chemokine receptors (INCB3284 by Incyte, MK0812 by Merck, CCX282 by ChemoCentryx, Maraviroc by Pfizer, TAK-779 by Takeda, Fingolimod by Novartis, MDX-1100 by Medarex).
‘Dynamic’ regulators acting in an immune response phase-related manner
Multiple new inducers of cell migration have been identified, but counterbalancing mechanisms (Friedl and Weigelin, 2008; Steevels and Meyaard, 2011; Steevels et al., 2011) have also been intensely studied, including down-regulation or desensitization of chemokine receptors, ligand competition, termination of chemoattractant activity through capture by neutralizing chemoattractant receptors and proteolytic degradation. Intracellularly, chemoattractant receptors are internalized, recycled to the leading edge or stored in vesicles, thus controlling the availability of both the chemoattractant and its receptor. Activation-induced down-regulation of the S1P1 receptor in T cells is another well-known example of chemoattractant signal tuning and was successfully targeted by drugs in multiple sclerosis (Kappos et al., 2006; Carroll, 2011; Cohen et al., 2011), organ transplantation (Habicht et al., 2006; Brinkmann, 2007; Lan et al., 2008) and allergic diseases (Marsolais et al., 2011).
Whereas many drugs have permanent silencing effects (e.g. conventional immunosuppressive drugs), this is not a common mode of action of endogenous regulators of the immune response, which instead act in a phase-related manner (Figure 4). This type of physiological regulators is commonly integrated into regulatory networks that have evolved to coordinate a sequence of processes towards optimal repair of injuries. Numerous cell migration and differentiation-regulating proteins are active during fetal development, become almost completely silenced in the healthy adult organism, but are typically re-induced in injured tissues. This type of re-induction of developmental proteins (Fechner et al., 2003; Perbal, 2004; Kubota and Takigawa, 2007; Hamilton, 2008; Llera et al., 2010), and also of fetal microRNA (miR) patterns (Thum et al., 2007), is often remarkably monomorphic irrespective of the specific type of injury or organ involved. Many typical examples are found in the large family of matricellular proteins (Vilmos et al., 2001), commonly characterized by re-expression during tissue injury and repair (Schellings et al., 2004; 2009; Leask and Abraham, 2006; Okamoto, 2007; Chen and Lau, 2009; Kyriakides and Maclauchlan, 2009; Norris et al., 2009; Chiodoni et al., 2011; Dobaczewski et al., 2011) and by multiple interactions with immune cells (Kuznetsova and Roberts, 2004; Frangogiannis, 2008; Sangaletti and Colombo, 2008). As opposed to dedicated chemokine–chemokine receptor or other specific ligand–receptor pairs, proteins of this type are commonly multimodular in structure and exert complex, context-dependent functions through multiple interacting proteins and signalling pathways. A following chapter on Endogenous Migration-Inhibiting Molecules as Parent Compounds for Drug Development discusses how a matricellular protein of this type, and a mimetic peptide, may have therapeutic potential in cardiovascular and other diseases associated with pathogenic inflammation. Whereas that chapter focuses on CCN1 as one paradigm for endogenous immune cell migration modulators to be considered as parent compounds for drug development, the full spectrum of proteins that dynamically and physiologically regulate immune responses is unknown. Remarkably, context dependency of endogenous immunomodulating proteins (such as the CCN protein family) has also been observed for adipocytokines (leptin, adiponectin and others). For a full discussion of their properties, we refer to excellent recent reviews (Lago et al., 2007; 2009; Lang and Ratke, 2009) and papers investigating the complex role of adiponectin in dilated (DCM) and inflammatory (DCMi) cardiomyopathy (Wittchen et al., 2007; Skurk et al., 2008; Bobbert et al., 2011). Briefly, leptin and adiponectin are involved in the regulation of migration of both leucocytes and tumour cells.
Figure 4.
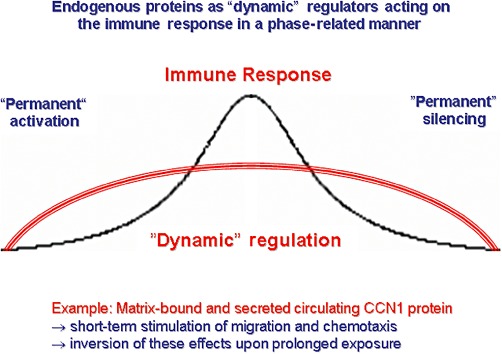
A coordinated immune response encompasses both activating and phase-related counterbalancing mechanisms. The latter are essential for proper responses to injury and include (i) down-regulation of chemokine receptors and desensitization of receptor-dependent signalling, (ii) termination of chemoattractant activity by neutralizing receptors and proteolytic degradation and (iii) chemoattractant receptor internalization and recycling or storage in vesicles.
Problems arising from biological redundancy and limited knowledge
Beyond specific issues relating to individual drugs, one general consideration relates to the fact that single-target approaches may be problematic due to the multitude of interacting and redundant factors involved in inflammatory processes. Any acute or chronic inflammation in the tissue in response to mechanical microbial injury, autoimmune disease or allograft rejection triggers tissue infiltration by effector cells: neutrophils, monocytes, T cells and in chronic states also B cells. This creates a highly complex network of interacting cells and signalling cascades that involve far more players than can be addressed by targeting of a single molecule. Serious problems of limited or lacking efficacy of single target drugs have indeed been encountered in multiple preclinical or clinical trials (Horuk, 2009a,b). It appears that multiple interactions and redundancy are commonplace in the biological systems regulating and balancing proper immune cell migration. It should be emphasized that the resulting limitation of narrowly targeted agents is likely to apply to any class of therapeutic agent (small molecule drugs, peptides, monoclonal antibodies, regulatory RNAs), unless a single unique process of outstanding importance can be identified.
The existence of unidentified subsets of immunological target cells may also confound the conclusions drawn from narrowly defined in vitro studies. One example are the monocytic cells that consist of ‘inflammatory’ and ‘resident’ subsets with differential functions and trafficking properties (Kamei and Carman, 2010). Notably, the spleen has recently been identified as a peculiar reservoir of ‘inflammatory’ monocytes that are readily recruited to injured myocardium and other tissues. In general, the complex architecture of the interstitial space and the full spectrum of phenotypic and functional changes of leucocytes resulting from their interactions with the endothelium during adhesion and transmigration cannot be modelled by any current in vitro system (Wong et al., 2010). Moreover, a single chemoattractant is frequently used in vitro, whereas multiple chemoattractants are regularly involved in vivo, which act upon intracellular signalling cascades in a hierarchical manner.
Focusing on chemokine receptors, Horuk has recently discussed possible reasons for the failures of multiple trials using single-chemokine receptor antagonists. He suggests that they may be attributable to trying to target a complex disease with an antagonist to a single receptor, whereas the more than 40 different chemokines and 19 chemokine receptors so far identified create an immensely complex immunoregulatory network. As an alternative approach, he suggests promiscuous non-peptide antagonists inhibiting multiple targets (Horuk, 2009a,b).
Current drug classes and clinical relevance in cardiac disease
Current single-target pharmacological approaches (Figure 3) include selectin inhibition (Ley et al., 2007; Barreiro et al., 2011; Fernandez-Borja et al., 2011), integrin targeting (Hehlgans et al., 2007) and chemokine or chemokine receptor blockade (Horuk, 2009a,b; Zernecke and Weber, 2011), which aim at cell surface targets involved in leucocyte rolling, adhesion and transendothelial migration. One important example for successful therapeutic integrin targeting is a monoclonal antibody (mAb) to α4β1 and α4β7 integrin for the treatment of multiple sclerosis and inflammatory bowel disease. The results of two phase 3 clinical trials showed that natalizumab markedly reduces the number of relapses in individuals with multiple sclerosis (Polman et al., 2006; Havrdova et al., 2009; Hutchinson et al., 2009).
In preclinical models in the context of cardiac diseases, the usage of monoclonal antibodies against integrins β2 and α4, either alone or in combination, showed a blockade of inflammatory cell migration into the ischaemic myocardium after myocardial infarction (MI) (Legare et al., 2007). Cai et al. investigated the role of T-cell selectin ligands on cardiac recruitment of CD8+ T cells during myocarditis and allograft rejection. Here, selectin ligand-deficient CD8+ T cells showed a reduction in their ability to interact with P- and E-selectins and a blockade of heart-directed migration (Cai et al., 2006). One of the earliest steps of an acute inflammatory response is the selectin-dependent rolling of leucocytes. Hicks et al. (2003) suggested recombinant P-selectin glycoprotein ligand-1-immunoglobulin (rPSGL-Ig) as characteristic ligand to influence leucocyte rolling in living blood vessels for an inhibition of neutrophil migration. Baron et al. further compared the effect of the chimeric antibody c7E3 Fab (abciximab) with the antibody LM609, which is directed specifically against integrin αvβ3, and observed comparable results in the prevention of smooth muscle cell adhesion to the extracellular matrix (ECM) proteins osteopontin and vitronectin, and of cell migration during the development of restenosis. Overall, combined administration of both antibodies represented the most effective treatment (Baron et al., 2000). Furthermore, the fibrin-derived peptide Bβ15-42 (FX06) was shown by Wiedemann et al. (2011) to reduce infarct size in a coronary artery occlusion/reperfusion model by inhibition of leucocyte migration and preservation of endothelial barrier function.
Intracellular migration-related signalling pathways have been directly addressed by inhibition of specific phosphoinositide-3-kinase (PI3K) isoforms (Barber et al., 2005; Camps et al., 2005), and significant progress has recently been made in understanding their differential functions with respect to cell migration. Pharmacological inhibition of PI3K-γ by synthetic small molecules has promoted infarct resorption and prevented adverse cardiac remodelling after MI in mice (Seropian et al., 2010). Loss of PI3K-γ has enhanced cAMP-dependent MMP remodelling of the myocardial N-cadherin adhesion complex and the ECM in response to biomechanical stress (Guo et al., 2011). At the molecular level, the interplay between class I PI3Ks and Rac signalling in phagocytic functions has been dissected (Costa et al., 2011), and negative feedback regulation of Rac in leucocytes from mice expressing a constitutively active PI3K-γ has been demonstrated (Costa et al., 2007). Leucocyte transmigration is also modulated by chemokine-mediated PI3K-γ-dependent phosphorylation of vimentin (Barberis et al., 2009). Signalling through PI3K-γ has functional relevance beyond immune cell migration, since it appears to be one common platform for leucocyte, platelet and cardiovascular stress sensing (Hirsch et al., 2006). For an overview on the role of PI3Ks in cardiovascular diseases and current PI3K-targeting drugs, see Eisenreich and Rauch (2011).
A therapeutic approach against autoimmune myocarditis was further suggested by Goser et al. (2005) who demonstrated that blockade of the chemokines MCP-1 or macrophage inflammatory protein-1α (MIP-1α) with monoclonal antibodies attenuates the pathogenesis of experimental autoimmune myocarditis (EAM), by inhibiting mononuclear cell (MNC) migration via the receptors CCR2 and CCR5.
Other preclinical studies have investigated drugs with migration-inhibiting properties. PPAR-γ ligands inhibit monocyte chemotactic protein-1 (MCP-1)-directed migration of monocytes (Kintscher et al., 2000), endothelial cells (Goetze et al., 2002) and vascular smooth muscle cells (VSMCs) (Goetze et al., 2001). In a chronic cardiac transplant rejection model, Ogawa et al. showed that clarithromycin, a macrolide antibiotic involved in MMP regulation, suppressed the development of graft arterial disease and myocardial remodelling. This treatment led to inhibition of MMP-9 and suppression of smooth muscle cell migration and proliferation (Ogawa et al., 2008). Positive effects on myocardial infarct size following left coronary ligation were observed by Nichols et al. after treatment of animals with the thromboxane A2 (TXA2) synthetase inhibitor U-63557A, and the TXA2 receptor antagonist SQ-29.548. Reduced neutrophil accumulation in the infarcted zone, together with a decrease in myocardial myeloperoxidase activity as a specific marker of neutrophil infiltration, and blockade on f-MLP-directed chemotaxis in vitro marked the protective effects in this study (Nichols et al., 1989).
A different therapeutic strategy is exemplified by the drug fingolimod (FTY-720) (Chiba et al., 1999), a receptor modulator that mimics the serum component sphingosine-1-phosphate (S1P) and acts as an agonist for four of the five members of the S1P family of GPCRs. The physiological role of S1P receptors on lymphocytes is control of their exit from lymphoid tissues (Schwab and Cyster, 2007), which is tightly regulated. Stimuli such as antigen challenge can block lymphocyte egress from lymph nodes. FTY-720 thus sequesters lymphocytes in lymphoid organs, prevents their migration to sites of inflammation and limits T-cell access to organ grafts and autoimmune lesions. In contrast to the synthetic selectin, integrin, chemokine or chemokine receptor blockers that antagonize endogenous migration activators, S1P-mimetic drugs mimic the action of an endogenous molecule that physiologically limits immune cell migration. In rodent models, S1P administration or FTY720 treatment were cardioprotective against ischaemia (Jin et al., 2002; Zhang et al., 2007; Karliner, 2009), improved recovery during reperfusion and reduced infarct size after coronary artery ligation (Hofmann et al., 2009; 2011). In clinical phase 2 and 3 trials of patients with multiple sclerosis, FTY-720 significantly reduced clinical relapse rates and infiltration of autoreactive lymphocytes into the CNS (Kappos et al., 2006). Side effects included an enhanced risk for respiratory tract infections and a reduction of total circulating lymphocytes.
Another example for endogenous limitation of migratory processes was found in the suppressors of cytokine signalling (SOCS). The eosinophil chemoattractant CCL11 interacts with CCR3, a chemokine receptor expressed by multiple cell types including macrophages (Menzies-Gow et al., 2002), resulting in SOCS induction and thereby blunted response to proinflammatory cytokines and microbial products (Yoshimura et al., 2007). SOCS controls signalling pathways downstream of integrins, for example, inhibiting focal adhesion kinase (FAK) that is indispensable for cell migration (Liu et al., 2003). Stevenson et al. (2011) showed that SOCS1 and 3 enhanced cell adhesion but strongly inhibited migration towards CCL11, and that inhibition of SOCS1 and 3 signalling pathway via FAK and RhoA blocked of immune cell infiltration to the site of allergic inflammation.
Endogenous migration-inhibiting molecules as parent compounds for drug development
Therapeutic tools employed for migration modulation by the various principles outlined above mainly comprise synthetic compounds: traditional small molecule drugs (Barber et al., 2005) (Camps et al., 2005), synthetic peptides (Rother et al., 2010; Jahns et al., 2011), monoclonal antibodies (Goser et al., 2005; Chan and Carter, 2011; Lee et al., 2011; Weiner et al., 2011) or bispecific protein–monoclonal antibody recombinant molecules (Langer et al., 2011). As possible future tools, we discuss here the possible use of endogenous proteins with inflammation-modulating, cell migration and chemotaxis blocking, or immune signalling properties, as parent compounds for the development of drugs mimicking effects of the endogenous protein or its subdomains. Such therapeutics comprised of, or mimicking, naturally occurring and often evolutionary ancient proteins are one approach towards the exploitation of biological resources. Of note, a number of biological systems such as innate immunity, or miRs with their dependent genes, may be considered as master regulators of fundamental biological processes including tissue repair and regeneration. Not surprisingly, such systems are often conserved during evolution back to ancient species, as illustrated impressively by phylogenetic studies of innate immunity components (Hoffmann et al., 1999; Christophides et al., 2002; Rast et al., 2006; Haine et al., 2008; Lee et al., 2011).
Against the background of more conventional drug discovery strategies, we illustrate this strategy using recent data from our group on a protein from the CCN family (Perbal, 2004; Rachfal and Brigstock, 2005; Kubota and Takigawa, 2007). The starting point for our study (Rother et al., 2010) was the fact that the CCN1 protein is an evolutionary highly conserved matricellular protein that modulates biological processes associated with tissue repair. Recently, we found first evidence of a novel function of CCN1 as a novel modulator of immune cell migration, with therapeutic potential in diseases associated with chronic pathogenic inflammation. In a proof-of-concept study, we used CCN1 gene transfer to evaluate its therapeutic potential in animal models of human inflammatory cardiomyopathy and of MI. CCN1 therapy significantly reduced immune cell infiltration in both models. Figure 5 shows data for the cardiomyopathy model and mechanistic studies demonstrating that the CCN1 treatment resulted in strongly suppressed random migration of immune cells both in vivo and in vitro, and in abrogation of their chemotactic response to various chemokines.
Figure 5.
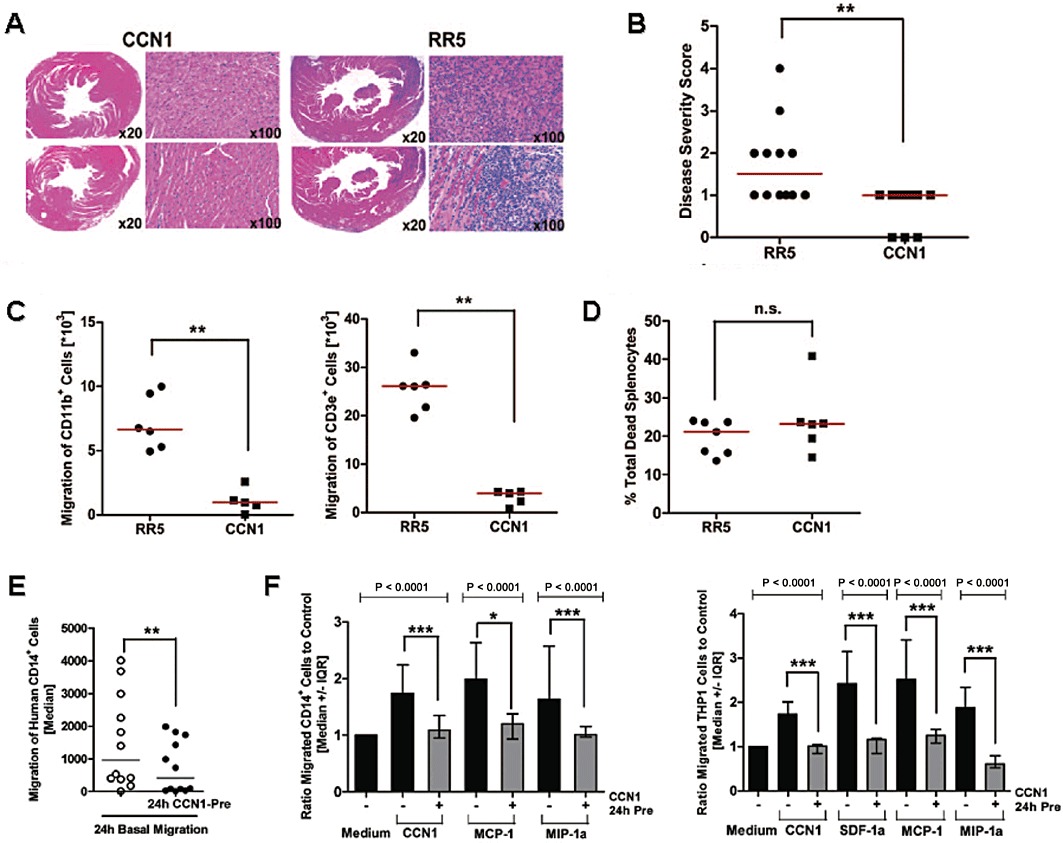
Systemic CCN1 therapy attenuates murine autoimmune cardiomyopathy (Rother et al. 2010) (reproduced by permission from Circulation). (A) Treatment with a CCN1 gene transfer vector (AdV-CCN1) significantly reduced cardiac immune cell infiltration as assessed by histological analysis of mouse hearts after 3 weeks, in comparison with RR5 control vector-treated animals. (B) The cardiac disease score was significantly reduced by this treatment. (C) Migration assays of splenocytes isolated from mice at peak inflammation showed significantly reduced ex vivo migration of CD11b+ macrophages (left) and CD3e+ T cells (right) from AdV-CCN1-treated mice compared with controls. (D) No difference in cell viability was detected. (E) Mechanistic studies of CCN1 effects in vitro showed a significantly reduced basal migration rate of human CD14+ monocytes. (F) Abrogation of the chemotactic response to chemokines (SDF-1α, MCP-1. MIP-1α) important in the pathogenesis of diverse cardiovascular and inflammatory diseases. SFD-1α, stromal cell-derived factor-1α; MIP-1α.
These data suggest that CCN1 has potential as a new broad-spectrum immune cell migration inhibitor, in contrast to specific chemokine- or chemokine receptor-blocking agents with their known limitations arising from the fact that in most inflammatory diseases, multiple chemokines and chemokine receptors are involved, and no single target of outstanding pathogenic importance exist (see above). From a clinical translational perspective, it was of particular interest that the effects of the endogenous protein CCN1 on immune cell chemotaxis and migration were partially mimicked by cyclic RGD (cRGD) peptides that are currently being evaluated in clinical trials, although as yet for cancer therapy only (Figure 6). Our proof-of-concept study therefore suggests further investigation of CCN1 as a new parent compound for immune cell migration modulation and of cRGD peptides as partial CCN1 mimetics with immediate potential for clinical evaluation in cardiac diseases associated with chronic pathogenic inflammation. At the same time, this study describes a novel migration-inhibiting effect for cRGD peptides, which should be relevant for both anti-cancer and anti-inflammatory treatment.
Figure 6.
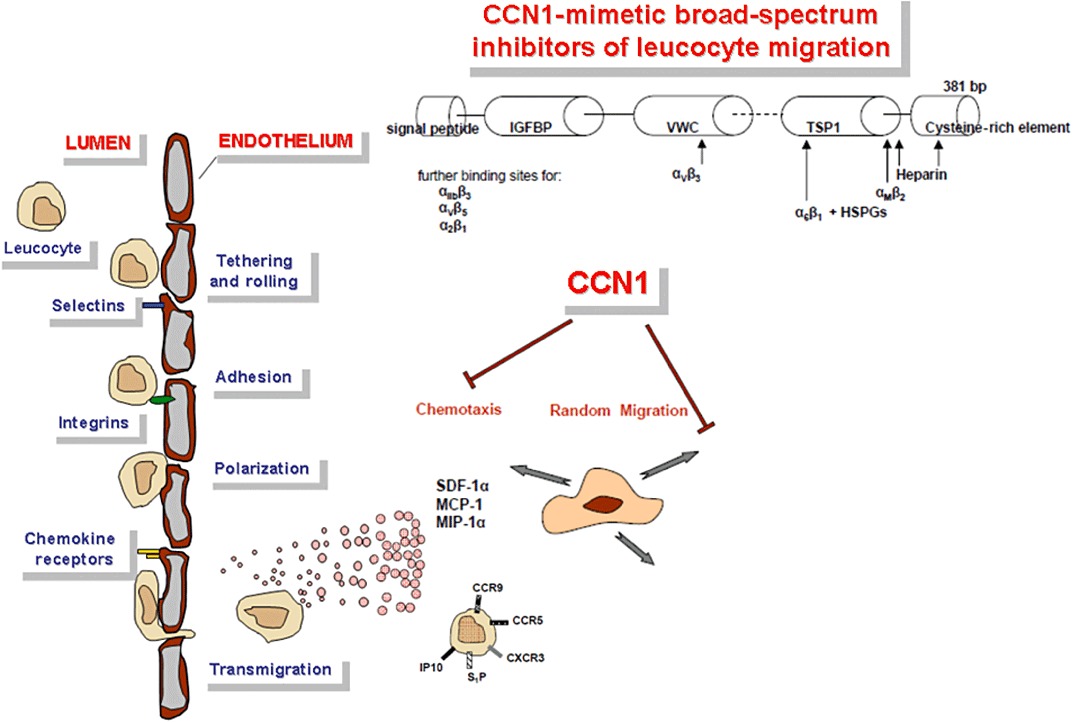
Migration modulation by CCN1: Within the context of other migration-modulating substances, the endogenous protein CCN1 showed significant inhibition of the random migration and chemotaxis of immune cells in vitro and in vivo. The complex multidomain protein CCN1 does not only interact with a broad spectrum of integrins including α6β1 (Chen et al., 2000), αvβ3 (Kireeva et al., 1998), αvβ5 (Monnier et al., 2008), αMβ2 (Schober et al., 2002), α2β1 (Lin et al., 2007) and αIIβ3 (Jedsadayanmata et al., 1999), but also with heparansulphate proteoglycans (HSPGs) (Chen et al., 2000). Nevertheless, immune cell preincubation with a structurally simple cRGD peptide binding selectively to αv type integrins only inhibited their chemotactic response in a similar way as CCN1 preincubation. Obviously, CCN1 effects on immune cells are in part mediated via integrins, and cRGD peptides may be evaluated as a first class of CCN1-mimetic drugs with immediate potential for clinical evaluation. CCN1 treatment certainly exerts more complex effects on inflammatory processes in vivo than cRGD peptides by way of the huge spectrum of integrin heterodimers that can be addressed by CCN1 on multiple cell types, but partial functions of therapeutic use may be extracted by further dissection of the proteins domain and subdomain functions. Regarding most matricellular proteins, our knowledge in this respect is only fragmentary.
As for most other migration modulating drugs, a full elucidation of the mechanisms by which CCN1 and cRGD peptide modulates immune cell migration at the molecular level has not yet been achieved. However, current data indicate that both random migration and directed migration along a chemotactic gradient are affected by these agents. We do not yet know if there are differential effects of CCN1 or cRGD peptide on the migration of immune cell subpopulations in vivo, if a T-cell response is generated in draining lymph nodes and if antigen-presenting cells (APCs) migrate there normally during these treatments. Differential effects of CCN1 and cRGD dose and timing with respect to disease course need to be evaluated further in the future. Despite these limitations of our current knowledge, however, CCN1 is likely to expand the spectrum of tools for chemotaxis modulation and offer new therapeutic perspectives for cardiovascular and autoimmune disorders. Beyond this proof-of-principle study using recombinant protein and a mimetic peptide, it certainly is necessary to better understand the metabolism of CCN1 (proteolytic cleavage, products with different biological functions) before a systematic search for the most useful mimetic drugs can be finalized. Interestingly, another group has arrived at a similar general strategy for drug development starting from thrombospondin-1 (TSP-1), another endogenous protein inhibiting angiogenesis (Colombo et al., 2011; Taraboletti et al., 2011). It is quite remarkable that this ‘parent compound’ TSP-1 is also a matricellular protein that is re-induced in the adult organism by tissue injury, similar to CCN1 with which it interacts.
Therapeutic interest in CCN1 was initially triggered by genomics studies in humans, which resembles the path of other investigations that have used genomic approaches to drive novel compound pipelines. Before any anti-inflammatory approach can be undertaken, it is crucial to delineate the distinction between acute inflammation supporting tissue repair and regeneration, as opposed to chronic inflammation without reparative advantage but instead inducing progressive tissue injury. With respect to the latter type of inflammation, further investigation of CCN1 as a new parent compound for immune cell migration modulation appears warranted, as well as of cRGD peptides as a first class of partially CCN1-mimetic drugs with immediate potential for clinical evaluation in cardiac disorders associated with chronic pathogenic inflammation. Cardiac transplant rejection and transplant injury in other organ transplants appear as particularly interesting disease targets because in these cases preconditioning of the host by CCN1 or cRGD treatment would be feasible, allowing modulation of the host before its immune response is initiated. Experimental investigations in transplant models (Nykänen et al., 2010) are currently in progress.
microRNAs as novel therapeutic tools and targets
In addition to innate immunity proteins and other ancient proteins involved in tissue repair and regeneration, with the regulatory (non-coding) RNA molecules, a completely new class of biological regulators has been discovered. These RNAs are distinct from the protein-coding messenger RNAs (mRNAs) and other RNAs essential for protein biosynthesis (ribosomal rRNAs, transfer tRNAs) and have long been neglected as dysfunctional molecules. The currently most intensely studies class of regulatory RNAs are miRs, which are capable of regulating complex gene networks, as well as short interfering RNAs (siRNAs) and short hairpin RNAs (shRNAs), which mediate gene silencing by the process of RNA interference (Poller and Fechner, 2010; Poller et al., 2010). Both classes, in particular shRNAs, have already been successfully employed as novel therapeutic tools in diverse diseases including cardiovascular (Suckau et al., 2009) and malignant disorders.
It has become evident that naturally occurring miRs are involved in the physiological regulation of the innate (Davidson-Moncada et al., 2011) and adaptive immune system (Baltimore et al., 2008; Carissimi et al., 2009; Sonkoly and Pivarcsi, 2009; Xiao and Rajewsky, 2009; O’Connell et al., 2011), and are deregulated in various diseases including autoimmune disorders (Pauley et al., 2009; Tang et al., 2009; Fulci et al., 2011; Furer et al., 2011; Liston et al., 2010), antiviral immunity (Cullen, 2006), organ transplant rejection (Harris et al., 2010) and atherosclerosis (Weber et al., 2010). Specifically, miRs are directly involved in immune cell migration (Pucci et al., 2009), function (Kohlhaas et al., 2009; Wei and Pei, 2010) and differentiation, (Schmeier et al., 2009), or mediate the effects of cell signalling molecules such as AKT1 (Androulidaki et al., 2009).
The importance of miRs in the immune response has also been demonstrated in studies that employed miR modulations to induce antigen-specific regulatory T cells (Tregs) and promote immunologic tolerance (Annoni et al., 2009), or to suppress the development of allergic airways disease (Mattes et al., 2009). Selective miR ablation in Treg cells has resulted in uncontrolled autoimmunity (Zhou et al., 2008), and inactivation of the miR-processing enzyme Dicer has disrupted invariant NKT cell development.
With respect to the regulation of cell migration in general, it has recently been discovered that miRs are not only synthesized and processed within individual cells, but that for certain miRs, there is active secretion via packaging into microvesicles (MVs) (Hunter et al., 2008; Yuan et al., 2009; Ogawa et al., 2011; Wang et al., 2011; Zhang et al., 2011), and that such vesicles can enter other cells and deliver miR-150 into, for example, endothelial cells where they then promote cell migration (Zhang et al., 2011). Based on these quite unexpected recent discoveries, microvesicle-encapsulated miR mimetics (Poller and Fechner, 2010; Poller et al., 2010) may also be considered as possible migration modulators.
Delivery systems for novel migration modulators
The above discussed novel migration modulators could be delivered as recombinant proteins as long as no mimetic small molecule drugs or peptides are available, or by gene transfer using vectors that continuously produce and deliver a therapeutic protein into the circulation. Use of a gene vector as a inexpensive ‘protein factory’ with the additional advantage that all posttranslational modifications are exerted by the host itself and thus in the most appropriate way is a rather old idea (Kay et al., 1993; 1994) first promoted in the field of haemophilia with its need for repetitive infusion of expensive blood-derived (and thus infection-prone) coagulation factors or recombinant proteins. Gene therapy has been significantly advanced by haemophilia researchers and with the employment of adeno-associated virus (AAV)-based vectors (Nathwani et al., 2007; Nathwani et al., 2011), an efficient system for long-term production of therapeutic proteins in liver (Mount et al., 2002; Niemeyer et al., 2009; Sabatino et al., 2011) or skeletal muscle (Arruda et al., 2011; Haurigot et al., 2011), has become available. Whereas most studies to date have been performed in rodents and non-human primates, clinical investigations have also been performed in humans (Arruda et al., 2001; Jiang et al., 2006) where vector dose reduction by use of advanced vector systems has been identified as a key determinant of long-term stability (Herzog et al., 2002; Grimm et al., 2008; Zhong et al., 2008). Given the fact that mimetic drugs are not yet available for numerous proteins of high clinical interest, the gene transfer option deserves continued attention.
When it comes to long-term therapies based on regulatory RNA molecules including RNAi-mediating sequences, efficient production and delivery is highly dependent upon gene transfer technology, due to the instability of these novel therapeutic agents in blood and tissues. For long-term treatments, they are either repetitively delivered in short time intervals, or continuously produced from tissue-targeted and long-term stable vectors. As a major breakthrough in the vector field has been achieved with the introduction of AAV vector systems (Muller et al., 2003; Waterkamp et al., 2006; Li et al., 2008; Schnepp et al., 2009; McPhee and Samulski, 2009), in particular the discovery of highly cardiotropic AAV serotypes (Inagaki et al., 2006; Pačak et al., 2006; Gray and Samulski, 2008). This has not only led to successful gene therapy (Sakata et al., 2007a,b; Muller et al., 2008; Raake et al., 2008; Goehringer et al., 2009; Rengo et al., 2009) and recently also to the first successful regulatory RNA therapy of a cardiac disease (Suckau et al., 2009) in vivo, by direct i.v. vector injection in animal models but is already in the status of clinical translational phase I and II trials (Jaski et al., 2009; Jessup et al., 2011). If perfect physical vector targeting to the diseased tissue cannot be achieved (transductional targeting), additional transcriptional confinement of the transgene may be achieved by using cardiac-specific promoters (Muller et al., 2006). A number of experimental papers on therapeutic cell migration modulation has successfully employed this delivery mode, for example gene transfer for IL-10 or chemokine MCP-1-7ND in autoimmune myocarditis (Goser et al., 2005; Kaya et al., 2011).
An issue of paramount importance before clinical translation of these experimental strategies is the safety of the delivery tools, which is high for AAV vectors and AAV-derived biological nanoparticles (BNPs), as well as an option to shut down the transgene in the case of serious adverse effects. In summary, current vector systems offer a realistic perspective to serve as relatively simple ‘drug factories’ for the delivery of biologicals such as proteins or RNA drugs in humans. Regulated delivery based on vector systems is still under development and has not yet reached the stage for clinical translation.
Side effects – general and specific
Any novel immune cell migration modulator for anti-inflammatory and cardiovascular therapy will inevitably interfere with a carefully balanced system of multiple migration regulators directing proper positioning of immune cells within the body. This intervention shall not, however, significantly impair pathogen elimination by background immune surveillance, thus provoking infectious complications. Due to the extraordinary complexity of the immune defence and our still limited knowledge of its regulation, it is impossible to predict the side effects of any new immune cell migration modulator by theoretical reasoning, but this needs to be investigated experimentally for each target disease. The need to conduct detailed in vivo studies on optimal drug doses and other issues is well exemplified by the stimulation of malignant tumour growth by low concentrations of a RGD-mimetic integrin inhibitor actually developed and clinically employed as an anti-tumour agent (Reynolds et al., 2009).
Conclusions
Because cardiovascular and immunological diseases that are not immediately fatal will be targeted by cell migration modulators as discussed above, issues of safety will be of paramount importance. Since leucocyte migration is an integral to immune surveillance and molecular recognition, including APC and target cell search and scanning of receptors expressed at cell surfaces, avoidance of unspecific chronic immunosuppression requires careful drug dose selection. Furthermore, cell migration targeting should ideally address specific leucocyte subsets and achieve functional effects related to immune response phase, possibly complementing interference with cell cycle progression (cytostatic drugs). In this regard, we have discussed a fundamental problem encountered with single-target approaches that arises from the complexity of any inflammation, with multiple interacting and often redundant factors (chemokines, receptors for chemokines and other ligands, intracellular signal proteins) being involved. Such discordance between simple synthetic tools versus complex natural networks is likely to cause problems for any class of therapeutic agent (small molecules, peptides, antibodies, regulatory RNAs) addressing single targets.
Against this background of single-target agents, we have discussed the potential of more complex, endogenous proteins addressing multiple receptors and signal pathways and acting on the immune response in a phase-specific manner, either a recombinant proteins or as parent compounds for the development of novel drugs. Based on recent evidence, we specifically suggest further investigation of matricellular proteins with their rich and context-dependent migration-migration modulating properties, and of their interaction partners, as novel starting points for anti-inflammatory and cardiovascular drug development.
Acknowledgments
This work has been supported by the German Research Foundation (DFG) through SFB Transregio 19 (grant C5 to WP and CScheibenbogen, grant B7N to CSkurk and CScheibenbogen). MR has been supported the Berlin School for Regenerative Therapies (BSRT).
Glossary
- APC
antigen-presenting cell
- CCN1
cysteine-rich angiogenic inducer 61
- cRGD
cyclic RGD peptide
- DCM
dilated cardiomyopathy
- DCMi
inflammatory cardiomyopathy
- EAM
experimental autoimmune myocarditis
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- mAb
monoclonal antibody
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- MIP-1α
macrophage inflammatory protein-1α
- MNC
mononuclear cell
- PI3K
phosphoinositide 3-kinase
- TSP
thrombospondin
- TXA
thromboxane
- VSMC
vascular smooth muscle cell
Conflicts of interest
None.
References
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2011;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogio C, et al. Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur J Immunol. 2009;39:1136–1146. doi: 10.1002/eji.200838884. [DOI] [PubMed] [Google Scholar]
- Baron JH, Moiseeva EP, de Bono DP, Abrams KR, Gershlick AH. Inhibition of vascular smooth muscle cell adhesion and migration by c7E3 Fab (abciximab): a possible mechanism for influencing restenosis. Cardiovasc Res. 2000;48:464–472. doi: 10.1016/s0008-6363(00)00201-7. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Martin P, Gonzalez-Amaro R, Sanchez-Madrid F. Molecular cues guiding inflammatory responses. Cardiovasc Res. 2011;86:174–182. doi: 10.1093/cvr/cvq001. [DOI] [PubMed] [Google Scholar]
- Bobbert P, Scheibenbogen C, Jenke A, Kania G, Wilk S, Krohn S, et al. Adiponectin expression in patients with inflammatory cardiomyopathy indicates favourable outcome and inflammation control. Eur Heart J. 2011;32:1134–1147. doi: 10.1093/eurheartj/ehq498. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Cai YH, Alvarez A, Alcaide P, Duramad P, Lim YC, Jarolim P, et al. Abrogation of functional selectin-ligand expression reduces migration of pathogenic CD8+ T cells into heart. J Immunol. 2006;176:6568–6575. doi: 10.4049/jimmunol.176.11.6568. [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Carissimi C, Fulci V, Macino G. MicroRNAs: novel regulators of immunity. Autoimmun Rev. 2009;8:520–524. doi: 10.1016/j.autrev.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Carroll WM. Oral therapy for multiple sclerosis – sea change or incremental step? N Engl J Med. 2011;362:456–458. doi: 10.1056/NEJMe0912019. [DOI] [PubMed] [Google Scholar]
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2011;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- Chiba K, Yanagawa Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating lymphocytes by acceleration of lymphocyte homing. Transplant Proc. 1999;31:1230–1233. doi: 10.1016/s0041-1345(98)01975-7. [DOI] [PubMed] [Google Scholar]
- Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2011;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2011;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Colombo G, Margosio B, Ragona L, Neves M, Bonifacio S, Annis DS, et al. Non-peptidic thrombospondin-1 mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J Biol Chem. 2011;285:8733–8742. doi: 10.1074/jbc.M109.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, et al. Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci U S A. 2007;104:14354–14359. doi: 10.1073/pnas.0703175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Germena G, Hirsch E. Dissection of the interplay between class I PI3Ks and Rac signaling in phagocytic functions. Scientificworldjournal. 2011;10:1826–1839. doi: 10.1100/tsw.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Is RNA interference involved in intrinsic antiviral immunity in mammals. Nat Immunol. 2006;7:563–567. doi: 10.1038/ni1352. [DOI] [PubMed] [Google Scholar]
- Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann N Y Acad Sci. 2011;1183:183–194. doi: 10.1111/j.1749-6632.2009.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2011;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich A, Rauch U. PI3K inhibitors in cardiovascular disease. Cardiovasc Ther. 2011;29:29–36. doi: 10.1111/j.1755-5922.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- Fechner H, Noutsias M, Tschoepe C, Hinze K, Wang X, Escher F, et al. Induction of coxsackievirus-adenovirus-receptor expression during myocardial tissue formation and remodeling: identification of a cell-to-cell contact-dependent regulatory mechanism. Circulation. 2003;107:876–882. doi: 10.1161/01.cir.0000050150.27478.c5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, van Buul JD, Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res. 2011;86:202–210. doi: 10.1093/cvr/cvq003. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, et al. miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2011;71:206–211. doi: 10.1016/j.humimm.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Furer V, Greenberg JD, Attur M, Abramson SB, Pillinger MH. The role of microRNA in rheumatoid arthritis and other autoimmune diseases. Clin Immunol. 2011;136:1–15. doi: 10.1016/j.clim.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Goehringer C, Rutschow D, Bauer R, Schinkel S, Weichenhan D, Bekeredjian R, et al. Prevention of cardiomyopathy in delta-sarcoglycan knockout mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res. 2009;82:404–410. doi: 10.1093/cvr/cvp061. [DOI] [PubMed] [Google Scholar]
- Goetze S, Kintscher U, Kim S, Meehan WP, Kaneshiro K, Collins AR, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit nuclear but not cytosolic extracellular signal-regulated kinase/mitogen-activated protein kinase-regulated steps in vascular smooth muscle cell migration. J Cardiovasc Pharmacol. 2001;38:909–921. doi: 10.1097/00005344-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Goetze S, Eilers F, Bungenstock A, Kintscher U, Stawowy P, Blaschke F, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun. 2002;293:1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Goser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, et al. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112:3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Samulski RJ. Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin Biol Ther. 2008;8:911–922. doi: 10.1517/14712598.8.7.911. [DOI] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Kassiri Z, Basu R, Chow FL, Kandalam V, Damilano F, et al. Loss of PI3Kgamma enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. 2011;107:1275–1289. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- Habicht A, Clarkson MR, Yang J, Henderson J, Brinkmann V, Fernandes S, et al. Novel insights into the mechanism of action of FTY720 in a transgenic model of allograft rejection: implications for therapy of chronic rejection. J Immunol. 2006;176:36–42. doi: 10.4049/jimmunol.176.1.36. [DOI] [PubMed] [Google Scholar]
- Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008;322:1257–1259. doi: 10.1126/science.1165265. [DOI] [PubMed] [Google Scholar]
- Halin C, Mora JR, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu Rev Cell Dev Biol. 2005;21:581–603. doi: 10.1146/annurev.cellbio.21.122303.133159. [DOI] [PubMed] [Google Scholar]
- Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Krams SM, Martinez OM. MicroRNAs as immune regulators: implications for transplantation. Am J Transplant. 2010;10:713–719. doi: 10.1111/j.1600-6143.2010.03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurigot V, Mingozzi F, Buchlis G, Hui DJ, Chen Y, Basner-Tschakarjan E, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2011;18:1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Hicks AE, Nolan SL, Ridger VC, Hellewell PG, Norman KE. Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: complete inhibition of rolling is not required for anti-inflammatory effect. Blood. 2003;101:3249–3256. doi: 10.1182/blood-2002-07-2329. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost. 2006;95:29–35. [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Hu K, Walter F, Burkard N, Ertl G, Bauersachs J, et al. Pharmacological pre- and post-conditioning with the sphingosine-1-phosphate receptor modulator FTY720 after myocardial ischaemia-reperfusion. Br J Pharmacol. 2011;160:1243–1251. doi: 10.1111/j.1476-5381.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009a;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- Horuk R. Promiscuous drugs as therapeutics for chemokine receptors. Expert Rev Mol Med. 2009b;11:e1. doi: 10.1017/S1462399409000921. [DOI] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M, Kappos L, Calabresi PA, Confavreux C, Giovannoni G, Galetta SL, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256:405–415. doi: 10.1007/s00415-009-0093-1. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns R, Schlipp A, Boivin V, Lohse MJ. Targeting receptor antibodies in immune cardiomyopathy. Semin Thromb Hemost. 2011;36:212–218. doi: 10.1055/s-0030-1251506. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3) J Biol Chem. 1999;274:24321–24327. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol. 2010;17:43–52. doi: 10.1097/MOH.0b013e3283333949. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc Res. 2009;82:184–192. doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M, Rothenberg S, Landen C, Bellinger D, Leland F, Toman C, et al. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kay M, Landen C, Rothenberg S, Taylor L, Leland F, Wiehle S, et al. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in haemophilia B dogs. Proceedings of the National Academy of Science USA. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya Z, Leib C, Werfel S, Goser S, Ottl R, Leuchs B, et al. Comparison of IL-10 and MCP-1-7ND gene transfer with AAV9 vectors for protection from murine autoimmune myocarditis. Cardiovasc Res. 2011;91:116–123. doi: 10.1093/cvr/cvr063. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Goetze S, Wakino S, Kim S, Nagpal S, Chandraratna RA, et al. Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. Eur J Pharmacol. 2000;401:259–270. doi: 10.1016/s0014-2999(00)00461-1. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Kuznetsova SA, Roberts DD. Functional regulation of T lymphocytes by modulatory extracellular matrix proteins. Int J Biochem Cell Biol. 2004;36:1126–1134. doi: 10.1016/j.biocel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Lago F, Gomez R, Gomez-Reino JJ, Dieguez C, Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009;34:500–510. doi: 10.1016/j.tibs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Lan YY, Tokita D, Wang Z, Wang HC, Zhan J, Brinkmann V, et al. Sphingosine 1-phosphate receptor agonism impairs skin dendritic cell migration and homing to secondary lymphoid tissue: association with prolonged allograft survival. Transpl Immunol. 2008;20:88–94. doi: 10.1016/j.trim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lang K, Ratke J. Leptin and Adiponectin: new players in the field of tumor cell and leukocyte migration. Cell Commun Signal. 2009;7:27. doi: 10.1186/1478-811X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer HF, von der Ruhr JW, Daub K, Schoenberger T, Stellos K, May AE, et al. Capture of endothelial progenitor cells by a bispecific protein/monoclonal antibody molecule induces reendothelialization of vascular lesions. J Mol Med (Berl) 2011;88:687–699. doi: 10.1007/s00109-010-0614-5. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol. 2011;125(Suppl. 2):S314–S323. doi: 10.1016/j.jaci.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Legare JF, Oxner A, Heimrath O, Issekutz T. Infiltration of polymorphonuclear cells into the post-ischaemic myocardium is dependent on beta2 and alpha4 integrins. Int J Exp Pathol. 2007;88:291–300. doi: 10.1111/j.1365-2613.2007.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, et al. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem. 2007;282:34594–34604. doi: 10.1074/jbc.M706600200. [DOI] [PubMed] [Google Scholar]
- Liston A, Linterman M, Lu LF. MicroRNA in the Adaptive Immune System, in Sickness and in Health. J Clin Immunol. 2010;30:339–346. doi: 10.1007/s10875-010-9378-5. [DOI] [PubMed] [Google Scholar]
- Liu E, Cote JF, Vuori K. Negative regulation of FAK signaling by SOCS proteins. EMBO J. 2003;22:5036–5046. doi: 10.1093/emboj/cdg503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llera AS, Girotti MR, Benedetti LG, Podhajcer OL. Matricellular proteins and inflammatory cells: a task force to promote or defeat cancer? Cytokine Growth Factor Rev. 2010;21:67–76. doi: 10.1016/j.cytogfr.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov. 2009;8:297–307. doi: 10.1038/nrd2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Yagi S, Kago T, Leaf N, Rosen H. Modulation of chemokines and allergic airway inflammation by selective local sphingosine-1-phosphate receptor 1 agonism in lungs. Mol Pharmacol. 2011;79:61–68. doi: 10.1124/mol.110.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee SW, Samulski RJ. Gene therapy for cardiomyocytes, a heart beat away. Gene Ther. 2009;16:707–708. doi: 10.1038/gt.2009.40. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, et al. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- Monnier Y, Farmer P, Bieler G, Imaizumi N, Sengstag T, Alghisi GC, et al. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res. 2008;68:7323–7331. doi: 10.1158/0008-5472.CAN-08-0841. [DOI] [PubMed] [Google Scholar]
- Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Muller O, Kaul F, Weitzman M, Pasqualini R, Arap W, Kleinschmidt J, et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat Biotechnol. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Leuchs B, Pleger ST, Grimm D, Franz WM, Katus HA, et al. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc Res. 2006;70:70–78. doi: 10.1016/j.cardiores.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Schinkel S, Kleinschmidt JA, Katus HA, Bekeredjian R. Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther. 2008;15:1558–1565. doi: 10.1038/gt.2008.111. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WW, Mehta J, Wargovich TJ, Franzini D, Lawson D. Reduced myocardial neutrophil accumulation and infarct size following thromboxane synthetase inhibitor or receptor antagonist. Angiology. 1989;40:209–221. doi: 10.1177/000331978904000309. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3:275–286. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykänen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2011;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Suzuki J, Hishikari K, Takayama K, Tanaka H, Isobe M. Clarithromycin attenuates acute and chronic rejection via matrix metalloproteinase suppression in murine cardiac transplantation. J Am Coll Cardiol. 2008;51:1977–1985. doi: 10.1016/j.jacc.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura K, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2011;398:723–729. doi: 10.1016/j.bbrc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Osteopontin and cardiovascular system. Mol Cell Biochem. 2007;300:1–7. doi: 10.1007/s11010-006-9368-3. [DOI] [PubMed] [Google Scholar]
- Pačak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Poller W, Fechner H. Development of novel cardiovascular therapeutics from small regulatory RNA molecules – an outline of key requirements. Curr Pharm Des. 2010;16:2252–2268. doi: 10.2174/138161210791792813. [DOI] [PubMed] [Google Scholar]
- Poller W, Hajjar R, Schultheiss HP, Fechner H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res. 2010;86:353–364. doi: 10.1093/cvr/cvq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood ‘resident’ monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- Raake PW, Hinkel R, Muller S, Delker S, Kreuzpointner R, Kupatt C, et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Rast JP, Smith LC, Loza-Coll M, Hibino T, Litman GW. Genomic insights into the immune system of the sea urchin. Science. 2006;314:952–956. doi: 10.1126/science.1134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, et al. Matricellular Signaling Molecule CCN1 Attenuates Experimental Autoimmune Myocarditis by Acting as a Novel Immune Cell Migration Modulator. Circulation. 2010;122:2688–2698. doi: 10.1161/CIRCULATIONAHA.110.945261. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Lange AM, Altynova ES, Sarkar R, Zhou S, Merricks EP, et al. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007a;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, et al. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol. 2007b;292:H1204–H1207. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Colombo MP. Matricellular proteins at the crossroad of inflammation and cancer. Cancer Lett. 2008;267:245–253. doi: 10.1016/j.canlet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeier S, MacPherson CR, Essack M, Kaur M, Schaefer U, Suzuki H, et al. Deciphering the transcriptional circuitry of microRNA genes expressed during human monocytic differentiation. BMC Genomics. 2009;10:595. doi: 10.1186/1471-2164-10-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp BC, Jensen RL, Clark KR, Johnson PR. Infectious molecular clones of adeno-associated virus isolated directly from human tissues. J Virol. 2009;83:1456–1464. doi: 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, et al. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Schulz C, Von Andrian UH, Massberg S. Trafficking of murine hematopoietic stem and progenitor cells in health and vascular disease. Microcirculation. 2009;16:497–507. doi: 10.1080/10739680902948351. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Seropian IM, Abbate A, Toldo S, Harrington J, Smithson L, Ockaili R, et al. Pharmacological inhibition of phosphoinositide 3-kinase gamma (PI3Kgamma) promotes infarct resorption and prevents adverse cardiac remodeling after myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;56:651–658. doi: 10.1097/FJC.0b013e3181f9a905. [DOI] [PubMed] [Google Scholar]
- Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, et al. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. Eur Heart J. 2008;29:1168–1180. doi: 10.1093/eurheartj/ehn136. [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Pivarcsi A. Advances in microRNAs: implications for immunity and inflammatory diseases. J Cell Mol Med. 2009;13:24–38. doi: 10.1111/j.1582-4934.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steevels TA, Meyaard L. Immune inhibitory receptors: Essential regulators of phagocyte function. Eur J Immunol. 2011;41:575–587. doi: 10.1002/eji.201041179. [DOI] [PubMed] [Google Scholar]
- Steevels TA, Lebbink RJ, Westerlaken GH, Coffer PJ, Meyaard L. Signal inhibitory receptor on leukocytes-1 is a novel functional inhibitory immune receptor expressed on human phagocytes. J Immunol. 2011;184:4741–4748. doi: 10.4049/jimmunol.0902039. [DOI] [PubMed] [Google Scholar]
- Stevenson NJ, McFarlane C, Ong ST, Nahlik K, Kelvin A, Addley MR, et al. Suppressor of cytokine signalling (SOCS) 1 and 3 enhance cell adhesion and inhibit migration towards the chemokine eotaxin/CCL11. FEBS Lett. 2011;584:4469–4474. doi: 10.1016/j.febslet.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Rusnati M, Ragona L, Colombo G. Targeting tumor angiogenesis with TSP-1-based compounds: rational design of antiangiogenic mimetics of endogenous inhibitors. Oncotarget. 2011;1:662–673. doi: 10.18632/oncotarget.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, Laake L, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- Vilmos P, Gaudenz K, Hegedus Z, Marsh JL. The Twisted gastrulation family of proteins, together with the IGFBP and CCN families, comprise the TIC superfamily of cysteine rich secreted factors. Mol Pathol. 2001;54:317–323. doi: 10.1136/mp.54.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2011;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterkamp DA, Muller OJ, Ying Y, Trepel M, Kleinschmidt JA. Isolation of targeted AAV2 vectors from novel virus display libraries. J Gene Med. 2006;8:1307–1319. doi: 10.1002/jgm.967. [DOI] [PubMed] [Google Scholar]
- Weber C, Schober A, Zernecke A. MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr Drug Targets. 2010;11:950–956. doi: 10.2174/138945010791591377. [DOI] [PubMed] [Google Scholar]
- Wei B, Pei G. MicroRNAs: critical regulators in Th17 cells and players in diseases. Cell Mol Immunol. 2010;7:175–181. doi: 10.1038/cmi.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2011;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann D, Schneeberger S, Friedl P, Zacharowski K, Wick N, Boesch F, et al. The fibrin-derived peptide Bbeta(15-42) significantly attenuates ischemia-reperfusion injury in a cardiac transplant model. Transplantation. 2011;89:824–829. doi: 10.1097/tp.0b013e3181ccd822. [DOI] [PubMed] [Google Scholar]
- Wittchen F, Suckau L, Witt H, Skurk C, Lassner D, Fechner H, et al. Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets. J Mol Med. 2007;85:257–271. doi: 10.1007/s00109-006-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Heit B, Kubes P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res. 2010;86:183–191. doi: 10.1093/cvr/cvq040. [DOI] [PubMed] [Google Scholar]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2011;86:192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2011;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]


