Abstract
Ischaemia is amongst the leading causes of death. Despite this importance, there are only a few therapeutic approaches to protect from ischaemia–reperfusion injury (IRI). In experimental studies, the amino acid glycine effectively protected from IRI. In the prevention of IRI by glycine in cells and isolated perfused or cold-stored organs (tissues), direct cytoprotection plays a crucial role, most likely by prevention of the formation of pathological plasma membrane pores. Under in vivo conditions, the mechanism of protection by glycine is less clear, partly due to the physiological presence of the amino acid. Here, inhibition of the inflammatory response in the injured tissue is considered to contribute decisively to the glycine-induced reduction of IRI. However, attenuation of IRI recently achieved in experimental animals by low-dose glycine treatment regimens suggests additional/other (unknown) protective mechanisms. Despite the convincing experimental evidence and the large therapeutic width of glycine, there are only a few clinical trials on the protection from IRI by glycine with ambivalent results. Thus, both the mechanism(s) behind the protection of glycine against IRI in vivo and its true clinical potential remain to be addressed in future experimental studies/clinical trials.
Keywords: glycine, ischaemia, reperfusion, hypoxia, reoxygenation, protection, therapy, alanine, taurine, clinical trial
Introduction
Glycine is the simplest amino acid, with just an amino group, a carboxyl group and two hydrogen atoms all bound to one carbon atom (Figure 1). Glycine is taken up by cells via a variety of glycine transporters; typically by a secondary active transport coupled to hydrogen, sodium and/or chloride ion uptake (Boll et al., 2004; Eulenburg et al., 2005; Zafra and Gimenez, 2008). Due to its small size, glycine is sterically extremely adaptable and the preferred candidate for an interior position in proteins. Besides being a decisive building block in many proteins, glycine is also a component of the tripeptide glutathione and of the bile acid glycocholic acid (Figure 2). In addition, it is an essential substrate for the synthesis of a variety of biomolecules such as creatine, porphyrins and purine nucleotides. Within cells, glycine is oxidatively (NAD+-dependently) degraded by the mitochondrial glycine cleavage system to CO2, NH4+, NADH and a methylene group, which is accepted by tetrahydrofolate, thus forming N5,N10-methylenetetrahydrofolate (Kikuchi et al., 2008; Tibbetts and Appling, 2010). Via methylenetetrahydrofolate, glycine is decisively involved in the metabolism of 1-carbon units and by that in further synthetic pathways. Using methylenetetrahydrofolate, mitochondrial and cytosolic serine hydroxymethyl transferase catalyses the formation of serine from glycine (Tibbetts and Appling, 2010). The reactions catalysed by the glycine cleavage system and by serine hydroxymethyl transferase are reversible and thus can also be used for the synthesis of glycine. Glycine is also formed from glyoxylate, a reaction catalysed by alanine : glyoxylate aminotransferase, an enzyme which is predominantly located in peroxisomes (Holmes and Assimos, 1998; Ichiyama, 2011).
Figure 1.
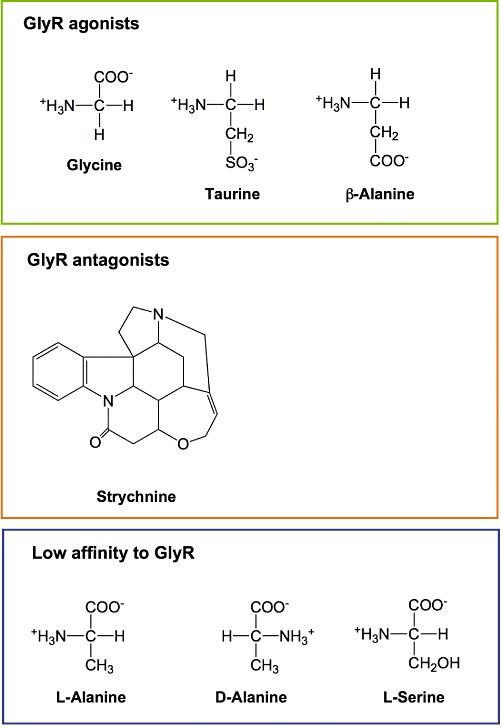
Glycine and other glycine receptor agonists, antagonists and compounds with low affinity to the glycine receptor. GlyR, glycine receptor.
Figure 2.
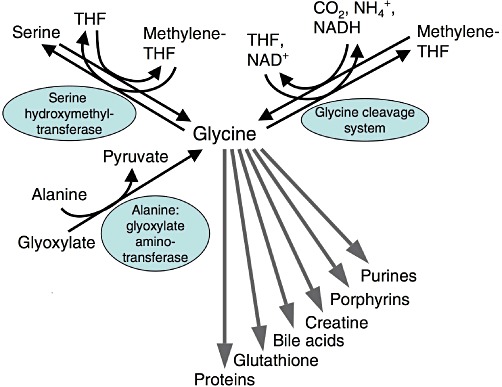
Metabolism of glycine. THF, tetrahydrofolate.
In addition to its fundamental role in metabolism, glycine represents the major inhibitory neurotransmitter in the adult CNS (preferentially in the brainstem and spinal cord). Binding of glycine to the glycine receptor (a ligand-gated chloride channel) causes chloride influx, membrane hyperpolarization, and thus inhibition of postsynaptic neurons (Figure 3) (Gundersen et al., 2005; Betz and Laube, 2006; Bowery and Smart, 2006; Webb and Lynch, 2007; Dresbach et al., 2008; Hernandes and Troncone, 2009; Lynch, 2009). That way glycine is, for example, involved in the generation of reflex responses, in the processing of sensorial inputs and in the sensation of pain. The alkaloid strychnine (Figure 1) is a potent competitive inhibitor of the glycine receptor acting already at micromolar concentrations. Structurally, the neuronal glycine receptor is a pentamer presumably being composed of two α and three β subunits. There are four different α subunits (α1 to α4) but only one β subunit, encoded by separate genes. The scaffolding protein gephyrin binds to the β subunits and connects the glycine receptor with filaments of the submembraneous cytoskeleton, mediating, among others, clustering of the receptor. In addition to its function as an inhibitory neurotransmitter, glycine facilitates neurotransmission mediated by glutamate, the major excitatory neurotransmitter in the CNS. The NMDA (glutamate) receptor does not only have a specific recognition site for glutamate but also a second one for glycine (Paoletti, 2011). To activate the receptor, both recognition sites need to be occupied. The specific binding site for glycine within the NMDA receptor cannot be blocked by strychnine (Gundersen et al., 2005), and an impaired function of NMDA receptors has been proposed to contribute to psychiatric disorders such as schizophrenia (Gaspar et al., 2009).
Figure 3.
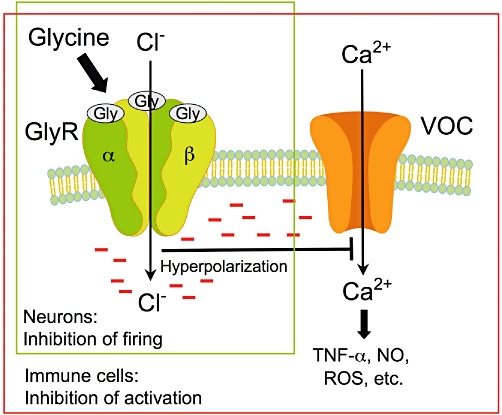
Glycine, an inhibitory neurotransmitter and an inhibitor of the activation of immune cells. Activation of GlyR by glycine results in influx of chloride ions, membrane hyperpolarization and thus, in neurons, in inhibition of the response to excitatory neurotransmitters. In immune cells, hyperpolarization inhibits opening of voltage-operated calcium channels (VOCs), thus influx of calcium ions and activation of these cells, resulting among others in attenuation of the formation of TNF-α, NO and ROS.
In ischaemia–reperfusion injury, intracellular processes but also a reaction of/within the surrounding tissues, usually referred to as inflammatory response (reaction), are involved in executing irreversible injury (Figure 4) (de Groot and Rauen, 2007; Abu-Amara et al., 2010; Turer and Hill, 2010; Vollmar and Menger, 2011). In the ischaemic phase, in the absence of any blood supply (no-flow ischaemia), hypoxic (anoxic) cell injury, that is, cell injury due to energy depletion (in the absence of O2), is the dominating pathogenetic event. Triggered by the impaired energy production, disturbances of the cellular sodium and calcium homeostasis, activation of hydrolases and increases in the permeability of intracellular membranes and of the plasma membrane may result in functional alterations of the cells or in cell death. With increasing residual blood supply (low-flow ischaemia), however, the inflammatory response increasingly contributes to the injurious process already during the ischaemic phase. Functionally altered and dead cells (cell fragments) are the trigger for the inflammatory response comprising, among others, activation of macrophages, granulocytes, the complement system and blood coagulation, the formation of reactive oxygen species (ROS) and disturbances of the microvascular perfusion. In the reperfusion phase, injury is dependent on the progression of the inflammatory response. Additional cell injury may occur (e.g. through excessive ROS formation or still by hypoxia due to impaired microvascular perfusion). On the other hand, at least part of the inflammatory response is a prerequisite for the regeneration of the injured tissue.
Figure 4.
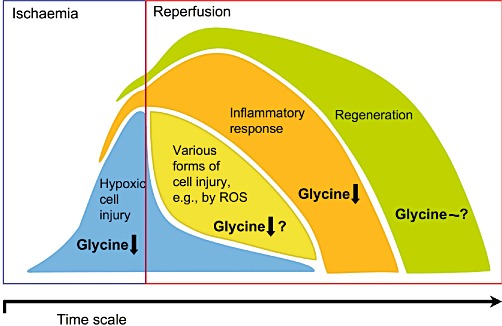
Cell injury, inflammatory response and regeneration during or following ischaemia–reperfusion. Protective effects of glycine. At concentrations around 1 mM and above, glycine decreases hypoxic cell injury by direct cytoprotection and inhibits the inflammatory response. A direct cytoprotective effect of glycine on other forms of cell injury occurring during reperfusion, such as those mediated by ROS, remains a matter of debate. The mechanism of protection by glycine at low-dose treatment regimens is unknown. The same is true for the effect of glycine on regeneration of the tissue injured by ischaemia–reperfusion.
Since its first description as a cytoprotective agent in 1987 (Weinberg et al., 1987), glycine has been shown to protect against ischaemia–reperfusion injury [including related injuries due to anoxia, hypoxia, ROS, chemically induced energy depletion, and mere resupply of oxygen (reoxygenation)] in a great variety of experimental models. There are already profound reviews covering specific aspects of this protective action such as protection of the liver (Habib et al., 2006; Wang et al., 2010) and mechanisms of cytoprotection and inhibition of inflammation (Wheeler et al., 1999; Zhong et al., 2003; Gundersen et al., 2005). In addition to protection from ischaemia–reperfusion injury, substantial experimental evidence has been presented that glycine may also protect against other injurious processes such as liver fibrosis and alcoholic liver disease, gastric ulcer, cyclosporine A-induced nephrotoxicity and arthritis (Wheeler et al., 1999; Zhong et al., 2003). Even inhibition of tumour growth in rats and mice has been reported (Rose et al., 1999a,b).
In the present review, we will first summarize the existing experimental data on the effects of glycine in ischaemia–reperfusion and related injuries, differentiating between experiments performed with cells, isolated perfused or stored organs and in vivo experiments. Subsequently, we will depict the presently discussed mechanisms of the protection provided by glycine and critically evaluate their relevance. Finally, we will delineate the potential of glycine to be applied clinically in the prevention of ischaemia–reperfusion injury.
Protection of primary cells and cell lines by glycine
In primary cells and cell lines, injury was induced by hypoxia (anoxia), hypoxia–reoxygenation, inhibitors of oxidative phosphorylation and/or glycolytic energy production (chemical hypoxia, chemical energy depletion) or by ROS such as hydrogen peroxide or tert-butylhydroperoxide. Glycine and related compounds were applied at concentrations of 1 mM and above, just before the induction of cell injury or during the injurious process, if not otherwise stated.
In experiments with isolated renal proximal tubules and epithelial cells isolated thereof, glycine clearly diminished hypoxic and hypoxia–reoxygenation injury, with its greatest effect during the hypoxic period (Weinberg et al., 1987; 1989; 1990b; 1995; Mandel et al., 1990; Paller and Patten, 1992; Wetzels et al., 1993a; Moran and Schnellmann, 1997; Tijsen et al., 1997), and a half-maximal protective effect at around 0.8 mM glycine (Weinberg et al., 1990b). In parallel, protection from hypoxic injury (Marsh et al., 1993; Brecht and de Groot, 1994; Nichols et al., 1994; Carini et al., 1997; Nagatomi et al., 1997; Frank et al., 2000; Bramey et al., 2009), with half-maximal effects at glycine concentrations between 0.2 and 0.4 mM (Nichols et al., 1994; Nagatomi et al., 1997), but also from hypoxia–reoxygenation injury (Brecht and de Groot, 1994) and injury due to restoration of pH from 6.2 (at hypoxia) to 7.4 (at reoxygenation) (Qian et al., 1997), was shown for isolated hepatocytes, in which glycine also prevented cold ischemic injury and injury due to warm reoxygenation after cold storage as used in transplantation (Marsh et al., 1991; 1993). In both, isolated renal proximal tubules (Weinberg et al., 1990a; 1991a,b; 1995; Aleo and Schnellmann, 1992; Garza-Quintero et al., 1993; Miller and Schnellmann, 1993; Miller et al., 1994) and isolated hepatocytes (Dickson et al., 1992; Marsh et al., 1993; Sakaida et al., 1996; Carini et al., 1997; Petrat et al., 2006), glycine was also protective upon energy depletion by inhibitors of oxidative phosphorylation and/or glycolytic energy production such as cyanide, antimycin A, NO or iodoacetate, again with half-maximal protective effects at around 0.4 mM (Aleo and Schnellmann, 1992; Dickson et al., 1992).
Protection by glycine against injury due to chemical energy depletion was demonstrated also in liver sinusoidal endothelial cells (Nishimura and Lemasters, 2001; Dehne et al., 2004), human umbilical vein endothelial cells (Weinberg et al., 1992), kidney epithelial cells (MDCK cells) (Venkatachalam et al., 1995; 1996; Pan et al., 2005; Jiang et al., 2011) and PC-12 cells (Zhang et al., 2003), a cell line with neuronal properties. In human umbilical vein endothelial cells, half-maximal protection was achieved at 0.3 mM and in PC-12 cells at 0.7 mM glycine. In liver sinusoidal endothelial cells, glycine also prevented injury induced by the combined effects of simulated ischaemia–reperfusion (presence of cyanide, washout of cyanide) and pH restoration (simulated ischaemia at pH 6.2, simulated reperfusion at pH 7.4) with half-maximal protection at about 0.3 mM glycine (Nishimura et al., 1998). In rat cortical neuron cultures, glycine decreased hypoxic injury in mature neurons (Zhao et al., 2005), and in HL-1 cardiac myocytes, glycine blocked injury induced by re-energization and pH normalization following simulated ischaemia (NaCN/2-deoxyglucose, pH 6.4) (Ruiz-Meana et al., 2004). Only in immature neurons (Zhao et al., 2005) and HEK-293 cells (Pan et al., 2005), glycine was without any effect on injury due to hypoxia or chemical energy depletion.
Protection from cell injury due to hypoxia, hypoxia–reoxygenation and chemical energy depletion has also been demonstrated for a variety of compounds structurally related to glycine such as l-alanine, β-alanine, d-alanine and l-serine (Figure 1) (Garza-Quintero et al., 1990; 1993; Mandel et al., 1990; Weinberg et al., 1990b; 1992; Dickson et al., 1992; Paller and Patten, 1992; Marsh et al., 1993; Brecht and de Groot, 1994; Nichols et al., 1994; Frank et al., 2000; Zhang et al., 2003). The actual effect depended on the cell type, the mode of induction of the insult and the respective compound. As a rule, these compounds were somewhat or clearly less effective than glycine, and/or higher concentration were required for the same protective effect.
The results with glycine being protective against injury mediated by ROS are contradictory. In experiments with renal proximal tubules, where cell injury was induced by tert-butylhydroperoxide, glycine either protected against cellular injury (Miller et al., 1994) or not (Sogabe et al., 1996). Similar to the latter finding, glycine did not prevent tert-butylhydroperoxide-induced injury in isolated rat hepatocytes (Marsh et al., 1993). In a human intestinal epithelial cell line (HCT-8), glycine protected only when cells were treated with glycine for several hours prior to tert-butylhydroperoxide application but not when glycine and tert-butylhydroperoxide were applied simultaneously (Howard et al., 2010); in these experiments, alanine was without any protective effect. In renal proximal tubules, glycine did not protect from injury induced by iron-loading either (Sogabe et al., 1996). In human umbilical vein endothelial cells, glycine protected against cell injury induced by hydrogen peroxide (Weinberg et al., 1992), while it failed to do so in CHO cells (Brandi et al., 1992).
In summary, with the exception of im-/pre-mature cells, glycine protects cells of different origin against injury mediated by hypoxia, hypoxia–reoxygenation or chemical energy depletion. Half-maximal protection occurred around 0.4 mM (0.2–0.8 mM); full protection was achieved by glycine concentrations of 1 mM and above. Whether glycine protects cells from injury induced by ROS remains controversial.
Protection of isolated perfused or stored organs by glycine
Isolated perfused organs
In isolated rat livers, glycine (3, 6 and 12 mM) dose-dependently prevented liver injury due to perfusion first with a hypoxic and subsequently an oxygenated buffered salt solution (Deters et al., 1997), and addition of 2 mM glycine upon reperfusion minimized reperfusion injury following a period of low-flow ischaemia (Zhong et al., 1996). In the latter study, the protective effect was half-maximal at already 0.13 mM glycine. In the isolated perfused rat liver, addition of 12 mM glycine to the perfusion buffer clearly decreased liver injury induced by tert-butylhydroperoxide (Deters et al., 1998).
In isolated kidneys perfused in the absence of erythrocytes or artificial oxygen carriers, hypoxic medullar injury already develops despite perfusion with oxygenated medium (95% O2, 5% CO2). This injury was largely prevented by glycine, l-alanine, d-alanine or β-alanine, with glycine being somewhat more protective than the other compounds (Baines et al., 1990; Silva et al., 1991; Heyman et al., 1992a). To achieve full protection, at least 2 mM of the protective compounds had to be present. l-serine had only a slightly protective effect, and taurine was not protective in these experiments.
In Langendorff-perfused rat hearts, 10 mM glycine prevented injury associated with pH normalization upon reperfusion following ischaemia (Ruiz-Meana et al., 2004).
Cold-stored organs
In renal transplantation experiments conducted on mongrel dogs, inclusion of 5 mM glycine to the preservation solution during cold storage clearly improved kidney function following transplantation (Mangino et al., 1991); in these experiments, 30 mM glycine, however, was toxic. On the other hand, in a pig lung transplantation model, supplementation of the preservation solution with 50 mM glycine resulted in a decreased injury of the transplanted lung (Gohrbandt et al., 2006), and in an ex vivo rat lung perfusion model, addition of 5 mM glycine to the preservation solution ameliorated lung injury upon warm reperfusion (Omasa et al., 2003). Likewise, in an in situ small intestine reperfusion model in dogs, inclusion of 5 mM glycine in the storage solution decreased intestinal injury (Mangino et al., 1996). Less clear-cut results were obtained with the liver. In rat livers stored for several hours in preservation solution [University of Wisconsin (UW) solution], replacement of glutathione by 10 mM glycine decreased injury of the transplanted liver and increased survival of the recipients (den Butter et al., 1993a). Such a protective effect, however, could not be demonstrated in the dog. Likewise, in rabbit livers, addition of 15 mM glycine to cold UW solution, again in the place of glutathione, did not diminish enzyme release or improve bile production upon mechanical reperfusion with a buffered salt solution (den Butter et al., 1994). In contrast, addition of 10 mM glycine to the reperfusion buffer decreased hepatocellular injury but again was without protective effect on bile formation. Similarly, in rat livers stored in UW solution and mechanically reperfused in the presence of 5 mM glycine, non-parenchymal cell injury was effectively prevented (half-maximal effect at about 0.1 mM glycine) (Currin et al., 1996).
In summary, with the exception of cold-stored livers, glycine (with a concentration dependency comparable with the one in primary cells and cell lines) protects not only isolated perfused but also cold-stored organs of different origin against ischaemia–reperfusion injury.
Protection by glycine in vivo
Liver
In rats and rabbits, pre-ischaemic i.v. infusion of glycine (5–200 mg·kg−1) clearly decreased ischaemia–reperfusion injury of the liver (Duenschede et al., 2006; Yamanouchi et al., 2007; Sheth et al., 2011). A comparable protective effect was obtained by injection of taurine (10 mg·kg−1) (Kincius et al., 2007). Pre-ischaemic injection of glycine (c. 150 mg·kg−1) or taurine (c. 240 mg·kg−1) even decreased ischaemia–reperfusion injury in pre-diseased (fatty) livers (Bruns et al., 2011).
In liver transplantation experiments in the rat, i.v. infusion of glycine to the donor (c. 130 mg·kg−1) before harvest decreased injury of the transplanted liver and increased survival of the recipient (Rentsch et al., 2005; Liu et al., 2006). Similar to glycine, taurine pre-treatment of the donor improved graft survival after transplantation (Schemmer et al., 2005). Here, maximal protection was already achieved by i.v. infusion of c. 80 mg taurine per kilogram before harvesting. Again in the rat, rinsing of the transplants prior to implantation with Carolina rinse solution additionally containing 5 mM glycine largely prevented liver injury following transplantation (Bachmann et al., 1995). In a pig model of a non-heart-beating donor, inclusion of 25 mg glycine per kilogram during normothermic reperfusion following warm ischaemia (still within the donor using a heart lung machine) decreased liver injury and improved outcome following transplantation (Barros-Schelotto et al., 2002). In dog liver transplantation experiments, glycine was not protective when included in the UW solution during cold preservation (see above). In these experiments, however, glycine (c. 30 mg·kg−1) turned out to protect the transplanted liver and to increase survival when given to the recipient after transplantation (den Butter et al., 1993a).
Glycine also protected the liver against in situ mechanical manipulation (Schemmer et al., 1998; 2001a) or resection (Ito et al., 2008; Benko et al., 2010), where ischaemia–reperfusion is not the primary event but contributes to liver injury.
Kidney
In rats, dietary glycine (5% glycine, for 2 days before ischaemia and continued for the whole experimental period of 2 weeks) combined with a glycine bolus injection (100 mg·kg−1) 5 min before the end of the ischaemic period decreased renal ischaemia–reperfusion injury (Yin et al., 2002). Protection by glycine occurred, however, only during short periods of ischaemia (15 min) and was lost with longer durations of ischaemia. No protection or even an increase in renal injury by glycine was observed with kidney ischaemia of 30 to 45 min duration (Heyman et al., 1992b; Wetzels et al., 1993b). In these experiments in rats, glycine was i.v. applied either at a dose of 1.035 g·kg−1 (for 90 min starting with the onset of ischaemia, resulting in plasma glycine concentrations of 3 to 4 mM) (Heyman et al., 1992b) or at a rate to increase serum glycine concentrations above 2 mM (starting 60 min before ischaemia) (Wetzels et al., 1993b). On the other hand, in renal transplantation experiments in mongrel dogs, glycine (c. 60 mg·kg−1) given to the recipient during surgery improved survival and post-transplant renal function (den Butter et al., 1993b).
Small intestine
In mice receiving two gastric gavages before the induction of ischaemia, accumulating to c. 4 g glycine per kilogram, intestinal injury was decreased and survival improved following reperfusion (Iijima et al., 1997). Attenuation of ischaemia–reperfusion injury of the small intestine was also achieved in rats by local i.a. infusion of c. 1.5 g glycine per kilogram each before ischaemia, before reperfusion or before both time points (Lee et al., 2001; 2002), and by i.v. infusion of 0.5, 0.75 or 1 g glycine per kilogram throughout the reperfusion period (Jacob et al., 2003; Kallakuri et al., 2003). Again in experiments in rats, we recently demonstrated that even a very low glycine dose (5, 10, 20 or 75 mg·kg−1, total doses, infused i.v. before ischaemia and during reperfusion) effectively diminished ischaemia–reperfusion injury; already upon infusion of 10 mg glycine per kilogram, maximal protection was obtained (Petrat et al., 2011). In these experiments, pre-ischaemic blood plasma glycine concentrations increased with increasing glycine doses from 280 to 330, 340, 380 and 680 µM respectively. In a rat small bowel transplantation model, glycine infusion in the donor before harvest and subsequently from the onset of reperfusion in the recipient (1 g glycine per kilogram, each) attenuated injury of the transplanted graft (Schaefer et al., 2008). As reported for the liver, glycine pre-treatment attenuated the inflammatory response to mechanical manipulation in the small intestine as well (Stoffels et al., 2011).
Other organs
In the dog, i.v. infusion of 750 mg·kg−1 glycine at the end of ischaemia and at the beginning of reperfusion preserved skeletal muscle function, decreased oedema and necrotic injury in the reperfused muscle (Ascher et al., 2001). In a porcine lung transplant model, donor preconditioning by intravenous infusion of 3.75 g glycine before organ procurement effectively improved graft function following transplantation (Gohrbandt et al., 2006). In a porcine right heart transplantation model, i.v. infusion of glycine (c. 136 mg·kg−1) to donor animals before harvest improved early post-ischaemic right ventricular compliance and decreased myocardial injury (Warnecke et al., 2006).
Haemorrhagic shock
A diet containing 5% glycine, fed for 4 days prior to the induction of haemorrhagic shock, increased survival in rats (Mauriz et al., 2001). In other experiments in rats, glycine proved to be protective even when injected intravenously just prior to resuscitation (Zhong et al., 1999). Glycine treatment largely increased survival, with a half-maximal effect at 25 mg·kg−1, and largely decreased injury of the lung, kidney and liver. An equimolar dose of alanine was without any effect under these conditions. The above results were confirmed in studies by Wang et al. (2004).
In summary, with the exception of the kidney, glycine (sometimes at very low doses) protects the liver, small intestine, lung, skeletal muscle and potentially the heart against injury mediated by ischaemia–reperfusion in vivo and increases survival following haemorrhagic shock and resuscitation.
Mechanism of protection by glycine
Direct cytoprotection
In cell injury due to hypoxia (anoxia) or chemical energy depletion (in the following collectively named as hypoxic cell injury), it is generally accepted that protection by glycine requires the presence of the amino acid but does not rely on its metabolism, on protein synthesis, on changes in cytosolic calcium or on the maintenance of the intracellular pH and GSH pool, and that glycine does neither support energy (ATP) generation nor help to diminish energy consumption (Baines et al., 1990; Weinberg et al., 1990a; 1991b; 1994; Dickson et al., 1992; Garza-Quintero et al., 1993; Brecht and de Groot, 1994; Churchill et al., 1995; Sakaida et al., 1996; Nagatomi et al., 1997). On the other hand, glycine (and also alanine) decreases proteolysis in the hypoxic cells, especially the one catalysed by Ca2+-dependent, non-lysosomal proteases (including calpains) (Dickson et al., 1992; Nichols et al., 1994; Tijsen et al., 1997). Inconsistent results have been reported for the effects of glycine on the accelerated phospholipid degradation (Venkatachalam et al., 1995; Sakaida et al., 1996) and on plasma membrane blebbing under conditions of hypoxia and reoxygenation (Dickson et al., 1992; Garza-Quintero et al., 1993; Brecht and de Groot, 1994; Venkatachalam et al., 1996).
In energy-depleted isolated renal proximal tubules, strychnine and other glycine receptor antagonists – at millimolar levels, well above micromolar and submicromolar concentrations that antagonize the neuronal glycine receptor – were as protective as glycine (Aleo and Schnellmann, 1992; Miller and Schnellmann, 1993; Moran and Schnellmann, 1997). Both, strychnine and glycine prevented chloride uptake, and chloride channel inhibitors provided protection as well. Based on these results, involvement of the glycine receptor in cytoprotection was proposed assuming that at high (cytoprotective) concentrations, glycine inhibits opening of the chloride channel. Protection by strychnine from hypoxic injury has subsequently been shown for several cell types, including hepatocytes, and for the isolated perfused liver (Currin et al., 1996; Zhong et al., 1996; Carini et al., 1997; Zhang et al., 2003). Moreover, in kidney epithelial (MDCK) cells, chloride channel blockers proved to be protective as well (Venkatachalam et al., 1996), and in energy-depleted hepatocytes, chloride-free medium was described to be protective (Carini et al., 1997). In further support of an essential role of the glycine receptor in the protective function of glycine, in HEK-293 cells lacking the glycine receptor, protection by glycine was restored by transfection with the α1-subunit of the glycine receptor (Pan et al., 2005). Furthermore, in the transfected HEK-293 cells and in MDCK cells (possessing the receptor), protection by glycine could be suppressed by RNA interference with the α1-subunit of the glycine receptor.
In obvious contradiction to the above proposal that glycine protects by inhibiting opening of a chloride channel of a putative glycine receptor, in hypoxic isolated (cultured) rat hepatocytes and energy-depleted MDCK cells and neuronal (PC-12) cells, removal of extracellular chloride did not protect while glycine was clearly protective (Venkatachalam et al., 1996; Frank et al., 2000; Zhang et al., 2003). Likewise, reperfusion of cold-stored rat livers with warm chloride-free buffer did not reduce reperfusion-induced injury of non-parenchymal cells and was without effect on the protection provided by glycine under these conditions (Currin et al., 1996). Furthermore, in hepatocytes and endothelial cells, even stimulation of chloride influx by glycine has been reported (at high, protective concentrations and inhibitable by strychnine at micromolar concentrations) (Yamashina et al., 2001; Qu et al., 2002; Yamashina et al., 2007). On the other hand, it is still unclear whether functional glycine receptors do exist in hepatocytes, endothelial and renal cells (Froh et al., 2002; van den Eynden et al., 2009).
Based on the results of their experiments with MDCK cells (protection by glycine, strychnine, and a variety of chloride channel blockers but lack of protection by chloride-free medium), Venkatachalam and coworkers proposed that glycine-gated chloride channel receptors are central components of multimeric proteins forming plasma membrane pores under injurious conditions (pathological pores), but that these pores are unrelated to the chloride channel activity of the glycine receptor (Venkatachalam et al., 1996). Glycine was suggested to prevent the formation of these pores. Using the same experimental model and studying the permeability characteristics of fluoresceinated dextrans of graded molecular size, they subsequently showed that the membrane defects evolve from small pores permeable only to propidium iodide (668 Da) and the smallest dextrane (4000 Da), before enlarging with time to become permeable to dextrans up to 145 000 Da (Dong et al., 1998). In these experiments, pore formation could not only be prevented by glycine but also by a membrane-impermeant homobifunctional ‘nearest-neighbour’ cross-linking agent and, in later experiments, by an impermeant strychnine derivative (Dong et al., 2001). Evidence for the formation of pathological pores, unrelated to the chloride channel activity of the glycine receptor, under conditions of energy deficiency and its prevention by glycine has also been presented in experiments with other cell types. In isolated cultured rat hepatocytes, glycine prevented a hypoxia-induced influx of the cations sodium, cobalt and nickel and an efflux of the anion Newport Green (Frank et al., 2000). In cultured hepatic sinusoidal endothelial cells, upon induction of chemical hypoxia, there was a delayed increase in the permeability of the plasma membrane to the anionic fluorophores calcein and lucifer yellow followed, with a time lag, by increased permeabilities to the cation propidium and to high molecular weight dextrans (40–2000 kDa) (Nishimura and Lemasters, 2001). These alterations were largely decreased or prevented by glycine. Entry of anions through the pathological pores (paralleled by sodium entry due to inhibition of Na,K-ATPase and opening of monovalent cation channels) was suggested to lead to cell swelling and bleb formation by colloid osmotic forces, ultimately resulting in plasma membrane rupture and thus full permeability to both low and high molecular weight solutes.
Overall, there is compelling evidence that cytoprotection mediated by glycine in hypoxic cell injury results from prevention of an increased permeability of the plasma membrane. The underlying structural alterations and the mechanism of their prevention by glycine, however, are largely unknown. Although most likely being formed, a pathological pore has never been identified, presumably because such a pore is not a fixed (permanent) entity, but several of such pores of different composition and characteristics develop in the course of injury (Figure 5). Since, however, agonists of the glycine receptor other than glycine but also antagonists of the glycine receptor as well as inhibitors of chloride channels proved to be protective as well, a relationship between the glycine receptor or components of this receptor and the formation of a pathological pore appears to be likely. However, all these protective compounds had to be applied at high concentrations, and cytoprotection was also achieved by compounds, such as alanine and serine (Figure 1), which are closely related to glycine but only poorly interact with the glycine receptor (Baines et al., 1990; Garza-Quintero et al., 1990; 1993; Mandel et al., 1990; Weinberg et al., 1990b; 1992; Silva et al., 1991; Dickson et al., 1992; Heyman et al., 1992a; Paller and Patten, 1992; Marsh et al., 1993; Brecht and de Groot, 1994; Nichols et al., 1994; Frank et al., 2000; Zhang et al., 2003). According to these results, and still in line with the other results, at least under certain conditions proteins only structurally related to components of the glycine receptor (but not components of the glycine receptor themselves) appear to be involved in the formation of the pathological pores.
Figure 5.
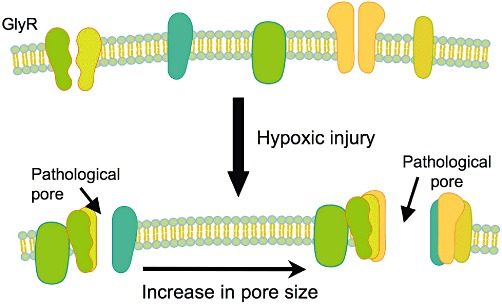
Formation of pathological plasma membrane pores during hypoxic injury and its prevention by glycine. During hypoxic cell injury, pathological pores are formed in the plasma membrane with increasing size. These pores are composed of different proteins, with components of GlyR presumably playing a central role. Glycine prevents the formation of these pores by binding to the GlyR components but possibly also to other structurally related proteins.
The formation of pathological plasma membrane pores and its prevention by glycine accounts for several of the effects of glycine on injurious alterations in hypoxic cells such as inhibition of the influx of sodium or calcium and, partly that way, of the activation of proteases and phospholipases. The formation of the plasma membrane pores (in their final forms), however, is a very late event in hypoxic cell injury (Dong et al., 1998; Frank et al., 2000; Nishimura and Lemasters, 2001) as also indicated by the observation that addition of glycine 1 h after energy depletion still provided effective protection (Dickson et al., 1992). This implies, on the other hand, that other injurious cellular alterations triggered by ATP depletion but not affected by glycine occur prior (upstream) to the formation of the pathological plasma membrane pores and/or independent of plasma membrane pore formation. An important example for the latter possibility appears to be the opening of the mitochondrial permeability transition pore and/or the development of other mitochondrial defects such as damage to complex I (Qian et al., 1997; Weinberg et al., 1997; 2000; Park et al., 2011). Due to these lesions, mitochondrial energy production may already be severely (irreversibly) impaired at a time point when the plasma membrane still maintains its selective permeability due to the protection provided by glycine, thus paradoxically indicating a still viable cell. Accordingly, withdrawal of glycine without recovery of the mitochondrial function led to a rapid loss of plasma membrane protection and thus cell death (Weinberg et al., 1997; 2000; Park et al., 2011).
There are, however, some observations, which, at least at first sight, are not compatible with the assumption that glycine protects from hypoxic cell injury by preventing the formation of plasma membrane pores. Examples are an involvement of ERK1/2 and Akt signalling pathways in glycine cytoprotection (Jiang et al., 2011) or direct inhibition of cytosolic proteases already at 2 mM glycine (Ferguson et al., 1993). The significance of these observations, however, remains to be established.
The results of the few studies on the protection by glycine against cell injury due to ROS or upon reoxygenation (reperfusion) provide only very limited and partly inconclusive information on the underlying protective mechanism. Thus, there is some evidence that glycine cannot protect from membrane injury due to lipid peroxidation (Sogabe et al., 1996). On the other hand, an increase in cellular GSH achieved by pre-treatment with glycine may contribute to its protective function against oxidative challenge due to tert-butylhydroperoxide treatment (Howard et al., 2010). In isolated hepatocytes where cell injury was induced by combining hypoxia–reoxygenation with restoration of the pH from 6.2 to 7.4, protection by glycine, added at reoxygenation/pH restoration, was independent of the presence of chloride in the medium, and no evidence for an effect of glycine on mitochondrial permeability transition pore opening was found (Qian et al., 1997). In contrast, under comparable conditions, opening of the permeability transition pore appears to be prevented by glycine in cardiomyocytes (Ruiz-Meana et al., 2004). In these experiments, glycine even blocked opening of the transition pore in the isolated mitochondria.
Inhibition of the inflammatory response
Thurman and coworkers were the first to suggest that glycine protects from ischaemia–reperfusion injury by inhibiting activation of macrophages and other cells of the immune system and thus the inflammatory response (Figure 4). In experiments with rat Kupffer cells, the resident macrophages in the liver, they demonstrated that their activation by lipopolysaccharides (LPS) was largely diminished by glycine with a half-maximal effect at c. 200 µM glycine (Ikejima et al., 1997). Since glycine blunted the LPS-induced increase in the cytosolic calcium concentration, and since the glycine effects were prevented by 1 µM strychnine or chloride-free buffer, they proposed that glycine activates a glycine-gated chloride channel, which hyperpolarizes the plasma membrane and thus inhibits calcium influx and activation of the Kupffer cells, similar to its action in neuronal cells (Figure 3); in these experiments, a high concentration of strychnine (1 mM) mimicked the glycine effects, while 1 mM alanine was without any effect.
Meanwhile, the existence of glycine-gated chloride channels on cells of the immune system, especially on macrophages and neutrophils, is generally accepted (Froh et al., 2002; van den Eynden et al., 2009). Inhibition of activation by glycine has been shown for neutrophils (half-maximal effect around 0.3 mM) (Wheeler et al., 2000), splenic macrophages (half-maximal inhibition at 0.55 mM) (Li et al., 2001), alveolar macrophages (half-maximal effect already at around 10 µM) (Wheeler and Thurman, 1999) and cultured mononuclear cells (CD4+ T lymphocytes) (half-maximal effect at c. 1 mM) (Bruck et al., 2003). On the other hand, for Kupffer cells, a lack of blockade of LPS-induced TNF-α formation by glycine has been reported (Currin et al., 1996), and in peritoneal macrophages, glycine pre-treatment for hours to days even improved TNF-α and NO formation following activation by LPS, an effect that was suggested to be mediated by neutral amino acid transporters (Carmans et al., 2010). In accordance with its capability of inhibiting cells of the immune system, glycine has been shown to protect from a variety of injurious processes where inflammation decisively triggers or amplifies the injurious process such as in endotoxin shock (Wheeler et al., 1999; Zhong et al., 2003).
Relevance
Blood plasma glycine concentration has been reported to vary between 170 and 330 µM both in humans and in experimental animals (Evins et al., 2000; Iresjöet al., 2006; Petrat et al., 2011). After a meal, plasma glycine concentration may increase from 250 to 330 µM (Iresjöet al., 2006). The structurally related amino acids alanine, serine and taurine are present in blood plasma at concentrations around 220 to 620, 70 to 180 and 40 to 100 µM respectively (Iresjöet al., 2006). Due to active uptake, the intracellular glycine concentrations are significantly higher than the extracellular levels (Weinberg et al., 1991c; Weinberg, 1992). Exceptionally high glycine concentrations exist in the kidney with more than 20 mM glycine in the tubular cells of the rabbit renal cortex. The glycine concentrations in blood plasma and the extracellular space equilibrate within minutes (Hahn et al., 1999; Hahn, 2006a). The half-life of glycine in the blood depends on the dose administered and may vary between half an hour and several hours. The majority of glycine administered is taken up by cells and metabolized, primarily in the liver. Only a minor amount is excreted in the urine.
Remarkably, the physiological presence of glycine under in vivo conditions has not been taken into consideration in the discussion of its protective mechanism(s) so far. The only exception is the kidney, where the very high glycine content of the tubule cells has been suggested to be responsible for the missing protective effect of glycine treatment under in vivo conditions (Weinberg, 1992). Upon energy depletion, glycine of the tubule cells is assumed to leak into the extracellular space reaching concentrations high enough to fully protect (still viable) cells from hypoxic injury. Upon reperfusion, however, this protection should be lost due to washout of glycine.
Studies on the protective properties of glycine and related compounds in primary cells, in cell lines and in isolated perfused or stored organs have been, as far as we can see, exclusively performed in the absence of blood, plasma or serum, using buffered salt solutions as incubation, perfusion or storage medium. Under these conditions, not only in cells but also in perfused or stored organs, direct cytoprotection should be the preferred mode of protection by glycine (Figure 4). In primary cells and cell lines, glycine necessarily prevents injury this way. In perfused or stored organs, protection is not only achieved by glycine but also by glycine receptor antagonists and glycine-related compounds, which only poorly activate the glycine receptor (Baines et al., 1990; Silva et al., 1991; Heyman et al., 1992a). In addition, in the absence of blood, the immune response is impaired and thus should contribute less to the injurious process. In marked contrast to the in vitro systems, clear evidence for the involvement of direct cytoprotection in the protection provided by glycine against ischaemia–reperfusion injury under in vivo conditions is missing.
In contrast to direct cytoprotection, protection from ischaemia–reperfusion injury provided by inhibition of the inflammatory response should mainly play a role in the presence of blood and thus under in vivo conditions, and here due to its requirement of oxygen especially in the reperfusion phase (Figure 4). In line with this mechanism of protection, in those in vivo experiments where glycine protected from ischaemia–reperfusion injury (and where parameters of the immune system were determined), a concomitant decrease in inflammation has been reported (Zhong et al., 1999; Mauriz et al., 2001; Wang et al., 2004; Rentsch et al., 2005; Duenschede et al., 2006; Liu et al., 2006; Yamanouchi et al., 2007; Schaefer et al., 2008; Bruns et al., 2011; Sheth et al., 2011). In all cases, however, the cause–effect relationship remained unclear. A decrease in the inflammatory response may merely result from a decrease in upstream injurious events such as a decrease in cell injury due to direct cytoprotection by glycine. On the other hand, protection by glycine receptor agonists other than glycine (Schemmer et al., 2005; Kincius et al., 2007; Bruns et al., 2011) and the lack of protection by alanine (Zhong et al., 1999) support the notion that inhibition of the inflammatory response is the decisive protective mechanism of glycine against ischaemia–reperfusion injury in vivo.
Both, direct cytoprotection and inhibition of the inflammatory response (Figure 4), occurred at half-maximal glycine concentrations of around 0.4 mM (see above), that is somewhat above the physiological range of the plasma (interstitial) glycine concentration. Accordingly, both protective mechanisms should be already operative under normal (physiological) in vivo conditions. Thus, to provide significant additional protection, glycine doses high enough to increase the plasma glycine concentration close to 1 mM should be required. In accordance with this postulation, plasma glycine concentrations of 1 mM and above were indeed achieved in the vast majority of the in vivo studies where protection by glycine was reported (see above). On the other hand, there is a significant number of studies where protection against ischaemia–reperfusion injury was already attained at low glycine or taurine doses (den Butter et al., 1993a; Zhong et al., 1999; Wang et al., 2004; Kincius et al., 2007; Petrat et al., 2011; Sheth et al., 2011). At these low-dose treatment regimens, the plasma glycine concentration does not increase to a level to elicit (additional) direct cytoprotection or inhibition of the inflammatory response. Thus, at least under these conditions, the mechanism of protection by glycine against ischaemia–reperfusion injury remains elusive, and alternative mechanisms of protection need to be considered such as stimulation of intracellular protective signalling pathways like those mediated by Akt (PKB) and glycogen synthase kinase 3β (GSK3β) (Heusch et al., 2008). This possibility was studied in intestinal ischaemia–reperfusion injury in rats, however, with a negative finding (unpubl. results). Ischaemia and reperfusion (10 min and 5 min) significantly increased phosphorylation of Akt without altering phosphorylation of GSK3β. Pre-treatment with 20 mg glycine per kilogram (i.v. infusion for 30 min before ischaemia) was without any effect on the phosphorylation of both kinases.
Clinical application of glycine
Application independent from ischaemia–reperfusion injury
Glycine is a standard component of each amino acid solution used in parenteral nutrition (Stein et al., 2009). The doses of glycine (per hour) used for parenteral feeding are clearly lower than the glycine doses applied for protection in most animal studies, but they are close to the doses achieved with the low-dose glycine treatment regimens (see above).
Administration of glycine has also been used in several clinical trials to ameliorate cognitive deficits and dementia, but especially to augment antipsychotic treatment of schizophrenia as an adjunct to conventional neuroleptic therapy (Heresco-Levy et al., 1996; Evins et al., 2000; Leung et al., 2008; Palmer et al., 2008). In these studies, glycine was given orally for days to weeks. Partly, high single glycine doses of up to 0.8 g·kg−1·day−1 were used. In some of these studies, serum/plasma glycine concentrations were determined as well. For instance, upon treatment with 60 g glycine (30 g twice a day), serum glycine increased from 0.239 mM to 1.390 mM at week 8 (Evins et al., 2000). In all these studies, glycine treatment was well tolerated (but in some studies, the primary end point was not achieved).
In endoscopic surgery, non-electrolyte solutions containing glycine at high concentrations are used as irrigating fluids with the potential complication of the systemic absorption of high amounts of glycine (Hahn, 2006a; Collins et al., 2007). From this complication and related experimental studies, valuable information on the dose and concentration dependency of glycine intoxication has been derived. In humans, i.v. (acute) uptake of at least 20 g of glycine is required to result in toxic symptoms, and the threshold plasma glycine concentration where adverse symptoms start to develop is around 5 mM (Sandfeldt and Hahn, 1999; Hahn, 2006a,b). Typical adverse effects of glycine include visual disturbances to transient blindness, prickling and burning sensations in the face and neck, transient confusion, arterial hypotension and cardiac impairment, as well as nausea and vomiting (Sandfeldt and Hahn, 1999; Hahn, 2006a; Collins et al., 2007).
Application in ischaemia–reperfusion injury
As compared with the large number of experimental studies, there have been only a few clinical trials performed with glycine to protect against ischaemia–reperfusion injury.
In 1999, Arora et al. reported that glycine rinse decreases injury of the transplanted liver (Arora et al., 1999). In their study with a total number of 50 patients, livers were cold-stored in UW solution. After completion of both vena cava anastomoses (i.e. immediately before reperfusion), the hepatic artery was flushed with 150 mL, and the portal vein with 350 mL of either an electrolyte solution or the same solution supplemented with 2 mM glycine. Glycine rinse decreased postoperative transaminase elevations and the occurrence of bile duct strictures, and none of the patients in the glycine group required retransplantation as compared with three patients in the no-glycine group.
Using a somewhat different approach, the effect of glycine on reperfusion injury of the liver following liver transplantation was also studied by Schemmer et al. (2001b; 2002). In their study, liver recipients were infused with glycine (5.6 g) 1 h prior to reperfusion of the transplanted organ and subsequently daily during the following week, resulting in a fivefold increase in the serum glycine concentration. In preliminary results from seven patients, serum transaminases were markedly lower compared with matched historic control patients. Based on these data, a prospective double-blinded multicenter clinical trial was started in 2005, using a comparable glycine treatment regimen but a daily glycine dose of 11 g (Luntz et al., 2005). As a preliminary result, decreased transaminases have been reported (Hoffmann et al., 2011). The final results, however, have not been published yet.
In a double-blinded, placebo-controlled trial, the efficacy of sublingual glycine treatment was studied in 200 patients with acute ischaemic stroke (0.5, 1.0 or 2.0 g·day−1 for 5 days). One and 2.0 g glycine per day significantly improved clinical (functional) outcome and tended to decrease the 30 day mortality (Gusev et al., 2000).
High-risk cardiac patients scheduled to undergo cardiac surgery with the use of extracorporal circulation were treated preoperatively with oral immune-enhancing nutrition supplement (OIENS) for at least 5 days; glycine (around 32 g·day−1) was added to the liquid supplement to prevent ischaemia–reperfusion injury (Tepaske et al., 2007). Glycine inclusion failed to improve organ function and postoperative recovery or to decrease postoperative infectious morbidity.
Conclusions
Glycine has a great potential to protect from ischaemia–reperfusion injury. This is clearly suggested by numerous studies with cells, isolated perfused or cold-stored organs and experimental animals. However, protection by glycine from ischaemia–reperfusion injury does not apply to all organs and under all conditions. For certain organs such as the liver and the small intestine, several reports demonstrating protection have been published, while for other organs, especially the heart, very limited information on protection by glycine is available. Protection of the kidney in vivo appears to be unlikely. The brain deserves special attention due to the fact that glycine acts as a neurotransmitter in the CNS.
Glycine may prevent ischaemia–reperfusion injury by direct cytoprotection, presumably by inhibition of the formation of plasma membrane pores and by inhibition of the inflammatory response. However, the relevance of both protective mechanisms in detail remains unclear. Direct cytoprotection is most likely responsible for and limited to the protection in cells and perfused/cold-stored organs in the absence of blood or plasma. In contrast, under in vivo conditions, inhibition of the inflammatory response is presumably involved in the protective action of glycine against ischaemia–reperfusion injury; this, however, only at glycine concentrations several times higher than the physiological values (i.e. close to 1 mM or above). The mechanism of protection achieved by low-dose glycine treatment regimens is unknown.
Glycine is a compound with very low toxicity. In addition, its adverse effects and their dose/concentration dependencies are well known. Comparison with those doses/concentrations providing protection reveals a wide therapeutic safety margin, especially with the low-dose treatment regimens. Clinical trials on the protection of glycine against ischaemia–reperfusion injury have been performed mainly in special surgical areas, and their results are only partly convincing. Trials in those areas where the protection by glycine against ischaemia–reperfusion injury has been suggested by experimental data most conclusively, such as intestinal ischaemia–reperfusion or haemorrhagic shock, are still missing and thus are urgently awaited.
Glossary
- Gly
glycine
- ROS
reactive oxygen species
Conflict of interest
Herbert de Groot is a consultant of Dr Franz Köhler Chemie GmbH (Bensheim, Germany). Rainer Schulz is on the advisory board of AstraZeneca and provides lectures for AstraZeneca, Merckle Recordati and SanofiAventis.
References
- Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks – a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- Aleo MD, Schnellmann RG. The neurotoxicants strychnine and bicuculline protect renal proximal tubules from mitochondrial inhibitor-induced cell death. Life Sci. 1992;51:1783–1787. doi: 10.1016/0024-3205(92)90048-t. [DOI] [PubMed] [Google Scholar]
- Arora AS, Nichols JC, DeBernardi M, Steers JL, Krom RA, Gores GJ. Glycine rinse protects against liver injury during transplantation. Transplant Proc. 1999;31:505–506. doi: 10.1016/s0041-1345(98)01729-1. [DOI] [PubMed] [Google Scholar]
- Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129:231–235. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Peng XX, Currin RT, Thurman RG, Lemasters JJ. Glycine in Carolina rinse solution reduces reperfusion injury, improves graft function, and increases graft survival after rat liver transplantation. Transplant Proc. 1995;27:741–742. [PubMed] [Google Scholar]
- Baines AD, Shaikh N, Ho P. Mechanisms of perfused kidney cytoprotection by alanine and glycine. Am J Physiol. 1990;259:F80–F87. doi: 10.1152/ajprenal.1990.259.1.F80. [DOI] [PubMed] [Google Scholar]
- Barros-Schelotto P, Net M, Valero R, Ruiz A, Almenara R, Capdevila L, et al. Reduced reperfusion injury by glycine in a porcine liver transplantation model with non-heart-beating donors. Transplant Proc. 2002;34:1114–1117. doi: 10.1016/s0041-1345(02)02636-2. [DOI] [PubMed] [Google Scholar]
- Benko T, Frede S, Gu Y, Best J, Baba HA, Schlaak JF, et al. Glycine pretreatment ameliorates liver injury after partial hepatectomy in the rat. J Invest Surg. 2010;23:12–20. doi: 10.3109/08941930903469466. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflugers Arch. 2004;447:776–779. doi: 10.1007/s00424-003-1073-4. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol. 2006;147(Suppl. 1):S109–S119. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramey T, Freitag P, Fandrey J, Rauen U, Pamp K, Erhard J, et al. No evidence for protective erythropoietin alpha signalling in rat hepatocytes. BMC Gastroenterol. 2009;9:26. doi: 10.1186/1471-230X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi G, Luzzi L, Giacomoni P, Albano A, Cattabeni F, Cantoni O. Differential effect of the amino acid cystine in cultured mammalian and bacterial cells exposed to oxidative stress. Mutat Res. 1992;281:157–161. doi: 10.1016/0165-7992(92)90002-y. [DOI] [PubMed] [Google Scholar]
- Brecht M, de Groot H. Protection from hypoxic injury in cultured hepatocytes by glycine, alanine, and serine. Amino Acids. 1994;6:25–35. doi: 10.1007/BF00808120. [DOI] [PubMed] [Google Scholar]
- Bruck R, Wardi J, Aeed H, Avni Y, Shirin H, Avinoach I, et al. Glycine modulates cytokine secretion, inhibits hepatic damage and improves survival in a model of endotoxemia in mice. Liver Int. 2003;23:276–282. doi: 10.1034/j.1600-0676.2003.00839.x. [DOI] [PubMed] [Google Scholar]
- Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, et al. Glycine and Taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011;18:205–213. doi: 10.1111/j.1549-8719.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- den Butter G, Lindell SL, Sumimoto R, Schilling MK, Southard JH, Belzer FO. Effect of glycine in dog and rat liver transplantation. Transplantation. 1993a;56:817–822. doi: 10.1097/00007890-199310000-00007. [DOI] [PubMed] [Google Scholar]
- den Butter G, Schilling MK, Lindell SL, Gandolf D, Southard JH, Belzer FO. Effect of glutathione and glycine in kidney preservation. Transplant Proc. 1993b;25:1633–1634. [PubMed] [Google Scholar]
- den Butter G, Marsh DC, Lindell SL, Belzer FO, Southard JH. Effect of glycine on isolated, perfused rabbit livers following 48-hour preservation in University of Wisconsin solution without glutathione. Transpl Int. 1994;7:195–200. doi: 10.1007/BF00327087. [DOI] [PubMed] [Google Scholar]
- Carini R, Bellomo G, de Cesaris MG, Albano E. Glycine protects against hepatocyte killing by KCN or hypoxia by preventing intracellular Na+ overload in the rat. Hepatology. 1997;26:107–112. doi: 10.1002/hep.510260114. [DOI] [PubMed] [Google Scholar]
- Carmans S, Hendriks JJ, Thewissen K, Van den Eynden J, Stinissen P, Rigo JM, et al. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J Neurosci Res. 2010;88:2420–2430. doi: 10.1002/jnr.22395. [DOI] [PubMed] [Google Scholar]
- Churchill TA, Green CJ, Fuller BJ. Protective properties of amino acids in liver preservation: effects of glycine and a combination of amino acids on anaerobic metabolism and energetics. J Hepatol. 1995;23:720–726. doi: 10.1016/0168-8278(95)80039-5. [DOI] [PubMed] [Google Scholar]
- Collins JW, Macdermott S, Bradbrook RA, Drake B, Keeley FX, Timoney AG. The effect of the choice of irrigation fluid on cardiac stress during transurethral resection of the prostate: a comparison between 1.5% glycine and 5% glucose. J Urol. 2007;177:1369–1373. doi: 10.1016/j.juro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Currin RT, Caldwell-Kenkel JC, Lichtman SN, Bachmann S, Takei Y, Kawano S, et al. Protection by Carolina rinse solution, acidotic pH, and glycine against lethal reperfusion injury to sinusoidal endothelial cells of rat livers stored for transplantation. Transplantation. 1996;62:1549–1558. doi: 10.1097/00007890-199612150-00004. [DOI] [PubMed] [Google Scholar]
- Dehne N, Li T, Petrat F, Rauen U, de Groot H. Critical O2 and NO concentrations in NO-induced cell death in a rat liver sinusoidal endothelial cell line. Biol Chem. 2004;385:341–349. doi: 10.1515/BC.2004.030. [DOI] [PubMed] [Google Scholar]
- Deters M, Strubelt O, Younes M. Protection by glycine against hypoxia-reoxygenation induced hepatic injury. Res Commun Mol Pathol Pharmacol. 1997;97:199–213. [PubMed] [Google Scholar]
- Deters M, Siegers CP, Strubelt O. Influence of glycine on the damage induced in isolated perfused rat liver by five hepatotoxic agents. Toxicology. 1998;128:63–72. doi: 10.1016/s0300-483x(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Bronk SF, Gores GJ. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992;102:2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA. Development of porous defects in plasma membranes of adenosine triphosphate-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Lab Invest. 1998;78:657–668. [PubMed] [Google Scholar]
- Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y. Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol. 2001;158:1021–1028. doi: 10.1016/S0002-9440(10)64049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresbach T, Nawrotzki R, Kremer T, Schumacher S, Quinones D, Kluska M, et al. Molecular architecture of glycinergic synapses. Histochem Cell Biol. 2008;130:617–633. doi: 10.1007/s00418-008-0491-y. [DOI] [PubMed] [Google Scholar]
- Duenschede F, Westermann S, Riegler N, Miesner I, Erbes K, Ewald P, et al. Different protection mechanisms after pretreatment with glycine or alpha-lipoic acid in a rat model of warm hepatic ischemia. Eur Surg Res. 2006;38:503–512. doi: 10.1159/000096061. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 2000;157:826–828. doi: 10.1176/appi.ajp.157.5.826. [DOI] [PubMed] [Google Scholar]
- van den Eynden J, Ali SS, Horwood N, Carmans S, Brone B, Hellings N, et al. Glycine and glycine receptor signalling in non-neuronal cells. Front Mol Neurosci. 2009;2:1–12. doi: 10.3389/neuro.02.009.2009. Article 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DM, Gores GJ, Bronk SF, Krom RA. An increase in cytosolic protease activity during liver preservation. Inhibition by glutathione and glycine. Transplantation. 1993;55:627–633. doi: 10.1097/00007890-199303000-00030. [DOI] [PubMed] [Google Scholar]
- Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32:58–66. doi: 10.1016/s0168-8278(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856–G863. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]
- Garza-Quintero R, Ortega-Lopez J, Stein JH, Venkatachalam MA. Alanine protects rabbit proximal tubules against anoxic injury in vitro. Am J Physiol. 1990;258:F1075–F1083. doi: 10.1152/ajprenal.1990.258.4.F1075. [DOI] [PubMed] [Google Scholar]
- Garza-Quintero R, Weinberg JM, Ortega-Lopez J, Davis JA, Venkatachalam MA. Conservation of structure in ATP-depleted proximal tubules: role of calcium, polyphosphoinositides, and glycine. Am J Physiol. 1993;265:F605–F623. doi: 10.1152/ajprenal.1993.265.5.F605. [DOI] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gohrbandt B, Fischer S, Warnecke G, Avsar M, Sommer SP, Haverich A, et al. Glycine intravenous donor preconditioning is superior to glycine supplementation to low-potassium dextran flush preservation and improves graft function in a large animal lung transplantation model after 24 hours of cold ischemia. J Thorac Cardiovasc Surg. 2006;131:724–729. doi: 10.1016/j.jtcvs.2005.09.049. [DOI] [PubMed] [Google Scholar]
- de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Gundersen RY, Vaagenes P, Breivik T, Fonnum F, Opstad PK. Glycine – an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol Scand. 2005;49:1108–1116. doi: 10.1111/j.1399-6576.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, Bashkatova VG, et al. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc Dis. 2000;10:49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- Habib MM, Hodgson HJ, Davidson BR. The role of glycine in hepatic ischemia-reperfusion injury. Curr Pharm Des. 2006;12:2953–2967. doi: 10.2174/138161206777947605. [DOI] [PubMed] [Google Scholar]
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006a;96:8–20. doi: 10.1093/bja/aei279. [DOI] [PubMed] [Google Scholar]
- Hahn RG. Glycine is toxic. Acta Anaesthesiol Scand. 2006b;50:261–262. doi: 10.1111/j.1399-6576.2006.00927.x. author reply 262. [DOI] [PubMed] [Google Scholar]
- Hahn RG, Nilsson A, Stahle L. Distribution and elimination of the solute and water components of urological irrigating fluids. Scand J Urol Nephrol. 1999;33:35–41. doi: 10.1080/003655999750016258. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry. 1996;169:610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- Hernandes MS, Troncone LR. Glycine as a neurotransmitter in the forebrain: a short review. J Neural Transm. 2009;116:1551–1560. doi: 10.1007/s00702-009-0326-6. [DOI] [PubMed] [Google Scholar]
- Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- Heyman S, Spokes K, Rosen S, Epstein FH. Mechanism of glycine protection in hypoxic injury: analogies with glycine receptor. Kidney Int. 1992a;42:41–45. doi: 10.1038/ki.1992.258. [DOI] [PubMed] [Google Scholar]
- Heyman S, Brezis M, Epstein FH, Spokes K, Rosen S. Effect of glycine and hypertrophy on renal outer medullary hypoxic injury in ischemia reflow and contrast nephropathy. Am J Kidney Dis. 1992b;19:578–586. doi: 10.1016/s0272-6386(12)80838-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Büchler MW, Schemmer P. Supplementation of amino acids to prevent reperfusion injury after liver surgery and transplantation – where do we stand today? Clin Nutr. 2011;30:143–147. doi: 10.1016/j.clnu.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol. 1998;160:1617–1624. [PubMed] [Google Scholar]
- Howard A, Tahir I, Javed S, Waring SM, Ford D, Hirst BH. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J Physiol. 2010;588:995–1009. doi: 10.1113/jphysiol.2009.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama A. Studies on a unique organelle localization of a liver enzyme, serine:pyruvate (or alanine:glyoxylate) aminotransferase. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:274–286. doi: 10.2183/pjab.87.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima S, Shou J, Naama H, Calvano SE, Daly JM. Beneficial effect of enteral glycine in intestinal ischemia/reperfusion injury. J Gastrointest Surg. 1997;1:61–67. doi: 10.1007/s11605-006-0011-0. discussion 67–68. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272:G1581–G1586. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- Iresjö BM, Körner U, Larsson B, Henriksson BA, Lundholm K. Appearance of individual amino acid concentrations in arterial blood during steady-state infusions of different amino acid formulations to ICU patients in support of whole-body protein metabolism. JPEN J Parenter Enteral Nutr. 2006;30:277–285. doi: 10.1177/0148607106030004277. [DOI] [PubMed] [Google Scholar]
- Ito K, Ozasa H, Noda Y, Koike Y, Arii S, Horikawa S. Effect of non-essential amino acid glycine administration on the liver regeneration of partially hepatectomized rats with hepatic ischemia/reperfusion injury. Clin Nutr. 2008;27:773–780. doi: 10.1016/j.clnu.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Jacob T, Ascher E, Hingorani A, Kallakuri S. Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery. 2003;134:457–466. doi: 10.1067/s0039-6060(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Jiang L, Qin X, Zhong X, Liu L, Jiang L, Lu Y, et al. Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur J Cell Biol. 2011;90:333–341. doi: 10.1016/j.ejcb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Kallakuri S, Ascher E, Pagala M, Gade P, Hingorani A, Scheinman M, et al. Protective effect of glycine in mesenteric ischemia and reperfusion injury in a rat model. J Vasc Surg. 2003;38:1113–1120. doi: 10.1016/s0741-5214(03)00939-x. [DOI] [PubMed] [Google Scholar]
- Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:246–263. doi: 10.2183/pjab/84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincius M, Liang R, Nickkholgh A, Hoffmann K, Flechtenmacher C, Ryschich E, et al. Taurine protects from liver injury after warm ischemia in rats: the role of Kupffer cells. Eur Surg Res. 2007;39:275–283. doi: 10.1159/000102982. [DOI] [PubMed] [Google Scholar]
- Lee MA, McCauley RD, Kong SE, Hall JC. Pretreatment with glycine reduces the severity of warm intestinal ischemic-reperfusion injury in the rat. Ann Plast Surg. 2001;46:320–326. doi: 10.1097/00000637-200103000-00020. [DOI] [PubMed] [Google Scholar]
- Lee MA, McCauley RD, Kong SE, Hall JC. Influence of glycine on intestinal ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr. 2002;26:130–135. doi: 10.1177/0148607102026002130. [DOI] [PubMed] [Google Scholar]
- Leung S, Croft RJ, O’Neill BV, Nathan PJ. Acute high-dose glycine attenuates mismatch negativity (MMN) in healthy human controls. Psychopharmacology (Berl) 2008;196:451–460. doi: 10.1007/s00213-007-0976-8. [DOI] [PubMed] [Google Scholar]
- Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA, et al. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun. 2001;69:5883–5891. doi: 10.1128/IAI.69.9.5883-5891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Yan LN, Li SW, You HB, Gong JP. Glycine blunts transplantative liver ischemia-reperfusion injury by downregulating interleukin 1 receptor associated kinase-4. Acta Pharmacol Sin. 2006;27:1479–1486. doi: 10.1111/j.1745-7254.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- Luntz SP, Unnebrink K, Seibert-Grafe M, Bunzendahl H, Kraus TW, Büchler MW, et al. HEGPOL: randomized, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312] BMC Surg. 2005;5:18. doi: 10.1186/1471-2482-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Mandel LJ, Schnellmann RG, Jacobs WR. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990;85:316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino JE, Kotadia B, Mangino MJ. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996;62:173–178. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- Mangino MJ, Murphy MK, Grabau GG, Anderson CB. Protective effects of glycine during hypothermic renal ischemia-reperfusion injury. Am J Physiol. 1991;261:F841–F848. doi: 10.1152/ajprenal.1991.261.5.F841. [DOI] [PubMed] [Google Scholar]
- Marsh DC, Hjelmhaug JA, Vreugdenhil PK, Belzer FO, Southard JH. Glycine prevention of cold ischemic injury in isolated hepatocytes. Cryobiology. 1991;28:105–109. doi: 10.1016/0011-2240(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Marsh DC, Vreugdenhil PK, Mack VE, Belzer FO, Southard JH. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology. 1993;17:91–98. [PubMed] [Google Scholar]
- Mauriz JL, Matilla B, Culebras JM, Gonzalez P, Gonzalez-Gallego J. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic Biol Med. 2001;31:1236–1244. doi: 10.1016/s0891-5849(01)00716-x. [DOI] [PubMed] [Google Scholar]
- Miller GW, Schnellmann RG. Cytoprotection by inhibition of chloride channels: the mechanism of action of glycine and strychnine. Life Sci. 1993;53:1211–1215. doi: 10.1016/0024-3205(93)90539-f. [DOI] [PubMed] [Google Scholar]
- Miller GW, Lock EA, Schnellmann RG. Strychnine and glycine protect renal proximal tubules from various nephrotoxicants and act in the late phase of necrotic cell injury. Toxicol Appl Pharmacol. 1994;125:192–197. doi: 10.1006/taap.1994.1064. [DOI] [PubMed] [Google Scholar]
- Moran JH, Schnellmann RG. Diverse cytoprotectants prevent cell lysis and promote recovery of respiration and ion transport. Biochem Biophys Res Commun. 1997;234:275–277. doi: 10.1006/bbrc.1997.6625. [DOI] [PubMed] [Google Scholar]
- Nagatomi A, Sakaida I, Matsumura Y, Okita K. Cytoprotection by glycine against hypoxia-induced injury in cultured hepatocytes. Liver. 1997;17:57–62. doi: 10.1111/j.1600-0676.1997.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Nichols JC, Bronk SF, Mellgren RL, Gores GJ. Inhibition of nonlysosomal calcium-dependent proteolysis by glycine during anoxic injury of rat hepatocytes. Gastroenterology. 1994;106:168–176. doi: 10.1016/s0016-5085(94)95147-0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8:850–858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Romer LH, Lemasters JJ. Mitochondrial dysfunction and cytoskeletal disruption during chemical hypoxia to cultured rat hepatic sinusoidal endothelial cells: the pH paradox and cytoprotection by glucose, acidotic pH, and glycine. Hepatology. 1998;27:1039–1049. doi: 10.1002/hep.510270420. [DOI] [PubMed] [Google Scholar]
- Omasa M, Fukuse T, Toyokuni S, Mizutani Y, Yoshida H, Ikeyama K, et al. Glycine ameliorates lung reperfusion injury after cold preservation in an ex vivo rat lung model. Transplantation. 2003;75:591–598. doi: 10.1097/01.TP.0000053200.98125.14. [DOI] [PubMed] [Google Scholar]
- Paller MS, Patten M. Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J Am Soc Nephrol. 1992;2:1338–1344. doi: 10.1681/ASN.V281338. [DOI] [PubMed] [Google Scholar]
- Palmer C, Ellis KA, O’Neill BV, Croft RJ, Leung S, Oliver C, et al. The cognitive effects of modulating the glycine site of the NMDA receptor with high-dose glycine in healthy controls. Hum Psychopharmacol. 2008;23:151–159. doi: 10.1002/hup.904. [DOI] [PubMed] [Google Scholar]
- Pan C, Bai X, Fan L, Ji Y, Li X, Chen Q. Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J. 2005;390:447–453. doi: 10.1042/BJ20050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33:1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol. 2011;301:F134–F150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, Li T, Dehne N, de Groot H, Rauen U. Sodium as the major mediator of NO-induced cell death in cultured hepatocytes. Life Sci. 2006;79:1606–1615. doi: 10.1016/j.lfs.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Petrat F, Drowatzky J, Boengler K, Finckh B, Schmitz KJ, Schulz R, et al. Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur Surg Res. 2011;46:180–187. doi: 10.1159/000324393. [DOI] [PubMed] [Google Scholar]
- Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- Qu W, Ikejima K, Zhong Z, Waalkes MP, Thurman RG. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1249–G1256. doi: 10.1152/ajpgi.00197.2002. [DOI] [PubMed] [Google Scholar]
- Rentsch M, Puellmann K, Sirek S, Iesalnieks I, Kienle K, Mueller T, et al. Benefit of Kupffer cell modulation with glycine versus Kupffer cell depletion after liver transplantation in the rat: effects on postischemic reperfusion injury, apoptotic cell death, graft regeneration and survival. Transpl Int. 2005;18:1079–1089. doi: 10.1111/j.1432-2277.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Rose ML, Cattley RC, Dunn C, Wong V, Li X, Thurman RG. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis. 1999a;20:2075–2081. doi: 10.1093/carcin/20.11.2075. [DOI] [PubMed] [Google Scholar]
- Rose ML, Madren J, Bunzendahl H, Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999b;20:793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Pina P, Garcia-Dorado D, Rodriguez-Sinovas A, Barba I, Miro-Casas E, et al. Glycine protects cardiomyocytes against lethal reoxygenation injury by inhibiting mitochondrial permeability transition. J Physiol. 2004;558:873–882. doi: 10.1113/jphysiol.2004.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaida I, Nagatomi A, Okita K. Protection by glycine against chemical ischemia produced by cyanide in cultured hepatocytes. J Gastroenterol. 1996;31:684–690. doi: 10.1007/BF02347617. [DOI] [PubMed] [Google Scholar]
- Sandfeldt L, Hahn RG. Comparison of urological irrigating fluids containing glycine and mannitol in volunteers. Prostate. 1999;41:89–98. doi: 10.1002/(sici)1097-0045(19991001)41:2<89::aid-pros3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Schaefer N, Tahara K, Schuchtrup S, Websky MV, Overhaus M, Schmidt J, et al. Perioperative glycine treatment attenuates ischemia/reperfusion injury and ameliorates smooth muscle dysfunction in intestinal transplantation. Transplantation. 2008;85:1300–1310. doi: 10.1097/TP.0b013e31816c576f. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Schoonhoven R, Swenberg JA, Bunzendahl H, Thurman RG. Gentle in situ liver manipulation during organ harvest decreases survival after rat liver transplantation: role of Kupffer cells. Transplantation. 1998;65:1015–1020. doi: 10.1097/00007890-199804270-00001. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Enomoto N, Bradford BU, Bunzendahl H, Raleigh JA, Lemasters JJ, et al. Activated Kupffer cells cause a hypermetabolic state after gentle in situ manipulation of liver in rats. Am J Physiol Gastrointest Liver Physiol. 2001a;280:G1076–G1082. doi: 10.1152/ajpgi.2001.280.6.G1076. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Golling M, Kraus T, Mayatepek E, Herfarth C, Klar E. Glycine reduces reperfusion injury in human liver transplantation: our first patients. Transplant Proc. 2001b;33:3750–3752. doi: 10.1016/s0041-1345(01)02588-x. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Golling M, Kraus T, Mehrabi A, Mayatepek E, Herfarth C, et al. Extended experience with glycine for prevention of reperfusion injury after human liver transplantation. Transplant Proc. 2002;34:2307–2309. doi: 10.1016/s0041-1345(02)03247-5. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Liang R, Kincius M, Flechtenmacher C, Bunzendahl H, Gutt CN, et al. Taurine improves graft survival after experimental liver transplantation. Liver Transpl. 2005;11:950–959. doi: 10.1002/lt.20501. [DOI] [PubMed] [Google Scholar]
- Sheth H, Hafez T, Glantzounis GK, Seifalian AM, Fuller B, Davidson BR. Glycine maintains mitochondrial activity and bile composition following warm liver ischemia-reperfusion injury. J Gastroenterol Hepatol. 2011;26:194–200. doi: 10.1111/j.1440-1746.2010.06323.x. [DOI] [PubMed] [Google Scholar]
- Silva P, Rosen S, Spokes K, Epstein FH. Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int. 1991;39:653–658. doi: 10.1038/ki.1991.78. [DOI] [PubMed] [Google Scholar]
- Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM. Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int. 1996;50:845–854. doi: 10.1038/ki.1996.384. [DOI] [PubMed] [Google Scholar]
- Stein J, Boehles HJ, Blumenstein I, Goeters C, Schulz R. Amino acids – guidelines on parenteral nutrition, Chapter 4. Ger Med Sci. 2009;7:1–8. doi: 10.3205/000083. Doc24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels B, Türler A, Schmidt J, Nazir A, Tsukamoto T, Moore BA, et al. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil. 2011;23:76–88. doi: 10.1111/j.1365-2982.2010.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepaske R, te Velthuis H, Oudemans-van Straaten HM, Bossuyt PM, Schultz MJ, Eijsman L, et al. Glycine does not add to the beneficial effects of perioperative oral immune-enhancing nutrition supplements in high-risk cardiac surgery patients. JPEN J Parenter Enteral Nutr. 2007;31:173–180. doi: 10.1177/0148607107031003173. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Tijsen MJ, Peters SM, Bindels RJ, van Os CH, Wetzels JF. Glycine protection against hypoxic injury in isolated rat proximal tubules: the role of proteases. Nephrol Dial Transplant. 1997;12:2549–2556. doi: 10.1093/ndt/12.12.2549. [DOI] [PubMed] [Google Scholar]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam MA, Weinberg JM, Patel Y, Hussong U, Davis JA. Effects of Ca++ and glycine on lipid breakdown and death of ATP-depleted MDCK cells. Kidney Int. 1995;48:118–128. doi: 10.1038/ki.1995.275. [DOI] [PubMed] [Google Scholar]
- Venkatachalam MA, Weinberg JM, Patel Y, Saikumar P, Dong Z. Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int. 1996;49:449–460. doi: 10.1038/ki.1996.64. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhao M, Wang EH. Effects of glycine and methylprednisolone on hemorrhagic shock in rats. Chin Med J (Engl) 2004;117:1334–1341. [PubMed] [Google Scholar]
- Wang YS, Yan YH, Zou XF. Protective effect of glycine on liver injury during liver transplantation. Chin Med J (Engl) 2010;123:1931–1938. [PubMed] [Google Scholar]
- Warnecke G, Schulze B, Steinkamp T, Haverich A, Klima U. Glycine application and right heart function in a porcine heart transplantation model. Transpl Int. 2006;19:218–224. doi: 10.1111/j.1432-2277.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Webb TI, Lynch JW. Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des. 2007;13:2350–2367. doi: 10.2174/138161207781368693. [DOI] [PubMed] [Google Scholar]
- Weinberg JM. Glutathione and glycine in acute renal failure. Ren Fail. 1992;14:311–319. doi: 10.3109/08860229209106635. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Davis JA, Abarzua M, Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987;80:1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Davis JA, Abarzua M, Kiani T. Relationship between cell adenosine triphosphate and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989;113:612–622. [PubMed] [Google Scholar]
- Weinberg JM, Davis JA, Abarzua M, Kiani T, Kunkel R. Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial ATP production. Am J Physiol. 1990a;258:C1127–C1140. doi: 10.1152/ajpcell.1990.258.6.C1127. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Garzo-Quintero R, Roeser NF, Davis JA. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990b;4:3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Buchanan DN, Davis JA, Abarzua M. Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol. 1991a;1:949–958. doi: 10.1681/ASN.V17949. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest. 1991b;87:581–590. doi: 10.1172/JCI115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S. Relationships between intracellular amino acid levels and protection against injury to isolated proximal tubules. Am J Physiol. 1991c;260:F410–F419. doi: 10.1152/ajprenal.1991.260.3.F410. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, et al. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140:457–471. [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA. Role of intracellular pH during cytoprotection of proximal tubule cells by glycine or acidosis. J Am Soc Nephrol. 1994;5:1314–1323. doi: 10.1681/ASN.V561314. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Goldberg H, Roeser NF, Davis JA. Modulation by Gly, Ca, and acidosis of injury-associated unesterified fatty acid accumulation in proximal tubule cells. Am J Physiol. 1995;268:F110–F121. doi: 10.1152/ajprenal.1995.268.1.F110. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA. Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int. 1997;52:140–151. doi: 10.1038/ki.1997.313. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000;279:F927–F943. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels JF, Wang X, Gengaro PE, Nemenoff RA, Burke TJ, Schrier RW. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol. 1993a;264:F94–F99. doi: 10.1152/ajprenal.1993.264.1.F94. [DOI] [PubMed] [Google Scholar]
- Wetzels JF, Yu L, Shanley PF, Burke TJ, Schrier RW. Infusion of glycine does not attenuate in vivo ischemic acute renal failure in the rat. J Lab Clin Med. 1993b;121:263–267. [PubMed] [Google Scholar]
- Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476–484. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277:L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Ikejema K, Enomoto N, Stacklewitz RF, Seabra V, Zhong Z, et al. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999;56:843–856. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi K, Eguchi S, Kamohara Y, Yanaga K, Okudaira S, Tajima Y, et al. Glycine reduces hepatic warm ischaemia-reperfusion injury by suppressing inflammatory reactions in rats. Liver Int. 2007;27:1249–1254. doi: 10.1111/j.1478-3231.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, et al. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001;40:197–204. doi: 10.1207/S15327914NC402_17. [DOI] [PubMed] [Google Scholar]
- Yamashina S, Ikejima K, Rusyn I, Sato N. Glycine as a potent anti-angiogenic nutrient for tumor growth. J Gastroenterol Hepatol. 2007;22(Suppl. 1):S62–S64. doi: 10.1111/j.1440-1746.2006.04655.x. [DOI] [PubMed] [Google Scholar]
- Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, et al. Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 2002;282:F417–F423. doi: 10.1152/ajprenal.00011.2001. [DOI] [PubMed] [Google Scholar]
- Zafra F, Gimenez C. Glycine transporters and synaptic function. IUBMB Life. 2008;60:810–817. doi: 10.1002/iub.128. [DOI] [PubMed] [Google Scholar]
- Zhang K, Weinberg JM, Venkatachalam MA, Dong Z. Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res. 2003;28:893–901. doi: 10.1023/a:1023275426637. [DOI] [PubMed] [Google Scholar]
- Zhao P, Qian H, Xia Y. GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci. 2005;22:289–300. doi: 10.1111/j.1460-9568.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Jones S, Thurman RG. Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol. 1996;270:G332–G338. doi: 10.1152/ajpgi.1996.270.2.G332. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Enomoto N, Connor HD, Moss N, Mason RP, Thurman RG. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12:54–62. doi: 10.1097/00024382-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]


