Abstract
Removal of disease-driving inflammatory leucocytes is central to resolution of inflammation. The current pharmacological dogma teaches leucocyte elimination through apoptosis followed by phagocytosis. However, actual resolving roles of apoptotic–phagocytic processes have been difficult to demonstrate in the major diseases that are characterized by mucosal tissue inflammation. Many current in vivo observations rather demonstrate that leucocyte elimination occurs by transepithelial locomotion. Findings in diseased gut and bladder mucosae support this notion. Respiratory disease data are particularly compelling. Eosinophils and neutrophils abound in sputum and tracheal aspirates during treatment-induced recovery from severe asthma. Prolonged sputum neutrophilia, along with clinical improvement, follows upon smoking cessation in COPD. Eosinophils, neutrophils, lymphocytes, mast cells and dendritic cells also move in large numbers into the bronchial lumen at spontaneous inflammation resolution following allergen challenge in allergic rhinitis and asthma. A corresponding reduction of infiltrated cells in the bronchial mucosal tissue demonstrates efficiency of the transepithelial elimination pathway. Underscoring its operational role, drugs impeding transepithelial elimination of leucocytes aggravate mucosal/parenchymal inflammation. Hence, relying on lumen cell data alone can lead to paradoxical conclusions regarding anti-inflammatory drug efficacy. Conversely, drugs promoting non-injurious transepithelial elimination of leucocytes could resolve mucosal inflammatory diseases.
Keywords: inflammation resolution, transepithelial migration, drug opportunity, drug toxicity, asthma, COPD, IBD
Introduction
Several of the major chronic diseases present mucosal inflammation as a central component. To switch off the inflammation, causative insults need to be removed and treatments instituted. The inflammation resolution that follows involves active healing and, importantly, active elimination of infiltrated inflammatory cells. The infiltrated cells can disappear by dying at the site or by emigration. The currently accepted notion is that the cells undergo apoptosis and are then engulfed by a process called efferocytosis, foremost executed by macrophages. Reflecting belief in the apoptotic–efferocytosis paradigm, efforts are aimed at finding pro-resolution drugs that induce apoptosis and/or facilitate efferocytosis. Increasing the interest further, glucocorticoids are thought to exert both these actions (Serhan et al., 2007; Perretti and D'Acquisto, 2009). However, despite the overwhelming developments in mechanistic research in this field of interest, the clinical importance of such actions in diseases involving hollow mucosal lined organs has not been compellingly demonstrated. Instead, another mode of leucocyte elimination can be discerned. This is evident not least from numerous in vivo data obtained in patient studies. As discussed further below, many of these so far little understood observations actually indicate that transepithelial elimination is a major mode of ridding diseased mucosal tissues of inflammatory cells. This vital role of epithelial traffic of leucocytes affects interpretation of the cell data that we gain from accessible lumen samples. Furthermore, drugs that target cell traffic may not exhibit the clinical efficacy that currently is expected.
During the last few decades, when inflammation resolution has become a topical research area, a transepithelial mode of leucocyte elimination seems largely to have been overlooked. The role of this ‘simple’ exit has likely been eclipsed by the interest in apoptotic–phagocytic removal of cells (Serhan et al., 2007). Contributing to the limited attention, another prevailing dogma has taught that the transepithelial movement of leucocytes is an injury-evoking, pathogenic component of mucosal inflammatory diseases. It is also thought that drugs should preferably inhibit this traffic (Chin and Parkos, 2007). Indeed, the view that airway lumen activity of granulocytes is a major pathogenic event in asthma has been widely disseminated (Lukacs, 2001). Considering this conceptual background paradoxical pharmacological observations abound in this field (Page, 2011). For example, several drugs (specified below) that inhibit the transepithelial passage of leucocytes in vivo have not been beneficial. Some have even severely aggravated an already established inflammatory condition. Conversely, markedly rising numbers of inflammatory leucocytes in the airway lumen have been associated with inflammation resolution.
A resolving role of luminal migration of leucocytes would profoundly affect interpretation of cell data that we gain from the accessible lumen cell samples. Appreciation of a resolving role of transepithelial leucocyte traffic would further have a significant bearing on current anti-inflammatory drug discovery and evaluation.
In this overview, we highlight observations that support a resolving role of transepithelial leucocyte locomotion. Aiming at identifying findings of pharmacological interest, we now put much of the data that we have discussed in respiratory journals (Persson and Uller, 2010a,b) in the broader context of mucosal diseases in general. We also include eosinophils, neutrophils, lymphocytes, mast cells and dendritic cells in the present discussion. Rather than reviewing the enormous literature on mechanisms involved in leucocyte traffic and apoptosis, respectively, we focus on actual observations in patients of relevance to resolution of inflammatory diseases. Most recent research developments in inflammation resolution have not dealt with transepithelial elimination of leucocytes. Hence, specific mechanistic data with proven in vivo relevance are as yet scarce in this field and are beyond the scope of this review. A majority of the human data on cell numbers, that we interpret, has been generated in subjects with allergic rhinitis or asthma. Similarly, lumen and tissue leucocyte numbers in murine (mouse and rat) models of asthma have been instrumental in building the present hypothesis. Supporting data further emanate from studies involving severe neutrophilic asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel diseases (IBD) and interstitial cystitis. Throughout this review, we put in vivo observations first (Persson et al., 2001).
Different paths for elimination of leucocytes from diseased mucosae (Figure 1)
Figure 1.
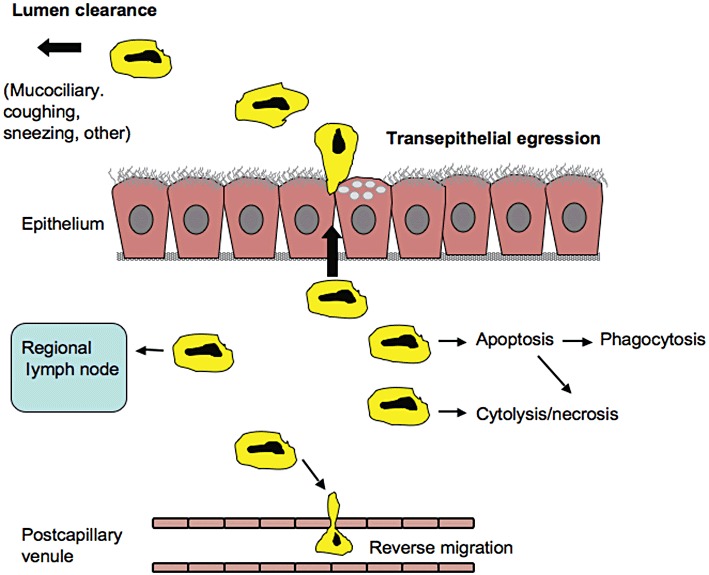
There are several possible elimination pathways by which infiltrated leucocytes could disappear from inflamed mucosal tissues. Cytolysis/necrosis and apoptosis – if phagocytic clearance is insufficient – are pro-inflammatory paths. Regional lymph nodes are essentially involved in two-way traffic of lymphocytes and dendritic cells, and potentially other leucocytes too, but a role of this traffic in quantitative elimination of infiltrated cells remains to be demonstrated. Similarly, it is not known if reverse migration of infiltrated leucocytes back into the mucosal microcirculation plays a significant role in leucocyte elimination. Transepithelial exit has been considered pathogenic but is depicted here as a major mode of non-injurious elimination of inflammatory leucocytes. Depending on the involved hollow organ, several clearance mechanisms contribute to final elimination of leucocytes from the lumen. The elimination paths are potential targets for pharmacological effects.
Efficient operation of the mucosal immune system requires rapid locomotion of leucocytes. This concerns recruitment from the circulation, motility through the interstitium to sites of insults and transepithelial migration. The transepithelial leucocyte egression is required for surveillance, sentinel and defence duties to be carried out in part on the mucosal surface and, likely, also in the lumen. Migration of neutrophils across the mucosa may be critical to a successful combating of mucosal infections (Godaly et al., 2001). Hence, it is reassuring that the transepithelial exit of granulocytes can occur without compromising the integrity of the mucosal epithelial barrier (Martin, 2002; Erjefalt et al., 2004). Indeed, an eosinophil-rich airway mucosa was emptied of its granulocytes within minutes by the transepithelial route. First, the subepithelial esinophils moved up between the columnar epithelial cells to an apical location. A quantitative elimination from the mucosa then occurred promptly without any sign of epithelial injury even at the ultrastructural level; also note worthy, the speedy and complete elimination of the mucosal tissue eosinophils occurred without any sign of eosinophil apoptosis (Erjefalt et al., 2004). Epithelial restitution after shedding-like epithelial damage, which does not injure the basement membrane, can be a very rapid process in vivo (Persson et al., 2008). However, for a well-functioning disease-resolving process, transepithelial elimination of leucocytes would have to occur without inflicting any injury at all.
To some extent, leucocytes may leave inflamed mucosal tissues by reverse migration back into the mucosal microcirculation. Reverse migration has been proposed as a mode of resolving inflammation in zebra fish (Huttenlocher and Poznansky, 2008). Buckley et al. (2006) have reported that a particular neutrophil phenotype prone to reverse migration occurs in human peripheral circulation. However, there is as yet no known role of reverse migration mechanisms in human mucosal inflammation. In a more recent discussion of inflammation resolution, Haworth and Buckley (2007) also do not mention this mechanism. Summers, Rankin and others discuss a particular homing in mice of senescent neutrophils to the bone marrow for destruction. However, the authors expressed doubts whether this mechanism also operates in humans (Summers et al., 2010). In a figure legend, these authors actually acknowledge that neutrophils in inflamed tissues either die or ‘are lost to the body following trans-epithelial migration’ (Summers et al., 2010). Pillay et al. (2010) recently reported that neutrophils have a lifespan of several days. This is much longer than previously thought and has aroused some controversy. Their data have in fact been re-interpreted by other authors to rather fit into the established view of a lifespan of about 10 h of these cells (Tofts et al., 2011). Neutrophils dwelling in inflamed tissues are considered to have quite long half-lives (Summers et al., 2010). However, as with other leucocytes, the turnover half-life of neutrophils in inflamed mucosal locations is not known in detail. Traffic between mucosal sites and regional lymph nodes is a core activity of dendritic cells and lymphocytes in immunity. As discussed below, this does not exclude the possibility that a significant portion of these cells is eliminated by exit into the lumen of hollow organs. Other leucocytes, including the eosinophil, can also move to regional lymph nodes (Korsgren et al., 1997). Quantitatively, this appears to be a minor route compared with the bulk elimination of these mucosal dwelling cells across the epithelial lining (Uller et al., 2006b).
On interpretation of leucocyte numbers in the airway lumen
During development of inflammation, newly recruited leucocytes may accumulate in the mucosal tissue rather than leaving promptly across the epithelial lining. Data based on lumen cells during this stage would underestimate the level of infiltration in the mucosal tissue. At chronic inflammation, leucocytes appearing in the lumen of hollow organs variably reflect the infiltrated state of the mucosal tissue (Persson and Uller, 2010a). Chronic inflammation in subgroups of patients with COPD may exhibit large numbers of granulocytes in the airway lumen with relatively low numbers in the airway wall. Given that removal from the lumen is unchanged, such data suggest that some stable disease conditions can be characterized by accelerated transepithelial granulocyte emigration (Persson and Uller, 2010b). Samples from the lumen in these cases will overestimate the infiltration of granulocytes in the airway wall. Acute allergen challenge of an allergic mucosa with constitutive eosinophilia, as in guinea pig trachea, can produce immediate elimination of the mucosal-dwelling eosinophils. There is then a brief period of tissue eosinophilopenia before de novo recruitment of these cells has built up an inflammatory eosinophilia. During this early post-challenge period, eosinophils have thus abounded in the lumen whilst the underlying mucosal tissue has lacked these cells (Erjefalt et al., 2004). Hence, timing seems essential to interpret in detail the occurrence of leucocytes in the lumen of mucosal-lined organs. Despite a significant variability, the ‘spill-over’ of airway wall eosinophils into the airway lumen has made sputum samples useful for monitoring disease severity and adjusting anti-inflammatory steroid treatment in asthma and COPD (Jayaram et al., 2006). Similarly, lumen eosinophils are often (but not always!) a useful measurement of airway allergic inflammation in animal models.
Transepithelial elimination of leucocytes and resolution of mucosal inflammation
Eosinophils, neutrophils, lymphocytes
The resolution phase after an attack of bronchial asthma has traditionally been associated with sputum eosinophilia (Salter, 1868). Furthermore, in a careful study, the gradual clinical improvement in severe neutrophilic asthma (requiring intubation) has been closely associated with markedly increased numbers of neutrophils in tracheal aspirates (Ordonez et al., 2000). Since severe asthma is treated with large doses of steroids, theses drugs evidently permit elimination of granulocytes into the airway lumen. Animal model data clearly demonstrate this permissive action in studies where steroid treatment resolves established airway mucosal eosinophilia. Also, this resolving effect occurs without inducing eosinophil apoptosis (Uller et al., 2001).
There are three reported clinical studies that involve examination of both bronchial lumen and wall eosinophils at the resolution of an allergic asthma induced by allergen challenge (Aalbers et al., 1993; Frew et al., 1996; Brown et al., 1998). Compellingly, the consistent picture emerging in these studies is increasing numbers of lumen cells (examined by bronchoalveolar lavage, BAL) along with decreasing numbers of mucosal tissue cells. The resolving role of transepithelial egression is thus indicated by a negative correlation between tissue and lumen eosinophils in these patients. In a study on the course of leucocyte infiltration in human allergen challenge-induced asthma, Lommatzsch et al. (2006) extended previous BAL findings and demonstrated that eosinophils, lymphocytes and neutrophils exhibited peak numbers in the airway lumen during a prolonged resolution phase post-allergen challenge.
Dendritic cells
A study involving asthmatic individuals examined occurrence of dendritic cells in the airway lumen following allergen challenge. There was little immediate entry of these cells, but the number of dendritic cells in the airway lumen had grown markedly several hours after the allergen exposure (Bratke et al., 2007). Dendritic cells in the lumen may be on their way to final elimination; they may also have a role to capture lumen allergens. Following sampling in the lumen, dendritic cells could migrate back into mucosal tissues and regional lymph nodes and present the allergen. Alternatively and importantly, the intraluminal ‘sampling’ carried out by dendritic cells and other phagocytes could be a significant mode of eliminating both offending pathogens/allergens and the capturer leucocytes by lumen clearance (Bellamy and Nielsen, 1974). This possibility is supported by recent observations on bacteria-capturing effects of dendritic cells in the gut lumen. These dendritic cells do not re-enter the tissue (Nicoletti et al., 2011).
Mast cells
Occurrence of mast cells in the bladder detrusor smooth muscle is almost a diagnostic in interstitial cystitis. Turnover of the bladder mast cells in this disease clearly involves traffic to and across the epithelial lining (Aldenborg et al., 1986). A corresponding traffic of mast cells also occurs in allergic rhinitis and asthma. Juliusson et al. (1992) observed a progressive increase of mast cells in the superficial nasal epithelium following allergen challenge in subjects with allergic rhinitis. After a delay of a couple of hours, mast cells increased progressively also in the nasal airway lumen. This early indication of elimination of airway wall mast cells by epithelial transmigration was followed by intriguing observations on mast cell traffic in asthmatic individuals. Gauvreau et al. (2000) thus made the ‘unexpected’ observation that the number of bronchial lumen mast cells correlated quite well with the magnitude of an allergen challenge-induced late phase asthmatic reaction occurring many hours prior to sampling of the lumen cells. These data actually extended previous observations by Crimi et al. (1991) who had obtained bronchial biopsies early during resolution of allergen challenge-induced late-phase reaction in asthmatics. Also at this earlier time point, there was a highly significant correlation between the number of superficial epithelial mast cells (but still remaining in the tissue) and the intensity of the late-phase bronchial reaction. These data, together with the lumen findings by Gavreau et al., strongly suggest that the epithelial mast cells were not only contributing to the late-phase reaction, but they were also effectively eliminated by migration into the bronchial lumen. There is little information on the role of apoptosis of these cells. Hence, epithelial transmigration currently emerges as a major mode of disappearance of mucosal tissue mast cells in resolving mucosal inflammation.
In summary, the resolution phase post-severe exacerbations (involving anti-inflammatory treatments) and post-allergen exposure (involving spontaneous resolution) is characterized by increasing lumen numbers of lymphocytes, dendritic cells and mast cells as well as eosinophils and neutrophils. The luminal route of clearance occurs in steroid-treated as well as in untreated patients. In this context, effects of smoking cessation in COPD are of interest: airway lumen neutrophils and lymphocytes have increased for prolonged periods of time along with clinical improvement of COPD (Willemse et al., 2005; Louhelainen et al., 2009). Similar loss of inflammatory cells across the gut mucosa may occur in IBD. Successful treatment of IBD has thus been associated with an early surge of faecal neutrophil indices (Malickova et al., 2010). Leucocytes in the mucus component of faecal cylinders have also been reported to be typical for IBD in remission (Swidsinski et al., 2008). On a related note, anti-TNF-α treatment of IBD has been clinically effective without evidence of the favoured mechanism of increased leucocyte apoptosis (Schreiber et al., 2007; Siegmund, 2009). These observations underscore the importance of studying the pharmacology of transepithelial traffic of leucocytes in resolving mucosal diseases.
Leucocyte apoptosis in resolving mucosal inflammation
Lumen samples
Guided by the apoptotic–phagocytic paradigm authors have looked for apoptotic granulocytes in airway–alveolar lumen samples obtained from patients with pulmonary inflammation–respiratory distress syndrome (Grigg et al., 1991), asthma (Gibson et al., 2001), COPD (Rytila et al., 2006), cystic fibrosis (Watt et al., 2005) and bronchiectasis (Watt et al., 2004). It is clear that apoptotic leucocytes can occur in the lumen samples. Occasional occurrence of macrophage engulfment of apoptotic cells has also been seen in this location in patients as well as in animal models. However, a role of apoptosis and phagocytosis of leucocytes in the lumen has not been compellingly demonstrated (Persson and Uller, 2010a,b). Not even treatment with acknowledged pro-apoptotic drugs (this property demonstrated in vitro) such as the glucocorticoids has produced the expected result. Contrary to predictions by the paradigm, steroid treatment of asthmatics that reduced sputum eosinophilia thus did not increase the number of apoptotic eosinophils, nor did it increase the number of macrophages that had ingested eosinophils (Gibson et al., 2001). To explain lumen neutrophilia in COPD, it has been hypothesised that components of airway surface liquids from these patients would increase survival of neutrophils, but this could not be demonstrated (Rytila et al., 2006). Matute-Bello and Martin (2003), who had unravelled anti-apoptotic actions of BAL fluid obtained from patients with respiratory distress syndrome, have since argued that neutrophil apoptosis has little to do with outcome in this disease.
It needs mentioning that apoptotic (dead) cells will not migrate. It should, therefore, be difficult to draw any conclusion at all about the role of apoptosis–phagocytosis in mucosal tissues from studies of lumen cell samples.
Mucosal tissue samples
Studies specifically addressing the role of leucocyte apoptosis in diseased mucosal tissues are warranted. This need is underscored by inconsistent data obtained in the relatively few studies that have addressed the occurrence of apoptotic leucocytes in the airway wall. As far as we can see, observations in vivo in human and animal mucosal tissues have not demonstrated the clear role of the apoptotic–phagocytic paradigm that others, and we, have been seeking.
The notion that steroid treatment induces apoptosis of T lymphocytes in human bronchial tissues has not been confirmed by studies involving asthmatic (Druilhe et al., 1998; O'Sullivan et al., 2004) and COPD patients (Hodge et al., 2005). One clinical experimental in vivo study focused on the effects of steroids, specifically, on resolution of airway tissue eosinophilia: Although treatment with glucocorticoids speeded up resolution of allergen challenge-induced eosinophilic rhinitis, any inducement of eosinophil apoptosis was not detected in the airway wall of these patients (Uller et al., 2010). Yet eosinophils do die in the airway tissues in asthma and rhinitis. However, death most commonly is then by primary cytolysis (without preceding apoptosis), causing the deposition of clusters of protein-rich eosinophil granules in the diseased tissue (Persson and Erjefalt, 1997; Uller et al., 2004). This is a pathogenic mode of death that is reduced by steroid treatment (Greiff et al., 1998). In agreement with human findings, there is a striking lack of apoptotic eosinophils in steroid-treated airway-pulmonary tissues in animal models of asthma (Uller et al., 2001; Uller et al., 2006a). Similarly, other drug compounds such as R-roscovitine, with promising pro-apoptosis actions on eosinophils in culture, currently fail to translate this effect into in vivo pro-apoptotic actions in animal models of asthma (Farahi et al., 2011; Rosenberg, 2011). R-roscovitine also failed to affect the resolution of eosinophilic airway inflammation in vivo. In an accompanying editorial to this work, Rosenberg (2011) highlights the role of transepithelial elimination of eosinophils.
A special example is the occurrence of airway tissue neutrophilia in association with steroid treatment of asthma and COPD. The favoured explanation for this effect has been the steroid-induced attenuation of neutrophil apoptosis that can be demonstrated in vitro. However, as discussed elsewhere (Persson and Uller, 2010b), in vivo data in patients, again, cannot support the notion of a role of steroid-induced inhibition of neutrophil apoptosis. The published human in vivo evidence rather indicates that steroid treatment can reduce airway epithelial transmigration of neutrophils (Persson and Uller, 2010b). Hence, lumen neutrophil numbers is probably a poor measure of anti-inflammatory efficacy of steroids.
Interventions impeding transepithelial elimination of leucocytes
Investigators carrying out experiments in humans and animals may have been surprised to find that airway lumen leucocytes have increased when other accepted signs of airway inflammation/disease have been reduced. Equally paradoxical and surprising, in vivo studies have indicated successful inhibition of the leucocytes in the airway–alveolar lumen without the expected beneficial effect on the targeted airway disease. Actually, the latter kind of observation should ring bells of warning. Studies in animal disease models have demonstrated that interventions (including anti-FAS mAb treatment, inhibition of ICAM-2 and knock-out of MMP-7 and −9), which reduce lumen leucocyte numbers, have produced quite severe aggravation of hollow organ (airways) inflammation. Explaining such deleterious effects, increased accumulation of infiltrated inflammatory cells in mucosal wall and/or parenchyma, indicating impeded transepithelial elimination, has been demonstrated (Gerwin et al., 1999; Corry et al., 2002; Uller et al., 2005) (Figure 2).
Figure 2.
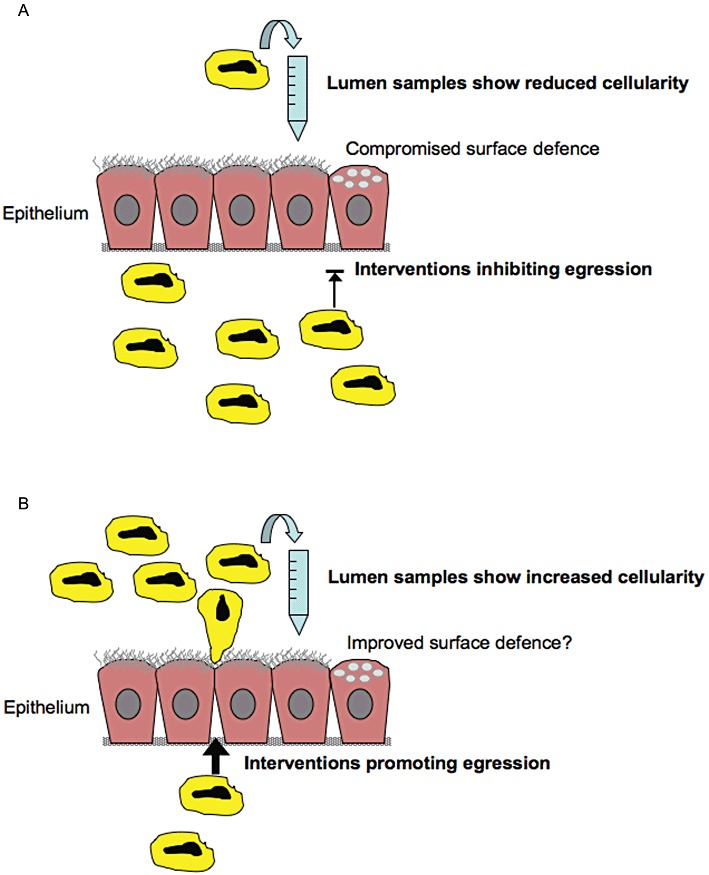
(A) When transepithelial exit from inflamed mucosae is inhibited by ‘anti-traffic’ drug treatment, leucocytes accumulate in mucosal tissues. In these cases, reduced cellularity in samples obtained from the lumen can be associated with aggravated mucosal tissue inflammation. This drug intervention effect also compromises immune defence at the mucosal surface. (B) It is suggested that drugs could be developed that speed up transepithelial exit of leucocytes and thus contribute to resolution of mucosal inflammation. It is currently unknown which molecular targets would be most suitable in this regard. (Several targets, integrin-dependent or -independent, are conceivably involved in the traffic through the tissue, across basement membranes, between epithelial cells and in release from the epithelial surface.) Spontaneous or drug-induced resolution of mucosal tissue inflammation is associated with a period of increased cellularity in the lumen of the involved hollow organ. During these periods, the occurrence of increased numbers of inflammatory cells in lumen samples is not a measure of inflammation but reflects inflammation resolution.
Animal model data further suggest that infectious agents can impede transepithelial egression/elimination of leucocytes. For example, aggravated responses to allergen challenges post viral infection (Sorkness et al., 2007) could be explained by impeded transepithelial migration causing increased airway tissue eosinophilia. Loughman and Hunstad (2011) demonstrated that uropathogenic bacteria markedly attenuated transepithelial neutrophil migration. This effect expectedly reduced anti-bacterial host defence. Preventing the epithelial passage of leucocytes may thus be a mechanism by which viruses and bacteria in part can escape immune surveillance and defence. This possibility makes it of interest to explore if a reduced transepithelial elimination of leucocytes contributes to the infection-evoked exacerbations of asthma and COPD that now lack effective treatment.
Along with effects on eosinophilopoieses and esinophil survival, eosinophil traffic may be under influence of IL-5. It is also well known that anti-IL-5 antibody (mepolizumab) treatment of asthmatic individuals effectively abolished eosinophils in the airway lumen and in the systemic circulation but had much less effect on the airway wall eosinophilia (Flood-Page et al., 2003). It cannot be excluded that the similar kinetics of eosinophils in airway lumen and circulating blood in part reflect a direct traffic between these two compartments. However, the location-dependent, anti-eosinophilic response to mepoluzimab is also what one could expect if transepithelial elimination of eosinophils had been inhibited and no pro-apoptosis effect (none has been demonstrated in vivo!) had been induced by the anti-IL-5 treatment. Recently, a CCR1 antagonist was given to patients with moderate COPD. A preceding study, involving an animal in vivo model of neutrophilic inflammation, had demonstrated that the CCR1 antagonist effectively reduced the occurrence of neutrophils in BAL fluid samples. This was obviously an accepted rationale for giving the drug to patients. However, instead of the expected clinical efficacy, the CCR1 antagonist significantly aggravated the COPD disease and induced one serious exacerbation (Kerstjens et al., 2010). This negative outcome report may have demonstrated the risk involved in giving agents that inhibit transepithelial leucocyte traffic to patients with established airway–pulmonary inflammation.
The pharmacological evidence of adverse effects evoked by inhibition of transepithelial elimination of leucocytes underscores the central role of this pathway in elimination of leucocytes from inflamed mucosal tissues. Interventions that may reduce transepithelial exit of inflammatory cells include drugs (established, in trials, in pipelines and in silico) that by various mechanisms inhibit adhesion molecules and chemokines involved in the transepithelial traffic of leucocytes in humans.
Experimental approaches and future directions
Transepithelial traffic of leucocytes in murine (mouse and rat) and guinea pig models of mucosal inflammation needs validation in corresponding human diseases. An iterative strategy of in vitro and in vivo approaches could then serve the unravelling of involved mechanisms and their pharmacological control. Advanced in vitro approaches involving increasingly complex three-dimensional techniques (Griffith and Swartz, 2006) are emerging. When epithelial cells are grown for a few weeks under air–liquid interface (ALI) conditions, the cells differentiate to a multilayered epithelium that includes ciliated cells and goblet cells. Such cultures have structural features in common with in vivo airway epithelium. They may also be more robust than a simple confluent monolayer of epithelial cells culture. Thus, the ALI culture technique may produce a barrier that is not so easily damaged and that contain several of the paracellular pathways, and associated junction molecules, that are important for the transepithelial migration of leucocytes. The use of ALI epithelial cultures has been considered for several specific purposes (Sabroe et al., 2007; Rothen-Rutishauser et al., 2008; Kesimer et al., 2009), but their utility in studies of non-injurious egression of cells has not received much attention. ALI epithelial co-cultures with leucocytes should be helpful for studies of the pharmacology of transepithelial elimination of leucocytes. Kato et al. (2002) have devised a model involving epithelial cells co-cultured with granulocytes and involving also different collagen gels. By this approach, they have started to explore mechanisms that stimulate and inhibit, respectively, the transmigration of eosinophils and neutrophils. Porter and colleagues have developed the notion of transepithelial elimination of leucocytes by demonstrating potentially important mechanisms of lymphocyte elimination by their test system of epithelial monolayer and lymphocyte co-culture (Porter, 2008; Porter et al., 2008). By different focused approaches, the pharmacology of inflammation resolution, as potentially accomplished by transepithelial elimination of leucocytes, could soon be significantly advanced.
The concept of a resolving role of transepithelial exit of leucocytes may have started with purging of the airways, an intervention that was considered therapeutic in ancient medicine. By mid-1800, Hyde (Salter (1868) noted that resolution of severe asthma was associated with generous production of cell-rich sputum, and Julius Cohnheim (1882) underscored the advantageous outward transport of inflammatory tissue infiltrates available in mucosal lined hollow organs. Still, in the 1970s, principal loss of leucocytes was thought to occur across mucosal epithelial linings (Bellamy and Nielsen, 1974). The challenge today, we think, is to critically assess the actual importance of transepithelial loss of leucocytes in different phases of mucosal inflammatory diseases. Much of the data that we have reviewed above have not been generated in studies designed specifically for the study of different modes of inflammation resolution. Thus, author and publication bias would be minimized, which is important in this field where a hegemonic paradigm exists. However, we need prospective studies addressing the relative roles of different leucocyte emigration pathways as well as any contribution of cell death and engulfment to effect inflammation resolution. We have not found compelling patient data supporting the alleged role of apoptosis–phagocytosis of mucosal leucocytes in spontaneous or drug-induced resolution of mucosal inflammatory diseases. However, this does not exclude the possibility that novel drugs can be produced that will contribute to resolution of such diseases through targeting apoptosis/phagocytosis-related molecular mechanisms. The molecular biology of leucocyte locomotion in tissues and across epithelial barriers has largely been addressed to unravel roles and mechanisms of leucocytes in immune processes and in pathogeneses of diseases. However, the molecular mechanisms involved in the transepithelial migration of tissue-dwelling leucocytes now need to be explored, in vitro and in vivo, with the additional focus on inflammation resolution.
Conclusion
Occurrence of inflammatory cells such as eosinophils, neutrophils, lymphocytes, dendritic cells and mast cells in the lumen of mucosal lined hollow organs has demonstrated that transepithelial elimination of such cells is operational at disease resolution. Many clinical observations and pharmacological in vivo evidence, accumulating in recent decades, are compellingly supportive. Hence, transepithelial migration emerges as a major mode of ridding diseased mucosal tissues of inflammatory cells. However, a resolving role of moving leucocytes into the lumen of hollow organs has limitations. For example, a distinction must be made between egression of infiltrated leucocytes across mucosal epithelia where a swift further elimination of the lumen cells can be expected to occur (nasal, tracheobronchial, gut and bladder mucosae) and the bronchiolar–alveolar epithelial linings where there is a risk of harmful accumulation of lumen cells. Drugs that somehow impede the transepithelial elimination of leucocytes may exhibit anything from reduced efficacy of their anti-inflammatory profile to lethal aggravation of existing inflammation. On the other hand, transepithelial elimination-promoting drugs, combined when needed with efforts to improve clearance of cells from the lumen, can have a role in resolving mucosal tissue inflammation in major respiratory and abdominal diseases.
Acknowledgments
Our work is supported by Swedish Medical Research Council, Swedish Heart and Lung Foundation and Vinnova.
Glossary
- ALI
air–liquid interface
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- IBD
inflammatory bowel disease
Conflict of interest
None.
References
- Aalbers R, de Monchy JG, Kauffman HF, Smith M, Hoekstra Y, Vrugt B, et al. Dynamics of eosinophil infiltration in the bronchial mucosa before and after the late asthmatic reaction. Eur Respir J. 1993;6:840–847. [PubMed] [Google Scholar]
- Aldenborg F, Fall M, Enerback L. Proliferation and transepithelial migration of mucosal mast cells in interstitial cystitis. Immunology. 1986;58:411–416. [PMC free article] [PubMed] [Google Scholar]
- Bellamy JE, Nielsen NO. Immune-mediated emigration of neutrophils into the lumen of the small intestine. Infect Immun. 1974;9:615–619. doi: 10.1128/iai.9.4.615-619.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratke K, Lommatzsch M, Julius P, Kuepper M, Kleine HD, Luttmann W, et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax. 2007;62:168–175. doi: 10.1136/thx.2006.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–146. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- Cohnheim J. 1882. Vorlesungen uber allgemeine Pathologie II. Entzundung.
- Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimi E, Chiaramondia M, Milanese M, Rossi GA, Brusasco V. Increased numbers of mast cells in bronchial mucosa after the late-phase asthmatic response to allergen. Am Rev Respir Dis. 1991;144:1282–1286. doi: 10.1164/ajrccm/144.6.1282. [DOI] [PubMed] [Google Scholar]
- Druilhe A, Wallaert B, Tsicopoulos A, Lapa e Silva JR, Tillie-Leblond I, Tonnel AB, et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–757. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS, Uller L, Malm-Erjefalt M, Persson CG. Rapid and efficient clearance of airway tissue granulocytes through transepithelial migration. Thorax. 2004;59:136–143. doi: 10.1136/thorax.2003.004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahi N, Uller L, Juss JK, Langton AJ, Cowburn AS, Gibson A, et al. Effects of the cyclin-dependent kinase inhibitor R-roscovitine on eosinophil survival and clearance. Clin Exp Allergy. 2011;41:673–687. doi: 10.1111/j.1365-2222.2010.03680.x. [DOI] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew AJS, Pierre J, Teran LM, Trefilieff A, Madden J, Peroni D, et al. Cellular and mediator responses twenty-four hours after local endobronchial allergen challenge of asthmatic airways. J Allergy Clin Immunol. 1996;98:133–143. doi: 10.1016/s0091-6749(96)70235-x. [DOI] [PubMed] [Google Scholar]
- Gauvreau GM, Lee JM, Watson RM, Irani AM, Schwartz LB, O'Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med. 2000;161:1473–1478. doi: 10.1164/ajrccm.161.5.9908090. [DOI] [PubMed] [Google Scholar]
- Gerwin N, Gonzalo JA, Lloyd C, Coyle AJ, Reiss Y, Banu N, et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyperresponsiveness. Immunity. 1999;10:9–19. doi: 10.1016/s1074-7613(00)80002-3. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Saltos N, Fakes K. Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2001;163:32–36. doi: 10.1164/ajrccm.163.1.9807061. [DOI] [PubMed] [Google Scholar]
- Godaly G, Bergsten G, Hang L, Fischer H, Frendeus B, Lundstedt AC, et al. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J Leukoc Biol. 2001;69:899–906. [PubMed] [Google Scholar]
- Greiff L, Erjefalt JS, Andersson M, Svensson C, Persson CG. Generation of clusters of free eosinophil granules (Cfegs) in seasonal allergic rhinitis. Allergy. 1998;53:200–203. doi: 10.1111/j.1398-9995.1998.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Grigg JM, Savill JS, Sarraf C, Haslett C, Silverman M. Neutrophil apoptosis and clearance from neonatal lungs. Lancet. 1991;338:720–722. doi: 10.1016/0140-6736(91)91443-x. [DOI] [PubMed] [Google Scholar]
- Haworth O, Buckley CD. Resolving the problem of persistence in the switch from acute to chronic inflammation. Proc Natl Acad Sci U S A. 2007;104:20647–20648. doi: 10.1073/pnas.0710633105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S, Hodge G, Holmes M, Reynolds PN. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J. 2005;25:447–454. doi: 10.1183/09031936.05.00077604. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Poznansky MC. Reverse leukocyte migration can be attractive or repulsive. Trends Cell Biol. 2008;18:298–306. doi: 10.1016/j.tcb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- Juliusson S, Pipkorn U, Karlsson G, Enerback L. Mast cells and eosinophils in the allergic mucosal response to allergen challenge: changes in distribution and signs of activation in relation to symptoms. J Allergy Clin Immunol. 1992;90(6 Pt 1):898–909. doi: 10.1016/0091-6749(92)90462-b. [DOI] [PubMed] [Google Scholar]
- Kato Y, Fujisawa T, Shibano M, Saito T, Gatto W, Kamiya H, et al. Airway epithelial cells promote transmigration of eosinophils in a new three-dimensional chemotaxis model. Clin Exp Allergy. 2002;32:889–897. doi: 10.1046/j.1365-2222.2002.01362.x. [DOI] [PubMed] [Google Scholar]
- Kerstjens HA, Bjermer L, Eriksson L, Dahlstrom K, Vestbo J. Tolerability and efficacy of inhaled AZD4818, a CCR1 antagonist, in moderate to severe COPD patients. Respir Med. 2010;104:1297–1303. doi: 10.1016/j.rmed.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CG. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher S, et al. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–97. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Loughman JA, Hunstad DA. Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect. 2011;13:555–565. doi: 10.1016/j.micinf.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhelainen N, Rytila P, Haahtela T, Kinnula VL, Djukanovic R. Persistence of oxidant and protease burden in the airways after smoking cessation. BMC Pulm Med. 2009;9:25. doi: 10.1186/1471-2466-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- Malickova K, Kalousova M, Fucikova T, Bortlik M, Duricova D, Komarek V, et al. Anti-inflammatory effect of biological treatment in patients with inflammatory bowel diseases: calprotectin and IL-6 changes do not correspond to sRAGE changes. Scand J Clin Lab Invest. 2010;70:294–299. doi: 10.3109/00365513.2010.485648. [DOI] [PubMed] [Google Scholar]
- Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest. 2002;110:1603–1605. doi: 10.1172/JCI17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti C, Arques JL, Bertelli E. CX(3)CR1 is critical for Salmonella-induced migration of dendritic cells into the intestinal lumen. Gut Microbes. 2011;1:131–134. doi: 10.4161/gmic.1.3.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan S, Cormican L, Burke CM, Poulter LW. Fluticasone induces T cell apoptosis in the bronchial wall of mild to moderate asthmatics. Thorax. 2004;59:657–661. doi: 10.1136/thx.2002.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- Page C. Paradoxical pharmacology: turning our pharmacological models upside down. Trends Pharmacol Sci. 2011;32:197–200. doi: 10.1016/j.tips.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Persson CG, Erjefalt JS. Eosinophil lysis and free granules: an in vivo paradigm for cell activation and drug development. Trends Pharmacol Sci. 1997;18:117–123. doi: 10.1016/s0165-6147(97)01042-0. [DOI] [PubMed] [Google Scholar]
- Persson C, Uller L. Transepithelial exit of leucocytes: inflicting, reflecting or resolving airway inflammation? Thorax. 2010a;65:1111–1115. doi: 10.1136/thx.2009.133363. [DOI] [PubMed] [Google Scholar]
- Persson CG, Uller L. Resolution of cell-mediated airways diseases. Respir Res. 2010b;11:75. doi: 10.1186/1465-9921-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson CG, Erjefalt JS, Uller L, Andersson M, Greiff L. Unbalanced research. Trends Pharmacol Sci. 2001;22:538–541. doi: 10.1016/s0165-6147(00)01839-3. [DOI] [PubMed] [Google Scholar]
- Persson C, Andersson M, Uller L. Epithelial repair and function. In: Proud D, editor. Pulmonary Epithelium. Chicester: Wiley; 2008. pp. 75–88. [Google Scholar]
- Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- Porter JC. Epithelial Rho GTPases and the transepithelial migration of lymphocytes. Methods Enzymol. 2008;439:205–217. doi: 10.1016/S0076-6879(07)00416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JC, Falzon M, Hall A. Polarized localization of epithelial CXCL11 in chronic obstructive pulmonary disease and mechanisms of T cell egression. J Immunol. 2008;180:1866–1877. doi: 10.4049/jimmunol.180.3.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF. Eosinophilic inflammation: life, death and apoptosis. Clin Exp Allergy. 2011;41:612–614. doi: 10.1111/j.1365-2222.2011.03727.x. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Blank F, Muhlfeld C, Gehr P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol. 2008;4:1075–1089. doi: 10.1517/17425255.4.8.1075. [DOI] [PubMed] [Google Scholar]
- Rytila P, Plataki M, Bucchieri F, Uddin M, Nong G, Kinnula VL, et al. Airway neutrophilia in COPD is not associated with increased neutrophil survival. Eur Respir J. 2006;28:1163–1169. doi: 10.1183/09031936.00149005. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Parker LC, Dockrell DH, Davies DE, Dower SK, Whyte MK. Targeting the networks that underpin contiguous immunity in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:306–311. doi: 10.1164/rccm.200606-777PP. [DOI] [PubMed] [Google Scholar]
- Salter HH. On Asthma, Its Pathology and Treatment. 2nd edn. Churchill: London; 1868. [Google Scholar]
- Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OO, Hanauer SB, McColm J, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B. Targeted therapies in inflammatory bowel disease. Dig Dis. 2009;27:465–469. doi: 10.1159/000233284. [DOI] [PubMed] [Google Scholar]
- Sorkness RL, Herricks KM, Szakaly RJ, Lemanske RF, Jr, Rosenthal LA. Altered allergen-induced eosinophil trafficking and physiological dysfunction in airways with preexisting virus-induced injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:L85–L91. doi: 10.1152/ajplung.00234.2006. [DOI] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14:147–161. doi: 10.1002/ibd.20330. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood. 2011;117:6050–6052. doi: 10.1182/blood-2010-10-310532. author reply 6053–6054. [DOI] [PubMed] [Google Scholar]
- Uller L, Persson CG, Kallstrom L, Erjefalt JS. Lung tissue eosinophils may be cleared through luminal entry rather than apoptosis: effects of steroid treatment. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1948–1956. doi: 10.1164/ajrccm.164.10.2011135. [DOI] [PubMed] [Google Scholar]
- Uller L, Andersson M, Greiff L, Persson CG, Erjefalt JS. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am J Respir Crit Care Med. 2004;170:742–747. doi: 10.1164/rccm.200402-240OC. [DOI] [PubMed] [Google Scholar]
- Uller L, Rydell-Tormanen K, Persson CG, Erjefalt JS. Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation. Respir Res. 2005;6:90. doi: 10.1186/1465-9921-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller L, Lloyd CM, Rydell-Tormanen K, Persson CG, Erjefalt JS. Effects of steroid treatment on lung CC chemokines, apoptosis and transepithelial cell clearance during development and resolution of allergic airway inflammation. Clin Exp Allergy. 2006a;36:111–121. doi: 10.1111/j.1365-2222.2006.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller L, Persson CG, Erjefalt JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci. 2006b;27:461–466. doi: 10.1016/j.tips.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Uller L, Ahlstrom Emanuelsson C, Andersson M, Erjefalt JS, Greiff L, Persson CG. Early phase resolution of mucosal eosinophilic inflammation in allergic rhinitis. Respir Res. 2010;11:54. doi: 10.1186/1465-9921-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AP, Brown V, Courtney J, Kelly M, Garske L, Elborn JS, et al. Neutrophil apoptosis, proinflammatory mediators and cell counts in bronchiectasis. Thorax. 2004;59:231–236. doi: 10.1136/thx.2003.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax. 2005;60:659–664. doi: 10.1136/thx.2004.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]


