Abstract
BACKGROUND AND PURPOSE
Bones are widely innervated, suggesting an important role for the sympathetic regulation of bone metabolism, although there are controversial studies. We investigated the effects of propranolol in a model of experimental periodontal disease.
EXPERIMENTAL APPROACH
Rats were assigned as follows: animals without ligature; ligated animals receiving vehicle and ligated animals receiving 0.1, 5 or 20 mg·kg−1 propranolol. After 30 days, haemodynamic parameters were measured by cardiac catheterization. Gingival tissues were removed and assessed for IL-1β, TNF-α and cross-linked carboxyterminal telopeptides of type I collagen (CTX) by elisa, or intercellular adhesion molecule 1 (ICAM-1), receptor activator of NF-κ B ligand (RANKL) and osteoprotegerin (OPG) by Western blot analysis. Sections from the mandibles were evaluated for bone resorption. Also, we analysed the ability of propranolol to inhibit osteoclastogenesis in vitro.
RESULTS
Propranolol at 0.1 and 5 mg·kg−1 reduced the bone resorption as well as ICAM-1 and RANKL expression. However, only 0.1 mg·kg−1 reduced IL-1β, TNF-α and CTX levels as well as increased the expression of OPG, but did not alter any of the haemodynamic parameters. Propranolol also suppressed in vitro osteoclast differentiation and resorptive activity by inhibiting the nuclear factor of activated T cells (NFATc)1 pathway and the expression of tartrate-resistant acid phosphatase (TRAP), cathepsin K and MMP-9.
CONCLUSIONS AND IMPLICATIONS
Low doses of propranolol suppress bone resorption by inhibiting RANKL-mediated osteoclastogenesis as well as inflammatory markers without affecting haemodynamic parameters.
Keywords: propranolol, periodontal disease, β-blocker, bone, inflammation
Introduction
The process of bone modelling and remodelling ensures adaptation of the size, shape, microarchitecture and mineral content of the skeleton, as well as the repair of bone damage, in response to growth, aging and mechanical constraints (Seeman and Delmas, 2006). The development and activation of osteoblasts and osteoclasts is controlled by growth factors and cytokines produced by bone cells themselves as well as by surrounding bone marrow cells. More recently, the neuroendocrine system has been implicated in the regulation of bone remodelling (Bonnet et al., 2008b).
β2-Adrenoceptors have been detected by RT-PCR in human periosteum-derived osteoblastic cells (SaM-1) (Togari et al., 1997), human osteosarcoma-derived cells (SaOS-2), mouse primary osteoblasts (Takeda et al., 2002; Bonnet et al., 2008a) and human osteoclastic cells (Togari, 2002). Also, bone-resorbing activity in mice was increased by activation of their sympathetic nervous system by the i.c.v. injection of leptin or LPS or by restraint stress (Takeda et al., 2002; Kondo and Togari, 2003).
β-Blocker drugs have a well-recognized antihypertensive action that is mediated through a reduction in cardiac output, the release of renin from the kidneys and inhibition of the action of endogenous catecholamines on β-adrenoceptors (Graham et al., 2008). This class of drugs is classified as one of the first-line choice for the treatment of hypertension and has been widely used in cardiovascular disease. Interestingly, a series of epidemiological studies (Mattila et al., 1989; De Stefano et al., 1993; Beck et al., 1996; Joshipura et al., 1996; Grau et al., 1997; Jansson et al., 2001; Cotti et al., 2011) has suggested an important relationship between periodontal disease and cardiovascular diseases. This association has been hypothesized to be the genesis of a common inflammatory response feature, which exposes individuals to the development of both periodontal disease and atherosclerosis. Thus, periodontal disease is believed to provide a massive release of inflammatory cytokines, which may contribute to atherosclerosis and thrombotic events (Cotti et al., 2011). On the other hand, our group previously demonstrated that the alveolar bone around teeth without ligature (experimental periodontal disease model in rats) in untreated spontaneous hypertensive rats (SHR) presented a higher expression of receptor activator of NK-κB ligand (RANKL) and a higher ratio of RANKL/osteoprotegerin (OPG), an elevated number of osteoclast cells, increased bone loss and decreased bone density, suggesting that a hypertensive status may directly affect alveolar bone, regardless of ligature challenge (Bastos et al., 2010).
There are several epidemiological studies that have demonstrated an association between high blood pressure and increased bone loss at the femoral neck and low bone mineral density, and that β-blockers are potential candidates of therapeutic drugs for osteoporosis and fracture healing (Cappuccio et al., 1999; Pasco et al., 2004; Schlienger et al., 2004; Graham et al., 2008). However, there are other studies showing that the use of β-blockers did not affect bone mineral density (Reid et al., 2005), indicating that the relationship between β-blocker use and bone remodelling needs further prospective studies (Bonnet et al., 2006).
Since the number of adult and ageing people suffering from cardiovascular associated with periodontal diseases is growing, the advantages of a dual-benefit effect of only one treatment on both heart and skeletal systems is of great interest. Thus, we hypothesized that a common β-blocker (propranolol) may be used to decrease the bone loss in a model of periodontal disease. To our knowledge, there is no specific information on low-, middle- and high-dose effects of propranolol on periodontal surrounding tissues or on its mechanism of action. The aim of this work was to investigate the effects of different doses of propranolol on the bone of rats with experimental periodontitis and to further elucidate its mechanism by measuring various bone/inflammatory markers.
Methods
All drug and molecular target nomenclature used in the manuscript is in accordance with the BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Animals
All animal care and experimental procedures were approved by The Institutional Committee for Animal Care and Use at University of Uberaba (previously approved the study protocol #048/2009). Thirty male Wistar rats were used in this study. Rats were 90 days of age and weighed 235 ± 20 g at the beginning of the study. During the acclimatization (5 days) and experimental periods (30 days), animals were housed in groups of five in plastic cages with access to food and drinking water ad libitum. The rats were kept in a room with a 12 h light/dark cycle and a temperature between 22°C and 24°C.
Experimental design and ligature placement
Experimental periodontitis was induced by a ligature placement. More specifically, under general anaesthesia obtained by i.m. administration of ketamine (1.0 mL·kg−1), a ligature was placed and immobilized around both mandible first molars of each animal. The ligature was left in position for the whole experimental period so that inflammation could be constantly induced by the colonization of bacteria inside of it. One day following ligature placement, the animals were randomly assigned to one of the following groups: (i) sham-ligated animals administered vehicle (control) (n= 6); (ii) animals with ligature administered vehicle (n= 6); (iii) animals with ligature administered propranolol 0.1 mg·kg−1·day−1 p.o. (n= 6); animals with ligature administered propranolol 5 mg·kg−1·day−1 p.o. (n= 6); and (iv) animals with ligature administered propranolol 20 mg·kg−1·day−1 p.o. (n= 6).
Haemodynamic parameters
Thirty days after ligature placement, the animals were anaesthetized (sodium pentobarbital, 40 mg·kg−1, i.p.) for cardiac catheterization with polyethylene catheters (PE-50) filled with saline solution (0.9%) into the carotid artery. The arterial catheter was connected to a pressure transducer (Statham P23Gb, Hato Rey, PR, USA) and the amplified (Hewlett-Packard amplifier, model 8805A, Waltham, MS, USA) signal was continuously sampled (1000 Hz) in an IBM/PC equipped with a 12 bit analogue to digital board (CAD12/36 Lynx Eletrônica, São Paulo, Brazil). All parameters, heart rate, arterial pressure, left ventricular systolic (LVSP) and end-diastolic pressures (LVEDP) and positive and negative LV dP/dt, were measured. After that, all animals were killed by an overdose of anaesthetic.
Histological procedures and histometric analyses
The right and left jaws were dissected, fixed in 10% buffered neutral formalin for 48 h and decalcified in a decalcifying solution of EDTA 10% for 3 months. After that, decalcified samples were briefly washed in running tap water, dehydrated and embedded in paraffin wax. Each sample was sliced into 6 µm sections in sagittal directions. After excluding the first and last sections in which the furcation area was totally evident 10 equally distant sections, which were selected every 30 µm, of each molar was chosen for histometric evaluations. The area between the inter-radicular bone crest and furcation roof was assessed for bone loss mm−2, as previously described (Napimoga et al., 2009). For the analysis of tooth-supporting alveolar bone density (BD), a standardized rectangular area (4.56 mm2) was delineated in the furcation area of the teeth. Subsequently, a checkered diagram was overlaid on this area, which constituted a drawing with 1200 intersections. The number of intersections under which bone tissue was present was counted. BD was calculated according to the following formula: BD × 100/1200. The analysis was performed by one trained, calibrated and (masked) examiner unaware of the different treatments (WFR) using image analysis software (Image-J, National Institute of Health, Bethesda, MD, USA).
Protein extraction from gingival tissue
The gingival tissues around the affected tooth of the animals were removed, triturated and homogenized in 300 µL of the appropriate buffer containing protease inhibitors (Sigma-Aldrich, St. Louis, MO) followed by centrifugation for 10 min at 10 000×g. The total amount of extracted proteins was measured by colorimetric analysis using the micro BCA protein assay kit (Thermo, Rockford, IL, USA). The supernatants were stored at −70°C until further analysis.
Cytokine and markers of bone resorption measurements
The levels of IL-1β and TNF-α (R&D Systems, Minneapolis, MN) and of cross-linked carboxyterminal telopeptides of type I collagen (CTX) (Rheabiotech, Paulinia, SP, Brazil) from the gingival tissue were evaluated by elisa. Briefly, 100 µL of detection antibody was added to all wells, except blank, mixed gently and incubated overnight (16–24 h) at room temperature. Plates were washed three times and standards and supernatants from gingival tissue (as described above) were added in the respective wells in duplicate. After 2 h at room temperature, the plates were washed again and incubated with 100 µL of conjugate for 60 min at room temperature. Plates were washed three times again, and 100 µL of avidin–peroxidase was added during 30 min at room temperature, followed by a new series of washes, and 100 µL of substrate was added and incubated for 15 min at room temperature in the dark. The reaction was stopped by the addition of 50 µL of stop solution, and colour was measured in an automated microplate spectrophotometer (Microplate Reader/Model 3550, Bio-Rad Laboratories, Hercules, CA, USA). The total amounts of cytokines were determined as pg·mg−1. Results were calculated using the standard curves created in each assay. The elisa assays were carried out by a person unaware of the different treatments.
Osteoclastogenesis
The murine monocyte/macrophage cell line RAW 264.7 was purchased from the American Type Culture Collection (Manasas, VA) and grown in DMEM supplemented with 10% heat-inactivated FBS, penicillin (40 U·mL−1) and gentamicin (40 µg·mL−1). All cells were grown in a humidified atmosphere containing 5% CO2 at 37°C. For osteoclastic differentiation, RAW264.7 cells were suspended in DMEM containing 10% FBS and then seeded at 1 × 105 cells per well in 24-well culture plates with 13 mm glass coverslips at a density of 1 × 103 cells per well in a 96-well culture plate and cultured with 50 ng·mL−1 soluble RANKL (PeproTech Inc., Rocky Hill, NJ, USA) for 4 days. Cells stimulated with RANKL were treated in a final concentration of 1, 3 or 10 µM of propranolol (in order to mimic the low-, middle- and high-dose used in vivo) and then activated by 2.5 µg·mL−1 of Aggregatibacter actinomycetemcomitans LPS (a gift from Dr Osamu Fujise, Kyushu University) for 48 h to mimic the influence of the bacterium. The culture medium was replaced with fresh medium every 2 days. After the culture, the cells were subjected to tartrate-resistant acid phosphatase (TRAP) staining.
Protein extraction from RAW 264.7 cells
Nuclear extracts were obtained from cultured RAW 264.7 cells by using a lysis buffer (1% Triton X-100, 100 mM Tris–HCl, pH 8.0, 10% glycerol, 5 mM EDTA, 200 mM NaCl, 1 mM dithiothreitol (DTT), 1 mM PMSF, 25 mM NaF, 2.5 mg·mL−1 leupeptin, 5 mg·mL−1 aprotinin and 1 mM sodium orthovanadate). Lysates were centrifuged at 16 000×g for 10 min at 4°C and quantified using the Bradford assay reagent from Bio-Rad.
Western blot
Equal amounts of protein (90 µg) from the gingival tissue or proteins isolated from RAW264.7 cells were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). A molecular weight standard (Bio-Rad Laboratories) was run in parallel to estimate molecular weight. Membranes were blocked, overnight at 4°C, in Tris-buffered saline–Tween (20 mM Tris–HCl, pH 7.5, 500 mM NaCl, 0.1% Tween 20; TBST) containing 5% of dried milk. After being blocked, the membranes were incubated, at 4°C overnight, with anti-ICAM-1 (1:1000), anti-RANKL (1:1000), anti-OPG (1:2000) or α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), used as an internal control (1:1000), diluted in TBST containing 5% of dried milk for the analyses of the gingival proteins. Anti-NF-κBp65 (1:1000) and anti-NFATc1 (1:1000) diluted in PBS containing 5% (w/v) BSA and 0.1% Tween-20 were also used for the analyses of the cell culture proteins. Membranes were then incubated with a secondary antibody conjugated with peroxidase (1:5000) diluted in TBS-T containing 5% of dried milk at room temperature for 60 min. Finally, the bands recognized by the specific antibody were visualized using a chemiluminescence-based ECL system (Amersham Biosciences, Piscataway, NJ, USA) and exposed to an X-ray film for 30 min (Eastman Kodak, Rochester, NY, USA). A computer-based imaging system (Image J) was used to measure the intensity of optical density of bands.
TRAP staining
A standard TRAP (Sigma-Aldrich) staining procedure was done according to the manufacturer's instructions. Briefly, cultured adherent cells in 13 mm glass coverslips were washed once with PBS and fixed in citrate/acetone solution for 30 s, then rinsed with deionized water and incubated in tartrate staining solution for 1 h at 37°C in the dark. After this, cells were rinsed in deionized water for 3 min and allowed to air dry. TRAP-positive cells appeared dark red, and TRAP-positive multinucleated cells containing three or more nuclei were counted as mature osteoclasts.
Resorption pit formation assay
Calcified matrix resorption activity of the osteoclasts was tested on calcium hydroxyapatite–coated slides (BioCoat Osteologic; BD Biosciences, Franklin Lakes, NJ, USA), using a culture setting identical to that described above. After 10 days, the cells were removed and the number of pits was counted. Data are expressed as number of pits per field.
Real-time quantitative PCR
Total RNA from the culture cells was isolated by the Trizol method (Gibco BRL, Life Technologies, Rockville, MD, USA) according to the manufacturer's instructions. RNA samples were resuspended in diethylpyrocarbonate-treated water and stored at −70°C. The RNA concentration was determined from the optical density using a micro-volume spectrophotometer (Nanodrop 1000, Nanodrop Technologies LLC, Wilmington, NC, USA).
Reverse transcription total RNA was DNase treated (Turbo DNA-frees, Ambion Inc., Austin, TX, USA), and 1 µg was used for cDNA synthesis. The reaction was carried out using the First-Strand cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA), following the manufacturer's instructions.
Primer sets for cathepsin K, collagenase (MMP-9), TRAP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed from sequences available from GenBank using Primer Express 3.0 probe design software (Applied Biosystems, Foster City, CA, USA). Primer sequences are as follows: MMP-9: forward: 5′-AGGCCTCTACAGAGTCTTTG-3′, reverse: 5′-CAGTCCAACAAGAAAGGACG-3′, length: 824 bp; cathepsin K: forward: 5′-TGCGACCGTGATAATGTGAACC-3′, reverse: 5′-ATGGGCTGGCTGGCTTGAATC-3′, length: 205 bp; TRAP: forward: 5′-CGCCAGAACCGTGCAGATTATG-3′, reverse: 5′-AAGATGGCCACGGTGATGTTCG-3′, length: 297 bp; and GAPDH: forward: 5′-GACTGTGGATGGCCCCTCTG-3′, reverse: 5′-CGCCTGCTTCACCACCTTCT-3′, length: 239 bp. Quantitative real-time PCR (qPCR) was performed in the 7300 Real Time PCR (Applied Biosystems) using the SYBR Green PCR Master Mix (Fermentas). The reaction product was quantified with the Relative Quantification tool, using GAPDH as the reference gene. Negative controls with SYBR Green PCR Master Mix and water were performed for all reactions.
Statistical analysis
Data are expressed as mean ± SD. Statistical comparisons among groups were made using anova followed by Bonferroni's test. Significance was accepted when the P-value was ≤0.05.
Results
Low dose of propranolol inhibits periodontal bone loss
All animals gained weight during the study; however, the mean body weight did not show statistically significant differences among groups at the end of the experimental period (data not shown). Experimental periodontitis induced by the ligature significantly increased the bone loss compared to the control group (Figure 1A. vs. B; P < 0.05). Oral administration of a low dose of propranolol (0.1 and 5 mg·kg−1) markedly reduced bone resorption (Figure 1C and D, P < 0.05). On the other hand, a high dose of propranolol (Figure 1E; 20 mg·kg−1) did not reduce the bone loss caused by the experimental periodontitis. Values of the resorption area of all groups are shown in Figure 1F. These results indicate that low doses of propranolol can indeed suppress the periodontal bone resorption caused by inflammatory reactions induced by ligature. Bone densities were significantly lower in tooth-supporting alveolar bone from ligature-induced periodontal disease (45.49 ± 7.24) and ligature-induced periodontal disease treated with 5 or 20 mg·kg−1 of propranolol (47.92 ± 3.85 and 38.13 ± 6.32, respectively) groups when compared with sham-ligated or ligature-induced periodontal disease treated with 0.1 mg·kg−1 of propranolol (77.08 ± 4.22 and 69.39 ± 6.56). Figure 2 illustrates the findings of bone densities.
Figure 1.
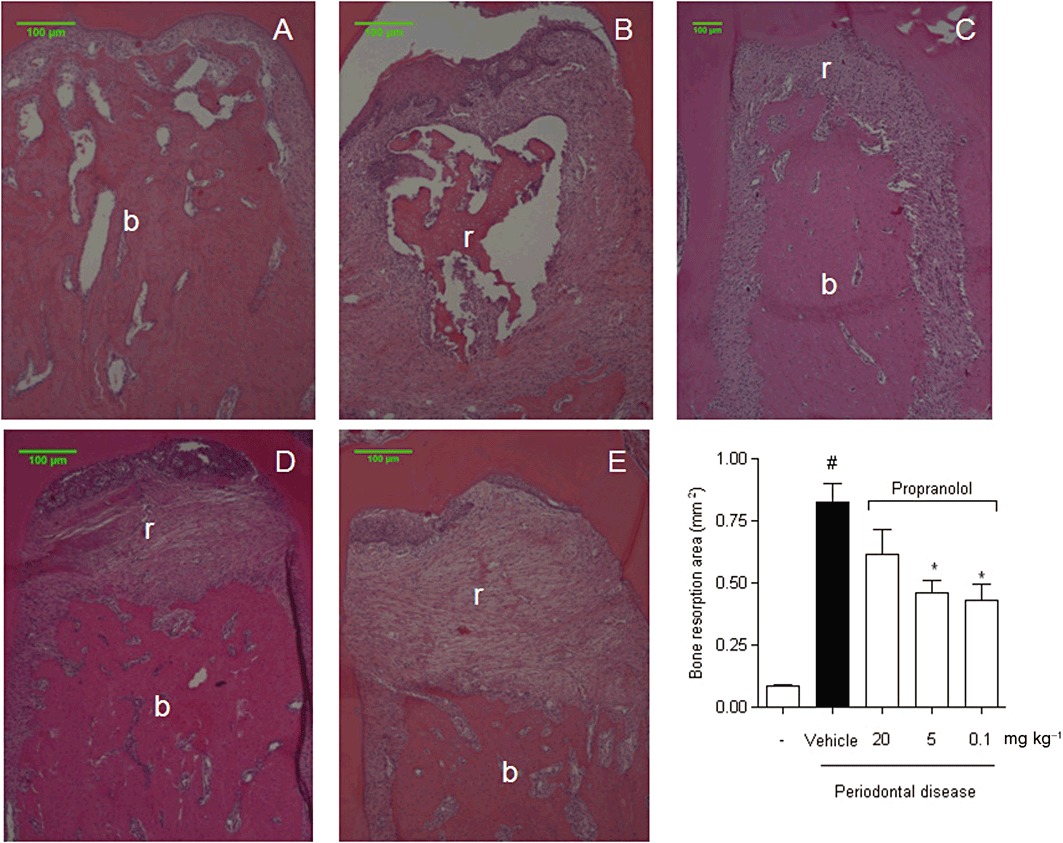
Propranolol decreases alveolar bone resorption. Histology at the furcation of first molars sampled from the rats killed after 30 days of experiments is shown (staining with haematoxylin–eosin, HE). (A) Sham-ligated animals. (B) Ligature-induced periodontitis treated with vehicle for 30 days. (C) Ligature-induced periodontitis treated with propranolol 0.1 mg·kg−1·day−1 for 30 consecutive days. (D) Ligature-induced periodontitis treated with propranolol 5 mg·kg−1·day−1 for 30 consecutive days. (E) Ligature-induced periodontitis treated with propranolol 20 mg·kg−1·day−1 for 30 consecutive days. (F) The bone resorption area measured at the furcation of first molars. Results are expressed as mean area (mm2) ± SD of six animals in each group. #P < 0.05 compared with sham-ligated animals; *P < 0.05 compared with ligature-induced periodontitis treated with vehicle (anova followed by Bonferroni's test). The letter b indicates the bone, and the letter r indicates the resorptive area.
Figure 2.
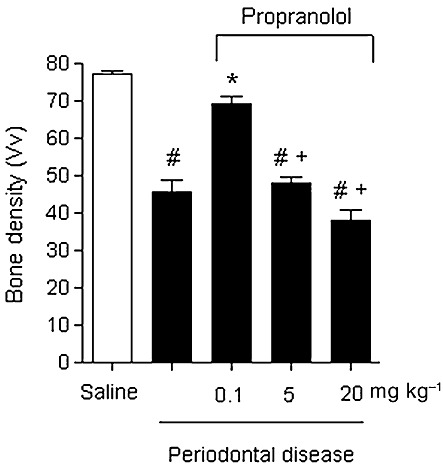
Mean and SD of the bone densities of all analysed groups. The number of intersections under which bone tissue was present was counted in a standardized area (see Methods). Results are expressed as mean bone density ± SD of six animals in each group. #P < 0.001 compared with sham-ligated animals; *P < 0.001 compared with ligature-induced periodontitis treated with vehicle; +P < 0.001 compared with ligature-induced periodontitis treated with 0.1 mg·kg−1 propranolol.
Haemodynamic parameters
Haemodynamic parameters measured by catheterization of the left ventricle were unaffected by 0.1 mg·kg−1·day−1 dose of propranolol (Table 1). In contrast, these parameters of cardiac function were severely affected in rats treated with higher doses particularly in the animals treated with 20 mg·kg−1 propranolol and to a lesser extent in the group treated with 5 mg·kg−1. The highest dose of propranolol (20 mg·kg−1) significantly decreased the cardiac output and ejection fraction in comparison with both placebo groups and the 0.1 mg·kg−1 propranolol-treated group (P < 0.05). Arterial pressure was also significantly lower in the group treated with 20 mg·kg−1 in comparison with all other groups (P < 0.05). The animals treated with 5 mg·kg−1 propranolol also had a lower arterial pressure in comparison with both non-treated groups but not compared to the 0.1 mg·kg−1-treated group (Table 1). LVEDP as well as the positive and negative left ventricular dP/dt were significantly altered in the groups treated with 5 and 20 mg·kg−1 propranolol (Table 1).
Table 1.
Heart rate, blood pressure and assessment of left ventricular function
| Sham-ligated | Periodontal disease+vehicle | Periodontal disease+0.1 mg·kg−1 | Periodontal disease+5 mg·kg−1 | Periodontal disease+20 mg·kg−1 | |
|---|---|---|---|---|---|
| HR (beats min−1) | 345.8 ± 2.4 | 347.3 ± 2.2 | 338.1 ± 6.2 | 323.8 ± 5.0 | 302.6 ± 9.2*#& |
| MAP (mmHg) | 104.9 ± 0.6 | 104.4 ± 0.3 | 102.6 ± 0.3 | 100.1 ± 0.8*# | 88.8 ± 0.5*#&§ |
| LVEDP (mmHg) | 7.82 ± 0.0 | 7.72 ± 0.0 | 8.17 ± 0.1 | 11.41 ± 0.1*#& | 17.21 ± 0.8*#&§ |
| dP/dtmax (mmHg s−1) | 7331 ± 403 | 7281 ± 305 | 6959 ± 364 | 5418 ± 286*# | 3155 ± 499*#&§ |
| dP/dtmin (mmHg s−1) | −6435 ± 83 | −6085 ± 77 | −6121 ± 160 | −4995 ± 60*#& | −2874 ± 146*#&§ |
Data are mean ± EPM. HR, heart rate; MAP, mean arterial pressure; dP/dtmax and dP/dtmin, average maximum and minimum values.
P < 0.05 versus sham-ligated;
P < 0.05 versus periodontal disease + vehicle;
P < 0.05 versus periodontal disease + 0.1 mg·kg−1; and
P < 0.05 versus periodontal disease + 5 mg·kg−1.
Cytokines and CTX measurements from gingival tissue
IL-1β and TNF-α are critical cytokines in the periodontal disease context. Thus, we evaluated the levels of both cytokines in the gingival tissue after the propranolol treatment. It was observed that for the group of ligature-induced periodontal disease, IL-1β and TNF-α were significantly increased. Interestingly, only the group of animals treated with 0.1 mg·kg−1 propranolol showed a significantly decreased expression of both cytokines (P < 0.05) (Figure 3A and B, respectively). In addition, the amount of CTX (a bone turnover biomarker) was significantly elevated in the gingival tissue of ligature-induced periodontal disease animals in comparison with non-ligated animals and the 0.1 mg·kg−1 propranolol-treated group. Both groups treated with 5 or 20 mg·kg−1 propranolol showed increased amounts of CTX, as demonstrated in Figure 3C.
Figure 3.
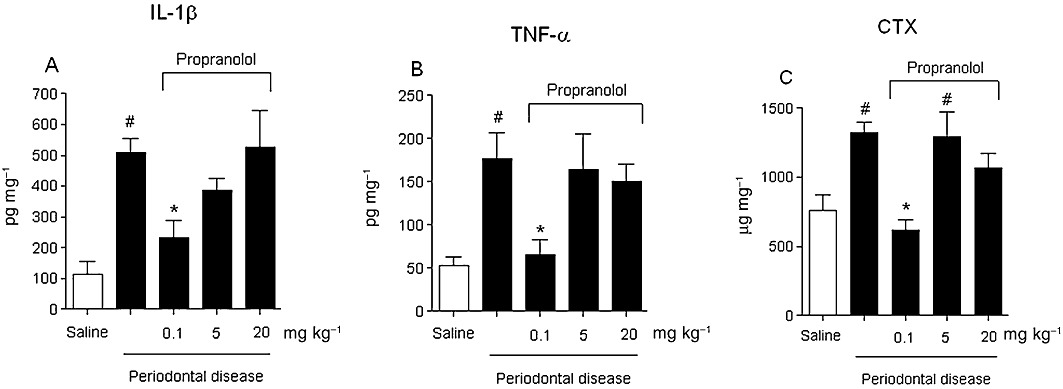
Concentration of pro-inflammatory cytokines IL-1β (A), TNF-α (B) or bone turnover marker CTX (C) in the gingival tissue was analysed using elisa. Results are expressed as means (pg·mg−1) ± SD of concentration of each cytokine in the tissue (n= 6 per group). #P < 0.05 compared with sham-ligated animals; *P < 0.05 compared with ligature-induced periodontitis treated with vehicle (anova, followed by Bonferroni's test).
ICAM-1 and RANKL/OPG expression
We also analysed the expression by Western blot of three important molecules: ICAM-1, which is involved in the leucocyte migration to the tissue; RANKL, a key molecule for activation of the osteoclast cells; and OPG, a decoy receptor for the RANKL. As demonstrated in the Figure 4A, ICAM-1 expression was up-regulated (P < 0.05) in the gingival tissue of the ligature-induced animals in comparison with the sham-ligated animals. In contrast, the animals that received 0.1 and 5 mg·kg−1 propranolol showed a significantly decreased expression of ICAM-1 (P < 0.05), while 20 mg·kg−1 propranolol did not alter the expression of this molecule. The same pattern was observed in the levels of RANKL expression in which the ligature-induced animals showed increased expression of RANKL in comparison with the sham-ligated animals, and 0.1 and 5 mg·kg−1 propranolol, but not 20 mg·kg−1 propranolol, significantly decreased the expression of this molecule (Figure 4B). On the other hand, the OPG expression was high in the sham-ligated animals as compared with ligature-induced animals (P < 0.05). Importantly, the ligature-induced animals treated with 0.1 mg·kg−1 propranolol had high levels of OPG expression high and statistically significant (P < 0.05) in comparison with ligature-induced animals and the groups treated with 5 and 20 mg·kg−1 propranolol (Figure 4C).The ratio of RANKL/OPG was significantly higher in the ligature-induced animals and the groups treated with 5 and 20 mg·kg−1 propranolol in comparison with the sham-ligated and the ligature-induced animals treated with 0.1 mg·kg−1 propranolol (Figure 4D).
Figure 4.
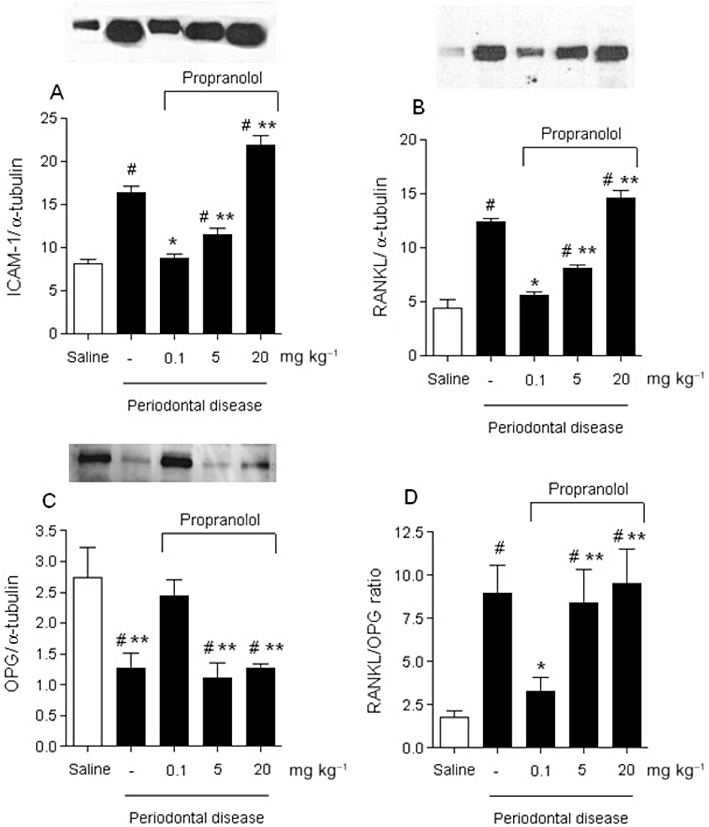
Effects of propranolol on ligature-induced periodontitis ICAM-1 (A), RANKL (B), OPG (C) and ratio of RANKL/OPG (D) expression on gingival tissue. Protein expression was analysed by Western blot, and the intensity of optical density of bands was measured from Western blots. Density of the both molecule bands were normalized to α-tubulin expression. Protein band intensity is represented as arbitrary units. The results are expressed as mean ± SD of six animals per group. #P < 0.05 compared with sham-ligated animals; *P < 0.05 compared with ligature-induced periodontitis treated with vehicle; **P < 0.05 compared with ligature-induced periodontitis treated with 0.1 mg·kg−1 of propranolol (anova, followed by Bonferroni's test).
In vitro osteoclastogenesis
In order to determine whether the propranolol can affect RANKL-mediated osteoclastogenesis, we examined the effects of this drug on in vitro osteoclast differentiation induced in RAW264.7 osteoclast precursor cells by stimulation with recombinant RANKL and LPS. The formation of TRAP-positive multinucleated cells induced in the culture of RANKL-stimulated RAW264.7 cells was inhibited by the addition of propranolol at all three doses tested (P < 0.05). Important, none of the tested doses (1, 3 and 10 µM) induced cell death as observed by the Trypan blue test and confirmed by MTT assay (data not shown). The number of TRAP-positive cells counted is shown in Figure 5A. In order to test if the presence of LPS in the culture could affect osteoclast differentiation and/or inhibition, we also performed the same culture conditions described above, but without the LPS in the last 48 h. The results observed were very similar to the ones observed in the presence of the LPS (data not shown).
Figure 5.
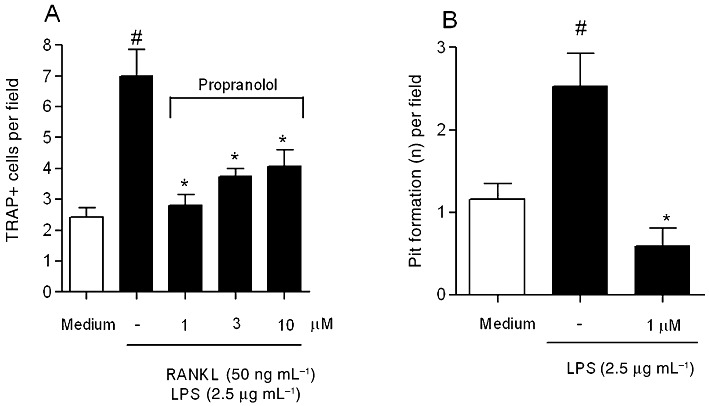
Effects of propranolol on in vitro osteoclastogenesis using mouse monocyte/macrophage cell line RAW264.7. (A) Propranolol inhibited the formation of TRAP-positive mononucleated cells compared with culture medium stimulated with RANKL and LPS. #P < 0.05 compared with RAW264.7 cells with medium alone; *P < 0.05 compared with RAW264.7 cells in the presence of RANKL and LPS. (B) RAW264.7 in the presence of propranolol (1 µM) inhibited the activity of the osteoclast cell after LPS stimulation. The number of pits was counted in each field of the Osteologic Biocoat. #P < 0.05 compared with RAW264.7 cells with medium alone; *P < 0.05 compared with RAW264.7 cells in the presence of LPS (anova followed by Bonferroni's test).
Since the lowest dose of propranolol (1 µM) demonstrated better results, we also analysed the ability of propranolol to inhibit osteoclast activity. As observed in Figure 5B, the number of pits formed, indicated by the number of erosive sites upon culture of osteoclast precursors with LPS, was significantly increased with LPS in comparison with medium alone. On the other hand, the presence of 1 µM propranolol significantly reduced the number of erosive sites (P < 0.05).
NFATc-1 but not NF-κBp65 expression was inhibited by propranolol in RAW264.7 cells
In an attempt to examine the intracellular pathway by which propranolol inhibits the osteoclast differentiation, we performed a Western blot assay of the proteins from the RAW 264.7 culture. As observed in the Figure 6, LPS significantly increased the expression of NF-κBp65, although all tested doses of propranolol did not altered the expression of NF-κBp65. However, LPS also significantly increased the expression of NFATc1 and 1 µM propranolol prevented (P < 0.05) this effect of LPS (Figure 7).
Figure 6.
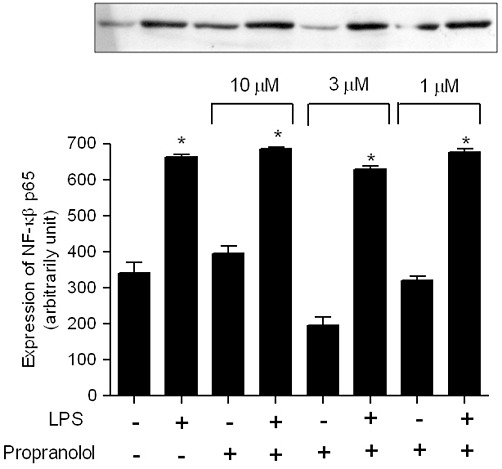
NF-κBp65 protein expression in the RAW264.7 cells in the presence or absence of propranolol. Cells were collected 48 h after LPS activation, and NF-κBp65 protein expression was analysed by Western blot. Intensity of optical density of bands was measured from Western blots. Protein band intensity is represented as arbitrary units. The results are expressed as mean ± SD of three culture wells. *P < 0.05 compared with cells in the absence of LPS stimulation.
Figure 7.
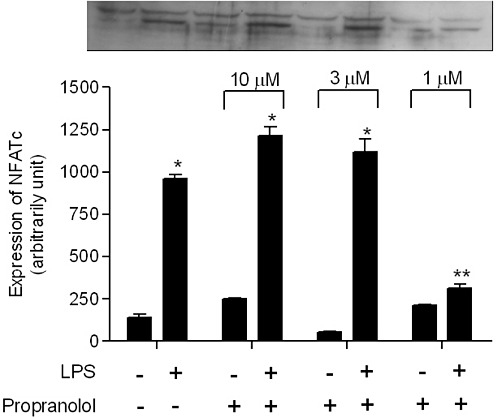
NFAT-c1 protein expression in the RAW264.7 cells in the presence or absence of propranolol. Cells were collected 48 h after LPS activation, and NFAT-c protein expression was analysed by Western blot. Intensity of optical density of bands was measured from Western blots. Protein band intensity is represented as arbitrary units. The results are expressed as mean ± SD of three culture wells. *P < 0.05 compared with cells in the absence of LPS stimulation; **P < 0.05 compared with cells in the presence of LPS stimulation.
Propranolol suppressed cathepsin K, TRAP and MMP-9 mRNA expression in RAW264.7 cells stimulated with RANKL
To elucidate the mechanism underlying the inhibition of RANKL-induced osteoclastogenesis by propranolol, we used RAW264.7 cells to test the effect of propranolol on the expression of genes that indicate osteoclast maturation, including TRAP, cathepsin K and MMP-9. Propranolol significantly suppressed the RANKL-induced osteoclast maturation genes TRAP (at doses of 1 and 3 µM; Figure 8A) and cathepsin K mRNA (at a dose of 1 µM; Figure 8B). Also, the increased expression of MMP-9 was inhibited by all three propranolol doses tested (1, 3 and 10 µM; Figure 8C).
Figure 8.
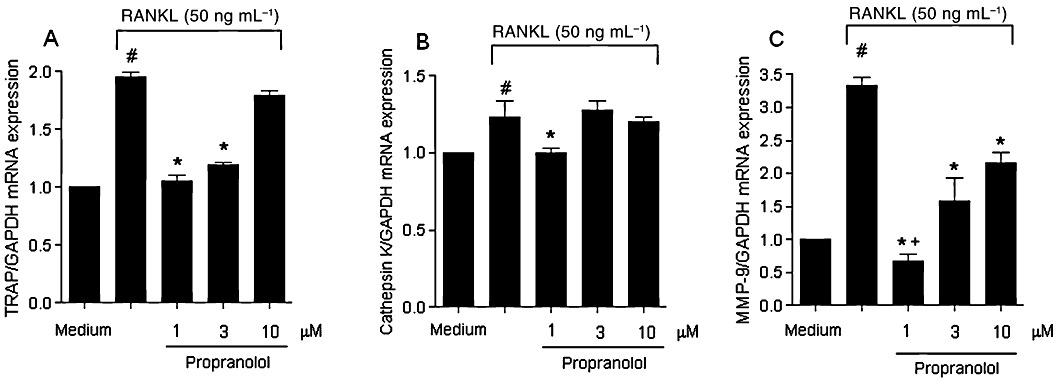
Propranolol attenuated the expression of TRAP (A), cathepsin K (B) and MMP-9 (C) mRNA in RAW 264.7 stimulated with RANKL. The results are expressed as mean ± SD of three culture wells. #P < 0.05 compared with RAW264.7 cells with medium alone; *P < 0.05 compared with RAW264.7 cells in the presence of RANKL. +P < 0.05 compared with RAW264.7 cells treated with 1 and 10 µM.
Discussion
The findings of the present study show that a low dose of a β-blocker (propranolol) can prevent periodontal bone loss by decreasing bone resorption, in agreement with a previous report (Okada et al., 2010). However, in this study, we demonstrated several results indicating the possible mechanisms by which a low dose of propranolol is therapeutically effective at preventing the periodontal bone resorption. Thus, we suggest that the effects of low doses of β-blockers on bone are partially due to the inhibition of inflammatory markers and osteoclastogenesis without affecting heart functions.
Some studies have indicated that periodontal tissue is under the control of the autonomic nervous system, particularly the sympathetic nervous system, and a dysfunction of this system can lead to periodontal breakdown (Breivik et al., 2005; Kim et al., 2009). Previous studies demonstrated that the down-regulation of bone formation is dependent on the activation of β2-adrenergic receptors, the only β-adrenergic receptors known to be expressed by osteoblasts (Togari et al., 1997). In our study, the ligated-animals treated with low doses of propranolol (0.1 and 5 mg·kg−1) had a bone-protective effect, avoiding inter-radicular bone resorption. Interestingly, pharmacological studies on adrenergic receptors have provided controversial results. Recent observations showed that propranolol was unable to completely rescue the bone loss in ovariectomized adult mice (Pierroz et al., 2006a). However, the beneficial effect of propranolol on bone formation was previously demonstrated in a surgically fractured rat model in which after 9 week period of propranolol treatment (0.1 mg·kg−1·day−1) induced an increase in the mineral apposition rate at the metaphysis and an increased bone formation rate at both the periosteum and endosteum (Minkowitz et al., 1991). Accordingly, Bonnet et al. (2008a) showed that low doses of β-blockers may have a therapeutic utility in the treatment of osteoporosis with high selectivity for bone tissues, corroborating our results for periodontal bone diseases.
Previous studies have provided strong evidence that the CNS not only receives messages from the immune system but also modulates immune function (Elenkov and Chrousos, 1999). The sympathetic nervous system, being part of the autonomic nervous system, has been shown to be an integrative interface between the two systems (Bedoui et al., 2003; Haug and Heyeraas, 2006). The inflammatory response is orchestrated by a large range of mediators able to promote vascular events, oedema and consequently the recruitment of inflammatory cells (Mackay, 2008). Our results demonstrated that animals treated with a low dose of propranolol have decreased levels of the pro-inflammatory cytokines, such as TNF-α and IL-1β, in the gingival tissue. This is an important finding since it is well documented that both cytokines are needed to induce the expression of endothelial molecules involved in the recruitment of leucocytes. Thus, our data suggest that the anti-inflammatory effect of propranolol in vivo could be as a result of the inhibition of pro-inflammatory cytokine production at inflammatory sites, which in turn prevents the interaction of leucocytes with endothelial cells that is dependent on the expression of ICAM-1. This hypothesis is supported by our results obtained with the gingival tissue (Figure 4A). To our knowledge, this is the first evidence implicating this mechanism of action in periodontal tissue. In addition, noradrenaline has been shown, both in vivo and in vitro, to inhibit the production of pro-inflammatory cytokines such as TNF-α, while stimulating the production of anti-inflammatory cytokines such as IL-10 (van der Poll et al., 1996; Hasko et al., 1998), suggesting that the sympathetic nervous system can alter the Th1/Th2 balance, shifting it from a pro-inflammatory (Th1 response) to an anti-inflammatory response (Th2 response) (Woiciechowsky et al., 1998; Elenkov and Chrousos, 1999).
Interestingly, we measured the CTX (a bone turnover marker) from the gingival tissue, and the amount of CTX was significantly elevated in the gingival tissue of ligature-induced periodontal disease animals in comparison with non-ligated animals and the 0.1 mg·kg−1 propranolol-treated group. Both groups treated with 5 or 20 mg·kg−1 of propranolol showed increased amounts of CTX. Previous studies have demonstrated that periodontal disease is more common in women with osteoporosis and is associated with higher concentrations of CTX, suggesting the underlying mechanism that links these two pathological conditions (Jabbar et al., 2011). It is interesting to note that both groups treated with 5 and 20 mg·kg−1 of propranolol had elevated levels of CTX as well as RANKL. This raised RANKL and RANKL/OPG ratio would then stimulate osteoclast activity and cause the observed significant elevations in CTX levels.
Another mechanism by which a low dose of propranolol can inhibit the bone resorption is by inhibition of osteoclastogenesis. A previous report has shown that activation of β-adrenoceptors increases the expression of RANKL and leads to stimulation of osteoclast differentiation in mouse bone marrow (Takeuchi et al., 2001; Aitken et al., 2009). In addition, the activation of osteoblasts can induce the expression of bone-active cytokines IL-6, IL-11 and PGE2, which in turn increase osteoclastogenesis (Kondo et al., 2001; Kondo and Togari, 2003). According to our results, the low dose of propranolol significantly decreased the expression of RANKL in the gingival tissue. This result is supported by previous data which showed that fenoterol (β2-agonist) stimulated RANKL mRNA expression nearly twofold and this was suppressed by propranolol (β-blocker), suggesting that β-adrenoceptors may play a role in modulation of bone turnover by the sympathetic nervous system (Huang et al., 2009). We also demonstrated that a low dose of propranolol increased the OPG expression in the ligature-induced periodontal disease model, which consequently down-modulates RANKL-mediated bone resorption. This result is supported by a previous finding, which showed that propranolol stimulates OPG on its own in osteoblast cells (Huang et al., 2009).
Further, we observed that osteoclast differentiation, as well its activity (pit formation in Biocoat osteologic assay) in vitro was decreased in the presence of a low dose of propranolol (β2-antagonist). In humans, osteoclast-like multinucleate cells constitutively express α1B-, α2B-, and β2-adrenoceptors. Also, β-agonists up-regulate the expression of characteristic markers of the mature osteoclast such as integrin, carbonic anhydrase II and cathepsin K and increased osteoclastic bone-resorbing activity (Arai et al., 2003). These findings suggest that β-adrenoceptor agonists directly stimulate bone-resorbing activity in mature osteoclasts, thus supporting our evidence. In addition, it was previously demonstrated that different β2-adrenoceptor agonists stimulate osteoclast formation and multinuclearity as well as bone resorption in bone marrow cultures, followed by an increased expression of RANKL by osteoblasts (Aitken et al., 2009). Interestingly, it has been demonstrated that treatment with a continuous low dose of isoprenaline (β2-agonist) induces bone loss due to increased osteoclast activity rather than inhibition of bone formation (Kondo and Togari, 2011).
We further analysed the intracellular pathway in osteoclast cells for a better understanding of this process. The essential role of NF-κB in the activation and differentiation of osteoclast has been demonstrated genetically. NF-κB p50 and p52 double-deficient mice develop severe osteopetrosis because of a defect in osteoclastogenesis (Franzoso et al., 1997; Iotsova et al., 1997). Although this recognized importance of NF-κB in the osteoclastogenesis, according to our results, the inhibitory effect of propranolol is not produced through this pathway; instead, the effect seems to be mediated by the inhibition of another important molecule, NFATc1. Importantly, RANKL specifically and strongly induces the expression of NFATc1, the master regulator of osteoclast differentiation (Takayanagi et al., 2002). The NFAT family of transcription factors was originally discovered in T cells, but they are involved in the regulation of various biological systems (Crabtree and Olson, 2002). Activation of NFATc1 is mediated by a specific phosphatase, calcineurin, which is activated by calcium–calmodulin signalling. The essential role of the Nfatc1 gene in osteoclastogenesis has been shown both in vitro and in vivo (Takayanagi et al., 2002).
We also evaluated some important protease genes synthesized by the osteoclast, cathepsin K, TRAP and MMP-9. We found that the low dose of propranolol (1 µM) was significantly decreased the expression of these three genes. These results have an important implication since cathepsin K is an important protease that promotes degradation of bone matrix molecules. The enzyme is activated intracellularly and secreted into the resorption lacunae, and it is speculated that cathepsin K is highly and relatively selectively expressed in osteoclasts (Inaoka et al., 1995). Importantly, in a previous study it was demonstrated that excessive production of cathepsin K results in a high turnover, osteopaenia, of the metaphyseal trabecular bone (Kiviranta et al., 2001). Moreover, TRAP-deficient mice develop an osteopetrotic phenotype due to deficient osteoclastic bone resorption with characteristic ultrastructural changes in the ruffled border area of osteoclasts and disturbed vesicular trafficking (Hollberg et al., 2002). Furthermore, we found that all three doses of propranolol decreased the expression of MMP-9; however, the lowest dose was the most effective. Interestingly, in accord with our results, MMP-9 is synthesized by the osteoclast and is also a rate limiting for osteoclast recruitment (Delaisséet al., 2003).
Thus, why does a low dose but not a high dose of propranolol suppress inflammation and osteoclastogenesis? Previous data in the literature demonstrated that mice lacking β2-adrenoceptors present a high bone mass phenotype (Elefteriou et al., 2005), while β1- and β2-adrenoceptor-deficient mice have reduced trabecular (Pierroz et al., 2005) and cortical bone mass (Pierroz et al., 2006b) and are not protected from ovariectomy-induced bone loss (Dhillon et al., 2004), suggesting that high doses of propranolol may somewhat imitate the double deletion phenotype, while low doses of propranolol may signal mainly via the β2-adrenoceptor to exert their beneficial effects.
In conclusion, our results indicate that a low dose of propranolol may suppress the periodontal bone resorption by inhibiting RANKL-mediated osteoclastogenesis as well inflammatory markers without affecting haemodynamic parameters.
Acknowledgments
The authors thank Jozafá C Campos-Júnior and Vilmar J Silva-Filho for their excellent technical support. This study was supported by CNPq 471305/2009-0 and 303080/2010-8, PAPE-UNIUBE 2009/001 and FAPEMIG 097/09.
Glossary
- AP
arterial pressure
- BD
bone density
- CTX
cross-linked carboxyterminal telopeptides of type I collagen
- DMEM
Dulbecco's modified Eagle medium
- DTT
dithiothreitol
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ICAM-1
intercellular adhesion molecule 1
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- NFAT
nuclear factor of activated T cells
- OPG
osteoprotegerin
- OVX
ovariectomized
- RANKL
receptor activator of NF-κ B ligand
- SaM-1
human periosteum-derived osteoblastic cells
- SaOS-2
human osteosarcoma-derived cells
- SHR
spontaneous hypertensive rats
- TBST
Tris-buffered saline–Tween
- TRAP
tartrate-resistant acid phosphatase
Conflict of interesting
There is no conflict of interest.
References
- Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys. 2009;482:96–103. doi: 10.1016/j.abb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A. Effects of β -adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta. 2003;1640:137–142. doi: 10.1016/s0167-4889(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Bastos MF, Brilhante FV, Gonçalves TE, Pires AG, Napimoga MH, Marques MR, et al. Hypertension may affect tooth-supporting alveolar bone quality: a study in rats. J Periodontol. 2010;81:1075–1083. doi: 10.1902/jop.2010.090705. [DOI] [PubMed] [Google Scholar]
- Beck JD, Garcia R, Heiss G, Vokonas P, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, von Horsten S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134:1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther. 2006;318:1118–1127. doi: 10.1124/jpet.106.105437. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, et al. Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol. 2008a;217:819–827. doi: 10.1002/jcp.21564. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008b;8:94–104. [PubMed] [Google Scholar]
- Breivik T, Gundersen Y, Opstad PK, Fonnum F. Chemical sympathectomy inhibits periodontal disease in Fischer 344 rats. J Periodontal Res. 2005;40:325–330. doi: 10.1111/j.1600-0765.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- Cotti E, Dessì C, Piras A, Mercuro G. Can a chronic dental infection be considered a cause of cardiovascular disease? A review of the literature. Int J Cardiol. 2011;148:4–10. doi: 10.1016/j.ijcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- De Stefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech. 2003;61:504–513. doi: 10.1002/jemt.10374. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Glatt V, Ferrari SL, Bouxsein ML. Beta-adrenergic receptor KO mice have increased bone mass and strength but are not protected from ovariectomy-induced bone loss. J Bone Miner Res. 2004;19:s32. [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Hammond-Jones D, Gamie Z, Polyzois I, Tsiridis E, Tsiridis E. The effect of β-blockers on bone metabolism as potential drugs under investigation for osteoporosis and fracture healing. Expert Opin Investig Drugs. 2008;17:1281–1299. doi: 10.1517/13543784.17.9.1281. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Buggle F, Siegler C. Association between acute cerebrovascular ischaemia and chronic and recurrent infection. Stroke. 1997;28:1724–1729. doi: 10.1161/01.str.28.9.1724. [DOI] [PubMed] [Google Scholar]
- Hasko G, Nemeth ZH, Szabo C, Zsilla G, Salzman AL, Vizi ES. Isoproterenol inhibits Il-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages. Brain Res Bull. 1998;45:183–187. doi: 10.1016/s0361-9230(97)00337-7. [DOI] [PubMed] [Google Scholar]
- Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. J Dent Res. 2006;85:488–495. doi: 10.1177/154405910608500602. [DOI] [PubMed] [Google Scholar]
- Hollberg K, Hultenby K, Hayman A, Cox T, Andersson G. Osteoclasts from mice deficient in tartrate-resistant acid phosphatase have altered ruffled borders and disturbed intracellular vesicular transport. Exp Cell Res. 2002;279:227–238. doi: 10.1006/excr.2002.5612. [DOI] [PubMed] [Google Scholar]
- Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J Cell Physiol. 2009;220:267–275. doi: 10.1002/jcp.21761. [DOI] [PubMed] [Google Scholar]
- Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M, Kokubo T. Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun. 1995;206:89–96. doi: 10.1006/bbrc.1995.1013. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Jabbar S, Drury J, Fordham J, Datta HK, Francis RM, Tuck SP. Plasma vitamin D and cytokines in periodontal disease and postmenopausal osteoporosis. J Periodontal Res. 2011;46:97–104. doi: 10.1111/j.1600-0765.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- Jansson L, Lavstedt S, Frithiof S, Theobald H. Relationship between oral health and mortality in cardiovascular diseases. J Clin Periodontol. 2001;28:762–768. doi: 10.1034/j.1600-051x.2001.280807.x. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Rimm EB, Douglass CV, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- Kim Y, Hamada N, Takahashi Y, Sasaguri K, Tsukinoki K, Onozuka M, et al. Cervical sympathectomy causes alveolar bone loss in an experimental rat model. J Periodontal Res. 2009;44:695–703. doi: 10.1111/j.1600-0765.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- Kiviranta R, Morko J, Uusitalo H, Aro HT, Vuorio E, Rantakokko J. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res. 2001;16:1444–1452. doi: 10.1359/jbmr.2001.16.8.1444. [DOI] [PubMed] [Google Scholar]
- Kondo A, Togari A. In vivo stimulation of sympathetic nervous system modulates osteoblastic activity in mouse calvaria. Am J Physiol Endocrinol Metab. 2003;285:E661–E667. doi: 10.1152/ajpendo.00026.2003. [DOI] [PubMed] [Google Scholar]
- Kondo A, Mogi M, Koshihara Y, Togari A. Signal transduction system for interleukin-6 and interleukin-11 synthesis stimulated by epinephrine in human osteoblasts and human osteogenic sarcoma cells. Biochem Pharmacol. 2001;61:319–326. doi: 10.1016/s0006-2952(00)00544-x. [DOI] [PubMed] [Google Scholar]
- Kondo H, Togari A. Continuous treatment with a low-dose β-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int. 2011;88:23–32. doi: 10.1007/s00223-010-9421-9. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- Mattila K, Nieminen MS, Valtonen VV, Rasi VP, Kesäniemi YA, Syrjälä SL, et al. Association between dental health and acute myocardial infarction. Br Med J. 1989;298:779–782. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkowitz B, Boskey AL, Lane JM, Pearlman HS, Vigorita VJ. Effects of propranolol on bone metabolism in the rat. J Orthop Res. 1991;9:869–975. doi: 10.1002/jor.1100090613. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-Dos-Santos DR, et al. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009;9:216–222. doi: 10.1016/j.intimp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hamada N, Kim Y, Takahashi Y, Sasaguri K, Ozono S, et al. Blockade of sympathetic b-receptors inhibits Porphyromonas gingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Arch Oral Biol. 2010;55:502–508. doi: 10.1016/j.archoralbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GE. β-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Bouxsein ML, Muzzin P, Rizzoli R, Ferrari SL. Bone loss following ovariectomy is maintained in absence of adrenergic receptor beta1 and beta2 signaling. J Bone Miner Res. 2005;20:s277. [Google Scholar]
- Pierroz DD, Bouxsein ML, Rizzoli R, Ferrari SL. Combined treatment with a beta-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone. 2006a;39:260–267. doi: 10.1016/j.bone.2006.01.145. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Baldock P, Bouxsein ML, Ferrari SL. Low cortical bone mass in mice lacking beta 1 and beta 2 adrenergic receptors is associated with low bone formation and circulating IGF-1. J Bone Miner Res. 2006b;21:s277. [Google Scholar]
- Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, et al. Effects of a beta- blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:5212–5216. doi: 10.1210/jc.2005-0573. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Kraenzlin ME, Lick SS, Meier CR. Use of β-blockers and risk of fractures. JAMA. 2004;292:1326–1332. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol. 2001;61:579–586. doi: 10.1016/s0006-2952(00)00591-8. [DOI] [PubMed] [Google Scholar]
- Togari A. Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech. 2002;58:77–84. doi: 10.1002/jemt.10121. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233:125–128. doi: 10.1016/s0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schoning B, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]


