Abstract
BACKGROUND AND PURPOSE
Tryptamine increases blood pressure by vasoconstriction, but little is known about its actions on the mesentery, in particular the resistance arteries. Tryptamine interacts with trace amine-associated receptors (TAARs) and because of its structural similarity to 5-HT, it may also interact with 5-HT receptors. Our hypothesis is therefore that the rat mesenteric arterial bed will exhibit vasopressor and vasodepressor responses to tryptamine via both 5-HT and TAARs.
EXPERIMENTAL APPROACH
Tryptamine-evoked responses were assayed from pressure changes of the rat-isolated mesenteric vasculature perfused at constant flow rate in the absence and presence of adrenoceptor and 5-HT receptor antagonists.
KEY RESULTS
Tryptamine caused dose-dependent vasoconstriction of the mesenteric arterial bed as increases in perfusion pressure. These were unaffected by the α1-adrenoceptor antagonist, prazosin, but were attenuated by the non-selective α-adrenoceptor antagonist, phentolamine. The 5-HT2A receptor antagonists, ketanserin and ritanserin, abolished the tryptamine-induced pressure increases to reveal vasodilator responses in mesenteric beds preconstricted with phenylephrine. These tryptamine-induced vasodilator responses were unaffected by the 5-HT7 receptor antagonist, SB269970, but were eliminated by the NOS inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME). Tyramine and β-phenylethylamine also caused vasodilatation in pre-constricted vasculature, which was also abolished by L-NAME.
CONCLUSIONS AND IMPLICATIONS
Tryptamine causes vasoconstriction of the mesenteric vasculature via 5-HT2A receptors, which when inhibited exposed vasorelaxant effects in pre-constricted tissues. The vasodilatation was independent of 5-HT2A and 5-HT7 receptors but like that for tyramine and β-phenylethylamine was due to NO release. Potency orders suggest TAAR involvement in the vasodilatation by these trace amines.
Keywords: mesenteric vasculature, rat, trace amines, trace amine-associated receptors, 5-HT receptors, tryptamine, tyramine, β-phenylethylamine
Introduction
Catecholamines (noradrenaline and dopamine), 5-HT and trace amines [β-phenylethylamine (β-PEA), tyramine, tryptamine, octopamine and synephrine] are classified as biogenic monoamines. Interest in the physiological effects of trace amines has been continuous for a century, ever since Sir Henry Dale and his colleagues (Dale and Dixon, 1909; Barger and Dale, 1910) began their pioneering investigations on amines. Subsequently, the pharmacological actions of trace amines led Burn and Rand (1958) to propose that they mediated the release of endogenous neuronal noradrenaline into the synaptic cleft, where it acted upon postsynaptic autologous receptors to mediate a response. This was defined as their indirect sympathomimetic activity (ISA). Although trace amines resemble other monoamines in terms of structure and function, important differences began to emerge in relation to their pharmacological effects (Hawthorn et al., 1985; Grandy, 2007), radioligand binding data (Grandy, 2007; and references therein) and molecular investigations (Borowsky et al., 2001; Bunzow et al., 2001; Grandy, 2007). These and other more recent investigations (Zucchi et al., 2006; Grandy, 2007; Herbert et al., 2008; Broadley et al., 2009) do not completely comply with the indirectly acting sympathomimetic thesis (Burn and Rand, 1958) in that the trace amines may interact directly with specific trace amine receptors (Bunzow et al., 2001; Broadley, 2010).
Since the discovery of trace amine-associated receptors (TAARs) at the beginning of the last decade, there has been a resurgence of interest in their physio-pharmacological properties (Zucchi et al., 2006; Grandy, 2007). Although TAAR-mediated effects of trace amines on the cardiovascular system have recently been proposed (Borowsky et al., 2001; Zucchi et al., 2006; Herbert et al., 2008; Broadley et al., 2009; Broadley, 2010), there is a paucity of data on the reactivity of resistance arteries to trace amines. Tryptamine is an important dietary and endogenous amine produced from decarboxylation of L-tryptophan by aromatic amino acid decarboxylase (Ruddick et al., 2006). It is structurally related to 5-HT and has been shown to cause vasoconstriction in rabbit aorta via 5-HT receptors and directly via α-adrenoceptors (Stollak and Furchgott, 1983). The presence of specific tryptamine receptors in the rat tail arteries has been described (Hicks and Langer, 1983). However, this notion was disputed by another group who suggested that tryptamine caused vasoconstriction solely through 5-HT2 receptors and the differential effects of 5-HT receptor antagonists against 5-HT and tryptamine could be attributed to different susceptibilities to degradation by monoamine oxidase (MAO; Bradley et al., 1985). To our knowledge, there are no investigations that have examined the actions of tryptamine on the rat mesenteric vascular bed.
Our hypothesis was that tryptamine can exert vascular responses in the rat mesentery through 5-HT receptors and TAARs. The first objective was to demonstrate the vascular reactivity of the rat mesenteric vascular bed to tryptamine. Secondly,we determine whether there were seasonal variations in tryptamine-induced vascular responses, and lastly, we sought to identify the receptors that mediate these responses. Preliminary results have been presented in abstract form (Anwar et al., 2009).
Methods
Animals
All animal care and experimental procedures complied with the Home Office Guidance on the operation of The Animals (Scientific Procedures) Act 1986 (H.M.S.O.) and were approved by the Local Animal Care and Use Committee at Cardiff University. Male Sprague–Dawley rats (250–350 g body weight; Harlan, Bicester, Oxfordshire, U.K.) were maintained in standard cages in a thermo-regulated (22 ± 1°C) colony room with fixed photoperiod of 12 h light and 12 h darkness, having access to water and rodent chow ad libitum.
Mesentery preparation
Rats were killed by cervical dislocation following stunning and the superior mesenteric artery cannulated (polyethylene tubing, PP50; Portex, Kent, U.K.). The mesentery was excised, isolated and placed in a perfusion chamber, based on the method of McGregor (1965). The bed was perfused at a constant flow rate (4 mL·min−1) with Krebs solution (composition in mM): NaCl 118, NaHCO3 25, glucose 11, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2 (pH 7.4), warmed to 37°C and gassed (95% O2, 5% CO2). Tissues were equilibrated for 1 h before experiments commenced. The perfusion pressure was monitored continuously throughout the duration of the experiment via a pressure transducer (Elcomatic EM 750; Elcomatic Ltd, Glasgow, UK) placed distally to the inflow cannula. Alterations in perfusion pressure, representing changes in resistance vessels of the mesentery, were displayed on a PowerLab/4SP computerized data acquisition system (AD Instruments, Charlgrove, Oxon, UK), and data were analysed using Chart version 5 software (AD Instruments).
Experimental design
Dose–response curves for tryptamine, tyramine and β-PEA were constructed after a 60 min equilibration period, by bolus injections of 100 µL (range: 0.1–1000 nmoles) into an injection port. All experiments involved exposure to no more than two dose–response curves and had a duration of no more than 3 h. Controls for time-dependent changes in sensitivity to 5-HT and tryptamine were obtained by repeating two dose–response curves in the same preparation. To examine possible indirectly acting sympathomimetic actions of tryptamine, dose–response curves were constructed first in the absence and repeated in the presence of the α1-adrenoceptor antagonist prazosin (10 nM) and the non-selective α1 and α2-adrenoceptor antagonist phentolamine (1 µM; receptor nomenclature follows Alexander et al., 2011). The above procedure was repeated with vasoconstrictor responses to the α1-adrenoceptor agonist, phenylephrine.
Dose–response curves to tryptamine were also constructed in the absence and presence of the selective 5-HT2A receptor antagonists, ketanserin (10 nM) or ritanserin (100 pM) (Conolan et al., 1986; Van Nueten et al., 1986). All antagonists were introduced 20 min before commencing the dose–response curves.
To examine vasodilator responses to tryptamine, tyramine and β-PEA, following a 60 min equilibrium period, the resting vascular tone was raised by continuous perfusion with phenylephrine (10 µM). Once a stable tone was established, a dose–response curve was constructed by administration of bolus doses of tryptamine, tyramine or β-PEA (0.1–1000 nmoles/100 µL) via an injection port. Between each dose the perfusion pressure was permitted to return to the pre-constricted tone. In the case of tryptamine, ritanserin (100 pM) was continuously perfused from 20 min prior to the perfusion with phenylephrine and for the duration of the experiment. Vasorelaxation to tryptamine (in the presence of ritanserin, 100 pM) was also assessed in the presence of the inhibitor of 5-HT7 receptors, SB 269970 (10 nM) (Hagan et al., 2000; Lovell et al., 2000). Dose–response curves for the vasodilator responses to tryptamine (in the presence of ritanserin, 100 pM), tyramine and β-PEA were examined before and repeated in the presence of Nω-nitro-L-arginine methyl ester (L-NAME) (1 mM), to inhibit nitric oxide synthase (NOS). To test for endothelial responses, prior to construction of vasodilator dose–response curves, acetylcholine (0.1 µM in 100 µL bolus) was added and only those preparations exhibiting a 50% reduction of the phenylephrine-induced vasoconstriction were used. This dose of acetylcholine was repeated at the end of the experiment to determine that L-NAME had abolished the response.
Data measurement and analysis
Vasoconstrictor responses were measured as the increase in perfusion pressure from the resting level immediately before each dose of amine and presented as the arithmetic mean ± SEM. Vasodilator responses were measured as the fall in perfusion pressure and expressed as a percentage of the increase in perfusion pressure to phenylephrine. Doses were administered as nmoles in 100 µL boluses. ED50 values (concentration of the agonist in nmoles required to produce half-maximal response, EMax) were calculated from individual dose–response curves using the Fig P software program (BioSoft, Cambridge, UK). Geometric mean ED50 values (antilog of mean log of individual ED50 values) with their 95% confidence intervals were calculated. Statistical comparisons between control and drug treatment dose–response curves were made by two-way anova with a Bonferroni post hoc test to compare individual doses. Student's t-test was used to compare ED50 values and EMax values. A level of P < 0.05 was considered statistically significant. n= numbers of animals.
Materials
Chemicals were purchased from Alliance Pharma (Chippenham, Wiltshire, UK) (phentolamine mesylate, Rogitine), Sigma–Aldrich Chemical Company (Poole, Dorset, UK) (acetylcholine chloride, ketanserin, L-NAME, β-PEA, phenylephrine hydrochloride, salts for Krebs solution, 5-HT hydrochloride, tryptamine hydrochloride, tyramine hydrochloride) and Tocris (Bristol, UK) {ritanserin, SB 269970 hydrochloride [(2R)-1-[(3-hydroxyphenyl)sulphonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride] and prazosin hydrochloride}. Stock solutions of prazosin and ritanserin were made in 100% ethanol, aliquoted, and stored in a freezer. Subsequent dilutions in Krebs solution were freshly prepared before each experiment. The remaining drugs were dissolved in the perfusion fluid, and all of these drugs were made up on the day of the experiment.
Results
Baseline perfusion pressure
The baseline perfusion pressure for the rat isolated perfused mesenteric bed was 20.1 ± 0.4 mmHg (n= 110). There were no differences between the baseline perfusion pressures prior to the first and second dose–response curves, including those in the presence of inhibitors.
Agonists
Tryptamine produced dose-dependent vasoconstriction of the mesenteric arterial bed, reflected by elevations in perfusion pressure (Figures 1A and 2). The α-adrenoceptor agonist, phenylephrine, was a more potent vasoconstrictor and produced a significantly greater maximum effect, with an Emax of 138 ± 12 mmHg and ED50 of 17.2 nmoles (8.6–34.4). In contrast, a bell-shaped dose–response curve to 5-HT was obtained (Figures 1B and 2). The order of maximum response (EMax, mmHg) was phenylephrine (138 ± 12) >5-HT (43 ± 14) ≅ tryptamine (37 ± 8), but the potency (ED50, nmoles) sequence was as follows: 5-HT (2.5 [1.3–4.8]) >tryptamine (31.9 [18.6–54.7) ≅ phenylephrine (17.2 [8.6–34.4]) (Figure 2). For comparison, β-PEA and tyramine were without effect in the rat mesentery (Figure 2).
Figure 1.
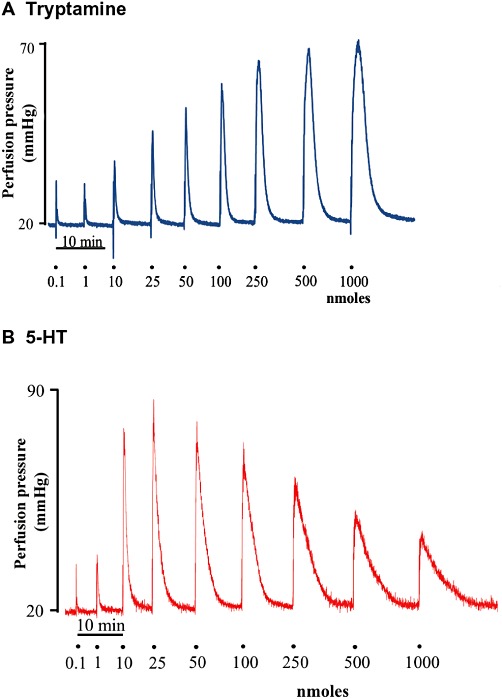
Representative tracings of the response to tryptamine (A) and 5-HT (B) in the isolated perfused mesenteric arterial bed of the rat. Doses shown are the bolus dose in nmoles.
Figure 2.
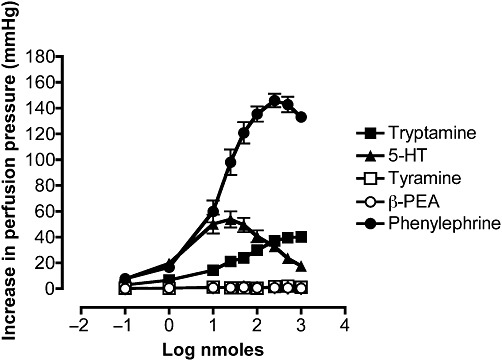
Mean dose–response curves for the increases in perfusion pressure of rat-isolated perfused mesenteric vascular beds to tryptamine (n= 6), phenylephrine (n= 6), 5-HT (n= 6), tyramine (n= 7) and β-PEA (n= 4). Results are expressed as mean ± SEM.
No seasonal variations in tryptamine-evoked maximum perfusion pressure responses in the rat mesentery were found over a 2 year period. The mean maximum increases in perfusion pressure for the summer (June to August, 31.0 ± 3.2, n= 33), autumn (September to November, 25.2 ± 1.5, n= 28), winter (December to February, 28.9 ± 3.3, n= 16) and spring (March to May, 24.8 ± 2.8, n= 33) were not significantly different.
When two consecutive dose–response curves for tryptamine were constructed, the first and second curves were identical [ED50 first curve: 31 nmoles (20–47); second curve: 47 nmoles (34–64). EMax first curve: 24 ± 5 mmHg; second curve: 26 ± 7 mmHg; Figure 3A]. In contrast, the peak of the bell-shaped dose–response curve for 5-HT (43 ± 14 mmHg) was significantly depressed (P < 0.05) in the second curve to 25 ± 10 mmHg (Figure 3B). The two curves for 5-HT, however, were identical in terms of sensitivity [ED50 first curve: 5 nmoles (3–9); second curve: 6 nmoles (3–16); Figure 3B].
Figure 3.
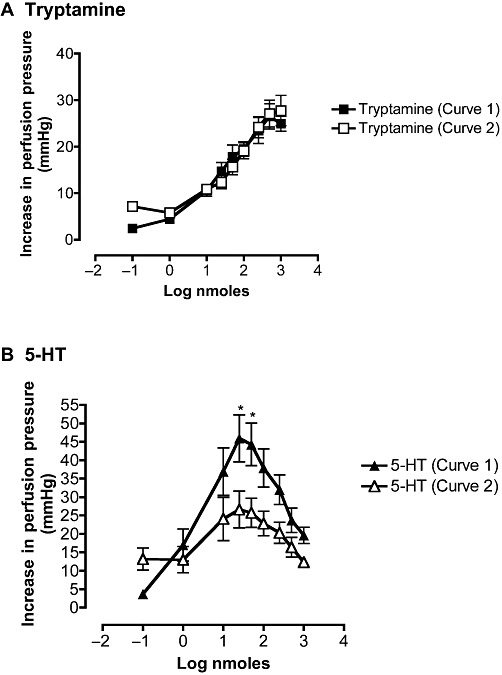
Mean first (curve 1, solid symbol) and second (curve 2, open symbol) dose–response curves for the increases on perfusion pressure of rat isolated perfused mesenteric vascular beds to tryptamine (A; n= 6) and 5-HT (B; n= 5). Points are means ± SEM. *P < 0.05, significantly different from curve 2; ANOVA followed by Bonferroni post-test.
Effects of antagonists
The vasoconstriction induced by phenylephrine in rat mesentery was antagonized by prazosin with a significant reduction of the response to the maximum dose (Figure 4C) and by phentolamine with a significant reduction of the response to the maximum dose from 110 ± 36 to 13 ± 4 mmHg (P < 0.001) (Figure 4D). However, the vasoconstrictor response to tryptamine was resistant to blockade by prazosin [ED50 before 37 nmoles (26–51); with prazosin 44 nmoles (27–70), NS; EMax before 15 ± 7 mmHg; with prazosin 18 ± 11 mmHg, NS; Figure 4A]. Phentolamine, however, reduced the vasoconstrictor responses of the perfused mesentery to tryptamine. The curve was shifted to the right, the ED50 increasing significantly (P < 0.05) from 39 (23–66) to 80 nmoles (58–110) and the EMax being decreased (P < 0.05) (Figure 4B). These changes, however, were not as marked as for the inhibition of phenylephrine, the reduction of the maximum for tryptamine (Figure 4B) was significantly less than for phenylephrine (Figure 4D).
Figure 4.
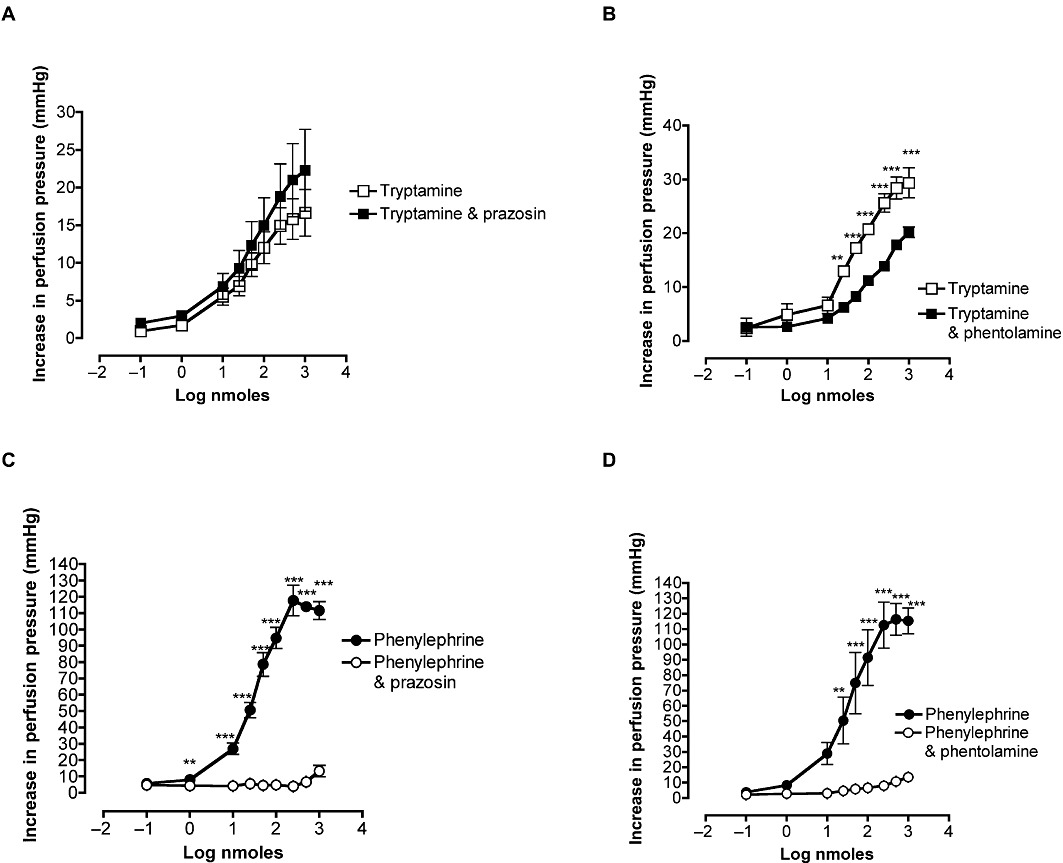
Effects of prazosin (A and C, 10 nM) and phentolamine (B and D, 1 µM) on the dose–response curves for increases in perfusion pressure of rat-isolated perfused mesenteric vascular beds to tryptamine (A; n= 4 and B; n= 3) and phenylephrine (C; n= 3 and D; n= 3). Points are means ± SEM. **P < 0.01 ***P < 0.001, significant effect of antagonist; ANOVA and Bonferroni post-test.
In the presence of the 5-HT2A receptor antagonists, ketanserin (10 nM) (Figure 5A) or ritanserin (100 pM) (Figure 5B), the tryptamine-induced vasoconstriction was abolished.
Figure 5.
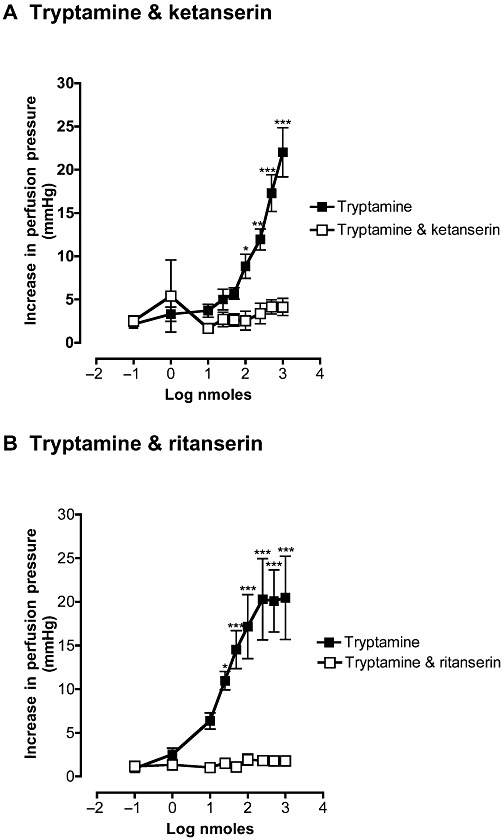
Effects of ketanserin (A, 10 nM,n= 4) and ritanserin (B, 100 pM, n= 4) on the dose–response curves for increases in perfusion pressure of rat-isolated perfused mesenteric vascular beds to tryptamine. Curves were obtained in the absence and presence of antagonists (□). Each point represents the mean ± SEM. *P < 0.05 **P < 0.01, significant effects of antagonists; ANOVA followed by Bonferroni post-test.
Vasodilator response to tryptamine, 5-HT, tyramine and β-PEA
To examine vasodilator responses of the mesenteric vasculature, vascular tone was raised by 52 ± 8 mmHg by perfusion with phenylephrine (10 µM). In the presence of ritanserin (100 pM) and preconstriction with phenylephrine (10 µM), low doses of tryptamine (0.01–10 nmoles) caused small further increases in perfusion pressure, whereas at higher doses of tryptamine (25–1000 nmoles), a prominent vasodilator effect was generated (Figures 6A and 7). The maximum relaxation reached was 71 ± 6% of the phenylephrine-induced vasoconstriction. When the mesentery was perfused with both ritanserin and the 5-HT7 receptor antagonist, SB269970 (10 nM), a similar relaxation response to tryptamine was produced, with a maximum of 56 ± 14% (Figure 7). 5-HT in preconstricted mesenteric beds and in the presence of ritanserin also caused vasoconstriction at lower doses but vasodilatation at higher doses (Figure 6B) and these responses were not modified by the additional presence of SB269970 (Figure 6C).
Figure 6.
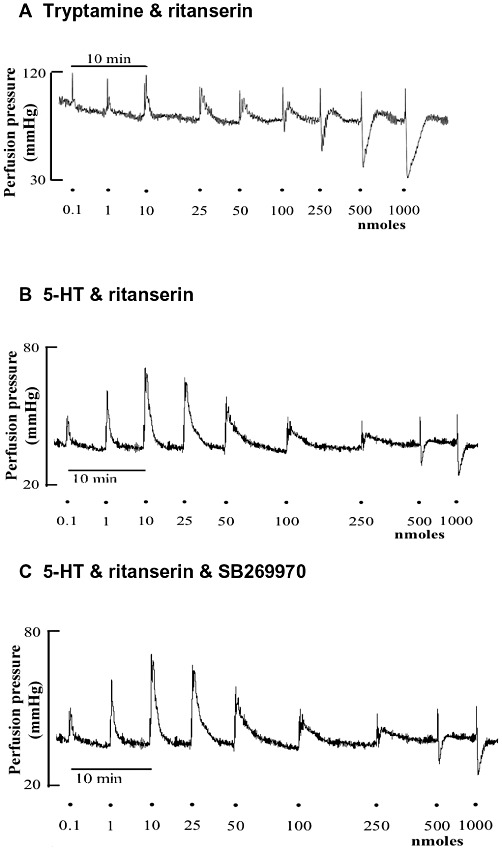
Representative tracings for dose–response curves for tryptamine (A) and 5-HT (B and C) in rat isolated perfused mesenteric arteries. (A) Biphasic profile for tryptamine (0.01–1000 nmoles) in the presence of ritanserin (100 pM). (B) Biphasic response profile for 5-HT (0.01–1000 nmoles) in the presence of ritanserin (100 pM) alone and (C) in the presence of ritanserin (100 pM) and SB269970 (10 nM). The tissues were submaximally pre-constricted with 10 µM phenylephrine and continuously perfused for the duration of the experiment. Doses are the bolus dose in nmoles.
Figure 7.
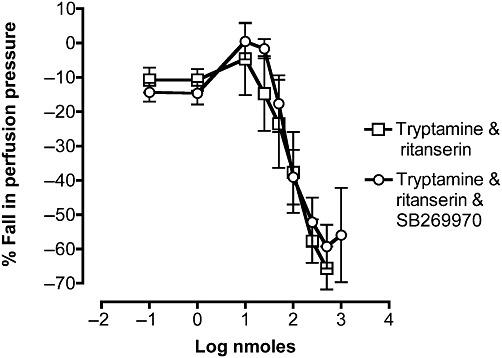
Mean dose-response curves for the falls in perfusion pressure of rat isolated perfused mesenteric vascular beds to tryptamine in tissues pre-constricted with 10 µM phenylephrine. Falls in perfusion pressure are shown as negative values, expressed as a percentage of the increase in perfusion pressure to phenylephrine. Dose-response curves were obtained in the presence of ritanserin (5-HT2A antagonist; 100 pM, n= 4,) and with both ritanserin (100 pM) and SB269970 (5-HT7 antagonist; 10 nM, n= 3), Each point represents the mean ± SEM.
Tyramine and β-PEA in mesenteric vascular beds preconstricted with phenylephrine also caused vasodilator responses and these responses, like those to tryptamine in the presence of ritanserin, were reproducible when repeated in a second dose–response curve (Figure 8). Octopamine produced vasodilator responses at high concentrations. The dose–response curves for four amines are compared in Figure 9. The potency orders for the vasodilator responses to these amines (tryptamine in the presence of ritanserin) was tyramine > β-PEA > tryptamine > octopamine, with their ED50 values shown in Table 1. To enable comparisons with EC50 values in the literature, these were converted to an approximate final concentration in the tissue, assuming that each bolus dose was distributed into 1 mL of perfusion fluid at the tissue. Thus, 1 nmole ≅ 1 µM and the corresponding geometric mean EC50 values (−LogM) are shown in Table 1.
Figure 8.
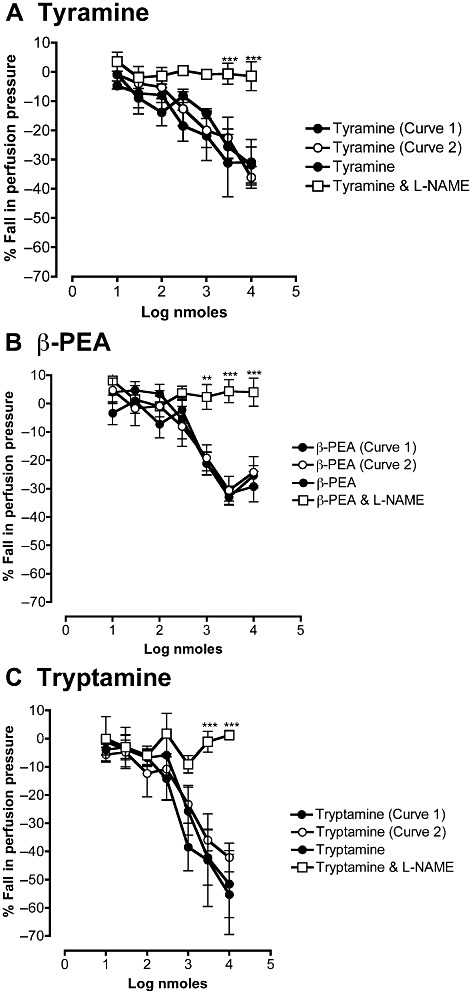
Effects of L-NAME (1 mM) on mean dose–response curves for the falls in perfusion pressure of rat isolated perfused mesenteric vascular beds in response to (A) tyramine, (B) β-PEA and (C). tryptamine. Tryptamine was examined in the continuous presence of ritanserin (100 pM). The perfusion pressure was raised by pre-constriction with phenylephrine (10 µM), which was continuously perfused for the duration of the experiment. Falls in perfusion pressure are shown as negative values, expressed as a percentage of the increase in perfusion pressure to phenylephrine. In control experiments, first (curve 1) and second (curve 2) dose–response curves for tyramine (A, n= 4), β-PEA (B, n= 6) and tryptamine (C, n= 4) were repeated to establish reproducibility. In separate experiments, dose–response curves for tyramine (A, n= 4), β-PEA (B, n= 5) and tryptamine in the presence of ritanserin (100 pM) (C, n= 4) were obtained in the absence and presence of L-NAME (1 mM). **P < 0.01 ***P < 0.001, significant effect of L-NAME; ANOVA followed byBonferroni post-test.
Figure 9.
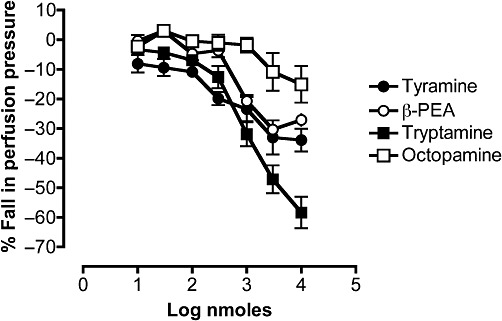
Comparison of the dose–response curves for the falls in perfusion pressure of rat isolated perfused mesenteric vascular beds in response to tyramine (n= 4), β-PEA (n= 8), tryptamine in the presence of ritanserin (100 pM) (n= 7) and octopamine (n= 4). The perfusion pressure was raised by pre-constriction with phenylephrine (10 µM), which was continuously perfused for the duration of the experiment. Falls in perfusion pressure are shown as negative values, expressed as a percentage of the increase in perfusion pressure to phenylephrine. Each point represents the mean ± SEM.
Table 1.
Potency orders of trace amines for the vasodilator responses of rat mesenteric vascular beds compared with published values for cAMP accumulation in rat TAAR-1 transfected cells, constriction of rat aorta and negative inotropy of rat isolated hearts
| −LogEC50 values (M) | ||||
|---|---|---|---|---|
| Vasodilatation in mesenteric bed | Tyramine | ß-PEA | Tryptaminea | Octopamine |
| 4.2 ± 1.5 | 3.63 ± 0.78 | 2.86 ± 0.09 | 2.59 ± 0.68 | |
| n= 4 | n= 8 | n= 7 | n= 4 | |
| Vasoconstriction in rat aorta Fehler et al. (2010) | Tryptaminea | ß-PEA | Octopamine | Tyramine (partial agonist) |
| 5.51 ± 0.12 | 4.46 ± 0.15 | 4.36 ± 0.17 | 3.71 ± 0.29 | |
| Negative inotropic responses in rat-isolated heartscFrascarelli et al. (2008) | Octopamine | ß-PEA | Tryptamine | Tyramine |
| 3.97 | 3.80 | 3.62 | 3.0 | |
| cAMP accumulation in rat TAAR-1 transfected cells Bunzow et al. (2001) | Tyramine | ß-PEA | Tryptamine | Octopamine |
| 7.16 | 6.62 | 6.51 | 5.89 | |
In the presence of ritanserin or ketanserin to block 5-HT2A receptors.
In preconstricted mesenteric beds, the vasodilator responses to tyramine (Figure 8A) and β-PEA (Figure 8B) were abolished by L-NAME (1 mM), as were those to tryptamine in the presence of ritanserin (Figure 8C) The fall in perfusion pressure in response to acetylcholine (0.1 µM) was virtually abolished in the presence of L-NAME (1 mM), being reduced from a 78 ± 10% inhibition of the phenylephrine vasoconstriction to only 7.6 ± 3.6% inhibition.
Discussion
Tryptamine produced dose-dependent vasoconstriction of the rat mesenteric vascular bed comparable with the responses induced by 5-HT. These responses were inhibited by the 5-HT2A receptor antagonists, ritanserin and ketanserin. In mesenteric vessels preconstricted with phenylephrine and in the presence of ritanserin, a prominent vasodilator response to tryptamine was revealed.
5-HT-evoked vascular responses
5-HT mediates a broad spectrum of physio-pharmacological effects by activating a large family of receptors that are distributed throughout the body, including the cardiovascular, central nervous, renal and gastrointestinal systems (Martin, 1994; Hoyer et al., 2002). The 5-HT receptors are classified into seven distinct families based on functional, structural and signalling information, with at least 14 different subtypes, including 5-HT1 (5-HT1A/1B/1D/1E/1F), 5-HT2 (5-HT2A/2B/2C), 5-HT3, 5-HT4, 5-HT5 (5-HT5A/5B), 5-HT6 and 5-HT7 receptors. Apart from the 5-HT3 receptor which is a ligand-gated ion channel, all the others belong to the G-protein-coupled receptor superfamily. The 5-HT receptors mediating changes in vascular tone are the 5-HT1, 5-HT2 and 5-HT7 subtypes (Martin, 1994; Hoyer et al., 2002; Watts, 2002).
5-HT-induced predominantly vasoconstrictor effects on the rat mesentery, the dose–response curves being bell-shaped or biphasic, which agrees with an earlier report (McLennan and Taylor, 1984). The vasoconstriction to 5-HT in the rat mesenteric arteries is inhibited by ketanserin and therefore due principally to 5-HT2A receptors (McLennan and Taylor, 1984; Watts, 2002). Moreover, agonists for 5-HT1B, 5-HT1D, 5-HT1F, and 5-HT2B receptors were unable to cause vasoconstriction of the thoracic aorta, superior mesenteric artery and resistance arteries from Sprague–Dawley rats (Watts, 2002). On repeating two dose–response curves for 5-HT in the same tissue, the EMax value was substantially reduced on the second curve. This may be explained by desensitization of 5-HT2A receptors. Several mechanisms are known to operate in desensitization of 5-HT2A receptors, including phosphorylation, arrestin binding and receptor internalization (Rahman and Neuman, 1993; Gray et al., 2003; Van Oekelen et al., 2003). No such desensitization was evident for the tryptamine-induced vasoconstriction, which suggests that tryptamine is not operating in the same way as 5-HT. The blockade of the vasoconstrictor responses of the mesentery to 5-HT by ritanserin confirms that it is via 5-HT2A receptors.
Tryptamine-induced vasoconstrictor responses
β-PEA and tyramine have been described as sympathomimetic amines that release noradrenaline from sympathetic neurons to exert effects such as vasoconstriction and increases in blood pressure (Trendelenburg, 1972). It has also been implied that tryptamine is an indirect sympathomimetic amine as it is also a trace amine (Zucchi et al., 2006). However, the contraction of the rabbit aorta by tryptamine was shown to be due to a direct stimulation of both α-adrenoceptors and 5-HT receptors and not an indirect effect (Stollak and Furchgott, 1983). The rat mesentery is heavily innervated with adrenergic neurones (Nyborg et al., 1986) and therefore an indirect sympathomimetic action should be possible. Moreover, α- and β-adrenoceptors are known to be present in the rat mesenteric arteries (Masuzawa et al., 1989; Bockman et al., 1996) and vasoconstriction occurred in this study with the α-adrenoceptor agonist, phenylephrine. The lack of vasoconstrictor responses of the mesentery to β-PEA and tyramine in the current investigation was therefore surprising. In contrast to our experiments, vasoconstriction to tyramine has been reported in the rat perfused mesentery (Elliott et al., 1989). The discrepancy between the two reports may be accounted for by differences in delivery of the drug and the strain of rats (Sprague–Dawley vs. Wistar). Tyramine and β-PEA have been shown to exert vasoconstrictor responses in other blood vessels including the rat aorta (Fehler et al., 2010) and porcine coronary artery (Herbert et al., 2008). Therefore, the mesentery may show a different response profile to other vessels.
Tryptamine, however, displayed substantial vasoconstrictor responses. There was no seasonal variation in this response, and indeed such differences would not be expected in animals housed under conditions of constant temperature, light/dark cycle and diet. As a result, responses could be compared throughout the year. Tryptamine produced vasoconstrictor responses in the µM range. This assumes that the threshold dose of 1 nmole reaches the tissue in a volume of 1 mL, yielding a concentration of 1 µM. Concentrations of tryptamine in the low nano-molar range have been found in blood and urine of human and other species; with brain concentrations being higher compared with the corresponding blood samples (Franzen and Gross, 1965; Sloan et al., 1975). These levels are a consequence of high turnover rates through metabolism via semi-carbazide sensitive amine oxidase (Kaitaniemi et al., 2009) and the intracellular MAO A and B (Sullivan et al., 1986). Tryptamine is obtained in the diet (Broadley, 2010) and levels can rise after tryptamine-rich foods, after inhibition of MAO or in disease states associated with low MAO activity such as schizophrenia (Sullivan et al., 1980). It is therefore possible that under these conditions tryptamine could induce vascular responses in vivo.
The vasoconstrictor responses to tryptamine observed in the present study concurs with a previous investigation in rat isolated mesenteric arteries (Watts et al., 1994), as opposed to the perfused vascular bed used here. Rat caudal arteries (Hicks and Langer, 1983; Bradley et al., 1985), rabbit aorta (Stollak and Furchgott, 1983) and rat aorta (Fehler et al., 2010) have also been shown to elicit vasoconstrictor responses to tryptamine. In dogs, tryptamine causes increases in blood pressure and hindlimb perfusion pressure which are enhanced by inhibition of MAO (Eble, 1965). Hicks and Langer (1983) implicated specific tryptaminergic receptors in the responses of rat-isolated perfused tail arteries, although this was contested by Bradley et al. (1985) who concluded that the response was mediated by the same receptor that mediated vasoconstriction to 5-HT. Stollak and Furchgott (1983) also concluded that vasoconstriction of rabbit aorta was mediated via both α-adrenoceptors and 5-HT receptors. In contrast, a vasoconstriction of rat aorta that is resistant to blockade by 5-HT and α-adrenoceptor antagonists has been attributed to stimulation of TAARs (Broadley et al., 2009; Fehler et al., 2010).
Trace amines such as tryptamine, are conventionally considered to be indirectly acting sympathomimetics, exerting their pharmacological responses via sympathetic nerves to release noradrenaline, which interacts with α1-adrenoceptors to induce vasoconstriction. Noradrenaline also induces vasodilator effects through post-synaptic α2- (Bockman et al., 1996), β-adrenoceptors (Masuzawa et al., 1989) and dopamine D1 receptors (Van der Graaf et al., 1995). 5-HT can also act indirectly via presynaptic 5-HT receptors to facilitate the neuronal release of noradrenaline from goat pial arteries (Marin and Sanchez, 1980) and rat mesenteric arteries (Su and Uruno, 1984). Hence, it is feasible that tryptamine, like other trace amines and 5-HT, may also interact with sympathetic neurons to secrete noradrenaline, which may initiate vascular responses. This, however, appears to be unlikely because the vasoconstrictor response to tryptamine was unaltered upon treatment of the mesentery with the α1-adrenoceptor antagonist, prazosin. Nevertheless, phentolamine, a non-selective α1- and α2-adrenoceptor antagonists decreased the maximum response to tryptamine. This was probably because phentolamine is known to bind to 5-HT receptors (Parsons et al., 1989; Hoffman and Lefkowitz, 1990), suggesting that 5-HT receptors mediate the vasoconstriction by tryptamine. Indeed, the 5-HT2A receptor antagonists, ritanserin and ketanserin (Conolan et al., 1986; Van Nueten et al., 1986), abolished the tryptamine-induced vasoconstrictor responses of the mesenteric arterial bed. That tryptamine should have affinity for 5-HT2A receptors, is not surprising because it is a structural congener of 5-HT. It has an affinity (−log molar EC50) of 6.59 ± 0.05 at human 5-HT2A receptors expressed in CHO-K1 cells compared with 7.51 ± 0.06 for 5-HT (Porter et al., 1999).
Vasodilator responses
When the perfusion pressure was raised by perfusion with the α-adrenoceptor agonist, phenylephrine and 5-HT2A receptors were inhibited with ritanserin, a small vasoconstrictor response remained, but at higher concentrations a vasodilator response to 5-HT was revealed. A relaxant action by 5-HT has been reported previously in rat-isolated perfused mesentery after ketanserin treatment (McLennan and Taylor, 1984). Clearly, this response is not mediated via 5-HT2A receptors as it occurred in the presence of ritanserin. 5-HT7 receptors have been shown to mediate vasodilatation in vivo (Martin and Humphrey, 1994) and in different vascular regions of various species, including dog (Cushing et al., 1996; Terrón and Falcón-Neri, 1999), rabbit (Morecroft and MacLean, 1998), pig (Ishine et al., 2000), monkey (Leung et al., 1996) and neonatal porcine vena cava (Trevethick et al., 1986). In common with 5-HT4 and 5-HT6 receptors, the 5-HT7 receptor subtype is positively coupled to adenylate cyclase through the G protein GS, leading to increased levels of cAMP (Hoyer et al., 2002). However, in the present study, the selective 5-HT7 receptor antagonist, SB26997, failed to modify the vasodilator response to 5-HT, suggesting that, in this vasculature and species, 5-HT7 receptors were not involved. The concentration of SB26997 (10 nM) should have been sufficient to block 5-HT7 receptor-mediated responses (Hagan et al., 2000). When tryptamine was examined in the presence of ritanserin in mesenteric vascular beds with perfusion pressure raised by phenylephrine infusion, there was also a marked vasodilator response. In common with 5-HT, this vasodilator response to tryptamine was also not inhibited by the selective 5-HT7 antagonist, SB26997 and therefore did not appear to be mediated via 5-HT7 receptors. A past study of the neonatal porcine isolated vena cava pre-contracted with the thromboxane mimetic, U46619, has also revealed relaxation responses induced by 5-HT, tryptamine, α-methyl-5-HT and 2-methyl-5-HT. This effect was also shown to be independent of 5-HT1A, 5-HT1B, 5-HT1C, 5-HT1D, 5-HT1-like (now regarded as the 5-HT7 receptor, Hoyer et al., 2002), 5-HT2, 5-HT3 and 5-HT4 receptors (Sumner, 1991). This response was functionally linked to NO formation by the endothelium.
We therefore examined the role of NO in the vasodilator response to tryptamine in the presence of ritanserin by use of the NOS inhibitor, L-NAME. The presence of an intact endothelium in our preparations and the effectiveness of the concentration of L-NAME, were demonstrated by abolition of the vasodilator responses to acetylcholine. We also compared tryptamine with tyramine and β-PEA, which had failed to exert vascular responses in unconstricted preparations. In agreement with our earlier studies (Broadley et al., 2009), when the perfusion pressure was raised, these trace amines exerted vasodilator responses similar to tryptamine. The vasodilator responses to tryptamine, tyramine and β-PEA were all abolished by L-NAME indicating that they were mediated through the release of NO, probably from the endothelium. Thus, the vasodilator responses to these trace amines were similar to those of 5-HT in the presence of ritanserin reported by Sumner (1991). Because the vasodilatation by tryptamine was not blocked by the 5-HT2A and 5-HT7 receptor antagonists, the question arises as to what receptor mediates this response. The most likely candidate is a TAAR (Sotnikova et al., 2009) because tryptamine, as well as tyramine, β-PEA and octopamine, have affinity for rat TA1 receptors transfected into HEK293 cells (Bunzow et al., 2001; Lindemann et al., 2005). The molar EC50 values were approximated from the ED50 values for comparison with published values. It is recognized that these are only approximations based on the volume of distribution of bolus doses. There will also be the uncertainty about metabolism by MAO, which may vary between the different amines and which was not prevented by use of a MAO inhibitor. Nevertheless, the approximated molar EC50 values yielded a potency order of tyramine > β-PEA > tryptamine > octopamine (Table 1). This compares with the published potency orders for the generation of cAMP in cell lines expressing rat TA1 receptors (Bunzow et al., 2001). However, the potencies are several orders of magnitude lower than for the transfected cells (Table 1). This difference cannot be accounted for by metabolism through MAO, because inclusion of a MAO inhibitor, tranylcypromine, in preliminary experiments had only minimal potentiating effects on the vasoconstrictor effects of lower doses of tryptamine. The potencies are, however, of the same magnitude as those obtained for other functional responses in rat isolated tissues including the contractions of rat aorta (Fehler et al., 2010) and negative inotropy of rat isolated hearts (Frascarelli et al., 2008). There were, however, differences in the potency orders, with tyramine showing partial activity in rat aorta and the weakest activity in the heart. The reasons for the discrepancies between functional responses of native receptors and those of transfected cells is probably because the cloned receptors are overexpressed and forced to couple with adenylyl cyclase so that their stimulation increases cAMP levels. This is unlikely to be the coupling pathway for the vasodilator response of the mesenteric bed, which we have shown is via NO release. Overexpression of the receptors will considerably magnify the transduction pathways for the responses and account for the greater apparent affinities in the transfected cells. The identical potency order to the cells transfected with TAAR-1, however, supports a role for these receptors in the vasodilator response of the mesenteric bed. Finally, the affinities of tryptamine (1.22 ± 0.7 µM) and 5-HT (5.24 ± 2.61 µM) are similar for rat transfected TAAR-1 (Lindemann et al., 2005). This suggests that in rats at least, 5-HT might also cause vasodilatation of the mesenteric bed through TAARs, which might explain its resistance to 5-HT receptor antagonists (Sumner, 1991).
The concentrations of tryptamine needed to induce vasodilator responses were in the high micromolar range. Whether endogenous tryptamine reaches these concentration is not known. However, as discussed earlier for the vasoconstriction, it is possible that local concentrations in the mesenteric circulation can achieve these levels after meals rich in tryptamine before enzymatic breakdown by MAO can occur.
In conclusion, tryptamine induced vasoconstriction of the rat mesenteric bed whereas tyramine and β-PEA were without effect unless perfusion pressure was raised, when they and octopamine caused vasodilator responses. In the presence of ritanserin to block 5-HT2A receptors, tryptamine caused a similar vasodilator response to tyramine and β-PEA, which was mediated via release of NO. It is suggested that the vasodilatation by these trace amines and by 5-HT was due to activation of TAARs.
Acknowledgments
This work was supported by a Research Fellowship to MAA from the Wellcome Trust (069301/Z/02/Z) and by the British Heart Foundation (PG/09/021).
Glossary
- ISA
indirectly acting sympathomimetic amine
- L-NAME
Nω-nitro-L-arginine methyl ester
- β-PEA
β-phenylethylamine
- TAARs
trace amine-associated receptors
Conflict of interest
The authors declare that no conflict of interest exists.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar MA, Ford WR, Broadley KJ. Constrictor and dilator responses to tryptamine in the isolated perfused mesenteric arterial circulation of the rat. Proc Br Pharmacol Soc. 2009;7:001P. pA2 online. [Google Scholar]
- Barger G, Dale HH. Chemical structure and sympathomimetic actions of amines. J Physiol. 1910;41:19–59. doi: 10.1113/jphysiol.1910.sp001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman CS, Gonzalez-Cabrera I, Abel PW. Alpha-2 adrenoceptor subtype causing nitric oxide-mediated vascular relaxation in rats. J Pharmacol Exp Ther. 1996;278:1235–1243. [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PB, Humphrey PPA, Williams RH. Tryptamine-induced vasoconstrictor responses in rat caudal arteries are mediated predominantly via 5-hydroxytryptamine receptors. Br J Pharmacol. 1985;84:919–925. doi: 10.1111/j.1476-5381.1985.tb17386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Ther. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Broadley KJ, Anwar MA, Herbert AA, Fehler M, Jones EM, Davies WE, et al. Effects of dietary amines on the gut and its vasculature. Br J Nutr. 2009;101:1645–1652. doi: 10.1017/S0007114508123431. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Burn JH, Rand MJ. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958;144:314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolan S, Quinn MJ, Taylor DA. In vivo and in vitro activity of selective 5-hydroxytryptamine2 receptor antagonists. Br J Pharmacol. 1986;89:129–135. doi: 10.1111/j.1476-5381.1986.tb11128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing DJ, Zgombick JM, Nelson DL, Cohen ML. LY215849, a high affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery. J Pharmacol Exp Ther. 1996;277:1560–1566. [PubMed] [Google Scholar]
- Dale HH, Dixon WE. The action of pressor amines produced by putrefaction. J Physiol. 1909;39:25–44. doi: 10.1113/jphysiol.1909.sp001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble JN. A study of the potentiation of tryptamine by monamine oxidase inhibitors in the dog. J Pharmacol Exp Ther. 1965;148:48–53. [PubMed] [Google Scholar]
- Elliott J, Callingham BA, Sharman DF. The influence of amine metabolizing enzymes on the pharmacology of tyramine in the isolated perfused mesenteric arterial bed of the rat. Br J Pharmacol. 1989;98:515–522. doi: 10.1111/j.1476-5381.1989.tb12625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehler M, Broadley KJ, Ford WR, Kidd EJ. Identification of trace amine-associated receptors (TAAR) in the rat aorta and their role in vasoconstriction by β-phenylethylamine. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:385–398. doi: 10.1007/s00210-010-0554-1. [DOI] [PubMed] [Google Scholar]
- Franzen F, Gross H. Tryptamine, N,N-dimethyltryptamine, N,N-dimethyl-5-hydroxytryptamine and 5-methoxytryptamine in human blood and urine. Nature. 1965;206:1052. doi: 10.1038/2061052a0. [DOI] [PubMed] [Google Scholar]
- Frascarelli S, Ghelardoni S, Chiellini G, Vargiu R, Ronca-Testoni S, Scanlan TS, et al. Cardiac effects of trace amines: pharmacological characterization of trace amine-associated receptors. Eur J Pharmacol. 2008;587:231–236. doi: 10.1016/j.ejphar.2008.03.055. [DOI] [PubMed] [Google Scholar]
- Grandy DK. Trace amine-associated receptor 1 – family archetype or iconoclast? Pharmacol Ther. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Compton-Toth BA, Roth BL. Identification of two serine residues essential for agonist-induced 5-HT2A receptor desensitization. Biochemistry. 2003;42:10853–10862. doi: 10.1021/bi035061z. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, et al. Characterization of SB-269970-A, a selective 5-HT7 anatagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorn MH, Broadley KJ, Gibbons CJ. Examination of the bronchoconstrictor response of guinea-pig isolated lung to beta-phenylethylamine. Gen Pharmacol. 1985;16:371–378. doi: 10.1016/0306-3623(85)90198-3. [DOI] [PubMed] [Google Scholar]
- Herbert AA, Kidd EJ, Broadley KJ. Dietary trace amine-dependent vasoconstriction in porcine coronary artery. Br J Pharmacol. 2008;155:525–534. doi: 10.1038/bjp.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks PE, Langer SZ. Antagonism by tetra-hydro-β-carboline of the vasoconstrictor responses to tryptamine in rat tail arteries. Eur J Pharmacol. 1983;96:145–149. doi: 10.1016/0014-2999(83)90543-5. [DOI] [PubMed] [Google Scholar]
- Hoffman BB, Lefkowitz RJ. Adrenergic receptor antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 8th edn. New York: Pergamon Press; 1990. pp. 221–243. [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ishine T, Bouchelet I, Hamel E, Lee TJ. Serotonin 5-HT7 receptors mediate relaxation of porcine pial veins. Am J Physiol. 2000;278:H907–H912. doi: 10.1152/ajpheart.2000.278.3.H907. [DOI] [PubMed] [Google Scholar]
- Kaitaniemi S, Elovaara H, Grön K, Kidron H, Liukkonen J, Salminen T, et al. The unique substrate specificity of human AOC2, a semicarbazide-sensitive amine oxidase. Cell Mol Life Sci. 2009;66:2743–2757. doi: 10.1007/s00018-009-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Walsh LKM, Pulido-Rios MT, Eglen RM. Characterization of putative 5-HT7 receptors mediating direct relaxation in Cynomolgus monkey isolated jugular vein. Br J Pharmacol. 1996;117:926–930. doi: 10.1111/j.1476-5381.1996.tb15282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, et al. A novel, potent, and selective 5-HT7 antagonist: (R)-3-(2-(2-(4-methyl-piperidin-1-yl)-ethyl) pyrrolidine-1-sulfonyl) phenol (SB-269970) J Med Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- Marin C, Sanchez CF. Influence of calcium on noradrenaline release evoked by 5-hydroxytryptamine and potassium from goat pial arteries. J Pharm Pharmacol. 1980;32:643–646. doi: 10.1111/j.2042-7158.1980.tb13021.x. [DOI] [PubMed] [Google Scholar]
- Martin GR. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Martin GR, Humphrey PPA. Receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neurophysiology. 1994;33:261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Masuzawa K, Matsuda T, Asano M. Decreased arterial responsiveness to multiple cyclic AMP-generating receptor agonists in spontaneously hypertensive rats. Br J Pharmacol. 1989;96:227–235. doi: 10.1111/j.1476-5381.1989.tb11804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgregor DD. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessels of the rat. J Physiol. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan PL, Taylor DA. Antagonism by ketanserin of 5-HT-induced vasoconstriction unmasks a 5-HT-induced vasodilation. Eur J Pharmacol. 1984;104:313–318. doi: 10.1016/0014-2999(84)90407-2. [DOI] [PubMed] [Google Scholar]
- Morecroft I, MacLean MR. 5-hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br J Pharmacol. 1998;125:69–78. doi: 10.1038/sj.bjp.0702055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg NC, Korsgaard N, Mulvany MJ. Neonatal sympathectomy of normotensive Wistar-Kyoto and spontaneously hypertensive rats with 6-hydroxydopamine: effects on resistance vessel structure and sensitivity to calcium. J Hypertens. 1986;4:455–461. doi: 10.1097/00004872-198608000-00010. [DOI] [PubMed] [Google Scholar]
- Parsons AA, Whalley ET, Feniuk W, Connor HE, Humphrey PPA. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br J Pharmacol. 1989;96:434–440. doi: 10.1111/j.1476-5381.1989.tb11835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Neuman RS. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur J Pharmacol. 1993;238:173–180. doi: 10.1016/0014-2999(93)90845-9. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Martin WR, Clements TH, Buchwald WF, Bridges SR. Factors influencing brain and tissue levels of tryptamine: species, drugs and lesions. J Neurochem. 1975;24:523–532. doi: 10.1111/j.1471-4159.1975.tb07670.x. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol. 2009;76:229–235. doi: 10.1124/mol.109.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollak JS, Furchgott RF. Use of selective anatagonists for determining the types of receptors mediating the actions of 5-hydroxytryptamine and tryptamine in the isolated rabbit aorta. J Pharmacol Exp Ther. 1983;224:215–221. [PubMed] [Google Scholar]
- Su C, Uruno T. Excitatory and inhibitory effects of 5-hydroxytryptamine in mesenteric arteries of spontaneously hypertensive rats. Eur J Pharmacol. 1984;106:283–290. doi: 10.1016/0014-2999(84)90715-5. [DOI] [PubMed] [Google Scholar]
- Sullivan JL, Coffey CE, Basuk B, Cavenar JO, Malthie AA, Zung WW. Urinary tryptamine excretion in chronic schizophrenics with low platelet MAO activity. Biol Psychiatry. 1980;15:113–120. [PubMed] [Google Scholar]
- Sullivan JP, McDonnell L, Hardiman OM, Farrell MA, Phillips JP, Tipton KF. The oxidation of tryptamine by the two forms of monoamine oxidase in human tissues. Biochem Pharmacol. 1986;35:3255–3260. doi: 10.1016/0006-2952(86)90421-1. [DOI] [PubMed] [Google Scholar]
- Sumner MJ. Characterization of the 5-HT receptor mediating endothelium-dependent relaxation in porcine vena cava. Br J Pharmacol. 1991;102:938–942. doi: 10.1111/j.1476-5381.1991.tb12280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrón JA, Falcón-Neri A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br J Pharmacol. 1999;127:609–616. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg U. Classification of sympathomimetic amines. In: Blaschko H, Muscholl E, editors. Catecholamines, Handbook of Experimental Pharmacology. Vol. 33. Berlin: Springer-Verlag; 1972. pp. 336–362. [Google Scholar]
- Trevethick MA, Fenuik W, Humphrey PPA. 5-Carboxamido-tryptamine: a potent agonist mediating relaxation and elevation of cyclic AMP in the isolated neonatal porcine vena cava. Life Sci. 1986;38:1521–1528. doi: 10.1016/0024-3205(86)90566-7. [DOI] [PubMed] [Google Scholar]
- Van der Graaf PH, Saxena PR, Shankley NP, Black JW. Exposure and characterization of the action of noradrenaline at dopamine receptors mediating endothelium-independent relaxation of rat isolated small mesenteric arteries. Br J Pharmacol. 1995;116:3237–3242. doi: 10.1111/j.1476-5381.1995.tb15130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nueten JM, Schuurkes JAJ, De Ridder WJE, Kuyps JJMD, Janssens WJ. Comparative pharmacological profile of ritanserin and ketanserin. Drug Dev Res. 1986;8:187–195. [Google Scholar]
- Van Oekelen D, Luyten WHML, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Watts SW. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in deoxycorticosterone acetate-salt hypertension. Hypertension. 2002;39:825–829. doi: 10.1161/hy0302.104668. [DOI] [PubMed] [Google Scholar]
- Watts SW, Gilbert L, Webb RC. 5-hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralcorticoid hypertensive rats. Hypertension. 1994;26:1056–1059. doi: 10.1161/01.hyp.26.6.1056. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]


